Abstract
In insects and other ectotherms, cold temperatures cause a coma resulting from loss of neuromuscular function, during which ionic and metabolic homeostasis are progressively lost. Cold adaptation improves homeostasis during cold exposure, but the ultimate targets of selection are still an open question. Cold acclimation and adaptation remodels mitochondrial metabolism in insects, suggesting that aerobic energy production during cold exposure could be a target of selection. Here, we test the hypothesis that cold adaptation improves the ability to maintain rates of aerobic energy production during cold exposure by using 31P NMR on live flies. Using lines of Drosophila melanogaster artificially selected for fast and slow recovery from a cold coma, we show that cold exposure does not lower ATP levels and that cold adaptation does not alter aerobic ATP production during cold exposure. Cold-hardy and cold-susceptible lines both experienced a brief transition to anaerobic metabolism during cooling, but this was rapidly reversed during cold exposure, suggesting that oxidative phosphorylation was sufficient to meet energy demands below the critical thermal minimum, even in cold-susceptible flies. We thus reject the hypothesis that performance under mild low temperatures is set by aerobic ATP supply limitations in D. melanogaster, excluding oxygen and capacity limitation as a weak link in energy supply. This work suggests that the modulations to mitochondrial metabolism resulting from cold acclimation or adaptation may arise from selection on a biosynthetic product(s) of those pathways rather than selection on ATP supply during cold exposure.
Keywords: chill coma, energetics, insect, oxygen limitation, thermal tolerance
INTRODUCTION
Temperature is a key factor determining the fundamental niche of an organism, and a predictive understanding of how temperature sets limits on life is essential because temperatures are rapidly shifting globally. Extreme low temperatures determine poleward range limits of many animals and plants (Overgaard et al. 2014; Williams et al. 2015), and mitigation of extreme low temperatures is facilitating poleward range shifts (Crozier 2004). However, what constitutes an “extreme” differs markedly between organisms, making it challenging to link projected changes in climate to organismal responses. An understanding of the functional mechanisms underlying responses to climate extremes is essential to predicting impacts of climate change on animal distributions, and can aid in identifying precise conditions under which populations will be most challenged under predicted climate changes (Pörtner 2001; Huey et al. 2012; Williams et al. 2017). Functional understanding is equally important to identifying the generality or lack thereof of specific mechanisms underlying loss of organismal function (Somero 2010). Pinpointing “weak links” in energy supply and demand chains could shed light on conditions that will have adverse effects on a wide range of organisms.
At temperatures below the lower thermal limit for coordinated movement (CTmin), insects enter a cold-induced coma (chill coma) resulting from loss of neuromuscular function, a state that is reversible upon the return of permissive temperatures (Overgaard & Mac-Millan 2017). While in chill coma, insects suffer a progressive loss of osmotic and ionic homeostasis that eventually leads to chill injury and death (Overgaard & MacMillan 2017). The time to recover coordinated movement after chill coma (chill coma recovery time) is a common metric of insect cold hardiness, and chill coma recovery time is directly related to the degree of osmotic and ionic disequilibrium incurred during cold. Cold acclimation, acclimatization and adaptation reduce the chill coma recovery time, but the ultimate cellular and biochemical targets of selection are unknown. In this study, we test the hypothesis that the ability to supply ATP aerobically during cold exposure is improved as a consequence of cold adaptation.
Variation in chill coma recovery time is associated with alterations to membrane composition, improved io-no-regulation and osmo-regulation, and protection or repair of macromolecular stability (Koštál 2010; MacMillan & Sinclair 2011; Overgaard & MacMillan 2017). All of these processes require energy in the form of ATP and building blocks in the form of proteins, carbohydrates and amino acids. Recovery from chill coma is energetically expensive, causing the metabolic rate to approximately double in a cricket species during 2-h recovery from a prolonged cold exposure, and energetic costs increase with the duration of cold exposure (Mac-Millan et al. 2012b). The mitochondria are the hub of cellular energy generation and biosynthesis, suggesting that failure of the mitochondrial electron transport chain to meet ATP demands during cold exposure and recovery at low temperature could compromise both energy and substrate availability, thus limiting the ability to maintain homeostasis and becoming a target of selection during cold adaptation. Cold acclimation improves mitochondrial function in the cold in Drosophila melanogaster Meigen, 1830 (Colinet et al. 2017). Similarly, lines selected for fast chill coma recovery time have evolved elevated rates of respiratory gas exchange, bio-synthesis and substrate oxidation at warm temperatures, and depressed rates during cold exposure, leading to greater plasticity of aerobic metabolism (Williams et al. 2016a,b). However, several studies have suggested that ATP levels do not drop during mild non-lethal cold exposure in insects, and in some cases increase (Pullin & Bale 1988; Pullin et al. 1990; Colinet 2011; MacMillan et al. 2012a). Physiological acclimation to cold and treatments that improve survival do not impact ATP levels in the cold, further suggesting that ATP supply is not limited in the cold (Koštál et al. 2004; Colinet 2011). However, no studies have tested for alterations to ATP homeostasis in the cold as a result of cold adaptation.
High-energy phosphorus-containing molecules including ATP are the primary energy currency of the cell. In aerobic organisms, ATP is chiefly supplied through oxidative phosphorylation in the electron-transport chain. During periods of high ATP demand or oxygen limitation, ATP concentrations can be buffered using phosphate transfer from the phosphagen pool, comprising creatine phosphate in vertebrates and arginine phosphate in many invertebrates, including insects (Ellington 2001; Nation 2008). This prevents ATP concentration from falling for a period of time, until the phosphagen pool becomes depleted. A failure of oxidative phosphorylation to meet the energetic needs of the cells can, thus, be detected by a decrease in the phosphagen pool and increase in inorganic phosphate. Levels of ATP, phosphagens and inorganic phosphate can be estimated using 31P nuclear magnetic resonance (NMR) spectroscopy and used to reconstruct the energetic status of cells or whole organisms (O’Neill & Richards 1980). This sensitive and non-invasive technique has previously been applied in live insects and amphibians to illustrate a decline in ATP and corresponding loss of energetic homeostasis during severe cold exposure and freezing (Pullin et al. 1990; Coulson et al. 1992; Layne & Kennedy 2002).
The main goal of this study was to assess whether the ability to maintain energy status during cold exposure and recovery was altered by cold-hardiness adaptation in lines of D. melanogaster artificially selected for fast and slow recovery from chill coma. We dynamically monitored phosphorus homeostasis using 31P-NMR during the course of cold exposure and recovery in cold-hardy versus cold-susceptible flies. If modulation of ATP supply during cold exposure and recovery underlies variation in chill coma recovery time, we predict that cold-hardy compared to cold-susceptible flies will maintain ATP homeostasis better during cold exposure and recovery, and will experience less of a transition to anaerobic metabolism (indicated by a transfer of phosphorus from phosphoarginine to inorganic phosphate). Alternatively, the metabolic remodeling that we have documented in D. melanogaster (Williams et al. 2014, 2016a,b) could primarily serve to increase metabolic turnover during cold exposure and recovery, without impacting energy state.
MATERIALS AND METHODS
Nuclear magnetic resonance experiments
Inbred lines of D. melanogaster flies originating from a mid-latitude population and selected for fast or slow recovery from chill coma were reared as previously described (Williams et al. 2014; Gerken et al. 2016). On day 3 of adulthood, mated females were sorted under light CO2 anesthesia, and allowed to recover for 2 days. All experiments were carried out in the Advanced Magnetic Resonance and Spectroscopy facility (AMRIS) at the University of Florida, on a Bruker Avance III 600 MHz spectrometer with a 5-mm Broadband probe operating at 242.89 MHz for 31P. Sixty live flies were aspirated into a 5-mm NMR tube (Wilmad LabGlass, Vineland, NJ, USA), held in place with deuterium oxide-matched susceptibility plugs (Doty susceptibility plugs, Wilmad LabGlass), and lowered into the temperature-controlled probe. We were not able to add a standard (e.g. methylene diphosphoric acid), because the solvents were toxic to the flies. We experimented with using concentric tubes to allow a standard to surround the flies without touching them, but evaporation precluded this arrangement. Therefore, we are not able to perform absolute quantitation, but instead normalize to total phosphorus and compare the relative size of the resonances. There was no difference in total phosphorus content between cold-hardy and cold-susceptible flies (ANOVA, F1,15 = 0.183, P = 0.675).
We constructed a nutation curve at 25 °C to find the correct 90° pulse angle, by sequentially increasing the pulse width and identifying the null at 180°. We measured T1 relaxation times at 0, 8.3, 16.7 and 25 °C on fly metabolites using an inversion recovery experiment with a T1IR pulse sequence and the following parameters: 0.0524 s acquisition, 16.5 μs (90°) pulse width at 33W, 2048 data points per free induction decay, 8 dummy scans, and 14 delay times varying from 0.1 to 5.0 s.
We performed an Ernst angle experiment to determine the optimal experimental parameters to maximize signal to noise within the constraints of our desired total experiment time (15 min, chosen to allow sufficient temporal resolution over the course of cold exposure and recovery). The delay time that optimized signal to noise at that sampling interval did not allow resonances to fully relax between pulses, which can lead to errors in quantitation if target resonances differ in T1 relaxation times because the magnetization does not return to equilibrium between pulses. We therefore conducted a series of experiments to correct for this effect and validate our results. Once the optimal pulse width and delay times had been determined, we carried out experiments at each of the 4 experimental temperatures (0, 8.3, 16.8 and 25 °C), wherein we collected spectra under experimental (short delay) conditions (described below), and then immediately afterwards conducted an experiment with identical parameters except with a 7-s delay (equal to 5 × T1 for the resonance with the longest T1 relaxation time) to allow complete relaxation (and, thus, accurate quantitation) of each resonance. The total experiment time for the completely relaxed experiment was 30 min. Processed data (processing described in next section) were normalized to total phosphorus, and then each peak was integrated to compare peak integrals between long-delay and short-delay conditions.
Experiment 1: Short delay acquisition
Experiment 1 was designed for high temporal resolution, but entails some inaccuracies in quantitation. We used 4–5 replicate pools of 60 flies from each line (2 cold-hardy and 2 cold-susceptible). Flies were aspirated into 5-mm NMR tubes as described above and lowered into a temperature-controlled probe at 25 °C. We collected 31P spectra on the live flies using the following parameters: 0.0319-s acquisition, 45° pulse (8.25 μs at 33W), 1248 data points per free induction decay, 4 dummy scans, 10 240 scans and 0.02-s relaxation delay. Each experiment took 14.5 min. After a spectrum was collected at 25 °C, we ramped the temperatures rapidly down to 0 °C in 8.3 °C increments, collecting a spectrum at each thermal increment during cooling (Fig. 1a). We collected 13 spectra at 0 °C (summing to 3 h 8 min cold exposure), then rewarmed in 8.3 °C increments, again collecting a spectrum at each thermal increment during rewarming (Fig. 1a). We performed a control experiment with 1 additional pool of 60 flies that were kept in a sealed 1-mL tube at 25 °C for 21 h during which time spectra were continuously collected as described above, to test the sensitivity of the instrument to detect ATP declines during a prolonged hypoxic period.
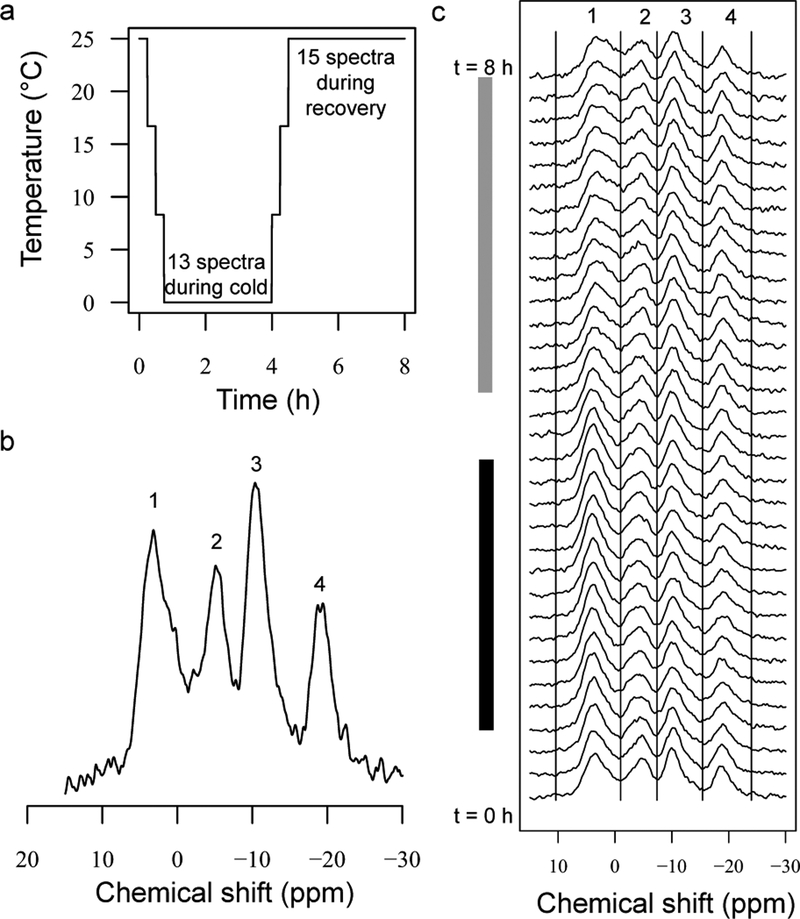
Figure 1.
Experimental design and example 31P spectra of live Drosophila melanogaster during cold exposure (Experiment 1). a) Temperatures were lowered from 25 to 0 °C in 8.3 °C increments, with 31P spectra (15 min/spectrum) taken at each increment. Each horizontal line portion represents one spectrum, unless otherwise noted. Spectra were collected continuously during a 3 h cold exposure at 0 °C and 3.75 h recovery at 25 °C. b) Annotated spectrum showing the four resolved resonances: Peak 1: sugar phosphates, phospholipids, inorganic phosphate; Peak 2: phosphoarginine, γATP, and βADP; Peak 3: αATP and αADP; Peak 4: βATP. c) Spectra collected throughout experiment for the same sample (group of flies) from panel B. Time 0 is at the bottom. The cold exposure at 0 °C is marked with a black bar on the left, recovery at 25 °C with a grey bar, and regions over which peaks were integrated are marked with vertical lines.
Experiment 2: Long delay acquisition
Experiment 2 has less temporal resolution than Experiment 1, but more accurate quantitation. All procedures were the same as Experiment 1, with the following modifications: 0.0509-s acquisition, 90° pulse (16.5 μs at 33 W), 1990 data points per free induction decay, 1 dummy scan, 256 scans and 7-s relaxation delay (5 × longest T1). Total experiment time was 30 min. We used 3 replicate pools of 60 flies from each line (2 cold-hardy and 2 cold-susceptible). We took 1 spectrum at 25 °C, immediately lowered the temperature to 0 °C and collected 6 spectra (3-h cold exposure), then rewarmed to 25 °C and took 1 spectrum.
Data processing and analysis
Data preprocessing was performed using TopSpin 3.2 (Bruker BioSpin, Rheinstetten, Germany). All downstream analyses were performed in R version 3.4.1 (R Core Team 2016). We Fourier-transformed the raw free induction decay into 2048 real data points following application of an exponential window function with 100 Hz line broadening, manually phased, and baseline corrected the spectra. For Experiment 1, pre-processed Bruker spectra were imported into R using the BATMAN package (Hao et al. 2012). Our intention was to use the package for peak deconvolution and quantitation so that we could estimate ATP, ADP and AMP separately, but due to inadequate reference spectra we were not able to deconvolute the overlapping peaks. We normalized each spectrum to total phosphorus to control for differences in the mass of flies in the radio-frequency coil. For each individual sample of flies in Experiment 1, we subtracted spectra of 1 time point from spectra taken at a subsequent time point, generating difference spectra that we used to visualize changes in metabolites between time points. We concentrated on the following differences: (1) beginning of cold exposure – control spectrum (Cooling); (2) end of cold – beginning of cold (Cold); (3) beginning of recovery – end of cold (Re-warming); and (4) end of recovery – end of cold (Recovery). We averaged the difference spectra within cold hardiness level (hardy and susceptible flies, 2 replicate lines with N = 4 pools of 60 flies/line), and calculated 95% confidence intervals. We integrated areas under each peak by summing all intensities across the range of chemical shifts encompassing each peak (marked in Fig. 1c), effectively collapsing each spectrum into a single value for each of 4 peaks for statistical analysis. Preliminary data quality checking was performed according to Zuur et al. (2010). We analyzed each time period separately (Cooling, Cold, Rewarming and Recovery as described above) using general linear mixed models fitted using the lme4 package (Bates et al. 2011) with P-values obtained using degrees of freedom obtained by Satterthwaite approximation in lmerTest (Kuznetsova et al. 2015). General models included fixed effects of time (continuous covariate) and cold hardiness (categorical: cold-hardy or cold-susceptible) and their interaction, as well as a random effect of sample nested in replicate selection line. Our general modeling approach was to start with maximal models containing all terms, and to simplify stepwise using the criteria that the simplified model should be retained if a likelihood ratio test showed a P-value >0.05, indicating no significant loss of information, until the minimal adequate model was reached (Crawley 2007). We checked for violations of model assumptions by plotting fitted values against residuals.
For Experiment 2, integrations of peak regions 1–4 were done manually using standard TopSpin 3.2 integration routines and the peak integrals imported into R for analysis. We summed the 4 peaks for each spectrum and normalized to total phosphorus to account for differences in mass of flies across samples. We analyzed effects of cold exposure and cold adaptation on the response of each peak to cold using the analysis framework previously described for Experiment 1.
RESULTS
Validation of nuclear magnetic resonance procedures
The 90° pulse width was 16.5 μs at 33 W. We obtained spectra with approximately 1000-Hz line widths that showed 4 distinct peaks, similar to spectra collected for other insect species (Fig. 1b) (Storey et al. 1984; Pullin et al. 1990; Coulson et al. 1992). Resonances were assigned with reference to previous studies: peak 1: sugar phosphates, inorganic phosphate, phosphomonoesters or diesters including membrane lipids; peak 2: phosphoarginine, γATP and βADP; Peak 3: αATP and αADP; peak 4: βATP (O’Neill & Richards 1980; Gard et al. 1985; Giblin et al. 1986; Storey et al. 1984; Cerdan & Seelig 1990; Pullin et al. 1990; Wegener et al. 1991). Peak 4 (βATP) provides the most unambiguous quantitation of ATP because it does not overlap with other resonances. A decrease in peak 2 (containing phosphoarginine) relative to peak 1 (containing inorganic phosphate) indicates that oxidative phosphorylation has become insufficient to meet ATP demands. During the control experiment during which flies were kept at 25 °C for 21 h, ATP and phosphoarginine declined while inorganic phosphate increased, showing that we have the power to detect biologically significant changes in energetic status (Fig. S1). Fifty percent of flies died after the 21-h exposure to 25 °C, presumably due to deleterious effects of hypoxia on energy balance.
Before examining biological responses to temperature, it was essential to determine and account for thermodynamic impacts on quantitation. T1 relaxation times were temperature-dependent and varied greatly among resonances: values were longer for peak 1 compared to peaks 2–4, and were longer at higher temperatures (Fig. S2, ANCOVA: main effect of temperature, F1,13 = 8.16, P = 0.0135; main effect of peak [best model had peaks 2–4 pooled, peak 1 separate], F1,13 = 66.03, P < 0.0001). This means that, for a given pulse delay, Peak 1 will relax to a smaller degree than the other peaks, possibly leading to underestimates of the peak integral. Indeed, this was supported empirically, because when we integrated peaks from spectra collected under short-delay (Experiment 1) and long-delay (Experiment 2) conditions (Table S1 and Fig. S3), it was apparent that under short-delay conditions, peak 1 was underestimated relative to peaks 2–4 (likely because of the longer T1 relaxation times for peak 1), and that the relative differences between peak 1 and peaks 2–4 increased with increasing temperature, due to lengthening T1s (Fig. S2). Peak 1 was underestimated relative to peaks 2–4 by 10.6% at 25 °C, dropping to 2.6% at 0 °C (Table S1), implying that decreasing the temperature from 25 to 0 °C would result in a relative increase in peak 1 of approximately 8% compared to peaks 2–4 (ATP). These thermodynamic effects should be kept in mind while interpreting the integrals from Experiment 1 (short delays, fine-scale time-course), but do not affect Experiment 2 because of the long relaxation times employed in that experiment. We thus use the 2 complementary experiments in parallel: Experiment 1 gives us better signal to noise and temporal resolution to test our core hypotheses about differences between cold-hardy and susceptible flies, and we use data from Experiment 2 to confirm that patterns are not caused by signal truncation.
Effects of cold exposure on phosphorus homeostasis
When flies were cooled from 25 to 0 °C, Peak 1 increased relative to peaks 2–4 (Figs 2a & 3 and Fig. S3; peak × time, P < 0.0001 in both experiments, Table 1). When peaks were analyzed separately to tease apart this interaction, peaks 1 and 2 both changed significantly over time in both experiments (P < 0.0001), reinforcing that this is a true biological change. Peaks 3–4, representing ATP and ADP, decreased slightly and significantly in Experiment 1 (Fig. 3, P < 0.0001), but the lack of a comparable decrease in Experiment 2 (Fig. S4, P > 0.100) suggests that this is an experimental artifact resulting from the differences in T1 relaxation times discussed above. Thus, ATP does not decrease during cooling to 0 °C, but an increase in inorganic phosphate (peak 1) relative to phosphoarginine (peak 2) indicates that the phosphagen pool was used to buffer ATP concentrations.
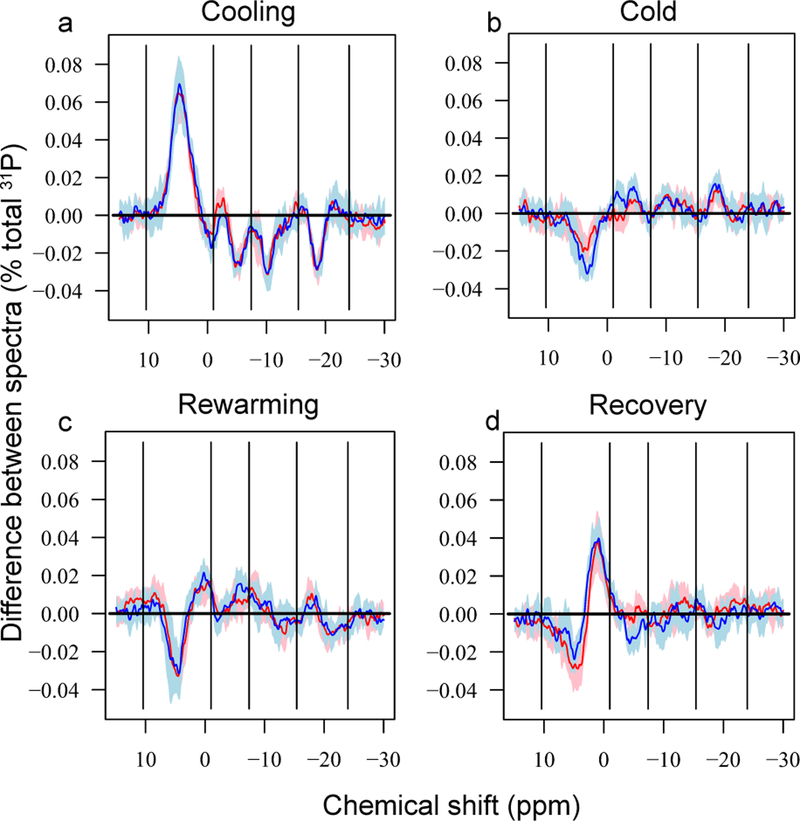
Figure 2.
Difference spectra illustrating average changes in Drosophila melanogaster phosphorus metabolites during cold exposure and recovery for hardy (pale blue 95% CIs and blue line, N = 8) and susceptible flies (pink 95% CIs and red line, N = 9). a) Immediate effects of cold exposure compared to pre-cold state, b) accumulated effects of cold during the 3 hours compared to state immediately after cooling, c) immediate effects of rewarming compared to state at the end of the cold exposure, and d) state at the end of the experiment, after 3.75 h recovery, compared to state immediately after rewarming. See methods for details of calculation of means and confidence intervals. Vertical lines mark regions that were integrated for quantitation of 4 main peaks, as indicated in Fig. 1C. Where confidence intervals overlap the horizontal line at 0, no change was documented at that resonance between those time points.
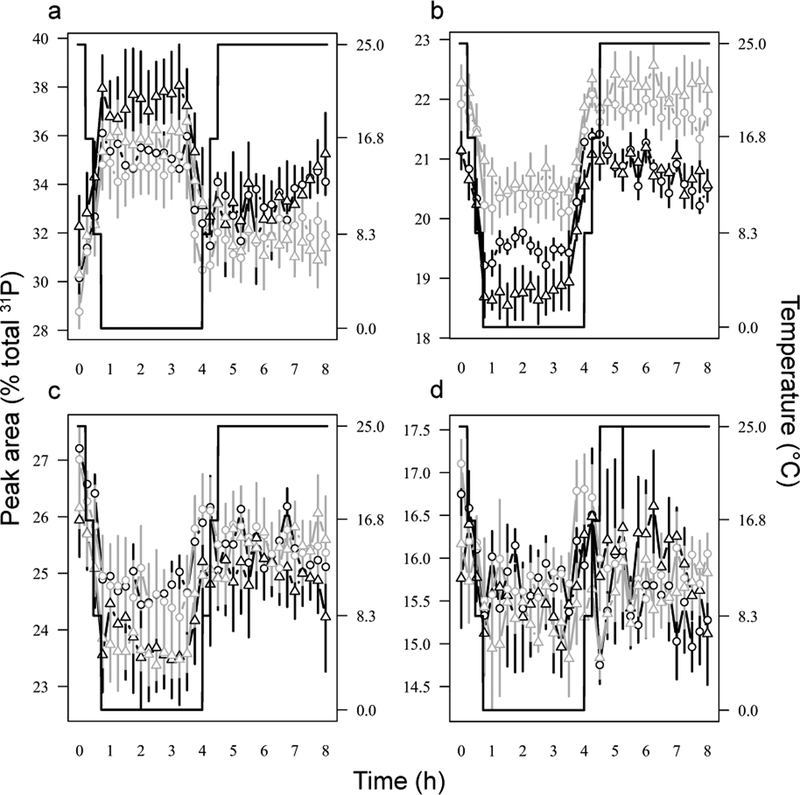
Figure 3.
Levels of 31P metabolites of cold-hardy (black) or cold-susceptible (grey) Drosophila melanogaster during cold exposure and recovery. Temperature is on the right axis (black solid line). Symbols denote replicate experimental evolution populations (circles = population 1, triangles = population 2). a) Peak 1 – sugar phosphates, phospholipids, inorganic phosphate, b) Peak 2 – phosphoarginine, γATP, and βADP, c) Peak 3 – αATP and αADP, d) Peak 4 – βATP.
Table 1.
Effects of cooling (25 to 0 °C), cold exposure (3 h at 0 °C), warming (0 to 25 °C) and recovery (3.75 h at 25 °C) on phosphorus metabolites (peaks 1–4) of female Drosophila melanogaster artificially selected for cold hardiness or cold susceptibility
| Terms in minimal adequate model | Experiment 1 | Terms in minimal adequate model | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F-value | df | P-value | F-value | df | P-value | ||||
| Cooling | |||||||||
| Cold hardiness | 0.1 | 1 | 260 | 0.936 | Cold hardiness | 0.1 | 1 | 2 | 0.789 |
| Time | 0.3 | 1 | 260 | 0.604 | Time | 1.2 | 1 | 74 | 0.280 |
| Peak | 482.4 | 3 | 260 | <0.0001 | Peak | 310.4 | 3 | 74 | <0.0001 |
| Cold hardiness × peak | 10.9 | 3 | 260 | <0.0001 | Cold hardiness × peak | 6.1 | 3 | 74 | 0.001 |
| Time × peak | 70.0 | 3 | 260 | <0.0001 | Time × peak | 22.7 | 3 | 74 | <0.0001 |
| Cold exposure | |||||||||
| Cold hardiness | 0 | 1 | 876 | 0.870 | Cold hardiness | 0 | 1 | 10 | 0.934 |
| Time | 0.3 | 1 | 266 | 0.596 | |||||
| Peak | 7310.8 | 3 | 876 | <0.0001 | Peak | 596.5 | 3 | 266 | <0.0001 |
| Cold hardiness × peak | 24.7 | 3 | 876 | <0.0001 | Cold hardiness × peak | 20.4 | 3 | 266 | <0.0001 |
| Time × peak | 3.6 | 3 | 266 | 0.014 | |||||
| Warming | |||||||||
| Cold hardiness | 0 | 1 | 260 | 0.977 | Cold hardiness | 1.9 | 1 | 10 | 0.201 |
| Time | 0 | 1 | 260 | 0.999 | Time | 0.1 | 1 | 74 | 0.706 |
| Peak | 9.8 | 3 | 260 | <0.0001 | Peak | 15.2 | 3 | 74 | <0.0001 |
| Cold hardiness × peak | 3.9 | 3 | 260 | 0.009 | Cold hardiness × peak | 7.5 | 3 | 74 | <0.001 |
| Time × peak | 2.2 | 3 | 260 | 0.093 | Time × peak | 5.7 | 3 | 74 | 0.001 |
| Recovery | No recovery in Experiment 2 | ||||||||
| Cold hardiness | 0 | 1 | 1004 | 0.950 | |||||
| Time | 0 | 1 | 1004 | 0.878 | |||||
| Peak | 204.2 | 3 | 1004 | <0.0001 | |||||
| Cold hardiness × time | 0 | 1 | 1004 | 0.903 | |||||
| Cold hardiness × peak | 1.2 | 3 | 1004 | 0.305 | |||||
| Time × peak | 3.6 | 3 | 1004 | 0.014 | |||||
| Cold hardiness × time × peak | 4.5 | 3 | 1004 | 0.004 | |||||
Bold indicates terms that were significant in both experiments. Statistics are from general linear models.
During cooling, cold-hardy flies had lower levels of peak 2 than cold-susceptible flies (cold hardiness × peak, P < 0.001 in both experiments, Table 1, Fig. 3, Fig. S4). There were no significant differences between cold-hardy and cold–susceptible flies in peaks 1, 3 or 4 in either experiment, and, thus, no differences between cold-hardy and cold-susceptible flies in maintenance of phosphorus homeostasis during cooling (Fig. 2). The lack of a standard and issues with signal truncation do not affect comparisons among lines, as all samples were affected equally by these issues.
During cold exposure and rewarming, the effects of cooling are gradually reversed (Fig. 2). There is no statistically significant effect of time on any of the individual peak integrals in Experiment 1 during cold exposure (Table 1, Fig. 3). In contrast, in Experiment 2 the peaks respond differently to cold (time × peak P = 0.014, Table 1), with separate analysis of the peaks confirming that peak 1 decreased significantly (P = 0.018), while peak 2 increased (P = 0.001, Fig. S4). This indicates that the phosphagen pool was gradually replenished during cold exposure. There were no changes in peaks 3–4 (and, thus, ATP) during cold exposure. During cold exposure, cold-hardy flies maintained lower levels of peak 2 than cold-susceptible flies (cold hardiness × peak, P < 0.0001 in both experiments, Table 1, Fig. 3, Fig. S4). No other peaks differed between cold-hardy and cold-susceptible flies during cold exposure. Upon rewarming, peak 1 again decreases relative to peak 2 (peak × time, P < 0.1 in both experiments, Table 1, Fig. 2c, Fig. 3, Fig. S4). Hardy flies continue to have lower levels of peak 2 in both experiments than do susceptible flies during rewarming (cold hardiness × peak, P < 0.01, Table 1, Figs 2 and 3), with no differences in peaks 1, 3 or 4.
During 3.75 h of recovery at 25 °C, a resonance on the right shoulder of peak 1 increases while the left resonance of peak 1 decreases (Fig. 2d). These compounds likely correspond to sugar phosphates and inorganic phosphate, but we are not able to deconvolute the complex peaks. Although no striking differences were observed between cold-hardy and cold-susceptible flies during recovery in the difference spectra (Fig. 2d), analysis of peak integrals revealed some subtle differences in phosphorus homeostasis during recovery (cold hardiness × peak × time, P = 0.004, Table 1, Fig. 3). When analyzed separately, peak 1 (P < 0.001), peak 2 (P = 0.001) and peak 4 (P = 0.031) all have significant cold hardiness × time interaction terms. Peak 1 increases during recovery in cold-hardy flies while staying constant in cold-susceptible flies, while peaks 2 and 4 decrease slightly in cold-hardy flies while staying constant in -susceptible flies (Fig. 3). Peak 2 remains lower in cold-susceptible compared to cold-hardy flies during recovery. The magnitude of change in peak 4 (ATP) is small relative to the variance, and both cold-hardy and cold-susceptible flies overlap considerably, suggesting that this change is not biologically relevant.
DISCUSSION
This is the first study to simultaneously examine the impacts of cold exposure and cold adaptation on ATP and phosphagen homeostasis in insects. Cold exposure did not compromise ATP levels, even in cold-susceptible flies. Flies transiently used the phosphagen pool (phosphoarginine) to buffer ATP levels during cooling, but replenished phosphagens during cold exposure, suggesting that oxidative metabolism was sufficient to meet ATP demand in the cold. Cold adaptation did not affect the ability to maintain ATP supply during cold exposure and recovery, nor the extent of reliance on anaerobic metabolism. It therefore appears that the variation in the ability to maintain ATP supply is not associated with genetic variation in chill coma recovery time in these flies, and possibly other insects. This adds to growing evidence that limitations to ATP supply do not set lower thermal limits in insects (Koštál et al. 2004; Colinet 2011; MacMillan et al. 2012a).
The oxygen and capacity limitation on thermal tolerance hypothesis states that organismal thermal limits for metazoans are set by the inability to deliver oxygen sufficient to supply oxidative metabolism, rather than cellular level failures such as protein denaturation (Pörtner 2001; Pörtner et al. 2017). Evidence supporting this hypothesis is weak in air-breathing invertebrates (Verberk et al. 2016), and the current study provides further evidence against a universal role of oxygen and capacity limitation in setting lower thermal limits. This suggests that other physiological processes likely constrain performance at low temperatures in insects (such as failure of protein function, ion homeostasis or membrane function; Verberk et al. 2016; Overgaard & MacMillan 2017).
The use of the phosphagen pool to maintain ATP levels during cooling, indicated by an increase in inorganic phosphate relative to phosphoarginine, suggests that oxidative metabolism temporarily becomes unable to meet ATP demands. There was no suggestion that reliance on anaerobic metabolism during cold exposure differed between cold-hardy and cold-susceptible flies, providing further evidence against oxygen and capacity limitations on thermal tolerance. The temporary disruption to oxidative metabolism likely occurs due to the effects of low temperature on enzymatic reactions, including ATP synthase (Somero et al. 2017). This pattern was reversed during cold exposure, indicating that both cold-hardy and cold-susceptible flies were able to replenish the phosphagen pool during cold exposure using ATP generated from oxidative phosphorylation, and that oxidative phosphorylation was sufficient to meet energetic demands during cold exposure. This rebalancing of energy supply and demand may have occurred through rapid modifications that enhance ATP supply, or reduce ATP demand. Cold acclimation enhances mitochondrial ATP synthesis in D. melanogaster (Colinet et al. 2017), supporting the hypothesis that ATP supply may be rapidly upregulated to counter the suppressing effects of cold exposure. This demonstrates that homeostasis can be recovered during constant temperatures, even if they are below the critical thermal minimum. More broadly, an upregulation of aerobic metabolism is a common response to cold adaptation and acclimatization (White et al. 2012; Somero et al. 2017).
In the lines of flies that were used in the current study, cold-hardy compared to cold-susceptible flies have higher metabolic rates and rates of glucose and leucine oxidation before cold exposure, lower metabolic rates and rates of nutrient flux in the cold (and, thus, greater metabolic suppression in the cold), and higher metabolic rates and rates of nutrient flux during recovery (Williams et al. 2016a,b). This raises the question of how we can see changes in metabolic flux (rates of ATP production) that are not mirrored in changes in steady-state ATP levels. ATP synthesis rates are controlled by adenylate energy charge, and steady-state ATP levels are homeostatically controlled by modulating glycolytic flux and oxidative phosphorylation in the face of fluctuations in ATP demand (Koebmann et al. 2002). No change in steady-state ATP concentrations shows that ATP production and removal remained in balance during cold exposure, but the lack of a change in the ATP concentration does not imply that there was no change in flux. Flux rates and steady-state metabolite levels are frequently decoupled, and inferences about flux from steady-state measurements should be treated with extreme caution.
The lack of fluctuations in ATP concentrations despite differences in rates of catabolism and biosynthesis thus suggest that the modulations to metabolic pathways we observe as a product of selection on cold hardiness likely came about as a function of selection on a bio-synthetic product(s) of those pathways rather than due to selection on energy balance per se. For example, our previous work showed that cold-hardy flies synthesized proline, an important cryoprotectant, more rapidly (Williams et al. 2016a), despite having lower steady-state levels of proline (Williams et al. 2014). Faster synthesis of molecules that function in cryoprotection or repair could, thus, be a target of selection that would result in a higher TCA cycle flux.
In conclusion, this work shows that ATP concentration is maintained in the cold in D. melanogaster without extensive reliance on anaerobic metabolism, and that genetically-based differences in chill coma recovery times are not associated with marked differences in adenylate homeostasis in the cold. This evidence, in combination with previous work showing no decline in ATP during cold exposure across deeply diverged insect lineages, suggests that neither oxygen limitation nor mitochondrial failure cause cold coma in insects.
Data archiving
All data and analysis scripts are archived in UC Berkeley DASH repository: Williams, Caroline (2018), Data for Cold adaptation does not alter ATP homeostasis during cold exposure in Drosophila melanogaster, Data-ONE Dash, https://doi.org/10.15146/R32X0G).
Supplementary Material
Table S1 Peak areas (as a percent of total phosphorus) for each of the 4 major peaks of the 31P spectra of live Drosophila melanogaster, collected under short (partially truncated) or long (fully relaxed) delay conditions. For details of experimental parameters, see text. Spectra are shown in Fig. S2.
Figure S1 31P spectra of a pool of 50 live Drosophila melanogaster held in a nuclear magnetic resonance (NMR) tube for 21 h at 25 °C. Peak integrals from Top-spin software are annotated below each peak. Between Time 0 and Time 21 h, ATP and phosphoarginine (P Arg) decline, and inorganic phosphate (Pi) increases, indicating that flies become hypoxic and transition towards anaerobic metabolism, which is insufficient to maintain ATP supply. Fifty percent of flies died after this experiment.
Figure S2 T1 (spin lattice) relaxation times as a function of temperature for the 4 distinct phosphorus resonances measured on live Drosophila melanogaster: Peak 1, open circles; peak 2, closed circles; peak 3, squares; peak 4, triangles. See Fig. 1 for peak assignments.
Figure S3 31P spectra of live Drosophila melanogaster collected under fully relaxed conditions (tau = 7 s, black lines) compared to those collected using a short delay (tau = 0.02 s, grey lines) over the range of temperatures used for the experiments: (a) 0 °C, (b) 8.3 °C, (c) 16.8 °C and (d) 25 °C. See Fig. 1 for peak assignments.
Figure S4 Levels of 31P metabolites of cold-hardy (black) or susceptible (grey) Drosophila melanogaster during cold exposure and recovery, collected under fully relaxed conditions (delay = 5 × T1). Temperature is on the right axis (black solid line). Symbols denote replicate experimental evolution populations (circles = population 1, triangles = population 2). (a) Peak 1: sugar phosphates, phospholipids, inorganic phosphate. (b) Peak 2: phosphoarginine, γATP and βADP. (c) Peak 3: αATP and αADP. (d) Peak 4: βATP.
ACKNOWLEDGMENTS
This work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida. This work was supported in part by an NIH award, S10RR031637, for magnetic resonance instrumentation, and by National Science Foundation (NSF) Grants IOS-1051770 (to T.J.M.), IOS-1051890 (to D.A.H, A.S.E and D.B.A.) and IOS-1558159 to CMW. A.S.E. received partial support from the Georgia Research Alliance, and D.A.H. received support from the IAEA/FAO CRP on Dormancy management to enable mass rearing and increase efficacy of sterile insects and natural enemies. Dan Plant provided valuable assistance setting up NMR experiments in AMRIS. Stephanie Dickinson checked the statistical analyses. D.B.A was supported by NIH grants U24AG056053 and P30AG050886.
Footnotes
SUPPLEMENTARY MATERIALS
Additional supporting information may be found in the online version of this article at the publisher’s website.
REFERENCES
- Bates DR, Maechler M, Bolker B (2011). lme4: Linear mixed-effects models using S4 classes. Journal of Statistical Software 67, 1–48. [Google Scholar]
- Cerdan S, Seelig J (1990). NMR studies of metabolism. Annual Review of Biophysics and Biophysical Chemistry 19, 43–67. [DOI] [PubMed] [Google Scholar]
- Colinet H (2011). Disruption of ATP homeostasis during chronic cold stress and recovery in the chill susceptible beetle (Alphitobius diaperinus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 160, 63–7. [DOI] [PubMed] [Google Scholar]
- Colinet H, Renault D, Roussel D (2017). Cold acclimation allows Drosophila flies to maintain mitochondrial functioning under cold stress. Insect Biochemistry and Molecular Biology 80 (Supplement C), 52–60. [DOI] [PubMed] [Google Scholar]
- Coulson SJ, Fisher J, Bale JS (1992). A 31P NMR investigation of the energy charge of the housefly Musca domestica (Diptera: Muscidae) during rapid cold hardening and cold shock. Cryo-Letters 13, 183–92. [Google Scholar]
- Crawley MJ (2007). The R Book. Wiley, New York. [Google Scholar]
- Crozier L (2004). Warmer winters drive butterfly range expansion by increasing survivorship. Ecology 85, 231–41. [Google Scholar]
- Ellington WR (2001). Evolution and physiological roles of phosphagen systems. Annual Review of Physiology 63, 289–325. [DOI] [PubMed] [Google Scholar]
- Gard JK, Kichura GM, Ackerman JJ et al. (1985). Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophysical Journal 48, 803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken AR, Mackay TFC, Morgan TJ (2016). Artificial selection on chill-coma recovery time in Drosophila melanogaster: Direct and correlated responses to selection. Journal of Thermal Biology 59, 77–85. [DOI] [PubMed] [Google Scholar]
- Giblin RM, Lee RWK, Platzer EG (1986). 31P NMR spectra of mosquito larvae parasitized with Romano-mermis culicivorax. Journal of Invertebrate Pathology 48, 52–9. [DOI] [PubMed] [Google Scholar]
- Hao J, Astle W, De Iorio M, Ebbels T (2012). BATMAN–An R package for the automated quantification of metabolites from nuclear magnetic resonance spectra using a Bayesian model. Bioinformatics 28, 2088–90. [DOI] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE (2012). Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society of London B: Biological Sciences 367, 1665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebmann BJ, Westerhoff HV, Snoep JL, Nilsson D, Jensen PR (2002). The glycolytic flux in Escherichia coli Is controlled by the demand for ATP. Journal of Bacteriology 184, 3909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koštál V (2010). Cell structural modifications in insects at low temperatures In: Denlinger DL, Lee RE, eds. Low Temperature Biology of Insects. Cambridge University Press, New York: pp. 116–41. [Google Scholar]
- Koštál V, Vambera J, Bastl J (2004). On the nature of pre-freeze mortality in insects: Water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. The Journal of Experimental Biology 207, 1509–21. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RH (2015). lmerTest: Tests in linear mixed effects models. Journal of Statistical Software 82, 1–26. [Google Scholar]
- Layne JR, Kennedy SD (2002). Cellular energetics of frozen wood frogs (Rana sylvatica) revealed via NMR spectroscopy. Journal of Thermal Biology 27, 167–73. [Google Scholar]
- MacMillan HA, Sinclair BJ (2011). Mechanisms underlying insect chill-coma. Journal of Insect Physiology 57, 12–20. [DOI] [PubMed] [Google Scholar]
- MacMillan HA, Williams CM, Staples JF, Sinclair BJ (2012a). Metabolism and energy supply below the critical thermal minimum of a chill-susceptible insect. Journal of Experimental Biology 215, 1366–72. [DOI] [PubMed] [Google Scholar]
- MacMillan HA, Williams CM, Staples JF, Sinclair BJ (2012b). Reestablishment of ion homeostasis during chill-coma recovery in the cricket Gryllus pennsyl-vanicus. PNAS 109, 20750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation JL (2008). Insect Physiology and Biochemistry. CRC Press, Taylor and Francis Group, Boca Raton, FL. [Google Scholar]
- O’Neill IK, Richards CP (1980). Biological 31P NMR Spectroscopy In: Webb GA, ed. Annual Reports on NMR Spectroscopy. Academic Press, London, pp. 133–6. [Google Scholar]
- Overgaard J, MacMillan HA (2017). The integrative physiology of insect chill tolerance. Annual Review of Physiology 79, 187–208. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Kearney MR, Hoffmann AA (2014). Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Global Change Biology 20, 1738–50. [DOI] [PubMed] [Google Scholar]
- Pörtner HO (2001). Climate change and temperature-dependent biogeography: Oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–46. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Bock C, Mark FC (2017). Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. Journal of Experimenal Biology 220, 2685–96. [DOI] [PubMed] [Google Scholar]
- Pullin AS, Bale JS (1988). Cause and effects of pre-freeze mortality in aphids. Cryo-Letters 9, 101–13. [Google Scholar]
- Pullin AS, Fontaine XLR, Bale JS (1990). Application of 31P-NMR to the study of pre-freeze mortality in aphids. Cryo-Letters 11, 127–36. [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Somero GN (2010). The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. Journal of Experimental Biology 213, 912–20. [DOI] [PubMed] [Google Scholar]
- Somero GN, Lockwood BL, Tomanek L (2017). Biochemical Adaptation: Responses to Environmental Challenges from Life’s Origin to the Anthropocene. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Storey KB, Miceli M, Butler KW, Smith ICP, Deslauriers R (1984). 31P-NMR studies of the freeze-tolerant larvae of the gall fly, Eurosta solidaginis. European Journal of Biochemistry 142, 591–5. [DOI] [PubMed] [Google Scholar]
- Verberk WCEP, Overgaard J, Ern R et al. (2016). Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comparative Bio-chemistry and Physiology Part A: Molecular & Inte-grative Physiology 192, 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener G, Bolas NM, Thomas AAG (1991). Locust flight metabolism studied in vivo by 31P NMR spectroscopy. Journal of Comparative Physiology B 161, 247–56. [Google Scholar]
- White CR, Alton LA, Frappell PB (2012). Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proceedings. Biological Sciences 279, 1740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Watanabe M, Guarracino MR et al. (2014). Cold adaptation shapes the robustness of metabolic networks in Drosophila melanogaster. Evolution 68, 3505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Henry HAL, Sinclair BJ (2015). Cold truths: How winter drives responses of terrestrial organisms to climate change. Biological Reviews 90, 214–35. [DOI] [PubMed] [Google Scholar]
- Williams CM, McCue MD, Sunny NE et al. (2016a). Cold adaptation increases rates of nutrient flow and metabolic plasticity during cold exposure in Drosophila melanogaster. Proceedings. Biological Sciences 283, 20161317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Szejner-Sigal A, Morgan TJ, Edison AS, Allison DB, Hahn DA (2016b). Adaptation to low temperature exposure increases metabolic rates independently of growth rates. Integrative and Comparative Biology 56, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Ragland GJ, Betini GS et al. (2017). Understanding evolutionary impacts of seasonality. Integrative and Comparative Biology 57, 921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Peak areas (as a percent of total phosphorus) for each of the 4 major peaks of the 31P spectra of live Drosophila melanogaster, collected under short (partially truncated) or long (fully relaxed) delay conditions. For details of experimental parameters, see text. Spectra are shown in Fig. S2.
Figure S1 31P spectra of a pool of 50 live Drosophila melanogaster held in a nuclear magnetic resonance (NMR) tube for 21 h at 25 °C. Peak integrals from Top-spin software are annotated below each peak. Between Time 0 and Time 21 h, ATP and phosphoarginine (P Arg) decline, and inorganic phosphate (Pi) increases, indicating that flies become hypoxic and transition towards anaerobic metabolism, which is insufficient to maintain ATP supply. Fifty percent of flies died after this experiment.
Figure S2 T1 (spin lattice) relaxation times as a function of temperature for the 4 distinct phosphorus resonances measured on live Drosophila melanogaster: Peak 1, open circles; peak 2, closed circles; peak 3, squares; peak 4, triangles. See Fig. 1 for peak assignments.
Figure S3 31P spectra of live Drosophila melanogaster collected under fully relaxed conditions (tau = 7 s, black lines) compared to those collected using a short delay (tau = 0.02 s, grey lines) over the range of temperatures used for the experiments: (a) 0 °C, (b) 8.3 °C, (c) 16.8 °C and (d) 25 °C. See Fig. 1 for peak assignments.
Figure S4 Levels of 31P metabolites of cold-hardy (black) or susceptible (grey) Drosophila melanogaster during cold exposure and recovery, collected under fully relaxed conditions (delay = 5 × T1). Temperature is on the right axis (black solid line). Symbols denote replicate experimental evolution populations (circles = population 1, triangles = population 2). (a) Peak 1: sugar phosphates, phospholipids, inorganic phosphate. (b) Peak 2: phosphoarginine, γATP and βADP. (c) Peak 3: αATP and αADP. (d) Peak 4: βATP.
Data Availability Statement
All data and analysis scripts are archived in UC Berkeley DASH repository: Williams, Caroline (2018), Data for Cold adaptation does not alter ATP homeostasis during cold exposure in Drosophila melanogaster, Data-ONE Dash, https://doi.org/10.15146/R32X0G).