Abstract
Idebenone is a well described drug that was initially developed against dementia. The current literature widely portrays this molecule as a potent antioxidant and CoQ10 analogue. While numerous papers seem to support this view, a closer look indicates that the pharmacokinetics of idebenone do not support these claims. A major discrepancy between achievable tissue levels, especially in target tissues such as the brain, and doses required to show the proposed effects, significantly questions our current understanding. This review explains how this has happened and highlights the discrepancies in the current literature. More importantly, based on some recent discoveries, a new framework is presented that can explain the mode of action of this molecule and can align formerly contradictory results. Finally, this new appreciation of the molecular activities of idebenone provides a rational approach to test idebenone in novel indications that might have not been considered previously for this drug.
Keywords: Idebenone, Antioxidant, Radical scavenger
1. Historical context
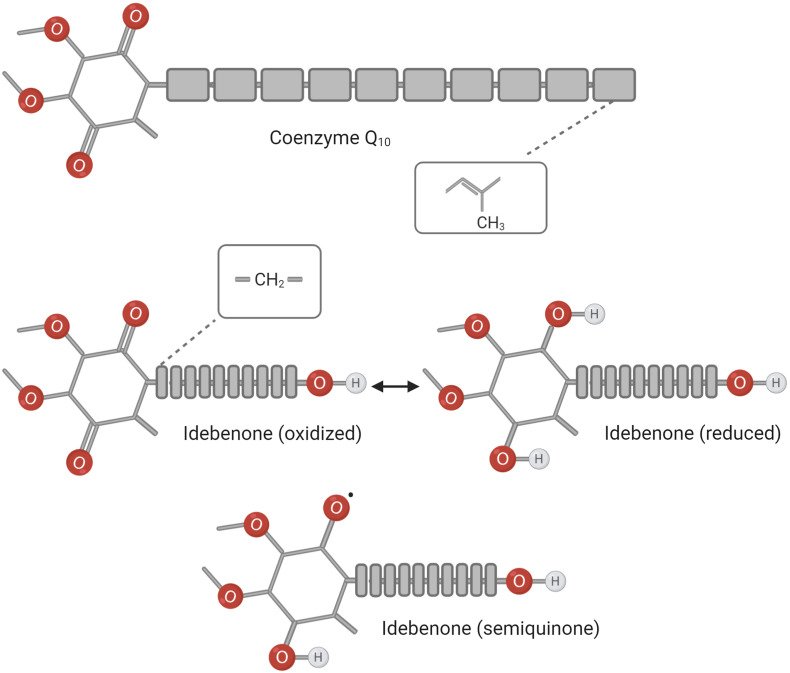
Idebenone is a well-known compound, developed in the early 1980s by Takeda Pharmaceuticals against cognitive decline/dementia. To understand the results and discrepancies in the contemporary literature around this molecule, it is imperative to understand its history. In 1970s and 80s far less was known about the molecular events associated with cognitive decline and dementia. At the time, one prominent theory to explain the pathology of dementia was an age-dependent irreversible change of vascular structure and function [1,2]. In particular, decreased cerebral blood flow, oxygen utilization or blood volume [3], a dysfunctional cholinergic system that restricts blood flow [4,5] as well as plaque deposition [6] or disease states such as cerebral atherosclerosis [4] were thought to be involved. Since the vascular changes in the brain were linked to the presence of free radicals (i.e. reactive oxygen species (ROS)) and lipid peroxidation [7], lipophilic antioxidants such as Coenzyme Q and Vitamin E promised to provide therapeutic effects [8]. It was known that CoQ10 acts as a catalytic antioxidant when chemically reduced from the ubiquinone to the ubiquinol form to enable its antioxidant activity. CoQ10 was also described to protect against lipid peroxidation in vitro [9] and in vivo [10]. However, CoQ10 is a large insoluble molecule with limited intestinal absorption and bioavailability. Therefore, a CoQ10 analogue with better pharmaco-chemical characteristics, but essentially the same molecular activity, was developed to a marketable drug that distanced the new molecule from the natural product CoQ10. Idebenone appeared ideal, with the same redox-active benzoquinone moiety as CoQ10, but increased solubility due to a significantly shorter lipophilic side chain that also contained a terminal hydroxyl group to increase polarity (Fig. 1).
Fig. 1.
Structural comparison of idebenone with CoQ10. In addition to the benzoquinone moiety that is shared by both molecules, the tail of CoQ10 contains ten isoprenyl (CH2–CH C(CH3)–CH2) subunits, while idebenone contains 10 methylene (CH2) subunits and a terminal hydroxyl group. A two-electron reduction of idebenone by NQO1 generates the active hydroquinone form, while a one-electron reduction generates the unstable semiquinone. Figure was made using www.biorender.com.
Based on an expectation that idebenone is a better CoQ10 analogue and therefore a better antioxidant to counteract the oxidative stress-induced vascular changes in dementia, multiple studies were published that confirmed the expected outcomes. Studies on isolated mitochondria demonstrated antioxidant activity and decreased lipid peroxidation and mitochondrial swelling [[11], [12], [13]], which were also confirmed in vivo [14]. Similarly, idebenone prevented ROS-induced prostaglandin synthesis and platelet aggregation indicative of an antioxidant function [15].
In line with the underlying hypothesis, idebenone was tested in a range of cerebrovascular disease models such as stroke [16,17], experimental ischemia [18], hypertension [19,20], hypoxia [21] and also behavioural models that reflected the disease pathophysiology in dementia [22,23]. Consistent with an anti-oxidant-driven increased cerebral blood flow [24], perhaps also as a consequence of ameliorated cholinergic deficits [23,25,26], pre-clinical results with idebenone appeared to support the therapeutic approach. This was further strengthened in several pre-clinical models of dementia where idebenone not only showed potent cytoprotective activity [20,21] and improved neurotransmitter turnover [23,26,27], but also reduced neurological deficits [19], improved memory [17,18,28] and normalised behaviour [22]. However, at a closer inspection, even these early studies appear contradictory. While micromolar idebenone doses for example were required to demonstrate activity on isolated mitochondria [13,29,30] only nano-molar concentrations were detected in the CNS and only for very short periods of time [16,31]. Up to this point, this discrepancy has not been widely appreciated or explained. In addition, only very limited information is available to what extent idebenone segregates into the different cellular compartments (i.e. cytoplasm, membranes, and mitochondria) and whether this distribution could be tissue specific and differ in vitro and in vivo.
2. Chemical context
Idebenone is a benzoquinone (Fig. 1), and like all quinones (including CoQ10) it can accept and donate electrons. It is this specific activity that forms the basis for the view that idebenone can act as an antioxidant and electron carrier in a cellular context. For quinones to be chemically reduced in cells and tissues, two principal pathways are available [32]. Reduction by a single electron can be performed by cellular reductases such as the p450 detoxification enzymes. However, this single electron reduction gives rise to an unstable semiquinone that produces superoxide and is therefore associated with significant toxicity. Given the many naturally occurring quinone compounds that organisms are exposed to, for example through the diet (i.e. Vitamin K) this possible source of toxicity is prevented in cells by a two-electron reduction mechanism. In the case of idebenone, this is done by NAD(P)H quinone oxidoreductase 1 (NQO1) that generates the stable hydroquinone form, which can be regarded as the active form of the molecule. This activated idebenone molecule can now donate electrons to detoxify radicals [32] as well as to the mitochondrial respiratory chain to aide ATP production [33,34]. These characteristics are supported by several reports that described idebenone toxicity and/or a lack of efficacy in test systems with low or absent NQO1 status [35]. It must be emphasized that NQO1 is part of the cells physiological response to stress and can be readily upregulated in many cell types and tissues via the Keap/Nrf2/ARE pathway [36], which in turn affects the biological activity of idebenone. In fact, idebenone itself was reported to upregulate NQO1 levels in vivo, which suggests that idebenone induces its own bioactivation system [37]. This begs the question: to what extent is idebenone a direct antioxidant?
3. Is idebenone a direct antioxidant?
Based on a large number of in vitro and in vivo studies, idebenone has been widely portrayed as a potent antioxidant [32,38], but is the evidence really conclusive? Many studies have reported that bioactivated idebenone can effectively protect against oxidative stress in numerous different test systems, which appears to substantiate its antioxidant activity. However, the interpretation of the results is heavily influenced by an expectation that idebenone acts as a direct antioxidant. It is undisputed that idebenone contains a redox-active quinone moiety that in principle could donate electrons to detoxify radicals [32]. However, there is in fact a scarcity of studies that succeeded to measure a direct antioxidant function of idebenone. Direct antioxidant function are best studied in cell free systems with defined mechanisms of ROS production, which differentiate the measured effects from the cellular environment with a multitude of potentially interfering redox reactions. In studies that aimed to formulate idebenone to increase its activity, high micromolar concentrations were required to measure direct antioxidant effects in cell-free systems [[39], [40], [41]], while other cell-free studies did not detect any antioxidant activity at all at concentrations of up to 600 μM [42]. Similarly, in isolated mitochondria micromolar concentrations were required for antioxidant activity, a process that is dependent on complex II activity to reduce idebenone to the activated hydroquinone form [12,13]. The only exception we are aware of is a report where idebenone reduced glycerophosphate-induced oxidative species with an IC50 of 50 nM in mitochondria isolated from brown adipose tissue [43]. Why this isolated report shows effects of idebenone at these low concentrations is so far unexplained, but if the benzoquinone moiety of idebenone acted directly as an acceptor for the glycerophosphate-derived electrons, it could have prevented ROS formation in the first place. Unfortunately, this report did not experimentally differentiate between the possibility of reduced ROS production versus a direct radical scavenging function of idebenone. Apart from this study, there is general agreement that at least micromolar, sometimes up to millimolar idebenone concentrations, combined with a test system that converts idebenone to the hydroquinone form, are required to see any direct antioxidant activity [26,42,44]. In contrast to cell-free studies, the majority of studies that reported “antioxidant activity” in cell culture used extended preincubation times with idebenone, typically overnight or over several days despite the fact that idebenone penetrates cells within a few minutes [[45], [46], [47]]. Although the majority of studies used concentrations in the low micromolar range, some surprisingly reported protective effects against ROS-induced toxicity at nanomolar concentrations [45,47] which are, unlike micromolar concentrations, drug concentrations that can be achieved in vivo.
In fact, studies in rodents consistently reported antioxidant effects of idebenone [[48], [49], [50]], while only very low nanomolar concentrations in target tissues could be detected for only short periods of time [16,31,51]. In addition, idebenone produces fluctuating plasma concentrations due to its short half-life, without significant evidence of drug accumulation in tissues after repeated dosing [16,31]. Although, data on idebenone tissue levels are not available for human patients, the pharmacokinetic characteristics of the compound in test animals are likely translatable to human patients based on reported plasma concentrations [52,53]. As these characteristics are clearly at odds with a direct and sustained antioxidant activity of idebenone, the obvious question is: How can idebenone protect against oxidative stress?
Effective physiological antioxidant systems in cells and tissues regulate ROS levels to the advantage of the organism. These endogenous antioxidants are typically present in micromolar to millimolar concentrations [54,55], making it therefore questionable how any antioxidant that only reaches nanomolar tissue concentrations can exert significant effects in the presence of much larger quantities of numerous endogenous antioxidants. To explain this contradiction, an amplification process can be postulated, where small quantities of drug induce effects that are several magnitudes larger. In fact, several in vitro and in vivo studies suggest that idebenone appears to do exactly that. In a model of galactose-induced cataract formation idebenone reduced oxidative damage by increasing total endogenous tissue antioxidant capacity [56]. This increased antioxidant capacity by idebenone is likely the consequence of an increased expression of a range of endogenous antioxidants such as superoxide dismutase (SOD), catalase, NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione (GSH), and glutathione peroxidase (GPx) [37,45,48,49,57], while also downregulating the superoxide-producing enzyme NOX2 [58]. Interestingly, some of the antioxidant genes reported to be activated by idebenone such as SOD, NQO1 and GPx are controlled by the transcription factor Nrf2. Consistent with the activation of Nrf2-dependent genes, Nrf2 activation by idebenone was observed in fibroblasts from Friedreich's Ataxia patients, although the effect was comparatively small [59]. While it is clear that idebenone requires NQO1 for its bioactivation, it is unclear if the idebenone-dependent activation of the Nrf2-NQO1 pathway occurs in all target tissues. It was therefore proposed to use idebenone in combination with other Nrf2-dependent NQO1-inducers to increase the therapeutic efficacy of idebenone in cells and tissues with low NQO1 levels [38].
Overall, the current data strongly suggest that, instead of being a direct antioxidant, idebenone increases the ability of cells to counteract oxidative stress by upregulating their physiological defence mechanisms and decreasing the production of oxidative radicals. However, there is significant doubt that protection against ROS-induced damage is the only molecular activity of idebenone that confers cytoprotection.
4. Does idebenone improve mitochondrial function and metabolism?
Several in vivo studies described protective effects of idebenone in different disease models without affecting oxidative stress. For example, idebenone protected brain function in a mouse model of Angelman syndrome without any evidence of antioxidant activity [60]. Similarly, in models of coronary syndrome, idebenone showed cardioprotective activity that was not associated with any antioxidant function but reportedly relied on an acute normalisation of mitochondrial respiration [61]; an activity that was previously described in vitro in the presence of dysfunctional complex I [33,34].
Based on the structural similarity between idebenone and CoQ10, it was always assumed that idebenone directly influences mitochondrial respiration and acts as a CoQ10 analogue. The fact that idebenone is not a CoQ10 analogue has been described in detail elsewhere [32] and is based on in vitro and in vivo data [62,63]. So, what is the evidence that idebenone affects mitochondrial function differently to CoQ10 and how does this activity protect mitochondrial function under pathological conditions? On the one hand, there is clear evidence that, unlike CoQ10, idebenone competitively inhibits complex I at higher concentrations (IC50 = 5.9 μM), while activating complex II-dependent respiration [[64], [65], [66]]. Furthermore, idebenone upregulates the glycerophosphate (G3PDH) shunt in isolated mitochondria in the presence of normal CoQ10 levels [[66], [67], [68]]. On the other hand, in line with its clinical use, idebenone (>10 μM) directly increased basal respiration in cell lines derived from mitochondrial disease patients with complex I deficiency [69].
How idebenone translates these changes to mitochondrial function into a therapeutic effect in vivo cannot, however, be explained by these activities. Similar to the notion of a direct “antioxidant” function, all direct effects of idebenone on mitochondrial function have been described for micromolar concentrations of the drug that are unlikely to be reached in the target tissues. How can this be reconciled with reports that nanomolar idebenone concentrations can alter brain energetics? In a LHON patient with the homoplastic 11,778 mtDNA mutation, P31-MRS studies indicated that idebenone reversibly improved bioenergetics in both muscle and brain [70]. Similarly, exposure of rats to idebenone significantly increased the respiratory control index (RCI) in brain mitochondria [71], which suggests that very low concentrations of idebenone can alter mitochondrial function in vivo, possibly by a similar mechanism to the described antioxidative activity involving altered gene expression. Primary hepatocytes isolated from idebenone-treated animals showed resistance to rotenone similar to cell lines cultured in vitro [33]. Interestingly, in this experiment the final treatment of animals with idebenone occurred 24 h before the cells were isolated, which implies that under these conditions idebenone was no longer present in the isolated cells [33]. This observation supports the hypothesis that transient exposure to idebenone activates a protective mechanism that persists beyond the time that the drug is present. In fact, there is some evidence that idebenone can not only upregulate mitochondrial copy numbers [72] but also affect the expression of respiratory complexes in vivo [60], which could counteract mitochondrial dysfunction to some extent. These effects alone, however, cannot easily explain the idebenone-dependent normalisation of mitochondrial function under a variety of pathological conditions such as oxidative damage [45,61], kinase inhibitors [73], mitochondrial toxins [33,34,68,74], the presence of metabolic toxins [75], hypoxia and reperfusion [21,49,61], amyloid-beta1-40 peptide [76] and different genetic defects [60,70,77].
Given the multitude of toxic stimuli that idebenone protects against, a single mitochondrial target is unlikely. Instead, it seems more likely that idebenone activates one or several fundamental pathways that confer this broad protective effect.
5. New insights
Only recently, several reports provided a completely different view on the mode of action of idebenone. These new insights could finally explain to a significant extent the at times inconsistent and contradictory literature, as well as rationalise new indications for this molecule.
5.1. Effect of idebenone metabolites
One of the main conceptual difficulties is to explain how idebenone can be protective, despite its rapid metabolic conversion. Interestingly, idebenone metabolites themselves might have some therapeutic activity. At least for the first idebenone metabolite, QS-10, where the terminal hydroxyl group of idebenone (Fig. 1) is oxidized to a carboxylic acid group, complex I bypass and CoQ10 substitution activity were reported [78]. Both activities are surprising since QS-10 is so water soluble that is should not be able to repeatedly enter the mitochondria [79]. In fact, in previous experiments QS-10 was unable to be reduced by NQO1 nor to restore ATP levels in the presence of complex I dysfunction in hepatocarcinoma cells [33]. At present it is unclear how these discrepancies can be explained. Although, cell and tissue type differences could be responsible, it must be noted that the quinone concentrations used by both studies differed significantly. While the former study [78] used 50 μM quinones (which were chemically reduced to compensate for the absence of NQO1 in the cells used), the latter used 5 μM for measuring the rescue of ATP levels. Intriguingly, protective effects by QS-10 were also observed in Zebrafish, where this metabolite was reported to be more protective against rotenone toxicity compared to idebenone which could indicate that in vivo effects are not necessarily dependent on chemical reduction [78]. Although cytoprotection is consistently reported despite a rapid metabolism of idebenone [80], this does not support the protective activity of idebenone metabolites since it is unclear at present what idebenone concentrations and for what period of time are required to initiate cytoprotection. Therefore, future studies will have to investigate the potential bioactivity of idebenone metabolites and their molecular targets in significantly more detail.
In fact, direct molecular protein targets have only recently been confirmed for idebenone. Although idebenone acts as a selective PPARα/γ agonist [81], only a small effect was observed in vivo at micromolar concentrations in zebrafish larvae, with idebenone reportedly sharing this activity with CoQ10 [81]. Since there is no evidence that idebenone itself can substitute for CoQ10 [32], its protective mode of action that sets it apart from CoQ10 must necessarily involve another molecular mechanism.
5.2. Inhibition of p52Shc
Recently, idebenone was reported to competitively inhibit the function of p52Shc [82], which acts as an adaptor protein required for a variety of molecular complexes. Most notably, p52Shc regulates signalling by protein tyrosine kinase receptors (PTK), such as the insulin receptor, where it binds to phosphorylated tyrosine residues at the cytoplasmic portion of the receptor. Idebenone was shown to bind to the phospho-tyrosine binding domain (PTB) of p52Shc, which dissociates it from the activated receptor [82]. Intriguingly, idebenone-p52Shc-binding was observed at low nanomolar concentrations and thus could for the first time explain how idebenone exerts its protective effects at physiological concentrations. As part of this molecular activity, idebenone reportedly reduced growth factor-induced extracellular signal-regulated kinase (Erk) signalling, while simultaneously over-activating Akt kinase via the IP3-kinase pathway [74,82]. Akt is a well described “survival-kinase” and controls a range of signalling pathways [83] that increase general resistance against stress [84], hypoxia [85], and drug exposure [86], while increasing insulin sensitivity [87], altering cellular metabolism [88], reducing inflammation [89], increasing lipid metabolism [90] and modifying mitochondrial function [91]; and could therefore account for some of the pleiotropic protective effects observed with idebenone.
5.3. Upregulation of Lin28A
Meanwhile, another publication provided a complementary new insight towards the mode of action of idebenone with wide ranging consequences. In a preclinical rodent model of hypoxia-reperfusion-induced vision loss, idebenone led to the retinal expression of the RNA-binding protein Lin28A, which was shown to be responsible for the observed neuroprotection [92]. Lin28A is a highly conserved regulator of many cellular RNAs that fundamentally affect metabolism, ageing, stress response, cell survival and also increase tissue repair [93,94]. The positive effect of Lin28A on tissue regeneration is mediated by increased mitochondrial function, improved glucose metabolism, increased insulin sensitivity, Akt activation and reduced autophagy [93,95,96]. Under physiological conditions, Lin28A is mostly expressed during embryogenesis but downregulated in postnatal tissues with the exception of stem cells and reproductive tissues. Since recombinant expression of Lin28A is used to generate induced pluripotent stem cells (iPSC) from adult tissues, it is tempting to speculate that an idebenone-dependent upregulation of Lin28A in adult tissue could induce some aspects of a stem cell-like phenotype associated with some regenerative capacity [93]. There is at least some evidence that idebenone can affect the fate of neural stem cells. While idebenone upregulated both neuronal (MAP2) and astrocytic (GFAP) markers in early neuronal progenitors, it downregulated MAP2 expression in neuronal stem cells and neural progenitors [72]. At present, it is unclear if this activity also translates into a rejuvenation of neuronal tissue. In support of this hypothesis, idebenone restored cortical nerve growth factor (NGF) levels in aged rats to the levels found in young rats, which was associated with restored cognitive function [97].
Interestingly, Lin28A overexpression was recently reported to promote axon regeneration in postmitotic neurons in the CNS [98]. The Lin28A-dependent regeneration of adult retinal ganglion cell axons from the retina back into the optic nerve [98] is of particular interest with regards to the clinical experience with idebenone in Leber's Hereditary Optic Neuropathy (LHON) patients [99]. Typically, response times in patients are slow, which is why the accepted consensus suggests to treat with idebenone for one year before deciding whether the drug is effective or not in a particular patient [100]. Neither restoration of mitochondrial function nor antioxidant function can explain the slow recovery typically seen in idebenone-treated LHON patients over several months. In contrast, axonal outgrowth, speculative as it might seem at present, would be consistent with the observed timelines of patient responses but at present there is no concrete experimental evidence for this possibility. It must be acknowledged that the effect of idebenone on Lin28A has only been reported by a single study [89]. Although, we could confirm this effect both in vitro and in vivo (unpublished observation), independent groups will have to reproduce and extend these observations to clarify if this effect is causally linked to the cytoprotection by idebenone.
5.4. Influence of hypoxia
One of the problems to conclusively demonstrate efficacy of idebenone in pre-clinical models as well as in patients remains a lack of consistency and especially a lack of measurable effects under normal physiological conditions. In this context, the upregulation of Lin28A by idebenone might also provide an answer to this problem since this effect seems strictly dependent on the prior presence of hypoxia-reperfusion injury [92]. This observation is in agreement with a large number of reports that demonstrated idebenone-dependent protection against hypoxia and hypoxia-reperfusion conditions [21,61,[101], [102], [103]]. Hypoxia is associated with numerous pathologies and diseases, which could explain why idebenone appears to show protective activity only in some animal models of disease and certain patients, while in other pre-clinical models and healthy individuals idebenone does not appear to have any effects. For the first time, this dependence on hypoxia could also explain the lack of consistency in pre-clinical in vitro models. Cell culture for example is notorious for the possibility of varying levels of hypoxia [104]. In vitro, hypoxia is influenced by many determinants such as metabolic activity and the number of cells per culture vessel, well geometry (volume to surface ratio), incubator vibration, handling of cultures, media age and preparation technique, which could therefore affect the activity of idebenone. The exact molecular details regarding just how the protective role of idebenone is controlled by hypoxia is unclear at present and requires further studies to delineate this interaction.
A recent study supported the hypoxia-dependent activity of idebenone in a neuroinflammatory model. Idebenone, at nanomolar to low micromolar concentrations, was only protective under hypoxia-reperfusion conditions, while under normoxic conditions it was ineffective [58]. When we confirmed the protective activity of idebenone in a rodent model of acute colitis, increased protection by idebenone was detected in the distal compared to the proximal colon [37]. The fact that oxygenation of the proximal colon is higher compared to the distal colon [105] supports the hypothesis that localised hypoxia could be a determinant for regional protection by idebenone within the same tissue. Future studies will need to investigate this possible connection in well-designed experiments to optimize the use of idebenone. Overall, p52Shc inhibition, Lin28A induction and a requirement for hypoxia provide a new theoretical framework to assess the suitability of idebenone in new indications.
5.5. Impact on inflammation
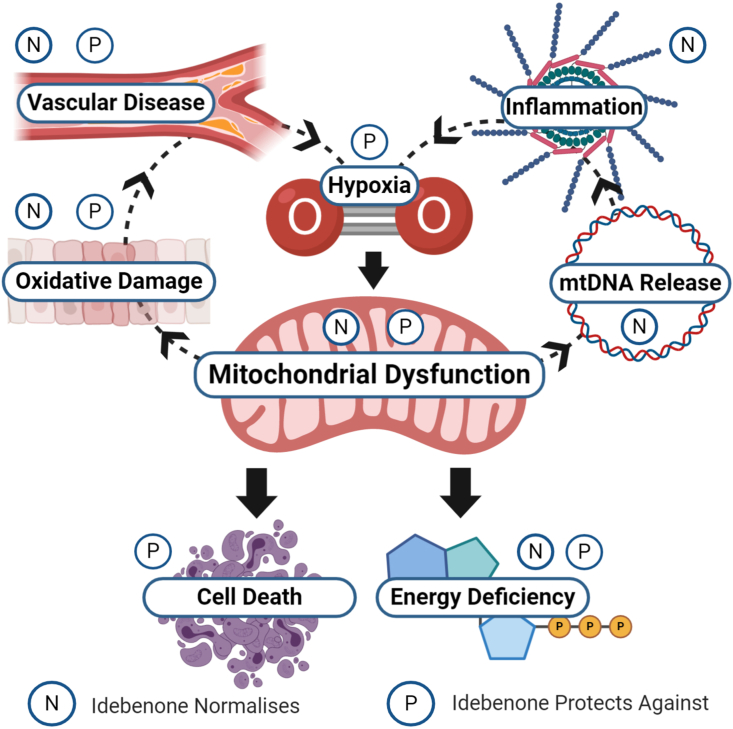
A growing body of evidence suggests that idebenone reduces inflammation in a variety of test systems such as lupus [106], neuroinflammation [58,74], ulcerative colitis [37], sepsis [107], nano-toxicity [108], atherosclerosis [48] and drug-induced inflammation [109]. This effect cannot be explained by antioxidant activity or a normalisation of energy supply alone. Instead, it was recently described that under hypoxia-reperfusion conditions, idebenone prevents the release of mtDNA and the subsequent activation of the inflammasome LNRP3, which enables idebenone to interfere with one of the earliest stages of the pro-inflammatory cascade [58]. It is important to note that inflammation is typically associated with hypoxia [110] and has a clear mitochondrial involvement [111], which suggests that inflammatory conditions could be clinically targeted with idebenone (Fig. 2).
Fig. 2.
Schematic representation of the interplay between hypoxia, mitochondrial dysfunction, oxidative damage, and inflammation. Figure was made using www.biorender.com.
5.6. Impact on ER stress
Inflammation is also tightly associated with the unfolded protein response [112] that is a characteristic physiological response to endoplasmic reticulum (ER) stress [113] and is observed in a large number of diseases. Given the close interactions between ER, mitochondria and ROS, it could be expected that idebenone might also be of benefit to patients that display ER stress. In fact, there is some evidence that idebenone might be beneficial in Wolfram Syndrome (WS), where, idebenone induced progressive but subjective visual recovery in a WS patient [114]. Given that WS cells are regarded as a perfect model to study and treat ER stress [115], this observation paved the way to test idebenone in other models associated with ER stress. In a mouse model of chronic colitis that is based on ER stress in intestinal Goblet cells, we recently observed that idebenone effectively restored tissue integrity and reduced disease symptoms [116]. This effect was associated with the downregulation of the ER stress markers CHOP, XBP-1 and ATF-6 in the distal colon and a near complete suppression of pro-inflammatory cytokines [116].
6. Conclusion
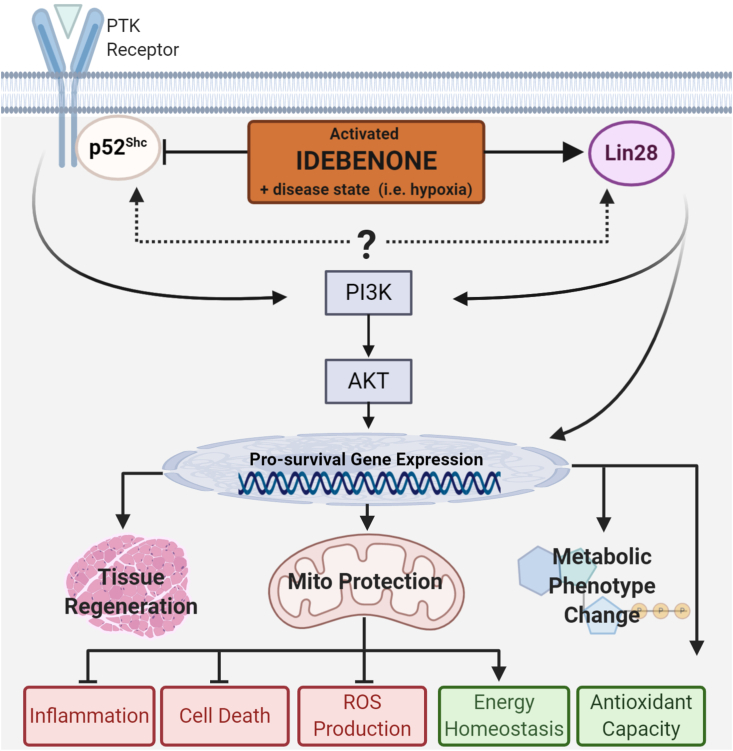
Overall, the idebenone-dependent activation of distinct signalling events that include inhibition of p52Shc, increased Lin28A expression, activation of Akt, and the subsequent transcriptional changes represent for the first time a convincing alternative to the long-held antioxidant hypothesis to explain the pleiotropic protective effects of idebenone in a large number of test systems (Fig. 3). Interestingly, the reported effects of Lin28A overexpression largely mirror the activities of p52Shc inhibition, such as Akt activation, reduced insulin resistance, altered metabolism, increased cytoprotection and changes to mitochondrial function. It is therefore tempting to speculate that both idebenone-induced events could be components of a shared pathway, which is the topic of ongoing studies. Based on a new understanding of the molecular activities and requirements of this drug, it can be expected that new indications for idebenone will be identified.
Fig. 3.
Schematic representation of the current evidence how idebenone could influence several cytoprotective mechanisms simultaneously. Figure was made using www.biorender.com.
Disclosures
NG and ER act as scientific consultants to Santhera Pharmaceuticals that developed idebenone for neuromuscular indications. Santhera had no role in the design, writing or publication of the manuscript.
Funding
This research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge the support of the University of Tasmania and Victoria University for the preparation of this manuscript.
References
- 1.Herzog A.G., Kemper T.L. Amygdaloid changes in aging and dementia. Arch. Neurol. 1980;37 doi: 10.1001/archneur.1980.00500590049006. [DOI] [PubMed] [Google Scholar]
- 2.Tachibana H., Meyer J.S., Kitagawa Y., Rogers R.L., Okayasu H., Mortel K.F. Effects of aging on cerebral blood flow in dementia. J. Am. Geriatr. Soc. 1984;32 doi: 10.1111/j.1532-5415.1984.tb05850.x. [DOI] [PubMed] [Google Scholar]
- 3.Grubb R.L., Raichle M.E., Gado M.H., Eichling J.O., Hughes C.P. Cerebral blood flow, oxygen utilization, and blood volume in dementia. Neurology. 1977;27 doi: 10.1212/wnl.27.10.905. [DOI] [PubMed] [Google Scholar]
- 4.Parkes J., Marsden C., Rees J., Curzon G., Kantamaneni B., Knill-Jones R., et al. Parkinson's disease, cerebral arteriosclerosis, and senile dementia: clinical features and response to levodopa. QJM: Int. J. Med. 1974;43 doi: 10.1093/oxfordjournals.qjmed.a067377. [DOI] [PubMed] [Google Scholar]
- 5.Marttila R.J., Rinne U.K. Dementia in Parkinson's disease. Acta Neurol. Scand. 1976;54 doi: 10.1111/j.1600-0404.1976.tb04375.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyakawa T., Sumiyoshi S., Murayama E., Deshimaru M. Ultrastructure of capillary plaque-like degeneration in senile dementia. Acta Neuropathol. 1974;29 doi: 10.1007/BF00685258. [DOI] [PubMed] [Google Scholar]
- 7.Demopoulos H., Flamm E., Pietronigro D., Seligman M. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol. Scand. 1980;110 [PubMed] [Google Scholar]
- 8.Clausen J. Demential syndromes and the lipid metabolism. Acta Neurol. Scand. 1984;70 doi: 10.1111/j.1600-0404.1984.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 9.Mellors A.a., Tappel A. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J. Biol. Chem. 1966;241 [PubMed] [Google Scholar]
- 10.Marubayashi S., Kiyohiko D., Kazuo Y., Takashi K. Changes in the levels of endogenous coenzyme Q homologs, α-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion, and the effect of pretreatment with coenzyme Q10. Biochim. Biophys. Acta Gen. Subj. 1984;797 doi: 10.1016/0304-4165(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 11.Suno M., Nagaoka A. Inhibition of brain mitochondrial swelling by idebenone. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90011-3. [DOI] [PubMed] [Google Scholar]
- 12.Suno M., Nagaoka A. Inhibition of lipid peroxidation by a novel compound (CV-2619) in brain mitochondria and mode of action of the inhibition. Biochem. Biophys. Res. Commun. 1984;125 doi: 10.1016/0006-291x(84)91389-5. [DOI] [PubMed] [Google Scholar]
- 13.Suno M., Nagaoka A. Inhibition of lipid peroxidation by idebenone in brain mitochondria in the presence of succinate. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90010-1. [DOI] [PubMed] [Google Scholar]
- 14.Suno M., Shibota M., Nagaoka A. Effects of idebenone on lipid peroxidation and hemolysis in erythrocytes of stroke-prone spontaneously hypertensive rats. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90012-5. [DOI] [PubMed] [Google Scholar]
- 15.Suno M., Terashita Z., Nagaoka A. Inhibition of platelet aggregation by idebenone and the mechanism of the inhibition. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90013-7. [DOI] [PubMed] [Google Scholar]
- 16.Nagai Y., Yoshida K., Narumi S., Tanayama S., Nagaoka A. Brain distribution of idebenone and its effect on local cerebral glucose utilization in rats. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90008-3. [DOI] [PubMed] [Google Scholar]
- 17.Kiyota Y., Hamajo K., Miyamoto M., Nagaoka A. Effect of idebenone (CV-2619) on memory impairment observed in passive avoidance task in rats with cerebral embolization. Jpn. J. Pharmacol. 1985;37 doi: 10.1254/jjp.37.300. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki N., Yomei T., Nagaoka A., Nagawa Y. Beneficial effect of idebenone (CV-2619) on cerebral ischemia-induced amnesia in rats. Jpn. J. Pharmacol. 1984;36 doi: 10.1254/jjp.36.349. [DOI] [PubMed] [Google Scholar]
- 19.Nagaoka A., Kakihana M., Fujiwara K. Effects of idebenone on neurological deficits following cerebrovascular lesions in stroke-prone spontaneously hypertensive rats. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90003-4. [DOI] [PubMed] [Google Scholar]
- 20.Nagaoka A., Shino A., Kakihana M., Iwatsuka H. Inhibitory effect of idebenone (CV-2619), a novel compound, on vascular lesions in hypertensive rats. Jpn. J. Pharmacol. 1984;36 doi: 10.1254/jjp.36.291. [DOI] [PubMed] [Google Scholar]
- 21.Kiyota Y., Miyamoto M., Nagaoka A. Protective effect of idebenone against hypoxia in mice. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90006-x. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto M., Nagaoka A. Effects of idebenone, a cerebral metabolism activator, on muricidal behavior in rats with raphe lesions. Pharmacol. Biochem. Behav. 1987;27 doi: 10.1016/0091-3057(87)90579-x. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki N., Nomura M., Nagaoka A., Nagawa Y. Idebenone improves learning and memory impairment induced by cholinergic or serotonergic dysfunction in rats. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90005-8. [DOI] [PubMed] [Google Scholar]
- 24.Nagaoka A., Suno M., Shibota M., Kakihana M. Effects of idebenone on neurological deficits, local cerebral blood flow, and energy metabolism in rats with experimental cerebral ischemia. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90002-2. [DOI] [PubMed] [Google Scholar]
- 25.Kakihana M., Yamazaki N., Nagaoka A. Effects of idebenone (CV-2619) on the concentrations of acetylcholine and choline in various brain regions of rats with cerebral ischemia. Jpn. J. Pharmacol. 1984;36 doi: 10.1254/jjp.36.357. [DOI] [PubMed] [Google Scholar]
- 26.Nagaoka A., Nagai Y., Yamazaki N., Miyamoto M., Kiyota Y. Beneficial effects of idebenone on memory impairment in rats. Drug Dev. Res. 1988;14 doi: 10.1254/jjp.36.349. [DOI] [Google Scholar]
- 27.Narumi S., Nagai Y., Kakihana M., Yamazaki N., Nagaoka A., Nagawa Y. Effects of idebenone (CV-2619) on metabolism of monoamines, especially serotonin, in the brain of normal rats and rats with cerebral ischemia. Jpn. J. Pharmacol. 1985;37 doi: 10.1254/jjp.37.235. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki N., Kiyota Y., Take Y., Miyamoto M., Nagawa Y., Nagaoka A. Effects of idebenone on memory impairment induced in ischemic and embolization models of cerebrovascular disturbance in rats. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90004-6. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama Y., Fujita T., Matsumoto M., Okamoto K., Imada I. Effects of idebenone (CV-2619) and its metabolites on respiratory activity and lipid peroxidation in brain mitochondria from rats and dogs. J. Pharmacobio-Dyn. 1985;8 doi: 10.1248/bpb1978.8.1006. [DOI] [PubMed] [Google Scholar]
- 30.Imada I., Fujita T., Sugiyama Y., Okamoto K., Kobayashi Y. Effects of idebenone and related compounds on respiratory activities of brain mitochondria, and on lipid peroxidation of their membranes. Arch. Gerontol. Geriatr. 1989;8 doi: 10.1016/0167-4943(89)90014-9. [DOI] [PubMed] [Google Scholar]
- 31.Torii H., Yoshida K., Kobayashi T., Tsukamoto T., Tanayama S. Disposition of idebenone (CV-2619), a new cerebral metabolism improving agent, in rats and dogs. J. Pharmacobio-Dyn. 1985;8 doi: 10.1248/bpb1978.8.457. [DOI] [PubMed] [Google Scholar]
- 32.Gueven N., Woolley K., Smith J. Border between natural product and drug: comparison of the related benzoquinones idebenone and coenzyme Q10. Redox Biology. 2015;4 doi: 10.1016/j.redox.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haefeli R.H., Erb M., Gemperli A.C., Robay D., Fruh I.C., Anklin C., et al. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PloS One. 2011;6 doi: 10.1371/journal.pone.0017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giorgio V., Petronilli V., Ghelli A., Carelli V., Rugolo M., Lenaz G., et al. The effects of idebenone on mitochondrial bioenergetics. Biochim. Biophys. Acta Bioenerg. 2012;1817 doi: 10.1016/j.bbabio.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaber S.M., Shealinna X.G., Milstein J.L., VanRyzin J.W., Waddell J., Polster B.M. Idebenone has distinct effects on mitochondrial respiration in cortical astrocytes compared to cortical neurons due to differential NQO1 activity. J. Neurosci. 2020;40 doi: 10.1523/JNEUROSCI.1632-17.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99 doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shastri S., Shinde T., Sohal S.S., Gueven N., Eri R. Idebenone protects against acute murine colitis via antioxidant and anti-inflammatory mechanisms. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaber S., Polster B.M. Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier? J. Bioenerg. Biomembr. 2015;47 doi: 10.1007/s10863-014-9571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montenegro L., Modica M.N., Salerno L., Panico A.M., Crascì L., Puglisi G., et al. In vitro antioxidant activity of idebenone derivative-loaded solid lipid nanoparticles. Molecules. 2017;22 doi: 10.3390/molecules22060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhai H., Cordoba-Diaz M., Wa C., Hui X., Maibach H.I. Determination of the antioxidative capacity of an antioxidant complex and idebenone: an in vitro rapid and sensitive method. J. Cosmet. Dermatol. 2008;7 doi: 10.1111/j.1473-2165.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso S.M., Pereira C., Oliveira C.R. The protective effect of vitamin E, idebenone and reduced glutathione on free radical mediated injury in rat brain synaptosomes. Biochem. Biophys. Res. Commun. 1998;246 doi: 10.1006/bbrc.1998.8563. [DOI] [PubMed] [Google Scholar]
- 42.Semsei I., Nagy K., Nagy I.Z.- vol. 11. Archives of Gerontology and Geriatrics; 1990. (In Vitro Studies on the OH• and O2−• Free Radical Scavenger Properties of Idebenone in Chemical Systems). [DOI] [PubMed] [Google Scholar]
- 43.Rauchová H., Vrbacký M., Bergamini C., Fato R., Lenaz G., Houštěk J., et al. Inhibition of glycerophosphate-dependent H2O2 generation in brown fat mitochondria by idebenone. Biochem. Biophys. Res. Commun. 2006;339 doi: 10.1016/j.bbrc.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 44.Mordente A., Martorana G.E., Minotti G., Giardina B. Antioxidant properties of 2, 3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1, 4-benzoquinone (idebenone) Chem. Res. Toxicol. 1998;11 doi: 10.1021/tx970136j. [DOI] [PubMed] [Google Scholar]
- 45.Lin P., Liu J., Ren M., Ji K., Li L., Zhang B., et al. Idebenone protects against oxidized low density lipoprotein induced mitochondrial dysfunction in vascular endothelial cells via GSK3β/β-catenin signalling pathways. Biochem. Biophys. Res. Commun. 2015;465 doi: 10.1016/j.bbrc.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 46.Fash D.M., Khdour O.M., Sahdeo S.J., Goldschmidt R., Jaruvangsanti J., Dey S., et al. Effects of alkyl side chain modification of coenzyme Q10 on mitochondrial respiratory chain function and cytoprotection. Bioorg. Med. Chem. 2013;21 doi: 10.1016/j.bmc.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 47.Ranganathan S., Harmison G.G., Meyertholen K., Pennuto M., Burnett B.G., Fischbeck K.H. Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2009;18 doi: 10.1093/hmg/ddn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang W., Geng H., Lv X., Ma J., Liu F., Lin P., et al. Idebenone protects against atherosclerosis in apolipoprotein E-deficient mice via activation of the Sirt3-SOD2-mtROS pathway. Cardiovasc. Drugs Ther. 2020 doi: 10.1007/s10557-020-07018-5. [DOI] [PubMed] [Google Scholar]
- 49.Baky N.A.A., Zaidi Z.F., Fatani A.J., Sayed-Ahmed M.M., Yaqub H. Nitric oxide pros and cons: the role of l-arginine, a nitric oxide precursor, and idebenone, a coenzyme-Q analogue in ameliorating cerebral hypoxia in rat. Brain Res. Bull. 2010;83 doi: 10.1016/j.brainresbull.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama H., Tsuchihashi N., Ogata T., Hiramatsu M., Mori N. An analysis of the intracerebral ability to eliminate a nitroxide radical in the rat after administration of idebenone by anin vivo rapid scan electron spin resonance spectrometer. Magnetic Resonance Materials in Physics, Biology and Medicine. 1996;4 doi: 10.1007/BF01772013. [DOI] [PubMed] [Google Scholar]
- 51.Heitz F.D., Erb M., Anklin C., Robay D., Pernet V., Gueven N. Idebenone protects against retinal damage and loss of vision in a mouse model of Leber's hereditary optic neuropathy. PloS One. 2012;7 doi: 10.1371/journal.pone.0045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boni J., Maugeri A., Zingali G., Ramelli L., Gherardi S. Steady-state pharmacokinetics of idebenone in healthy volunteers. Arch. Gerontol. Geriatr. 1992;15 doi: 10.1016/0167-4943(92)90055-9. [DOI] [PubMed] [Google Scholar]
- 53.Becker C., Bray-French K., Drewe J. Pharmacokinetic evaluation of idebenone. Expet Opin. Drug Metabol. Toxicol. 2010;6 doi: 10.1517/17425255.2010.530656. [DOI] [PubMed] [Google Scholar]
- 54.Kucharská J., Gvozdjáková A., Simko F. Simvastatin decreased coenzyme Q in the left ventricle and skeletal muscle but not in the brain and liver in L-NAME-induced hypertension. Physiol. Res. 2007;56 doi: 10.33549/physiolres.931397. [DOI] [PubMed] [Google Scholar]
- 55.Wu G., Fang Y.-Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134 doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 56.Sadik N.A.H., El‐Boghdady N.A., Omar N.N., Al‐Hamid H.A. Esculetin and idebenone ameliorate galactose‐induced cataract in a rat model. J. Food Biochem. 2020 doi: 10.1111/jfbc.13230. [DOI] [PubMed] [Google Scholar]
- 57.Nagy K., Zs-Nagy I. The effects of idebenone on the superoxide dismutase, catalase and glutathione peroxidase activities in liver and brain homogenates, as well as in brain synaptosomal and mitochondrial fractions. Arch. Gerontol. Geriatr. 1990;11 doi: 10.1016/0167-4943(90)90073-F. [DOI] [PubMed] [Google Scholar]
- 58.Peng J., Wang H., Gong Z., Li X., He L., Shen Q., et al. Idebenone attenuates cerebral inflammatory injury in ischemia and reperfusion via dampening NLRP3 inflammasome activity. Mol. Immunol. 2020;123 doi: 10.1016/j.molimm.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Petrillo S., D'Amico J., La Rosa P., Bertini E.S., Piemonte F. Targeting NRF2 for the treatment of Friedreich's ataxia: a comparison among drugs. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Llewellyn K.J., Nalbandian A., Gomez A., Wei D., Walker N., Kimonis V.E. Administration of CoQ10 analogue ameliorates dysfunction of the mitochondrial respiratory chain in a mouse model of Angelman syndrome. Neurobiol. Dis. 2015;76 doi: 10.1016/j.nbd.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Perry J.B., Davis G.N., Allen M.E., Makrecka-Kuka M., Dambrova M., Grange R.W., et al. Cardioprotective effects of idebenone do not involve ROS scavenging: evidence for mitochondrial complex I bypass in ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2019;135 doi: 10.1016/j.yjmcc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 62.López L.C., Quinzii C.M., Area E., Naini A., Rahman S., Schuelke M., et al. Treatment of CoQ10 deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PloS One. 2010;5 doi: 10.1371/journal.pone.0011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haginoya K., Miyabayashi S., Kikuchi M., Kojima A., Yamamoto K., Omura K., et al. Efficacy of idebenone for respiratory failure in a patient with Leigh syndrome: a long-term follow-up study. J. Neurol. Sci. 2009;278 doi: 10.1016/j.jns.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Watzke N., Diekert K., Obrdlik P. Electrophysiology of respiratory chain complexes and the ADP−ATP exchanger in native mitochondrial membranes. Biochemistry. 2010;49 doi: 10.1021/bi1011755. [DOI] [PubMed] [Google Scholar]
- 65.Brière J.-J., Schlemmer D., Chretien D., Rustin P. Quinone analogues regulate mitochondrial substrate competitive oxidation. Biochem. Biophys. Res. Commun. 2004;316 doi: 10.1016/j.bbrc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Esposti M.D., Ngo A., Ghelli A., Benelli B., Carelli V., McLennan H., et al. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch. Biochem. Biophys. 1996;330 doi: 10.1006/abbi.1996.0267. [DOI] [PubMed] [Google Scholar]
- 67.Rauchová H., Drahota Z., Bergamini C., Fato R., Lenaz G. Modification of respiratory-chain enzyme activities in brown adipose tissue mitochondria by idebenone (hydroxydecyl-ubiquinone) J. Bioenerg. Biomembr. 2008;40 doi: 10.1007/s10863-008-9134-1. [DOI] [PubMed] [Google Scholar]
- 68.Rauchová H., Vokurková M., Drahota Z. Idebenone-induced recovery of glycerol-3-phosphate and succinate oxidation inhibited by digitonin. Physiol. Res. 2012;61 doi: 10.33549/physiolres.932318. [DOI] [PubMed] [Google Scholar]
- 69.Chin R.M., Panavas T., Brown J.M., Johnson K.K. Patient-derived lymphoblastoid cell lines harboring mitochondrial DNA mutations as tool for small molecule drug discovery. BMC Res. Notes. 2018;11 doi: 10.1186/s13104-018-3297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortelli P., Montagna P., Pierangeli G., Lodi R., Barboni P., Liguori R., et al. Clinical and brain bioenergetics improvement with idebenone in a patient with Leber's hereditary optic neuropathy: a clinical and 31P-MRS study. J. Neurol. Sci. 1997;148 doi: 10.1016/S0022-510X(96)00311-5. [DOI] [PubMed] [Google Scholar]
- 71.Sugiyama Y., Fujita T. Stimulation of the respiratory and phosphorylating activities in rat brain mitochondria by idebenone (CV-2619), a new agent improving cerebral metabolism. FEBS Lett. 1985;184 doi: 10.1016/0014-5793(85)80650-5. [DOI] [PubMed] [Google Scholar]
- 72.Augustyniak J., Lenart J., Zychowicz M., Stepien P.P., Buzanska L. Mitochondrial biogenesis and neural differentiation of human iPSC is modulated by idebenone in a developmental stage-dependent manner. Biogerontology. 2017;18 doi: 10.1007/s10522-017-9718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gil J., Almeida S., Oliveira C.R., Rego A.C. Cytosolic and mitochondrial ROS in staurosporine-induced retinal cell apoptosis. Free Radic. Biol. Med. 2003;35 doi: 10.1016/j.freeradbiomed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Yan A., Liu Z., Song L., Wang X., Zhang Y., Wu N., et al. Idebenone alleviates neuroinflammation and modulates microglial polarization in LPS-stimulated BV2 cells and MPTP-induced Parkinson's disease mice. Front. Cell. Neurosci. 2019;12 doi: 10.3389/fncel.2018.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H., Xu Z., Wu A., Dong Y., Zhang Y., Yue Y., et al. 2-Deoxy-D-Glucose enhances anesthetic effects in mice. Anesth. Analg. 2015;120 doi: 10.1213/ane.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirai K., Hayako H., Kato K., Miyamoto M. Idebenone protects hippocampal neurons against amyloid β-peptide-induced neurotoxicity in rat primary cultures. N. Schmied. Arch. Pharmacol. 1998;358 doi: 10.1007/pl00005296. [DOI] [PubMed] [Google Scholar]
- 77.Angebault C., Gueguen N., Desquiret-Dumas V., Chevrollier A., Guillet V., Verny C., et al. Idebenone increases mitochondrial complex I activity in fibroblasts from LHON patients while producing contradictory effects on respiration. BMC Res. Notes. 2011;4 doi: 10.1186/1756-0500-4-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giorgio V., Schiavone M., Galber C., Carini M., Da Ros T., Petronilli V., et al. The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects. Biochim. Biophys. Acta Bioenerg. 2018/09/01/2018;1859 doi: 10.1016/j.bbabio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Erb M., Hoffmann-Enger B., Deppe H., Soeberdt M., Haefeli R.H., Rummey C., et al. Features of idebenone and related short-chain quinones that rescue ATP levels under conditions of impaired mitochondrial complex I. PloS One. 2012;7 doi: 10.1371/journal.pone.0036153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng Z., Smith J., Güven N., Quirino J. Metabolic stability of new mito-protective short-chain naphthoquinones. Pharmaceuticals. 2020;13 doi: 10.3390/ph13020029. 02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tiefenbach J., Magomedova L., Liu J., Reunov A.A., Tsai R., Eappen N.S., et al. Idebenone and coenzyme Q10 are novel PPARα/γ ligands, with potential for treatment of fatty liver diseases. Disease Models & Mechanisms. 2018;11 doi: 10.1242/dmm.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomilov A., Allen S., Hui C.K., Bettaieb A., Cortopassi G. Idebenone is a cytoprotective insulin sensitizer whose mechanism is Shc inhibition. Pharmacol. Res. 2018;137 doi: 10.1016/j.phrs.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 83.Ersahin T., Tuncbag N., Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015;11 doi: 10.1039/C5MB00101C. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z., Yao L., Yang J., Wang Z., Du G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol. Med. Rep. 2018;18 doi: 10.3892/mmr.2018.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kovacs K., Vaczy A., Fekete K., Kovari P., Atlasz T., Reglodi D., et al. PARP inhibitor protects against chronic hypoxia/reoxygenation-induced retinal injury by regulation of MAPKs, HIF1α, Nrf2, and NFκB. Invest. Ophthalmol. Vis. Sci. 2019;60 doi: 10.1167/iovs.18-25936. [DOI] [PubMed] [Google Scholar]
- 86.Gallyas F., Jr., Sumegi B., Szabo C. Role of Akt activation in PARP inhibitor resistance in cancer. Cancers. 2020;12 doi: 10.3390/cancers12030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang N., Liu X., Zhuang L., Liu X., Zhao H., Shan Y., et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: dual regulation of the PI3K/AKT and MAPK pathways. Regul. Toxicol. Pharmacol. 2020;110 doi: 10.1016/j.yrtph.2019.104544. [DOI] [PubMed] [Google Scholar]
- 88.Yang Q., Vijayakumar A., Kahn B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018;19 doi: 10.1038/s41580-018-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y., Tie L. Apolipoprotein M and sphingosine-1-phosphate complex alleviates TNF-α-induced endothelial cell injury and inflammation through PI3K/AKT signaling pathway. BMC Cardiovasc. Disord. 2019;19 doi: 10.1186/s12872-019-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krycer J.R., Sharpe L.J., Luu W., Brown A.J. The Akt–SREBP nexus: cell signaling meets lipid metabolism. Trends in Endocrinology & Metabolism. 2010;21 doi: 10.1016/j.tem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Chae Y.C., Vaira V., Caino M.C., Tang H.-Y., Seo J.H., Kossenkov A.V., et al. Mitochondrial Akt regulation of hypoxic tumor reprogramming. Canc. Cell. 2016;30 doi: 10.1016/j.ccell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lei D., Shao Z., Zhou X., Yuan H. Synergistic neuroprotective effect of rasagiline and idebenone against retinal ischemia-reperfusion injury via the Lin28-let-7-Dicer pathway. Oncotarget. 2018;9 doi: 10.18632/oncotarget.24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shyh-Chang N., Zhu H., Yvanka de Soysa T., Shinoda G., Seligson Marc T., Tsanov Kaloyan M., et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155 doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peter W. Reddien, "Lin28: time for tissue repair. Cell. 2013;155 doi: 10.1016/j.cell.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 95.Zhu H., Shyh-Chang N., Segrè Ayellet V., Shinoda G., Shah Samar P., Einhorn William S., et al. The lin28/let-7 Axis regulates glucose metabolism. Cell. 2011;147 doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burkhalter M.D., Morita Y., Rudolph K.L. Lin28a – boost your energy for youthful regeneration. EMBO J. 2014;33 doi: 10.1002/embj.201387363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nabeshima T., Nitta A., Fuji K., Kameyama T., Hasegawa T. Oral administration of NGF synthesis stimulators recovers reduced brain NGF content in aged rats and cognitive dysfunction in basal – forebrain – lesioned rats. Gerontology. 1994;40 doi: 10.1159/000213627. suppl 2. [DOI] [PubMed] [Google Scholar]
- 98.Wang X.-W., Li Q., Liu C.-M., Hall P.A., Jiang J.-J., Katchis C.D., et al. Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell Rep. 2018;24 doi: 10.1016/j.celrep.2018.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klopstock T., Yu-Wai-Man P., Dimitriadis K., Rouleau J., Heck S., Bailie M., et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134 doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carelli V., Carbonelli M., de Coo I.F., Kawasaki A., Klopstock T., Lagrèze W.A., et al. International consensus statement on the clinical and therapeutic management of leber hereditary optic neuropathy. J. Neuro Ophthalmol. 2017;37 doi: 10.1097/wno.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 101.Wieland E., Oellerich M., Braun F., Schütz E. c-fos and c-jun mRNA expression in a pig liver model of ischemia/reperfusion: effect of extended cold storage and the antioxidant idebenone. Clin. Biochem. 2000;33 doi: 10.1016/S0009-9120(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 102.Chapela S.P., Burgos H.I., Salazar A.I., Nievas I., Kriguer N., Stella C.A. Biochemical study of idebenone effect on mitochondrial metabolism of yeast. Cell Biol. Int. 2008;32 doi: 10.1016/j.cellbi.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Ali S.A., Aly H.F., Faddah L.M., Zaidi Z.F. Dietary supplementation of some antioxidants against hypoxia. World J. Gastroenterol. 2012;18 doi: 10.3748/wjg.v18.i44.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Place T.L., Domann F.E., Case A.J. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017;113 doi: 10.1016/j.freeradbiomed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 2015;309 doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blanco L.P., Pedersen H.L., Wang X., Lightfoot Y.L., Seto N., Carmona-Rivera C., et al. Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis & Rheumatology. 2020;72 doi: 10.1002/art.41128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hill A.L., Lowes D.A., Webster N.R., Sheth C.C., Gow N.A.R., Galley H.F. Regulation of pentraxin-3 by antioxidants. Br. J. Anaesth. 2009;103 doi: 10.1093/bja/aep298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fadda L.M., Hagar H., Mohamed A.M., Ali H.M. Quercetin and idebenone ameliorate oxidative stress, inflammation, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in rat liver. Dose-Response. 2018;16 doi: 10.1177/1559325818812188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lauro F., Ilari S., Giancotti L.A., Ventura C.A., Morabito C., Gliozzi M., et al. Pharmacological effect of a new idebenone formulation in a model of carrageenan-induced inflammatory pain. Pharmacol. Res. 2016;111 doi: 10.1016/j.phrs.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 110.McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018;125 doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 111.Aguilar-López B.A., Moreno-Altamirano M.M.B., Dockrell H.M., Duchen M.R., Sánchez-García F.J. Mitochondria: an integrative hub coordinating circadian rhythms, metabolism, the microbiome, and immunity. Frontiers in Cell and Developmental Biology. 2020;8 doi: 10.3389/fcell.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li W., Cao T., Luo C., Cai J., Zhou X., Xiao X., et al. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020;104 doi: 10.1007/s00253-020-10614-y. [DOI] [PubMed] [Google Scholar]
- 113.Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016;16 doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bababeygy S., Wang M., Khaderi K., Sadun A. Visual improvement with the use of idebenone in the treatment of Wolfram syndrome. J. Neuro Ophthalmol. 2012;32 doi: 10.1097/WNO.0b013e318273c102. [DOI] [PubMed] [Google Scholar]
- 115.Pallotta M.T., Tascini G., Crispoldi R., Orabona C., Mondanelli G., Grohmann U., et al. Wolfram syndrome, a rare neurodegenerative disease: from pathogenesis to future treatment perspectives. J. Transl. Med. 2019;17 doi: 10.1186/s12967-019-1993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shastri S., Shinde T., Perera A.P., Gueven N., Eri R. Idebenone protects against spontaneous chronic murine colitis by alleviating endoplasmic reticulum stress and inflammatory response. Biomedicines. 2020 doi: 10.3390/biomedicines8100384. [DOI] [PMC free article] [PubMed] [Google Scholar]