Abstract
Objective
To examine associations between birth defects and cancer from birth into adulthood.
Design
Population based nested case-control study.
Setting
Nationwide health registries in Denmark, Finland, Norway, and Sweden.
Participants
62 295 cancer cases (0-46 years) and 724 542 frequency matched controls (matched on country and birth year), born between 1967 and 2014.
Main outcome measures
Relative risk of cancer in relation to major birth defects, estimated as odds ratios with 99% confidence intervals from logistic regression models.
Results
Altogether, 3.5% (2160/62 295) of cases and 2.2% (15 826/724 542) of controls were born with major birth defects. The odds ratio of cancer for people with major birth defects compared with those without was 1.74 (99% confidence interval 1.63 to 1.84). For individuals with non-chromosomal birth defects, the odds ratio of cancer was 1.54 (1.44 to 1.64); for those with chromosomal anomalies, the odds ratio was 5.53 (4.67 to 6.54). Many structural birth defects were associated with later cancer in the same organ system or anatomical location, such as defects of the eye, nervous system, and urinary organs. The odds ratio of cancer increased with number of defects and decreased with age, for both non-chromosomal and chromosomal anomalies. The odds ratio of cancer in people with any non-chromosomal birth defect was lower in adults (≥20 years: 1.21, 1.09 to 1.33) than in adolescents (15-19 years: 1.58, 1.31 to 1.90) and children (0-14 years: 2.03, 1.85 to 2.23). The relative overall cancer risk among adults with chromosomal anomalies was markedly reduced from 11.3 (9.35 to 13.8) in children to 1.50 (1.01 to 2.24). Among adults, skeletal dysplasia (odds ratio 3.54, 1.54 to 8.15), nervous system defects (1.76, 1.16 to 2.65), chromosomal anomalies (1.50, 1.01 to 2.24), genital organs defects (1.43, 1.14 to 1.78), and congenital heart defects (1.28, 1.02 to 1.59) were associated with overall cancer risk.
Conclusions
The increased risk of cancer in individuals with birth defects persisted into adulthood, both for non-chromosomal and chromosomal anomalies. Further studies on the molecular mechanisms involved are warranted.
Introduction
Globally, in 2017, birth defects and childhood cancer were the third and ninth top causes of childhood disease burden, respectively (excluding injuries and perinatal diseases).1 Approximately 3% of liveborn children in the Nordic countries are born with major birth defects.2 Birth defects, particularly chromosomal anomalies but also non-chromosomal defects, are one of the strongest and most consistent risk factors for childhood cancers.3 4 5 6 This suggests that birth defects and childhood cancer may have a common aetiology—genetic, environmental, or a combination. Few established risk factors exist for both birth defects and childhood cancer,6 7 and identifying specific birth defects and childhood cancer associations can facilitate further research on common factors that affect disease development.
The reported excess risk of cancer in children with birth defects varies by type of anomaly. Children with Down’s syndrome are, for instance, at increased risk of developing leukaemia, whereas the elevated risk of cancer in children with non-chromosomal defects seems to be driven mostly by embryonal tumours.3 4 Several specific associations have been observed in previous studies, and the gradient in risk seems to increase with number of birth defects.3 5 8 Risk of cancer is highest in young children, but few studies have investigated risk beyond childhood and adolescence.8 9 10 11 12 13 14 Thus, the contribution of birth defects to risk of cancer in adulthood is to a large degree unknown.15
The rarity of both birth defects and childhood cancers makes studying these associations challenging, and very large studies are needed to identify enough individuals with birth defects to allow stable estimates of cancer risk. In this large population based nested case-control study of children, adolescents, and adults (age 0-46 years), we linked national health registries in four Nordic countries to examine the association between major birth defects and cancer, both overall and for specific types, and stratified by age at diagnosis of cancer. We aimed to identify associations between birth defects and cancer, assess whether risk of cancer changed with the number of birth defects, and determine whether these associations persisted into adulthood.
Methods
Data sources
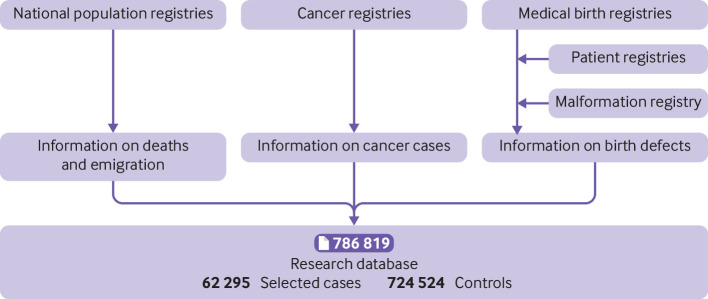
All Nordic countries have national population based health registries that are based on compulsory notification from healthcare providers, and access to healthcare is universal and independent of income. Information on birth defects came from the medical birth registries, containing information on all births in Denmark, Finland, Norway, and Sweden since 1973, 1987, 1967, and 1973, respectively.16 The Danish National Patient Registry (since 1977), the Register of Congenital Malformations at the Finnish Institute for Health and Welfare (since 1963), and the Swedish National Patient Register at the Swedish National Board of Health and Welfare (since 1964) provided additional information on birth defects.17 18 19 As we were interested in major birth defects, we used only inpatient diagnoses during the first year of life from the patient registries. We obtained information on cancer from the cancer registries in Denmark, Finland, Norway, and Sweden, covering the entire populations since 1943, 1953, 1953, and 1958, respectively.20 Information on deaths and emigration came from the national population registries. Figure 1 shows the data sources for the research database.
Fig 1.
Data sources in four Nordic countries. Controls were frequency matched on birth year in each country (1:10 case-control ratio with 100% successful matching). Some benign cases (for example, cervical precursor lesions) were later excluded from research database, resulting in final case-control ratio of 1:12
Study population
Every resident in the Nordic countries is assigned a country specific unique identification number used in all administrative and medical registries, which makes accurate record linkage possible. Cases were defined as liveborn individuals in the birth registries, with a subsequent cancer diagnosis recorded in the cancer registries. We selected controls from among people who were alive, living in the country, and with no cancer diagnosis by the end of follow-up (2013 in Denmark, Finland, and Norway; 2014 in Sweden). We frequency matched them on country and year of birth (case-control ratio 1:10). After exclusion of ineligible cases (but keeping the controls), the study population included 62 295 cases and 724 542 controls.
Classification of cancer
In Norway and Finland, and for leukaemia and lymphoma in Denmark, cases of cancer were classified according to the ICD-O-3 (international classification of diseases for oncology, third edition).21 In Denmark, except for leukaemia and lymphoma, we used the ICD-10 (international classification of diseases, 10th revision) codes and ICD-O-3 morphology codes.22 In Sweden, we used ICD-7 codes, combined with morphology diagnosis coded by ICD-O-2/3 or the WHO/HS/CANC/24.1 classification.23 We excluded non-malignant neoplasms, except for tumours in the urinary tract or central nervous system and other intracranial tumours (other endocrine glands), and cases without verified morphology, except for central nervous system and other intracranial tumours. We also excluded basal cell carcinomas. We classified cases in ICD-10 groups,24 except for leukaemia and lymphoma, which we classified in ICD-O-3 morphology groups 25 (supplementary table A).
Classification of major birth defects
The exposure of interest was major birth defects, classified in subgroups, registered in the birth registries, congenital malformation registry, or patient registries. We classified birth defects, and excluded minor birth defects, by using the definitions applied by the European network of population-based registries for the epidemiological surveillance of congenital anomalies (EUROCAT)26 (using ICD-10 codes, but not including the British Paediatric Association extensions to ICD-10 as these codes were not available in all countries). In Denmark, the birth defects were coded according to ICD-8 throughout 1993 and ICD-10 thereafter.17 The Finnish Register of Congenital Malformations coded birth defects according to ICD-9 Atlanta modification from 1986 onwards with the retrospective inclusion of ICD-10 codes from 1996. In Norway, the birth defects were coded according to ICD-8 during 1967-98, with the addition of some internally generated codes, and ICD-10 from 1999. In Sweden, the birth defects were coded according to the Swedish versions of ICD-8 during 1973-86, ICD-9 during 1987-96, and ICD-10 from 1997 onwards. We defined single birth defects, multiple defects within the same anatomical subgroup, and multiple defects when these were part of a sequence as isolated birth defects. We defined multiple birth defects from different anatomical subgroups, and not part of a sequence, as multiple birth defects according to the algorithm described by Garne et al.27
Statistical analysis
We used unconditional logistic regression models to obtain odds ratios of overall and specific types of cancer with 99% confidence intervals comparing individuals with major birth defects with those without major birth defects.28 Because cancer is relatively rare among both exposed (individuals with major birth defects) and unexposed people, we interpreted the odds ratios as approximations of relative risks.29 30 We adjusted odds ratios for the matching factors (country and birth year) and sex. Other possible confounders evaluated were in vitro fertilisation, maternal age, and smoking. We did not adjust for intermediate factors (birth weight and preterm birth) in order to estimate the total effect of birth defects on risk of cancer. Confounder selection is illustrated in a directed acyclic graph (supplementary figure A). We stratified by age at cancer diagnosis to evaluate risk of cancer at different ages. We assessed the association between number of major birth defects (1, 2, 3, or ≥4) as a categorical exposure and cancer and tested for linear trend by using orthogonal polynomial contrasts.31 We analysed chromosomal anomalies and non-chromosomal birth defects separately. For selected analyses with enough cases, we stratified by country to evaluate whether the findings were consistent. When evaluating smoking as possible confounder, in the time period when this information was available, we used a complete case approach for handling missing data.32 We chose 99% confidence intervals to reduce the probability of false positive results. We used Stata version 16 for all analyses.
Patient and public involvement
No patients or members of the public were involved in the study design, interpretation of results, or development of dissemination strategy. This study was entirely based on data already recorded in mandatory population based registers and databases.
Results
Table 1 shows characteristics of the population. Age at diagnosis of cancer ranged from 0 to 46 years, with a median of 23 (interquartile range 10-31) years. Thirty two per cent (19 881/62 295) of the cases were below 15 years of age, and 58% (36 068/62 295) were above 20. As the registries were established in different years, the age distribution differed between countries, with the oldest population in Norway. The median maternal age at delivery was 27 (23-31) years. Altogether, 2160 (3.5%) of cases and 15 826 (2.2%) of controls were registered with a major birth defect. The most common were congenital heart defects, limb defects, and genital anomalies (table 2). The three largest malignancy groups were lymphoid and haematopoietic malignancies, genitourinary cancers, and central nervous system tumours (fig 2).
Table 1.
Characteristics of study population in Denmark (1977-2013), Finland (1987-2013), Norway (1967-2013), and Sweden (1973-2014). Values are numbers (percentages)
| Characteristics | Cases (n=62 295) | Controls (n=724 542) |
|---|---|---|
| Major birth defects | 2160 (3.5) | 15 826 (2.2) |
| Sex*: | ||
| Male | 30 352 (48.7) | 371 313 (51.2) |
| Female | 31 943 (51.3) | 353 229 (48.8) |
| Birth weight, g: | ||
| <2500 | 2565 (4.1) | 29 464 (4.1) |
| 2500-3999 | 48 211 (77.4) | 570 204 (78.7) |
| ≥4000 | 11 353 (18.2) | 123 009 (17.0) |
| Missing | 166 (0.3) | 1865 (0.3) |
| Gestational age, weeks: | ||
| <37 | 3329 (5.3) | 37 173 (5.1) |
| 37-40 | 38 833 (62.3) | 460 388 (63.5) |
| ≥41 | 18 220 (29.2) | 207 066 (28.6) |
| Missing | 1913 (3.1) | 19 915 (2.7) |
| Maternal smoking†: | ||
| No | 14 745 (23.7) | 197 724 (27.3) |
| Yes | 3869 (6.2) | 57 622 (8.0) |
| Missing | 43 681 (70.1) | 469 196 (64.8) |
| Missing‡ | 1702/20 316 (8.4) | 24 291/279 291 (8.7) |
| Maternal age, years: | ||
| <25 | 20 460 (32.8) | 236 312 (32.6) |
| 25-29 | 22 137 (35.5) | 260 778 (36.0) |
| 30-34 | 13 603 (21.8) | 159 422 (22.0) |
| ≥35 | 6095 (9.8) | 68 030 (9.4) |
| In vitro fertilisation§: | ||
| No | 12 356 (19.8) | 126 859 (17.5) |
| Yes | 159 (0.3) | 1265 (0.2) |
| Missing | 49 780 (79.9) | 596 418 (82.3) |
| Year of birth: | ||
| <1970 | 5596 (9.0) | 48 412 (6.7) |
| 1970-79 | 23 858 (38.3) | 253 884 (35.0) |
| 1980-89 | 17 413 (28.0) | 250 660 (34.6) |
| 1990-99 | 10 071 (16.2) | 115 998 (16.0) |
| 2000-09 | 4612 (7.4) | 47 621 (6.6) |
| ≥2010 | 745 (1.2) | 7967 (1.1) |
| Age at primary cancer diagnosis, years¶: | ||
| 0-4 | 10 362 (16.6) | - |
| 5-9 | 5057 (8.1) | - |
| 10-14 | 4462 (7.2) | - |
| 15-19 | 6346 (10.2) | - |
| 20-29 | 16 977 (27.3) | - |
| 30-39 | 15 692 (25.2) | - |
| ≥40 | 3399 (5.5) | - |
| Year of primary cancer diagnosis¶: | ||
| <1980 | 1320 (2.1) | - |
| 1980-89 | 3970 (6.4) | - |
| 1990-99 | 10 424 (16.7) | - |
| 2000-09 | 24 924 (40.0) | - |
| 2010-14 | 21 657 (34.8) | - |
Differences between cases and controls were due to sex ratio at birth and different cancer risk for males and females in study population (aged 0-46 years).
Available from 1991 in Denmark, 1987 in Finland, 1998 in Norway, and 1982 in Sweden.
Percentage missing in time period when this information was recorded.
Reported in 1990-2013 in Finland, 1984-2013 in Norway, and 1995-2014 in Sweden; not included for Denmark. Missingness in registration period cannot be calculated.
Reported only for cases.
Table 2.
Risk of overall cancer in people with any, or specific, major birth defects
| Birth defect* | No (%) | Odds ratio (99% CI) | |
|---|---|---|---|
| Cases† (n=62 295) | Controls† (n=724 542) | ||
| All anomalies | 2160/62 295 (3.47) | 15 826/724 542 (2.18) | 1.74 (1.63 to 1.84) |
| All anomalies excluding chromosomal anomalies | 1818/61 953 (2.93) | 15 067/723 783 (2.08) | 1.54 (1.44 to 1.64) |
| Specific sites | |||
| Nervous system | 225/60 360 (0.37) | 593/709 309 (0.08) | 4.76 (3.89 to 5.83) |
| Neural tube defects | 90/60 225 (0.15) | 216/708 932 (0.03) | 5.00 (3.61 to 6.92) |
| Eye | 60/60 195 (0.10) | 373/709 089 (0.05) | 2.07 (1.44 to 2.96) |
| Ear, face, and neck | 8/60 143 (0.01) | 92/708 808 (0.01) | 1.13 (0.44 to 2.93) |
| Congenital heart defects | 381/60 516 (0.63) | 3512/712 228 (0.49) | 1.42 (1.24 to 1.64) |
| Respiratory system | 24/60 159 (0.04) | 239/708 955 (0.03) | 1.23 (0.71 to 2.15) |
| Oro-facial clefts | 116/60 251 (0.19) | 1242/709 958 (0.17) | 1.12 (0.87 to 1.44) |
| Cleft palate only | 32/60 167 (0.05) | 397/709 113 (0.06) | 0.97 (0.60 to 1.56) |
| Cleft lip with/without cleft palate | 84/60 219 (0.14) | 846/709 562 (0.12) | 1.18 (0.88 to 1.59) |
| Digestive system | 111/60 246 (0.18) | 764/709 480 (0.11) | 1.85 (1.43 to 2.41) |
| Abdominal wall defects | 16/60 151 (0.03) | 119/708 835 (0.02) | 1.51 (0.76 to 3.01) |
| Urinary system | 104/60 239 (0.17) | 782/709 498 (0.11) | 1.76 (1.34 to 2.30) |
| Genital organs | 242/60 377 (0.40) | 2538/711 254 (0.36) | 1.30 (1.09 to 1.55) |
| Limb | 292/60 427 (0.48) | 2803/711 519 (0.39) | 1.27 (1.09 to 1.49) |
| Skeletal dysplasia | 30/60 165 (0.05) | 114/708 830 (0.02) | 3.34 (1.97 to 5.67) |
| Genetic syndromes and microdeletions | 54/60 189 (0.09) | 125/708 841 (0.02) | 5.44 (3.57 to 8.28) |
| Chromosomal | 342/60 477 (0.57) | 759/709 475 (0.11) | 5.53 (4.67 to 6.54) |
| Down’s syndrome | 301/60 436 (0.50) | 604/709 320 (0.09) | 6.08 (5.06 to 7.30) |
| Other anomalies/syndromes | 424/60 559 (0.70) | 2790/711 506 (0.39) | 1.95 (1.70 to 2.23) |
Odds ratios adjusted for matching variables (birth year and country) and sex. In all analyses for specific sites, other than for chromosomal anomalies, individuals with chromosomal anomalies were excluded. In all analyses, unexposed group was composed of individuals without major birth defects. Percentages of cases and controls are reported per analysis; study population consists of exposed (cases and controls with specific birth defect being analysed) and unexposed people (cases and controls without major birth defects).
Categorised according to EUROCAT.
Individuals with more than one diagnosis can be included in more than one sub-category; thus, the totals do not sum up to 2160.
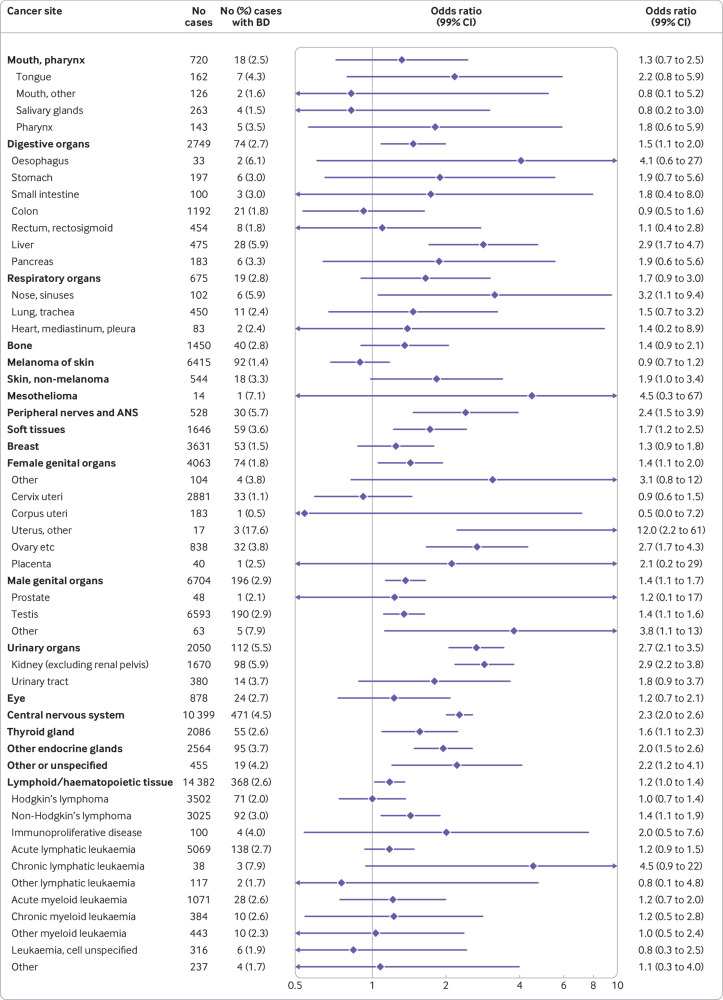
Fig 2.
Risk of specific cancers in people with any major non-chromosomal birth defects among 61 953 cases and 723 783 controls. Odds ratios (ORs) adjusted for matching variables (birth year and country) and sex. Cancer sites classified in ICD-10 groups; sites with no co-occurring birth defects and cancers are not included. ORs for cancer of urinary systems, central nervous system, and other endocrine glands are presented for benign and malignant cases combined. Separate effect estimates for malignant cases are 3.2 (2.4 to 4.1), 1.5 (1.2 to 1.9), and 2.8 (1.9 to 4.1) , respectively; estimates for benign cases are 0.7 (0.2 to 3.2), 3.3 (2.8 to 3.9), and 1.4 (0.9 to 2.1). ANS=autonomic nervous system; BD=birth defect
Risk of overall cancer in people with birth defects
We observed an increase in overall cancer risk in people with any major birth defect compared with those without major birth defects (odds ratio 1.74, 99% confidence interval 1.63 to 1.84) (table 2). The odds ratio was highest for people with chromosomal anomalies (5.53, 4.67 to 6.54), with the highest overall relative cancer risk for those with Down’s syndrome (6.08, 5.06 to 7.30). Risk of cancer was also elevated in people with non-chromosomal birth defects (odds ratio 1.54, 1.44 to 1.64), with the highest relative risks of any cancer in individuals with genetic syndromes/microdeletions (5.44, 3.57 to 8.28), nervous system defects (4.76, 3.89 to 5.83), and skeletal dysplasia (3.34, 1.97 to 5.67). Furthermore, we observed an increased risk of cancer for people with birth defects of the eye, digestive system, urinary organs, heart, genital organs, and limbs and other anomalies/syndromes.
Risk of specific cancer types in people with birth defects
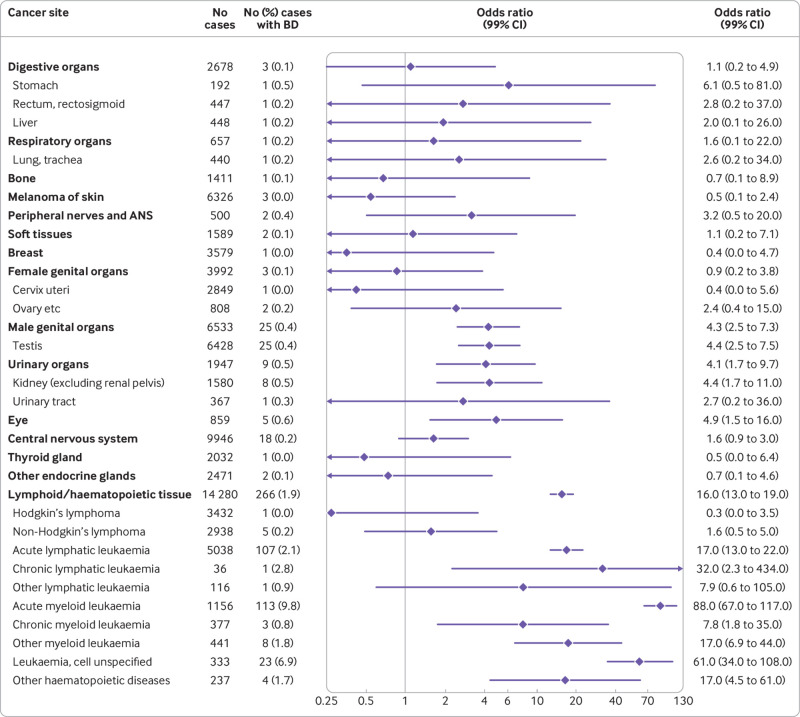
Among people with non-chromosomal birth defects, we observed the highest relative risks of cancers of urinary organs (mainly kidney cancer) (odds ratio 2.7, 2.1 to 3.5), peripheral nerves and autonomic nervous system (2.4, 1.5 to 3.9), and central nervous system (2.3, 2.0 to 2.6) compared with people without major birth defects (fig 2). In addition, we observed increased risks of cancers of digestive organs (mainly liver), soft tissue, genital organs, nose/sinuses, thyroid and other endocrine glands, and lymphoid and haematopoietic tissue (non-Hodgkin’s lymphoma in particular) and other or unspecified cancer. For people with chromosomal anomalies, we observed an increased risk of cancers of lymphoid and haematopoietic tissue, with the highest risk observed for acute myeloid leukaemia (odds ratio 88, 67 to 117) (fig 3). In addition, we saw increased risks for eye, testicular, and kidney cancer.
Fig 3.
Risk of specific cancers in people with chromosomal birth defects (n=1101; 905 Down’s syndrome) among 60 477 cases and 709 475 controls. Odds ratios (ORs) adjusted for matching variables (birth year and country) and sex. Cancer sites classified in ICD-10 groups; sites with no co-occurring chromosomal anomalies and cancers are not included. ANS=autonomic nervous system; BD=birth defect
Risk of overall cancer in people with birth defects stratified by age at diagnosis
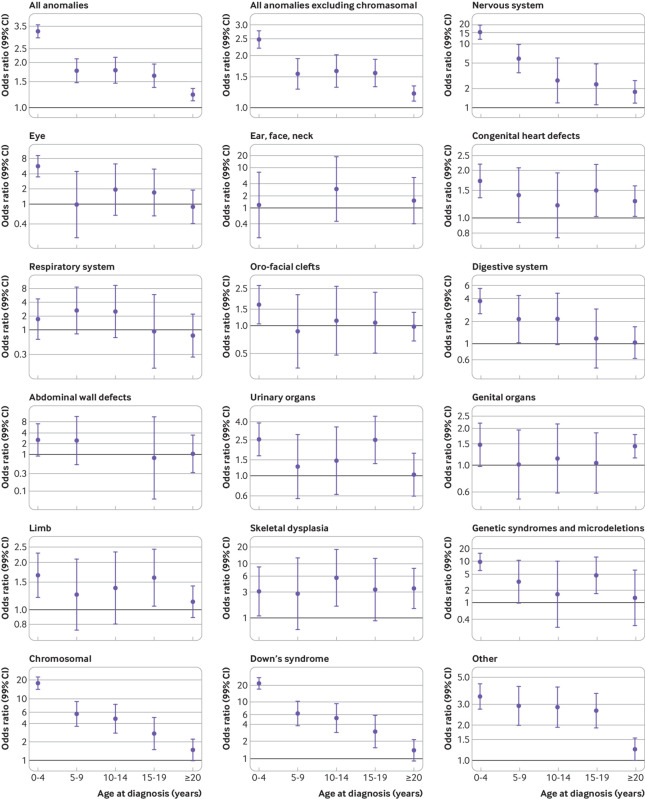
The overall risk of cancer associated with birth defects was elevated in all age groups (0-4, 5-9, 10-14, 15-19, ≥20 years) (fig 4). However, the odds ratios decreased with age at diagnosis for both non-chromosomal and chromosomal anomalies. The overall odds ratio of cancer in people with non-chromosomal birth defects was lower in adults (≥20 years: 1.21, 1.09 to 1.33) than in adolescents (15-19 years: 1.58, 1.31 to 1.90) and children (0-14 years: 2.03, 1.85 to 2.23) (supplementary table B). For skeletal dysplasia and congenital heart defects, the reduction in odds ratio in adults compared with children was less pronounced than for most other defects (skeletal dysplasia: adults 3.54 (1.54 to 8.15) versus children 3.59 (1.74 to 7.42); congenital heart defects: adults 1.28 (1.02 to 1.59) versus children 1.53 (1.26 to 1.86)). The relative overall cancer risk among adults with chromosomal anomalies was markedly reduced (odds ratio 1.50 (1.01 to 2.24) in adults versus 11.3 (9.35 to 13.8) in children). In contrast, genital birth defects were associated with a higher relative risk of cancer among adults (odds ratio 1.43, 1.14 to 1.78) than adolescents (1.04, 0.59 to 1.83) and children (1.25, 0.92 to 1.70). The highest relative risk of cancer among adults was for people with skeletal dysplasia (3.5-fold) followed by those with nervous system defects (odds ratio 1.76, 1.16 to 2.65). For birth defects of the eye, digestive system, respiratory system, limbs, abdominal wall, and urinary organs and oro-facial clefts, we found no association with adult cancer.
Fig 4.
Risk of any cancer in people with any, or specific, major birth defects, stratified by age at diagnosis. Note that scales differ across figures. Odds ratios (ORs) are adjusted for matching variables (birth year and country) and sex. In all analyses for specific sites, other than for chromosomal anomalies, people with chromosomal anomalies were excluded. In all analyses, the unexposed group was composed of people without major birth defects. Some age groups do not have an estimated OR owing to no co-occurring birth defect and cancer cases
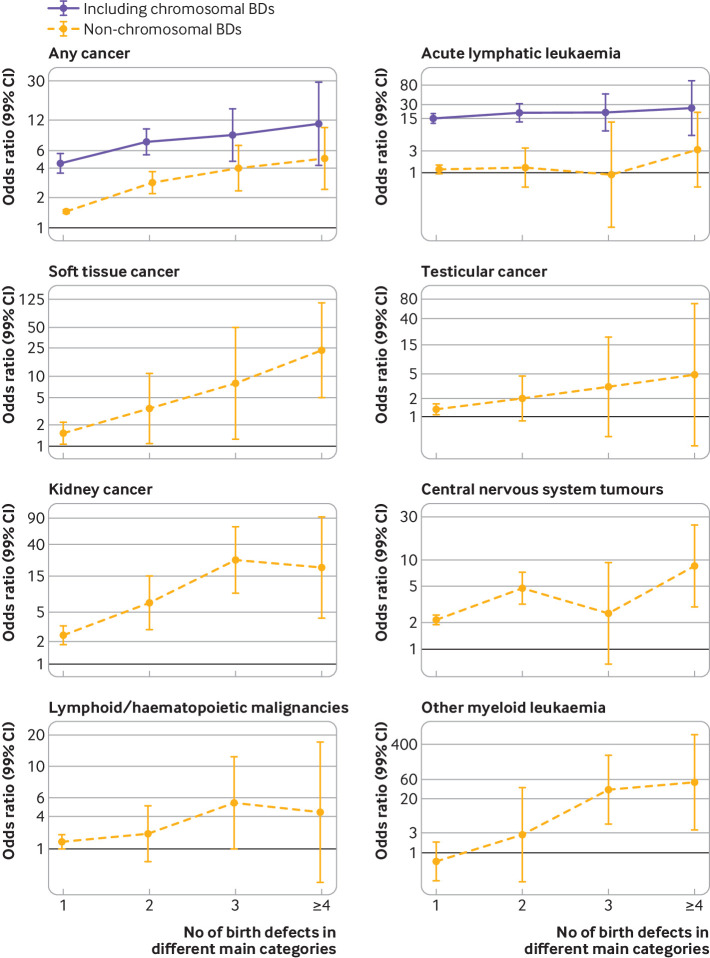
Risk of overall and specific cancer types in people with multiple birth defects
The risk of overall cancer in people with four or more non-chromosomal birth defects in different anatomical subgroups was nearly five times (odds ratio 4.9, 2.4 to 10.1) the risk in those without major birth defects (fig 5). Among people with non-chromosomal birth defects, the odds ratio of overall cancer increased with the number of birth defects in different subgroups (P for trend<0.001), as did the odds ratios of soft tissue cancer, kidney cancer, and central nervous system tumours (P for trend<0.001). Among people with chromosomal anomalies, we observed an increase in risk of overall cancer and acute lymphatic leukaemia as the number of birth defects in different subgroups increased (P for trend<0.001).
Fig 5.
Risk of selected cancers in people with major birth defects according to number of major birth defects in different anatomical subgroups. Results are presented separately for people with any birth defect (including chromosomal birth defects) and those with non-chromosomal defects only. Odds ratios (ORs) are adjusted for matching variables (birth year and country) and sex. Results are presented for all cancers in individuals with 1, 2, 3, and ≥4 birth defects (BDs)
Associations between specific birth defects and specific types of cancers
We further explored the associations between specific major birth defects and specific cancers both in the entire study population and among adults (table 3). In the total population, the strongest associations were between defects involving genetic syndromes and microdeletions and cancers of urinary organs (odds ratio 35, 18 to 69), soft tissue (17, 5.6 to 49), and other endocrine glands (9.6, 3.0 to 31); between Down’s syndrome and lymphoid/ haematopoietic malignancies (19, 16 to 23); between anomalies of the eye and eye cancer (18, 7.5 to 44); between nervous system defects and central nervous system tumours (16, 13 to 21); and between urinary organs defects and cancer of urinary organs (8.0, 4.5 to 14). In the adult population, the strongest associations were between nervous system defects and cancers of urinary organs (odds ratio 14, 4.7 to 40) and other endocrine glands (5.8, 1.8 to 19); between Down’s syndrome and cancer of male genital organs (testicular cancer) (4.8, 2.7 to 8.6); between congenital heart defects and non-melanoma skin cancer (4.6, 1.6 to 13); between urinary organs defects and cancer of digestive organs (4.0, 1.2 to 13); between genital defects and cancer of digestive organs (2.3, 1.2 to 4.4) and male genital organs (testicular cancer) (1.9, 1.3 to 2.6); and between oro-facial clefts (mainly cleft lip) and breast cancer (2.3, 1.0 to 5.2).
Table 3.
Associations between specific major birth defects and specific cancer groups (with ≥5 co-occurring cases) among total study population and among adults (≥20 years). Altogether, 104 associations, significant at 1% significance level, are reported after 264 analyses
| Birth defect* and cancer site† | Total study population | Adults (≥20 years) | |||||
|---|---|---|---|---|---|---|---|
| No of cases | No (%) cases with birth defects | Odds ratio (99% CI) | No of cases | No (%) cases with birth defects | Odds ratio (99% CI) | ||
| Nervous system | |||||||
| Main groups: | |||||||
| Central nervous system‡ | 10 067 | 139 (1.4) | 16 (13 to 21) | 3612 | 6 (0.2) | 2.4 (0.83 to 6.9) | |
| Other endocrine glands | 2484 | 15 (0.6) | 7.7 (3.9 to 15) | 1281 | 5 (0.4) | 5.8 (1.8 to 19) | |
| Eye | 859 | 5 (0.6) | 6.7 (2.1 to 22) | - | - | - | |
| Urinary organs | 1948 | 10 (0.5) | 6.2 (2.7 to 14) | 690 | 6 (0.9) | 14 (4.7 to 40) | |
| Thyroid gland | 2038 | 7 (0.3) | 4.6 (1.7 to 12) | - | - | - | |
| Soft tissues | 1593 | 6 (0.4) | 4.4 (1.5 to 13) | - | - | - | |
| Subgroups: | |||||||
| Urinary tract | 371 | 5 (1.3) | 18 (5.6 to 59) | 279 | 5 (1.8) | 26 (8.1 to 86) | |
| Kidney (excluding renal pelvis) | 1577 | 5 (0.3) | 3.8 (1.2 to 12) | - | - | - | |
| Neural tube defects | |||||||
| Main groups: | |||||||
| Central nervous system | 9979 | 51 (0.5) | 16 (11.0 to 24) | - | - | - | |
| Urinary organs | 1944 | 6 (0.3) | 10 (3.6 to 30) | 689 | 5 (0.7) | 26 (8.1 to 86) | |
| Other endocrine glands | 2476 | 7 (0.3) | 9.5 (3.5 to 26) | - | - | - | |
| Subgroups: | |||||||
| Urinary tract | 371 | 5 (1.3) | 46 (14 to 151) | 279 | 5 (1.8) | 62 (19 to 204) | |
| Eye | |||||||
| Main groups: | |||||||
| Eye | 863 | 9 (1.0) | 18 (7.5 to 44) | - | - | - | |
| Urinary organs | 1951 | 13 (0.7) | 12 (6.0 to 26) | - | - | - | |
| Subgroups: | |||||||
| Kidney (excluding renal pelvis) | 1585 | 13 (0.8) | 14 (6.9 to 30) | - | - | - | |
| Congenital heart defects | |||||||
| Main groups: | |||||||
| Skin, non-melanoma | 533 | 7 (1.3) | 3.5 (1.3 to 9.3) | 412 | 6 (1.5) | 4.6 (1.6 to 13) | |
| Lymphoid/ haematopoietic tissue | 14 223 | 209 (1.5) | 2.5 (2.1 to 3.0) | 4700 | 19 (0.4) | 1.1 (0.58 to 1.9) | |
| Urinary organs | 1963 | 25 (1.3) | 2.3 (1.4 to 3.9) | - | - | - | |
| Female genital organs | 4015 | 26 (0.6) | 1.9 (1.1 to 3.1) | 3705 | 23 (0.6) | 1.9 (1.1 to 3.3) | |
| Male genital organs | 6545 | 37 (0.6) | 1.6 (1.1 to 2.5) | 5740 | 31 (0.5) | 1.7 (1.0 to 2.6) | |
| Central nervous system§ | 10 010 | 82 (0.8) | 1.5 (1.2 to 2.1) | 3625 | 19 (0.5) | 1.6 (0.87 to 2.9) | |
| Subgroups: | |||||||
| Acute myeloid leukaemia | 1092 | 49 (4.5) | 7.8 (5.3 to 11) | - | - | - | |
| Leukaemia, cell unspecified | 322 | 12 (3.7) | 6.6 (3.1 to 14) | - | - | - | |
| Liver | 459 | 12 (2.6) | 4.5 (2.1 to 9.5) | - | - | - | |
| Ovary etc. | 817 | 11 (1.3) | 3.1 (1.4 to 6.7) | 558 | 8 (1.4) | 4.0 (1.6 to 10) | |
| Kidney (excluding renal pelvis) | 1596 | 24 (1.5) | 2.6 (1.5 to 4.4) | - | - | - | |
| Acute lymphatic leukaemia | 5021 | 90 (1.8) | 2.5 (1.9 to 3.4) | - | - | - | |
| Testis | 6439 | 36 (0.6) | 1.6 (1.0 to 2.5) | 5667 | 30 (0.5) | 1.6 (1.0 to 2.6) | |
| Oro-facial clefts | |||||||
| Main groups: | |||||||
| Breast | 3589 | 11 (0.3) | 2.3 (1.0 to 5.1) | 3578 | 11 (0.3) | 2.3 (1.0 to 5.2) | |
| Subgroups: | - | - | - | ||||
| Ovary etc | 811 | 5 (0.6) | 4.3 (1.3 to 14) | - | - | - | |
| Cleft palate only | |||||||
| Subgroups: | |||||||
| Ovary etc | 811 | 5 (0.6) | 11 (3.4 to 36) | - | - | - | |
| Cleft lip with without cleft palate | |||||||
| Main groups: | |||||||
| Other endocrine glands | 2477 | 8 (0.3) | 2.8 (1.1 to 7.1) | - | - | - | |
| Breast | 3587 | 9 (0.3) | 2.8 (1.1 to 6.7) | 3576 | 9 (0.3) | 2.8 (1.1 to 6.7) | |
| Digestive system | |||||||
| Main groups: | |||||||
| Urinary organs | 1947 | 9 (0.5) | 4.0 (1.7 to 9.4) | - | - | - | |
| Other endocrine glands | 2479 | 10 (0.4) | 3.7 (1.6 to 8.5) | - | - | - | |
| Digestive organs | 2683 | 8 (0.3) | 3.1 (1.2 to 7.7) | - | - | - | |
| Lymphoid/ haematopoietic tissue | 14 064 | 50 (0.4) | 2.9 (2.0 to 4.2) | 4688 | 7 (0.1) | 1.5 (0.58 to 4.1) | |
| Subgroups: | |||||||
| Liver | 1050 | 7 (0.7) | 5.5 (2.0 to 15) | - | - | - | |
| Acute myeloid leukaemia | 1580 | 8 (0.5) | 4.2 (1.7 to 11) | - | - | - | |
| Kidney (excluding renal pelvis) | 2945 | 12 (0.4) | 3.5 (1.7 to 7.5) | - | - | - | |
| Non-Hodgkin’s lymphoma | - | - | - | ||||
| Acute lymphatic leukaemia | 4951 | 20 (0.4) | 3.0 (1.7 to 5.4) | - | - | - | |
| Urinary | |||||||
| Main groups: | |||||||
| Urinary organs | 1958 | 20 (1.0) | 8.0 (4.5 to 14) | - | - | - | |
| Other endocrine glands | 2480 | 11 (0.4) | 4.2 (1.9 to 9.2) | - | - | - | |
| Digestive organs | 2684 | 9 (0.3) | 3.9 (1.6 to 9.3) | 2028 | 5 (0.2) | 4.0 (1.2 to 13) | |
| Subgroups: | |||||||
| Kidney (excluding renal pelvis) | 1589 | 17 (1.1) | 8.0 (4.2 to 15) | - | - | - | |
| Genital | |||||||
| Main groups: | |||||||
| Urinary organs | 1957 | 19 (1.0) | 2.9 (1.6 to 5.2) | - | - | - | |
| Digestive organs | 2692 | 17 (0.6) | 2.0 (1.0 to 3.7) | 2038 | 15 (0.7) | 2.3 (1.2 to 4.4) | |
| Male genital organs | 6576 | 68 (1.0) | 1.8 (1.3 to 2.5) | 5770 | 61 (1.1) | 1.9 (1.3 to 2.6) | |
| Subgroups: | |||||||
| Rectum, rectosigmoid | 451 | 5 (1.1) | 3.5 (1.1 to 11) | 438 | 5 (1.1) | 3.7 (1.1 to 12) | |
| Liver | 452 | 5 (1.1) | 3.3 (1.0 to 11) | - | - | - | |
| Kidney (excluding renal pelvis) | 1588 | 16 (1.0) | 3.2 (1.7 to 6.2) | - | - | - | |
| Testis | 6469 | 66 (1.0) | 1.8 (1.3 to 2.5) | 5698 | 61 (1.1) | 1.9 (1.3 to 2.6) | |
| Limb | |||||||
| Main groups: | |||||||
| Thyroid gland | 2048 | 17 (0.8) | 2.4 (1.3 to 4.5) | 1624 | 9 (0.6) | 1.6 (0.69 to 3.9) | |
| Urinary organs | 1956 | 18 (0.9) | 2.3 (1.2 to 4.2) | - | - | - | |
| Other endocrine glands | 2489 | 20 (0.8) | 2.1 (1.2 to 3.8) | 1284 | 8 (0.6) | 1.7 (0.7 to 4.4) | |
| Subgroups: | |||||||
| Kidney (excluding renal pelvis) | 1588 | 16 (1.0) | 2.5 (1.3 to 4.8) | - | - | - | |
| Skeletal dysplasia | |||||||
| Main groups: | |||||||
| Lymphoid/ haematopoietic tissue | 14 026 | 12 (0.1) | 4.3 (1.9 to 9.4) | - | - | - | |
| Central nervous system | 9934 | 6 (0.1) | 3.4 (1.2 to 10) | - | - | - | |
| Subgroups: | |||||||
| Non-Hodgkin’s lymphoma | 2940 | 7 (0.2) | 13 (4.9 to 37) | - | - | - | |
| Genetic syndromes and microdeletions | |||||||
| Main groups: | |||||||
| Urinary organs | 1955 | 17 (0.9) | 35 (18 to 69) | - | - | - | |
| Soft tissues | 1593 | 6 (0.4) | 17 (5.6 to 49) | - | - | - | |
| Other endocrine glands | 2474 | 5 (0.2) | 9.6 (3.0 to 31) | - | - | - | |
| Central nervous system | 9935 | 7 (0.1) | 3.1 (1.1 to 8.3) | - | - | - | |
| Lymphoid/ haematopoietic tissue | 14 025 | 11 (0.1) | 2.9 (1.3 to 6.5) | - | - | - | |
| Subgroups: | |||||||
| Kidney (excluding renal pelvis) | 1589 | 17 (1.1) | 39 (20 to 77) | - | - | - | |
| Down’s syndrome | |||||||
| Main groups: | |||||||
| Lymphoid/ haematopoietic tissue | 14 269 | 255 (1.8) | 19 (16 to 23) | 4689 | 8 (0.2) | 2.2 (0.86 to 5.4) | |
| Male genital organs | 6532 | 24 (0.4) | 4.8 (2.7 to 8.3) | 5730 | 21 (0.4) | 4.8 (2.7 to 8.6) | |
| Subgroups: | |||||||
| Acute myeloid leukaemia | 1155 | 112 (9.7) | 111 (84 to 148) | - | - | - | |
| Leukaemia, cell unspecified | 333 | 23 (6.9) | 80 (45 to 141) | - | - | - | |
| Acute lymphatic leukaemia | 5034 | 103 (2.0) | 22 (16 to 29) | - | - | - | |
| Other myeloid leukaemia | 440 | 7 (1.6) | 18 (6.8 to 49.0) | - | - | - | |
| Testis | 6427 | 24 (0.4) | 4.8 (2.8 to 8.4) | 5658 | 21 (0.4) | 4.9 (2.7 to 8.7) | |
| Other anomalies/ syndromes | |||||||
| Main groups: | |||||||
| Central nervous system¶ | 10 084 | 156 (1.5) | 4.3 (3.4 to 5.3) | 3629 | 23 (0.6) | 1.9 (1.1 to 3.2) | |
| Peripheral nerves and autonomic nervous system | 505 | 7 (1.4) | 3.6 (1.3 to 9.6) | - | - | - | |
| Urinary organs | 1961 | 23 (1.2) | 3.2 (1.8 to 5.4) | 690 | 6 (0.9) | 2.4 (0.84 to 7.0) | |
| Soft tissues | 1605 | 18 (1.1) | 3.0 (1.6 to 5.6) | - | - | - | |
| Bone | 1421 | 11 (0.8) | 2.2 (1.0 to 4.8) | - | - | - | |
| Lymphoid/ haematopoietic tissue | 14 100 | 86 (0.6) | 1.6 (1.2 to 2.1) | 4705 | 24 (0.5) | 1.3 (0.79 to 2.3) | |
| Male genital organs | 6547 | 39 (0.6) | 1.5 (1.0 to 2.4) | 5741 | 32 (0.6) | 1.5 (0.92 to 2.3) | |
| Subgroups: | |||||||
| Kidney (excluding renal pelvis) | 1595 | 23 (1.4) | 4.0 (2.3 to 6.9) | 416 | 6 (1.4) | 4.4 (1.5 to 13) | |
| Acute myeloid leukaemia | 1053 | 10 (0.9) | 2.5 (1.1 to 5.7) | - | - | - | |
| Acute lymphatic leukaemia | 4961 | 30 (0.6) | 1.6 (1.0 to 2.6) | - | - | - | |
| Testis | 6442 | 39 (0.6) | 1.6 (1.0 to 2.4) | 5669 | 32 (0.6) | 1.5 (0.93 to 2.3) | |
Chromosomal anomalies are excluded from all birth defect groups other than Down’s syndrome. In all analyses, unexposed group was composed of individuals without major birth defects. Odds ratios adjusted for matching variables (birth year and country) and sex.
Categorised according to EUROCAT.
Categorised according to Cancer in Norway (2017)/NORDCAN.
Separate odds ratios and 99% CIs for malignant and benign cases: 7.8 (4.9 to 13) and 24 (18 to 33), respectively, in total study population; 3.9 (1.2 to 12), only benign cases, among adults.
Separate odds ratios and 99% CIs for malignant and benign cases: 1.3 (0.8 to 2.0) and 2.0 (1.4 to 3.1), respectively, in total study population; 1.7 (0.8 to 3.9) and 1.5 (0.6 to 3.5), respectively, among adults.
Separate odds ratios and 99% CIs for malignant and benign cases: 2.3 (1.5 to 3.4) and 8.0 (6.2 to 10.3), respectively, in total study population; 0.8 (0.3 to 2.6) and 3.0 (1.6 to 5.5), respectively, among adults.
Discussion
In this large population based nested case-control study in four Nordic countries, people with chromosomal and non-chromosomal birth defects were at increased risk of overall cancer into adulthood (investigated for individuals up to the age of 46). People with non-chromosomal birth defects had an increased risk of cancer in several different organ systems, whereas the dominant malignancy for those with chromosomal anomalies was leukaemia. Many structural birth defects were associated with later cancer in the same organ system or anatomical location, and the relative risk of cancer increased with number of birth defects. Although the associations generally were stronger in children than adults, they persisted into adulthood. For instance, compared with people without major birth defects, those with two of the most common birth defect groups, congenital heart defects and genital defects, had an increased risk of cancer as adults (≥20 years).
Strengths and limitations of study
Among the strengths of our study were the large number of cancer cases (including all cases among births registered in the medical birth registries in four Nordic countries) and the ability to assess risk of cancer in adulthood and adolescence, as well as childhood in the same population. The large population meant that we could also study the associations between several specific birth defects and specific cancers. The linkages of comprehensive and compulsory population based databases gave reliable and almost complete information on cancer diagnoses.20 From the patient registries, we used only diagnoses of birth defects from inpatient registrations because of low validity of outpatient diagnoses.19 In addition, we limited diagnoses to those occurring in the first year of life for consistency of exposure criteria in all four countries. For Finland, the data are from the Register of Congenital Malformation, which uses diagnoses given in hospital inpatient and outpatient care. However, all cases with major birth defect are validated from the hospitals before being entered in the register. We did a sensitivity analysis in which we stratified on country during 1987-2013 when all countries had available data and found similar risk estimates for any cancer among children with non-chromosomal anomalies (odds ratios from 1.8 to 2.7). Also, the risk estimates for larger cancer groups were in the same direction, supporting the reported associations.
In our study, ascertainment of birth defects may have differed both over time and between countries. Ascertainment depends on type and severity, so most studies, including ours, exclude minor birth defects. Variation also exists in the degree of ascertainment of major birth defects, especially if defects are registered only at or immediately after birth. Visibility of the defect at birth is associated with higher ascertainment than for less visible birth defects.33 34 However, under-ascertainment of birth defects is unlikely to be associated with later diagnosis of cancer and should generally bias associations towards the null. On the other hand, if cases among individuals aged under 1 year are more likely to be diagnosed as having a birth defect than controls, the results may be biased away from the null. Although adjustments for in vitro fertilisation, maternal age, and maternal smoking habits did not change the results substantially (supplementary tables C, D, and E), we may lack information for other unknown confounders. For instance, we could not include information on parental income or education owing to strict data regulations in some study countries. Also, if the missingness of data on maternal smoking was not completely at random, this analysis may be biased. For some of the analyses of combinations of specific birth defects and cancers, statistical power was limited. Spurious associations resulting from multiple comparisons may also be a concern. Therefore, we attempted to evaluate patterns of associations with regard to aetiology and relevant biological mechanisms.
Comparison with other studies
Previous studies have reported declining risk of cancer with age, but most were limited by size, shorter follow-up time, or both, and few were able to assess specific birth defects.8 9 10 11 12 13 35 36 Only three studies included adults, and these evaluated only nervous and circulatory system defects and congenital heart defects.14 35 36 In our study, we found that although the increase in overall cancer risk declined with age, it persisted into adulthood for both non-chromosomal and chromosomal anomalies. Furthermore, we were able to look at anatomical subgroups of birth defects and observed that the increased risk at younger ages was more pronounced for some subgroups, such as nervous system defects, genetic syndromes and microdeletions, and chromosomal anomalies. Most cancers associated with birth defects appear during childhood owing to the exposure being congenital and the typical latency of cancer. This is supported by odds ratios for cancer being higher during childhood (0-14 years) than adulthood (20 years or older). The exception was for people with defects in genital organs relative those without such defects, for which the odds ratio for cancer (one third of which were testicular) was 1.43 (99% confidence interval 1.14 to 1.78) for adults compared with 1.25 (0.92 to 1.70) for children. The long latency could be explained by the current model for this tumour’s development, comprising genetic susceptibility for both genital organ defects and testicular cancer, combined with environmental factors exerting their effect during fetal life.37 Incidence of testicular cancer rises with the testosterone surge in puberty and peaks at 30-35 years. In addition to testicular cancer, our study provided evidence for other associations between birth defects and cancer diagnosed in adulthood. For example, the odds ratio for congenital heart defects and overall cancer was 1.28 (1.02 to 1.59), similar to or lower than those previously suggested for adults.14 35 36 Another example was nervous system defects, with a 15-fold increased risk of cancer before the age of 5, whereas the odds ratio for adults was reduced to 1.76 (1.16 to 2.65). This trend has been suggested previously but was limited to the first 12 years of life and/or with few co-occurring cases.13 14
An increasing number of (non-chromosomal) birth defects in different organ systems have been associated with increased risk of cancer overall.3 5 8 9 14 Our results support this, and we also saw the same trend for chromosomal birth defects. We observed an increase in relative risk of overall cancer with increasing number of birth defects and, in addition, for some specific cancers such as acute lymphatic leukaemia (for chromosomal birth defects), soft tissue cancer, kidney cancer, central nervous system tumours, and other myeloid leukaemia (for non-chromosomal birth defects).
As expected, the increased overall cancer risk was lower than in previous studies limited to childhood cancer, but the results for children were in line with previous findings when stratified by age at diagnosis.3 4 The associations between chromosomal birth defects (driven mainly by Down’s syndrome) and cancer are well known, such as the high risks for leukaemia. Specifically, our estimated odds ratios of 111 and 22 for acute myeloid leukaemia and acute lymphatic leukaemia, respectively, are in concordance with the corresponding hazard ratio estimates of 125 and 28 recently published by Lupo et al.3 In addition, adults with Down’s syndrome were at increased risk of testicular cancer (odds ratio 4.9, 2.7 to 8.7), which has also been suggested previously but with less precision.38
Implications of findings and future research
Our study showed that birth defects are associated with risk of cancer in adulthood as well as in adolescence and childhood, a finding of clinical importance for healthcare workers responsible for follow-up of people with birth defects. Surveillance for cancer in children with birth defects has been discussed, but thus far the absolute cancer risk has been regarded as too low. In the Nordic countries, for instance, the cumulative risk of any cancer in the 0-44 year age group was 2.3% for males and 3.8% for females in 2016.39 Thus, the most important implication of our results is to provide further rationale for additional studies on the molecular mechanisms involved in the developmental disruptions underlying both birth defects and cancer.
What is already known on this topic
Being born with a birth defect is one of the strongest risk factors for childhood cancer
Several specific birth defect-cancer associations have been identified, and increasing risk with increasing number of birth defects has been reported
The risk of cancer is higher at younger ages, but few studies have investigated cancer risk beyond childhood and adolescence
What this study adds
Many structural birth defects were associated with later cancer in the same organ system or anatomical location
The increased cancer risk in individuals with birth defects persisted into adulthood
In particular, the increased risk in adults remained for those born with congenital heart defects, genital organs defects, chromosomal anomalies, nervous system defects, and skeletal dysplasia
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: TB, AE, and KK designed and planned the study. TB, IG, MG, and HTS obtained access to data. DSD did the data analysis and wrote the first draft of the manuscript with support from TB, AE, and KK. SMMS did preliminary analyses. All authors were involved in interpreting the results, revising the manuscript, and approving the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. DSD is the guarantor.
Funding: This study was supported by the Norwegian Cancer Society (agreement No 5703714-2014). The research was designed, conducted, analysed, and interpreted by the authors independently of the funding sources.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from Norwegian Cancer Society; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by ethics committees in Norway (2015/317/REK vest) and Stockholm, Sweden (2015/1642-31/2), and by the Data Protection Agency in Denmark (2015-57-0002). Permission to use health register data in Finland was granted by the Finnish Institute of Health and Welfare after consultation with the data protection authority (THL/68/5.05/2014 and THL/909/5.05/2015).
Data sharing: The datasets analysed during the current study are not freely available owing to national regulations, but similar data can be obtained from the register authorities.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of this study will be disseminated to relevant user organisations (Norwegian Cancer Society), patient groups, and healthcare workers.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. GBD 2017 Childhood Cancer Collaborators The global burden of childhood and adolescent cancer in 2017: an analysis of the Global Burden of Disease Study 2017. Lancet Oncol 2019;20:1211-25. 10.1016/S1470-2045(19)30339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EUROCAT. Prevalence charts and tables. 2020. https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en.
- 3. Lupo PJ, Schraw JM, Desrosiers TA, et al. Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. JAMA Oncol 2019;5:1150-8. 10.1001/jamaoncol.2019.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson KJ, Lee JM, Ahsan K, et al. Pediatric cancer risk in association with birth defects: A systematic review. PLoS One 2017;12:e0181246. 10.1371/journal.pone.0181246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norwood MS, Lupo PJ, Chow EJ, et al. Childhood cancer risk in those with chromosomal and non-chromosomal congenital anomalies in Washington State: 1984-2013. PLoS One 2017;12:e0179006. 10.1371/journal.pone.0179006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spector LG, Pankratz N, Marcotte EL. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am 2015;62:11-25. 10.1016/j.pcl.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feldkamp ML, Carey JC, Byrne JLB, Krikov S, Botto LD. Etiology and clinical presentation of birth defects: population based study. BMJ 2017;357:j2249. 10.1136/bmj.j2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjørge T, Cnattingius S, Lie RT, Tretli S, Engeland A. Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomarkers Prev 2008;17:500-6. 10.1158/1055-9965.EPI-07-2630 [DOI] [PubMed] [Google Scholar]
- 9. Agha MM, Williams JI, Marrett L, To T, Zipursky A, Dodds L. Congenital abnormalities and childhood cancer. Cancer 2005;103:1939-48. 10.1002/cncr.20985 [DOI] [PubMed] [Google Scholar]
- 10. Botto LD, Flood T, Little J, et al. Cancer risk in children and adolescents with birth defects: a population-based cohort study. PLoS One 2013;8:e69077. 10.1371/journal.pone.0069077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carozza SE, Langlois PH, Miller EA, Canfield M. Are children with birth defects at higher risk of childhood cancers? Am J Epidemiol 2012;175:1217-24. 10.1093/aje/kwr470 [DOI] [PubMed] [Google Scholar]
- 12. Dawson S, Charles AK, Bower C, de Klerk NH, Milne E. Risk of cancer among children with birth defects: a novel approach. Birth Defects Res A Clin Mol Teratol 2015;103:284-91. 10.1002/bdra.23364 [DOI] [PubMed] [Google Scholar]
- 13. Janitz AE, Neas BR, Campbell JE, et al. Childhood cancer in children with congenital anomalies in Oklahoma, 1997 to 2009. Birth Defects Res A Clin Mol Teratol 2016;106:633-42. 10.1002/bdra.23494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun Y, Overvad K, Olsen J. Cancer risks in children with congenital malformations in the nervous and circulatory system-A population based cohort study. Cancer Epidemiol 2014;38:393-400. 10.1016/j.canep.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 15. Spector LG, Olshan AF. Birth Defects and Cancer in Childhood-Dual Diseases of Development. JAMA Oncol 2019;5:1105-7. 10.1001/jamaoncol.2019.1207 [DOI] [PubMed] [Google Scholar]
- 16. Langhoff-Roos J, Krebs L, Klungsøyr K, et al. The Nordic medical birth registers--a potential goldmine for clinical research. Acta Obstet Gynecol Scand 2014;93:132-7. 10.1111/aogs.12302 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449-90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ritvanen A. Epämuodostumat 1993-2011 – Congenital anomalies 1993-2011. National Institute for Health and Welfare in Finland, 2014. [Google Scholar]
- 19. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic Cancer Registries - an overview of their procedures and data comparability. Acta Oncol 2018;57:440-55. 10.1080/0284186X.2017.1407039 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. International classification of diseases for oncology (ICD-O) – 3rd edition, 1st revision, 3rd ed. 2013. https://apps.who.int/iris/handle/10665/96612.
- 22.World Health Organization. International statistical classification of diseases and related health problems, 10th revision, Fifth edition, 2016. 2015. https://apps.who.int/iris/handle/10665/246208.
- 23.Socialstyrelsen. Kodning i cancerregistret 2017. 2016. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2016-12-9.pdf.
- 24. Cancer Registry of Norway Cancer in Norway 2017 - Cancer incidence, mortality, survival and prevalence in Norway. Cancer Registry of Norway, 2018. [Google Scholar]
- 25.NORDCAN. Comparable cancer statistics for Denmark, Finland, Iceland, Norway, Sweden, the Faroe Islands, and Greenland. https://nordcan.iarc.fr/en.
- 26. EUROCAT EUROCAT Guide 1.4: Instruction for the registration of congenital anomalies. EUROCAT Central Registry. University of Ulster, 2013. [Google Scholar]
- 27. Garne E, Dolk H, Loane M, et al. EUROCAT Working Group Paper 5: Surveillance of multiple congenital anomalies: implementation of a computer algorithm in European registers for classification of cases. Birth Defects Res A Clin Mol Teratol 2011;91(Suppl 1):S44-50. 10.1002/bdra.20777 [DOI] [PubMed] [Google Scholar]
- 28. Pearce N. Analysis of matched case-control studies. BMJ 2016;352:i969. 10.1136/bmj.i969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cornfield J. A method of estimating comparative rates from clinical data; applications to cancer of the lung, breast, and cervix. J Natl Cancer Inst 1951;11:1269-75. [PubMed] [Google Scholar]
- 30. Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol 1993;22:1189-92. 10.1093/ije/22.6.1189 [DOI] [PubMed] [Google Scholar]
- 31. Mitchell MN. Interpreting and Visualizing Regression Models Using Stata. Stata Press, 2012: 558. [Google Scholar]
- 32. Karahalios A, Baglietto L, Carlin JB, English DR, Simpson JA. A review of the reporting and handling of missing data in cohort studies with repeated assessment of exposure measures. BMC Med Res Methodol 2012;12:96. 10.1186/1471-2288-12-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kubon C, Sivertsen A, Vindenes HA, Åbyholm F, Wilcox A, Lie RT. Completeness of registration of oral clefts in a medical birth registry: a population-based study. Acta Obstet Gynecol Scand 2007;86:1453-7. 10.1080/08037050701645090 [DOI] [PubMed] [Google Scholar]
- 34. Melve KK, Lie RT, Skjaerven R, et al. Registration of Down syndrome in the Medical Birth Registry of Norway: validity and time trends. Acta Obstet Gynecol Scand 2008;87:824-30. 10.1080/00016340802217184 [DOI] [PubMed] [Google Scholar]
- 35. Mandalenakis Z, Karazisi C, Skoglund K, et al. Risk of Cancer Among Children and Young Adults With Congenital Heart Disease Compared With Healthy Controls. JAMA Netw Open 2019;2:e196762. 10.1001/jamanetworkopen.2019.6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gurvitz M, Ionescu-Ittu R, Guo L, et al. Prevalence of Cancer in Adults With Congenital Heart Disease Compared With the General Population. Am J Cardiol 2016;118:1742-50. 10.1016/j.amjcard.2016.08.057 [DOI] [PubMed] [Google Scholar]
- 37. Facchini G, Rossetti S, Cavaliere C, et al. Exploring the molecular aspects associated with testicular germ cell tumors: a review. Oncotarget 2017;9:1365-79. 10.18632/oncotarget.22373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patja K, Pukkala E, Sund R, Iivanainen M, Kaski M. Cancer incidence of persons with Down syndrome in Finland: a population-based study. Int J Cancer 2006;118:1769-72. 10.1002/ijc.21518 [DOI] [PubMed] [Google Scholar]
- 39.NORDCAN. Incidence, Males, in 2016: All sites. https://nordcan.iarc.fr/en/dataviz/tables.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials