Key Points
Question
What is the magnitude and duration of driving impairment following vaporization of cannabis containing varying concentrations of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD)?
Findings
In this crossover clinical trial that included 26 healthy participants who underwent on-road driving tests, the standard deviation of lateral position (SDLP, a measure of lane weaving, swerving, and overcorrecting) at 40 to 100 minutes following vaporized consumption was 18.21 cm for CBD-dominant cannabis, 20.59 cm for THC-dominant cannabis, 21.09 cm for THC/CBD-equivalent cannabis, and was 18.26 cm for placebo. At 240 to 300 minutes, the SDLP was 19.03 cm for CBD-dominant cannabis, 20.59 cm for THC-dominant cannabis, 19.88 cm for THC/CBD-equivalent cannabis, and 19.37 cm for placebo. Compared with placebo, SDLP with THC-dominant and THC/CBD-equivalent cannabis was significantly greater at 40 to 100 minutes but not 240 to 300 minutes after consumption; there were no significant differences between CBD-dominant cannabis and placebo.
Meaning
Although this study did not find statistically significant differences in driving performance during experimental on-road driving tests between CBD-dominant cannabis and placebo, the effect size may not have excluded clinically important impairment, and the doses tested may not necessarily represent common usage.
Abstract
Importance
Cannabis use has been associated with increased crash risk, but the effect of cannabidiol (CBD) on driving is unclear.
Objective
To determine the driving impairment caused by vaporized cannabis containing Δ9-tetrahydrocannabinol (THC) and CBD.
Design, Setting, and Participants
A double-blind, within-participants, randomized clinical trial was conducted at the Faculty of Psychology and Neuroscience at Maastricht University in the Netherlands between May 20, 2019, and March 27, 2020. Participants (N = 26) were healthy occasional users of cannabis.
Interventions
Participants vaporized THC-dominant, CBD-dominant, THC/CBD-equivalent, and placebo cannabis. THC and CBD doses were 13.75 mg. Order of conditions was randomized and balanced.
Main Outcomes and Measures
The primary end point was standard deviation of lateral position (SDLP; a measure of lane weaving) during 100 km, on-road driving tests that commenced at 40 minutes and 240 minutes after cannabis consumption. At a calibrated blood alcohol concentration (BAC) of 0.02%, SDLP was increased relative to placebo by 1.12 cm, and at a calibrated BAC of 0.05%, SDLP was increased relative to placebo by 2.4 cm.
Results
Among 26 randomized participants (mean [SD] age, 23.2 [2.6] years; 16 women), 22 (85%) completed all 8 driving tests. At 40 to 100 minutes following consumption, the SDLP was 18.21 cm with CBD-dominant cannabis, 20.59 cm with THC-dominant cannabis, 21.09 cm with THC/CBD-equivalent cannabis, and 18.28 cm with placebo cannabis. SDLP was significantly increased by THC-dominant cannabis (+2.33 cm [95% CI, 0.80 to 3.86]; P < .001) and THC/CBD-equivalent cannabis (+2.83 cm [95% CI, 1.28 to 4.39]; P < .001) but not CBD-dominant cannabis (−0.05 cm [95% CI, −1.49 to 1.39]; P > .99), relative to placebo. At 240 to 300 minutes following consumption, the SDLP was 19.03 cm with CBD-dominant cannabis, 19.88 cm with THC-dominant cannabis, 20.59 cm with THC/CBD-equivalent cannabis, and 19.37 cm with placebo cannabis. The SDLP did not differ significantly in the CBD (−0.34 cm [95% CI, −1.77 to 1.10]; P > .99), THC (0.51 cm [95% CI, −1.01 to 2.02]; P > .99) or THC/CBD (1.22 cm [95% CI, −0.29 to 2.72]; P = .20) conditions, relative to placebo. Out of 188 test drives, 16 (8.5%) were terminated due to safety concerns.
Conclusions and Relevance
In a crossover clinical trial that assessed driving performance during on-road driving tests, the SDLP following vaporized THC-dominant and THC/CBD-equivalent cannabis compared with placebo was significantly greater at 40 to 100 minutes but not 240 to 300 minutes after vaporization; there were no significant differences between CBD-dominant cannabis and placebo. However, the effect size for CBD-dominant cannabis may not have excluded clinically important impairment, and the doses tested may not represent common usage.
Trial Registration
EU Clinical Trials Register: 2018-003945-40
This crossover randomized clinical trial evaluated driving test performance of healthy young adult volunteers after vaporized consumption of cannabis (Δ9-tetrahydrocannabinol [THC] and cannabidiol [CBD]) vs placebo.
Introduction
Epidemiological studies have indicated that cannabis is associated with increased crash risk and culpability.1,2 Acute cannabis intoxication increases the standard deviation of lateral position (SDLP),3 an index of lane weaving, swerving, and overcorrecting that is a validated measure of alcohol- and drug-induced driving impairment.4
Cannabis chemovars can be broadly categorized into 3 chemotypes: tetrahydrocannabinol (THC)-dominant, cannabidiol (CBD)-dominant, and THC/CBD-equivalent.5 THC-dominant products are typically used for intoxication while CBD-dominant products, which are presumed not to be intoxicating, are prescribed for the treatment of epilepsy, anxiety, psychosis, and neurological disorders.6 THC/CBD-equivalent products are sometimes consumed with the expectation that CBD can ameliorate THC-related symptoms such as anxiety, paranoia, and cognitive impairment.7 Although some research has suggested an absence of cognitive, psychomotor, or subjective effects with oral and vaporized CBD,8 sedation and somnolence are sometimes reported with CBD, albeit usually in the presence of other drugs,8,9 but which nonetheless could affect driving.
Cannabis can be smoked or ingested, but vaporization is an increasingly popular method of administration.10,11 The present study investigated the effects of vaporized THC-dominant (THC), THC/CBD-equivalent (THC/CBD) and CBD-dominant (CBD) cannabis on driving performance, cognitive function, and subjective experiences.
Methods
The study was approved by the medical ethics committee of Maastricht University and conducted in accordance with the ethical standards of the Declaration of Helsinki. The trial protocol including the statistical analysis plan is provided in Supplement 1.
Participants
Healthy volunteers with a history of occasional cannabis use were recruited via advertisement, social media, and word of mouth. Inclusion criteria were age between 20 and 50 years, self-reported cannabis use less than 2 times per week in the past 12 months and more than 10 lifetime exposures, possession of a valid driver’s license with at least 2 years’ driving experience and driving more than 2000 km per year, and body mass index (calculated as weight in kilograms divided by height in meters squared) between 20 and 28.
Exclusion criteria were presence of any major medical, endocrine, or neurological condition; history of drug abuse or addiction; current or history of psychiatric disorder; current use of medications known to affect driving; active hypertension; pregnancy; history of cardiac dysfunction; and any serious prior adverse response to cannabis. Participants meeting eligibility criteria underwent a comprehensive medical examination involving a medical history review, electrocardiogram, blood testing (hematology and serology), and physical examination. All participants provided written informed consent prior to participation.
Study Design and Procedures
This double-blind, within-participants, crossover study included 4 experimental sessions that were scheduled at least 1 week apart to avoid potential drug carryover effects. Participants were required to abstain from use of cannabis and other drugs for the duration of the study and from use of alcohol for 24 hours prior to each session. Prior to the first experimental session, participants completed a practice session to familiarize them with the on-road driving test and cognitive test procedures. For experimental sessions, participants vaporized cannabis containing 13.75 mg THC (THC condition), 13.75 mg THC and 13.75 mg CBD (THC/CBD condition), 13.75 mg CBD (CBD condition), or placebo (placebo condition). Study drugs were prepared in advance (J.R. and E.T.) according to a computer-generated balanced, randomization schedule with a block size of 6 (based on expected recruitment of 24 participants). Investigators conducting test days (T.A. and F.V.) and participants were blind to the randomization schedule. The study was conducted between May 2019 and March 2020 at the Faculty of Psychology and Neuroscience at Maastricht University.
Experimental Sessions
The order of events during the 4 experimental sessions is shown in eTable 1 in Supplement 2. Upon participant arrival, a zero breath alcohol concentration was confirmed via breathalyzer (Alcotest 5510, Dräger), and oral fluid was screened (DrugTest 5000, Dräger) to identify any recent use of cannabis, cocaine, opiates, amphetamine, methamphetamine, or 3,4-methylenedioxymethamphetamine (MDMA [otherwise known as ecstasy]). Following baseline measurements of cardiovascular measures and self-reported drug effects, a catheter was inserted into the participant’s nondominant arm and the first blood sample was collected. Participants then inhaled THC, THC/CBD, CBD, or placebo. Driving tests occurred at 40 to 100 minutes and 240 to 300 minutes postvaporization. Cognitive tests were conducted at 5, 135, and 205 minutes postvaporization. Blood samples, blood pressure, and heart rate were obtained at baseline (indicates predrug administration), and at minute 0 (indicates the end of drug administration), and at 25, 130, 200, and 320 minutes postvaporization. Subjective drug effects were assessed at baseline and at 0, 25, 130, 200, and 240 minutes postvaporization.
Study Drugs
THC-dominant (THC 22% and CBD<1%), CBD-dominant (THC<1% and CBD 9%) and placebo (<0.2% total cannabinoid content) cannabis varieties (Bedrocan) were used to deliver target doses of 13.75 mg THC, 13.75 mg THC/CBD, and 13.75 mg CBD. Placebo cannabis was added to active cannabis varieties so that each treatment contained target doses of THC and CBD within 215 mg total plant material. Study drugs were vaporized at 200 °C (Mighty Medic, Storz & Bickel) according to a standardized procedure (inhale 5 seconds, hold 3 seconds, exhale, and rest for 30 seconds; minimum of 10 inhalations and repeated if necessary until vapor no longer visible).
Subjective Drug Effects
Subjective drug effects were assessed using 7 visual analog scales (VAS) with 10 cm lines ranging from 0 (lowest score) to 10 (highest score).12 Participants rated the following: Strength of drug effect (No effect to Very strong), Liking of drug effect (Dislike very much to Like very much), Stoned (Not stoned to Very stoned), Sedated (Not sedated to Very sedated), Relaxed (Not relaxed to Very relaxed), Anxious (Not anxious to Very anxious), and Confident to drive (Not confident to Very confident). Perceived driving quality was assessed after each driving test using the following VAS items: How would you rate the quality of your driving just now? (Very poor to Very good) and Do you think your driving was impaired? (Not at all to Very much). Anxiety was further assessed using the state subscale of the State Trait Anxiety Inventory, which consists of 20 statements that are rated on a 4-point Likert scale (range, 1-4 [Not at all to Very much so]). Possible score totals range from 20 to 80 with higher scores indicating greater anxiety.13
Driving Tests
The on-road driving test (road-tracking test14) ran for approximately 60 minutes. Participants drove a specially instrumented vehicle over a 100-km highway circuit while maintaining a constant speed (95 km/h [59 mph]) and a steady lateral position in the right (slower) traffic lane. Participants were accompanied by a licensed driving instructor who had access to dual vehicle controls (accelerator and brake pedals).
Cognitive and Psychomotor Measures
Cognitive and psychomotor performance was assessed using 4 computerized tasks that have proven sensitive to THC impairment.12,15,16 These were the Digit Symbol Substitution Task,17 Divided Attention Task,18 Paced Serial Addition Task,19 and Tower of London.20 Participants also completed the Emotional Stroop Task.21
These tasks assess processing speed (Digit Symbol Substitution Task; Paced Serial Addition Task), divided attention (Divided Attention Task), psychomotor function (Digit Symbol Substitution Task; Divided Attention Task), working memory (Paced Serial Addition Task), and decision-making and cognitive flexibility (Tower of London; Emotional Stroop Task). The Digit Symbol Substitution Task, Divided Attention Task, and Paced Serial Addition Task were completed in this order at 5-minutes postvaporization and at 205 minutes postvaporization. The Emotional Stroop Task and Tower of London were completed once in each session at 5 minutes postvaporization and at 135 minutes postvaporization. Further details are provided in eMethods 1 in Supplement 2.
Blood Collection and Plasma Cannabinoid Analyses
Blood was collected via indwelling peripheral venous catheter into 10-mL purple-top (EDTA) Vacutainer tubes (Becton, Dickinson and Company) and centrifuged at 3000g for 10 minutes. The supernatant plasma was then decanted and stored in 2-mL cryotubes at −20 °C. Plasma was subsequently thawed for analysis via liquid chromatography-tandem mass spectrometry (LC-MS/MS) according to published methods.22,23 Target analytes included THC, 11-OH-THC, 11-COOH-THC, and CBD. Further details of these analyses are provided in eMethods 2 in Supplement 2.
Outcomes
The prespecified primary end point was mean SDLP during the on-road driving test. Lateral position, which is the distance between the vehicle and the lane boundary to the left of the vehicle, was recorded by a camera mounted onto the roof of the vehicle and sampled continuously at 4 Hz. Measurements of lateral position over the time of the driving test were averaged to yield the mean lateral position, and standard deviation was calculated to determine the mean SDLP. Larger numbers indicate greater variability (ie, reduced stability) in lane positioning. A 2.4-cm drug vs placebo increase in SDLP is typical of a driver with a blood alcohol concentration (BAC) of 0.05% and is thought to indicate the lower limit of clinically relevant driving impairment.4
Other end points for the primary outcome were mean speed and standard deviation of speed, which were recorded electronically by an on-board computer. Secondary outcomes included cognitive and psychomotor performance measures (previously described), subjective drug effects (0-10 cm VAS items as previously described), cardiovascular measures (blood pressure; heart rate), and plasma cannabinoid concentrations (ng/mL).
Post hoc outcomes were the proportions of participants showing impairment or improvement in relation to SDLP changes associated with BACs of 0.02% (1.12 cm)24 and 0.05% (2.4 cm),4 2 common legal driving limits.
Statistical Analysis
Sample size was determined by power calculation using the effect size obtained in a previous study of dronabinol (10-20 mg THC) on SDLP during on-road driving.25 This indicated that 20 participants were needed to detect an equivalent effect (Cohen f = 0.62; ΔSDLP = 1.94 cm; approximately 0.04% BAC26) with 95% power.
Available data from all 26 participants were analyzed according to randomization group using SPSS version 25 (IBM Corporation) using linear mixed-effects models. Model parameters included condition, time and condition × time as fixed effects, and a random intercept. A first-order autoregressive residual covariance structure was used as it consistently provided the lowest Schwarz Bayesian Information Criterion model fit values. The restricted maximum likelihood method was used as it provides an unbiased estimation of the variance parameters when the data are unbalanced. Missing data were handled using listwise deletion.
If a significant main effect of condition or a significant condition × time interaction was observed, 2-sided pairwise comparisons compared means across conditions at each level of time. To control the family-wise type I error rate, a Bonferroni correction was applied such that significance values were multiplied by 6, the total number of comparisons. The predefined comparisons of interest were THC vs placebo, THC/CBD vs placebo, CBD vs placebo and THC vs THC/CBD. Statistical significance was set at a P value of less than .05. Analyses including only completing participants (n = 22) did not differ meaningfully from the full results presented here (eTable 2 in Supplement 2).
Results
The Table presents the characteristics of the 26 participants who were enrolled into the study and randomized. Complete results of statistical analyses are found in eTable 3 (Supplement 2), and pairwise comparisons are found in eTables 4, 5, 6, 7, and 8 (Supplement 2). Figure 1 shows the flow of participants through the study.
Table. Participant Demographics and Characteristics.
| Demographic/characteristic | Participants, No. (%) |
|---|---|
| No. | 26 |
| Women | 16 |
| Men | 10 |
| Age, mean (SD), y | 23.2 (2.6) |
| Body mass index, mean (SD)a | 21.4 (2.4) |
| Participants with at least some tertiary education, % | 100 |
| Episodes of cannabis use in past 3 mo, median (IQR) | 4.5 (1-20) |
| Years in possession of driver’s license, median (IQR) | 5 (4-7) |
| Average No. of km driven per year, median (IQR) | 4500 (3000-8000) |
| Ever driven while under the influence of cannabis | 5 (19.2) |
| Weekly use of alcohol | 10 (38.5) |
| Prior use of other drugs | |
| Psilocybin | 7 (26.9) |
| Ecstasy/MDMA | 6 (23.1) |
| Cocaine | 4 (15.4) |
| LSD | 3 (11.5) |
| Other | 2 (7.7) |
| Amphetamine | 1 (3.8) |
Abbreviations: IQR, interquartile range; LSD, lysergic acid diethylamide; MDMA, 3,4-methylenedioxymethamphetamine.
Conversion factor: To convert kilometers to miles, divide the value by 1.609.
Calculated as weight in kilograms divided by height in meters squared.
Figure 1. Flow of Participants Through the Study of the Effects of CBD and THC on Driving Performance.
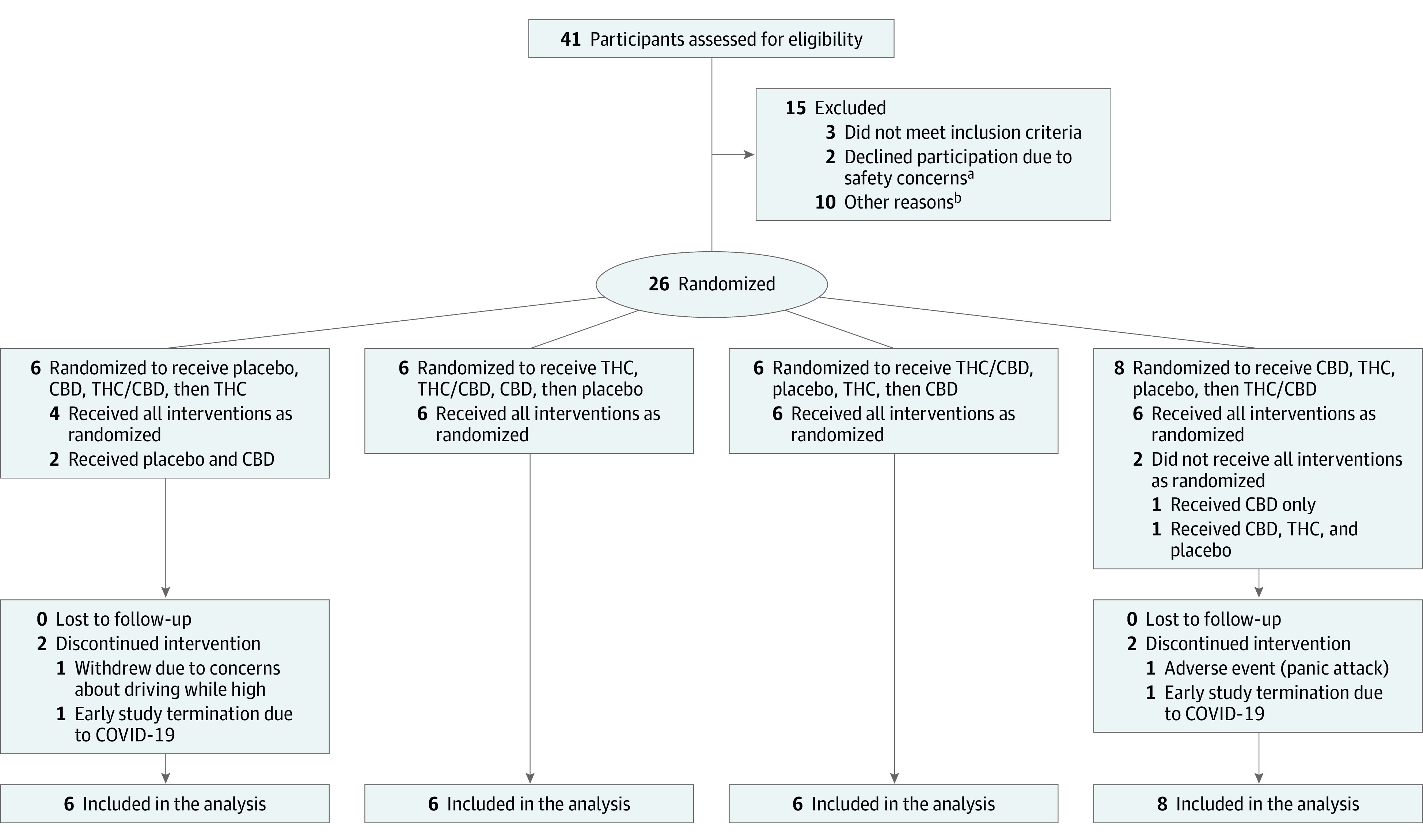
CBD indicates cannabidiol condition; COVID-19, coronavirus disease 2019; THC, Δ9-tetrahydrocannabinol condition; THC/CBD, Δ9-tetrahydrocannabinol/cannabidiol condition.
aSafety concerns regarded driving under the influence of cannabis.
bOther reasons: 6 participants became unresponsive and could not be contacted, 3 were unable to meet study time commitments, and 1 underwent a medical screening that revealed a low red blood cell count.
Primary Outcome
From 40 to 100 minutes, the mean lateral position was 86.94 cm (95% CI, 81.50 to 91.48) in the THC condition, 85.51 cm (95% CI, 81.81 to 89.21) in the THC/CBD condition, 84.07 cm (95% CI, 79.40 to 88.74) in the CBD condition, and 84.41 cm (95% CI, 80.01 to 88.82) in the placebo condition; from 240 to 300 minutes, the mean lateral position was 85.03 cm (95% CI, 80.88 to 89.17) in the THC condition, 84.04 cm (95% CI, 80.64 to 87.54) in the THC/CBD condition, 84.25 cm (95% CI, 79.85 to 88.65) in the CBD condition, and 83.68 cm (95% CI, 79.45 to 87.91) in the placebo condition. The overall range of mean lateral position values was 53.62 cm. A significant main effect of condition was found for SDLP (P < .001) (Figure 2). Pairwise comparisons revealed increased SDLP at 40 to 100 minutes in the THC condition compared with placebo (2.33 cm [95% CI, 0.08 to 3.86]; P < .001) and the THC/CBD condition compared with placebo (2.83 cm [95% CI, 1.28 to 4.39]; P < .001) but not at 240 to 300 minutes in the THC condition compared with placebo (0.51 cm [95% CI, −1.01 to 2.02]; P > .99) or the THC/CBD condition compared with placebo (1.22 cm [95% CI, −0.29 to 2.72]; P = .20). CBD did not affect SDLP compared with placebo at 40 to 100 minutes (−0.05 cm [95% CI, −1.49 to 1.39]; P > .99) or at 240 to 300 minutes (−0.34 cm [95% CI, −1.77 to 1.10]; P > .99), and there was no significant difference between the THC/CBD and THC conditions at 40 to 100 minutes (0.50 cm [95% CI, −1.10 to 2.10]; P > .99) or at 240 to 300 minutes (0.71 cm, [95% CI, −0.83 to 2.25]; P > .99). No significant differences were observed across conditions for mean speed (P = .56) or standard deviation of speed (P = .67). At 40 to 100 minutes, mean speed was 92.53 km per hour for CBD, 91.82 km per hour for THC, 92.86 km per hour for THC/CBD, and 92.65 km per hour for placebo. At 240 to 300 minutes, mean speed was 92.64 km per hour for CBD, 93.00 km per hour for THC, 93.01 km per hour for THC/CBD, and 92.75 km per hour for placebo. At 40 to 100 minutes, mean standard deviation of speed was 3.06 km per hour for CBD, 3.32 km per hour for THC, 3.18 km per hour for THC/CBD, and 2.93 km per hour for placebo. At 240 to 300 minutes, mean standard deviation of speed was 3.29 km per hour for CBD, 3.26 km per hour for THC, 3.37 km per hour for THC/CBD, and 3.40 km per hour for placebo.
Figure 2. The Standard Deviation of Lateral Position During On-Road Driving Tests.
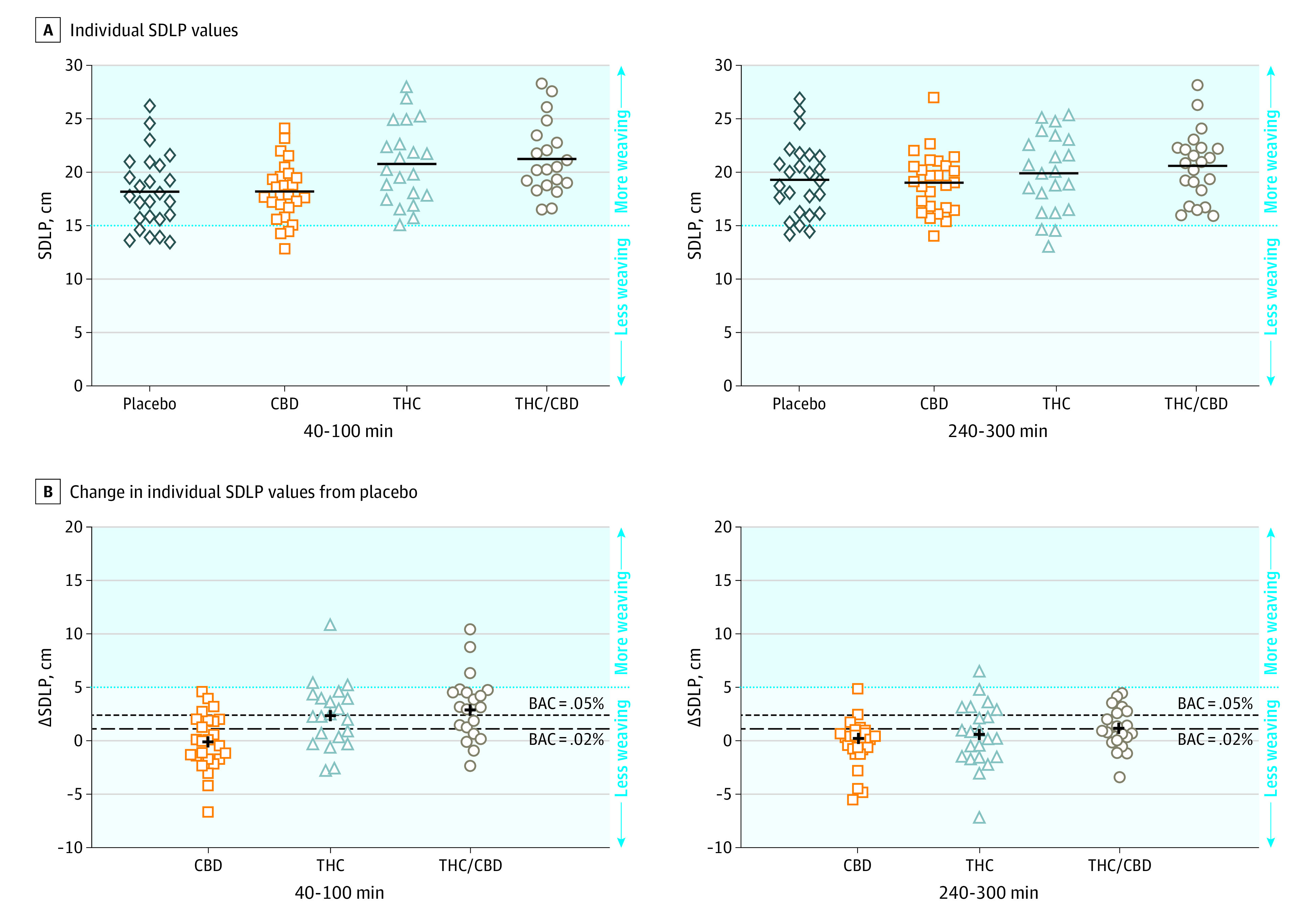
The x-axes indicate minutes postvaporization. Higher values on the y-axes indicate more weaving vs less weaving for lower values. A, The horizontal black bars indicate the mean standard deviation of lateral position (SDLP) in each condition. B, The dashed lines indicate the mean SDLP increase associated with a blood alcohol level (BAC) of 0.02% and 0.05%. The plus symbol shows the mean change in SDLP in each condition. CBD indicates cannabidiol; THC, Δ9-tetrahydrocannabinol.
Secondary Outcomes
At the end of each driving test, participants rated their driving as significantly more impaired compared with placebo in the THC condition (at 100 minutes, 4.15 [95% CI, 2.29 to 6.02]; P < .001, and at 300 minutes, 2.27 [95% CI, 0.41 to 4.12]; P = .008) and the THC/CBD condition (at 100 minutes, 4.09 [95% CI, 2.20 to 5.98]; P < .001, and at 300 minutes, 2.70 [95% CI, −0.93 to 4.57]; P = .001) (Figure 3). Participants rated the quality of their driving as significantly worse compared with placebo at 100 minutes (the end of the first driving test only) (THC, −1.95 [95% CI, −3.64 to −0.26]; P = .01, and for THC/CBD, −2.14 [95% CI, −3.83 to −0.44]; P = .006) (eFigure 1 in Supplement 2).
Figure 3. Confidence in Driving Ability Over Time and Perceived Driving Impairment at 2 Time Points.
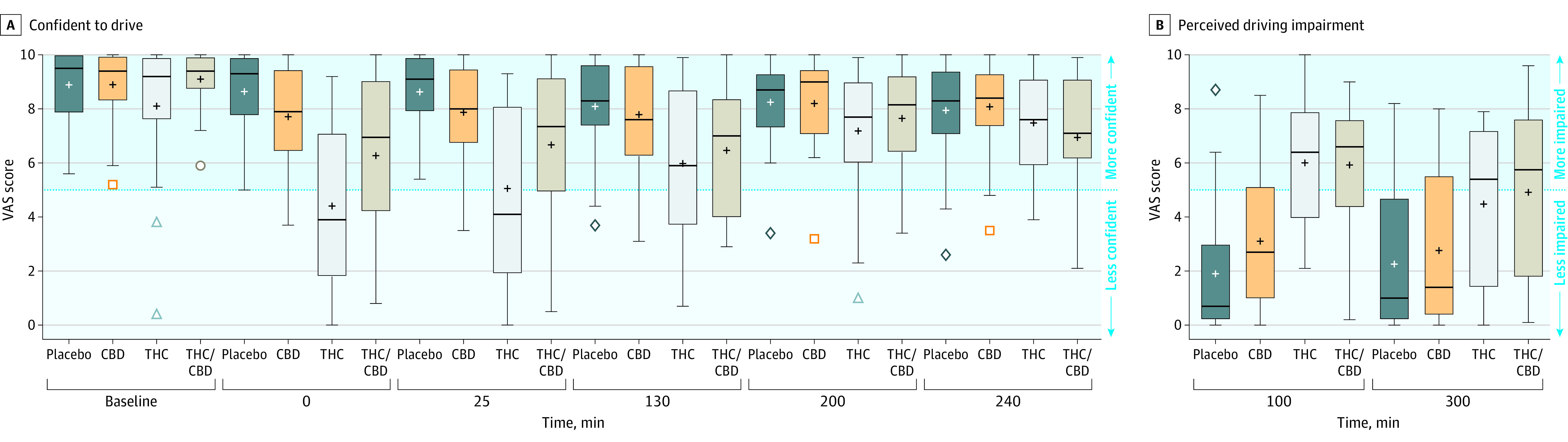
A, Baseline on the x-axis indicates predrug administration, minute 0 indicates the end of drug administration, all other values indicate time since vaporization. The visual analog scale (VAS) indicates mean values (range, 0-10 [not confident to very confident]). B, The VAS indicates mean values (range, 0-10 [less impaired to more impaired]) as assessed at the end of each on-road driving test.
Boxplot edges indicate the 25th and 75th quartile values. Horizontal bars indicate the median, and the plus signs indicate the mean. If there are no outliers (Q1 − 1.5 × [Q3 − Q1] and Q3 + 1.5 × [Q3 − Q1]), the whiskers indicate minimum and maximum values. Outliers (if present) are shown as colored symbols, the whiskers indicate the lowest and highest values that are not outliers. CBD indicates cannabidiol; THC, Δ9-tetrahydrocannabinol.
There was a main effect of condition for Confident to Drive (P < .001), with ratings decreased in the THC condition compared with placebo (at 0 minutes, −4.3 [95% CI, −5.61 to −2.98] [P < .001]; at 25 minutes, −3.65, [95% CI, −4.96 to −2.33] [P < .001]; and at 130 minutes, −2.18 [95% CI, −3.49 to −0.86] [P < .001]), decreased in the THC/CBD condition compared with placebo (at 0 minutes, −2.48 [95% CI, −3.81 to −1.14] [P < .001]; 25 minutes, −2.08 [95% CI, −3.41 to −0.75] [P < .001]; and at 130 minutes, −1.74 [95% CI, −3.07 to −0.41] [P = .003]) and with ratings greater in the THC/CBD condition compared with the THC condition (at 0 minutes, 1.82 [95% CI, −0.47 to 3.17] [P = .002]; and at 25 minutes, 1.57 [95% CI, 0.22 to 2.92] [P = .01]) (Figure 4). Results for other subjective drug effect measures are shown in eFigure 3 in Supplement 2, and results for the state subscale of the State Trait Anxiety Inventory are shown in eFigure 4 in Supplement 2. The rating of the Strength of Drug Effect was significantly lower in the THC/CBD condition than in the THC condition at 0 minutes (−1.67 [95% CI, −2.97 to −0.37]; P = .004) and at 25 minutes (−1.57 [95% CI, −2.87 to −0.27]; P = .01), and the rating of Anxious was significantly lower in the THC/CBD condition than in the THC condition at 0 minutes (−1.88 [95% CI, −2.99 to −0.76]; P < .001) and at 25 minutes (−1.14 [95% CI, −2.26 to −0.02]; P = .04).
Figure 4. Performance on the Digit Symbol Substitution Task, Divided Attention Task, and Paced Serial Addition Task.
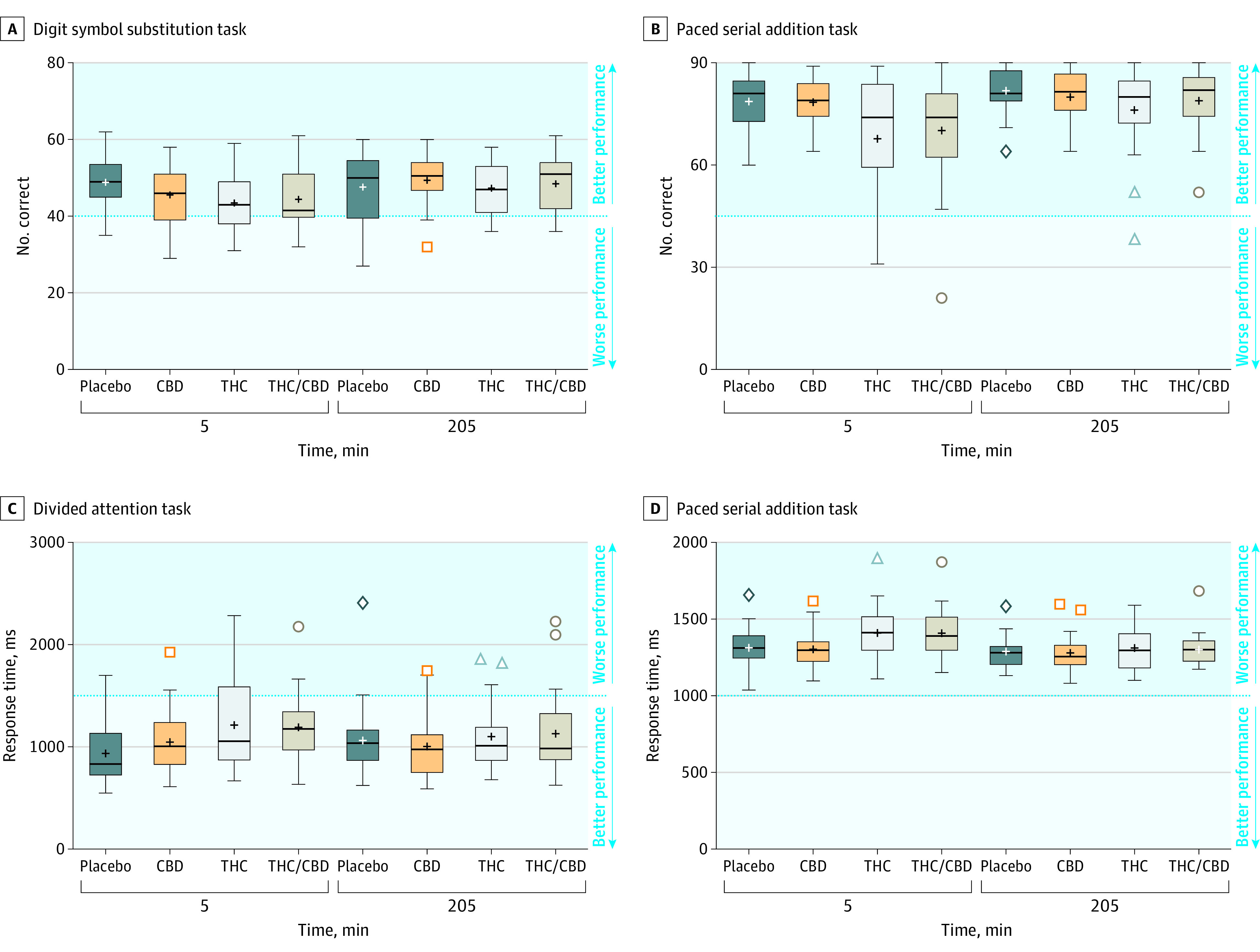
Time points on the x-axis indicate time since vaporization. Boxplot edges indicate the 25th and 75th quartile values. Horizontal bars indicate the median, and the plus signs indicate the mean. If there are no outliers (Q1 − 1.5 × [Q3 − Q1] and Q3 + 1.5 × [Q3 − Q1]), the whiskers indicate minimum and maximum values. Outliers (if present) are shown as colored symbols, the whiskers indicate the lowest and highest values that are not outliers. CBD indicates cannabidiol; THC, Δ9-tetrahydrocannabinol. Additional outcome measures are shown in eFigure 2 in Supplement 2.
Cognitive performance results are shown in Figure 4 and in eFigure 2 in Supplement 2. There was a significant main effect of condition for number correct and percent correct on the Digit Symbol Substitution Task (P = .04; P = .03) but not number attempted (P = .26); tracking error and response time on the Divided Attention Task (P = .02; P = .003); response time, number correct, and percent correct on the Paced Serial Addition Task (P = .001; P < .001; P = .002); and number correct and response time on the Tower of London (P = .03; P = .02). There was no effect of condition for either number correct or response time on the Emotional Stroop Task (P = .62; P = .82). The THC and THC/CBD conditions did not differ from placebo on any measures at 205 minutes, and the CBD condition did not differ from placebo on any measures at either time point (eTable 6 in Supplement 2).
Heart rate and blood pressure data are shown in eFigure 5 in Supplement 2. There was a significant condition × time interaction for systolic blood pressure (P = .001), although pairwise comparisons showed that neither THC nor THC/CBD differed significantly from placebo at any point in time (eTable 8 in Supplement 2). There was a main effect of condition on heart rate (P < .001) and a significant condition × time interaction (P < .001). eFigure 6 in Supplement 2 shows median (interquartile range) plasma cannabinoid concentrations over time. There was a significant main effect of condition, time, and condition × time for all analytes (eTable 3 in Supplement 2).
Post Hoc Outcomes
The proportions of participants showing impairment at 40 to 100 minutes at the 0.02% BAC criterion were 40% (CBD), 62% (THC), and 75% (THC/CBD). At 240 to 300 minutes, the proportions showing impairment were 16% (CBD), 36% (THC), and 50% (THC/CBD). The proportions of participants showing impairment at 40 to 100 minutes at the 0.05% BAC criterion were 16% (CBD), 48% (THC), and 60% (THC/CBD). At 240 to 300 minutes, the proportions were 8% (CBD), 27% (THC), and 32% (THC/CBD). As shown in eTable 9 in Supplement 2, symmetry analysis revealed no significant difference in the proportion of participants showing impaired or improved driving in the CBD condition at either BAC criterion (0.02%, ΔSDLP = 1.12 cm; 0.05%, ΔSDLP = 2.4 cm). There was a significant difference for the THC and THC/CBD conditions at 40 to 100 minutes, with most participants showing impairment at both BAC criterion levels.
Adverse Events
One participant had a panic attack shortly after cannabis administration in the THC condition, leading to termination of that test day and withdrawal from the study. Out of 188 test drives that commenced, 16 (8.5%) were terminated by the driving instructor due to safety concerns. Of these terminated drives, 9 occurred during the first driving test (placebo [2], CBD [2], THC [2], THC/CBD [3]) and 7 during the second test (placebo [1], CBD [1], THC [2], THC/CBD [3]). All terminations in the second test were due to the participant appearing heavily fatigued while driving. There were no significant differences in terminations across conditions. In addition, 3 drives were cancelled prior to commencement (THC [2] and THC/CBD [1]) due to participant concerns about their ability to drive safely.
Discussion
In this randomized clinical trial, THC-dominant and THC/CBD-equivalent cannabis produced a short-term impairment during experimental on-road driving, as indexed by a significant increase in SDLP measured 40 to 100 minutes following vaporization. In agreement with previous studies involving smoked cannabis or oral THC (dronabinol),26,27 this impairment was modest in magnitude and similar to that seen in drivers with a 0.05% BAC (≈2.4-2.5 cm28). SDLP in the placebo and CBD conditions did not differ, indicating that CBD, when administered in a bolus dose via vaporization, did not impair driving. During these driving tests, the overall range of lateral position values (ie, the actual distance between the vehicle and the lane boundary to the left of the vehicle) was approximately 54 cm.
This finding was validated by a post hoc symmetry analysis, which showed that drivers in the CBD condition were no more likely to show impairment than they were improvement relative to placebo at SDLP thresholds corresponding to BACs of 0.02% and 0.05%. Consistent with prior research,29 CBD-dominant cannabis also failed to produce significant cognitive or psychomotor impairment compared with placebo. While the doses of THC in the current study (13.75 mg) were moderate, they caused strong subjective effects including reduced confidence to drive. The presence of CBD did not reduce THC impairment of driving, although there were subtle differences in the subjective effects of THC-dominant and THC/CBD-equivalent cannabis despite near-identical THC plasma concentrations. THC/CBD-equivalent cannabis appeared to cause less anxiety, reduced strength of drug effects, and greater confidence to drive than THC-dominant cannabis, particularly at earlier time points. This agrees with prior, albeit limited, evidence that coadministered CBD can reduce the euphoric, anxiogenic and subjective drug effects of THC.30,31 Other studies have failed to find such modulatory effects,7,12 suggesting they may be subtle and ephemeral in nature.
Previous on-road26,32 and simulator12,33 studies have described increased SDLP for up to 3 hours following inhaled cannabis. Consistent with this, the present study failed to detect changes in SDLP at 240 to 300 minutes. Impairment could be extended with use of oral products15 or with higher inhaled doses, and so these results should not be considered definitive. Confidence to drive only tracked SDLP to a limited extent while post hoc evaluation of driving ability appeared more accurate, suggesting that participants were better able to evaluate their driving performance after the fact than predict it. This same pattern has been observed with other drugs known to impair driving, such as alcohol, alprazolam, and zolpidem.34 Participants considered their driving at 240 to 300 minutes to be significantly more impaired in the THC and THC/CBD conditions than in the placebo condition, despite there being no difference across conditions in SDLP at that point in time. Participants may have retrospectively overrated their impairment, or this may have indicated subtle persistence of THC-induced impairment, perhaps combined with fatigue, causing subclinical SDLP increments (ie, <1.5 cm) that likely have limited real-world relevance.
Limitations
This study has several limitations. First, it was limited to healthy volunteers who were occasional cannabis users. The applicability of these findings to more frequent users, including medical cannabis patients, is unclear given that daily cannabis use may produce at least partial tolerance to the impairing effects of THC.35 Second, only 1 dose of CBD and a single 1:1 ratio of CBD and THC were tested. The CBD dose used was also lower than that used in clinical practice for conditions such as pediatric epilepsy in which oral administration of CBD oils at doses of approximately 10 to 20 mg/kg is common.8 Driving outcomes may differ with higher CBD and THC doses and different CBD:THC ratios. Retail CBD products in North America and other regions are not strictly regulated and so actual CBD content may be unknown or misrepresented.36 Third, the confidence limits associated with change in SDLP in the CBD condition suggested the possibility of subclinical impairment similar to that seen at low BACs. While symmetry analysis suggested no difference in the proportion of impaired vs improved drivers in the CBD condition, these findings are exploratory and based on a small number of drivers and a single CBD dose. Fourth, this study was limited to a sample of young drivers with similar driving experience. Degree of driving impairment may differ as a function of driving experience as well as experience with cannabis and the driving task. Fifth, this study was powered to detect an effect of THC on driving and may have been underpowered to detect a difference between the THC and THC/CBD conditions.
Conclusions
In a crossover clinical trial that assessed driving performance during on-road driving tests, the SDLP following vaporized THC-dominant and THC/CBD-equivalent cannabis compared with placebo was significantly greater at 40 to 100 minutes but not 240 to 300 minutes after vaporization; there were no significant differences between CBD-dominant cannabis and placebo. However, the effect size for CBD-dominant cannabis may not have excluded clinically important impairment, and the doses tested may not represent common usage.
Trial Protocol
eMethods 1. Cognitive and Psychomotor Performance
eMethods 2. Plasma Cannabinoid Analysis
eTable 1. Test Day Order of Events
eTable 2. Results of Linear Mixed-Effects Model Analyses With Data From Study Completers Only (N=22)
eTable 3. Results of Linear Mixed-Effects Model Analyses With Data From All Participants (N=26)
eTable 4. Results of Post Hoc Tests for Subjective Drug Effect Measures
eTable 5. Results of Post Hoc Tests for Driving Measures
eTable 6. Results of Post Hoc Tests for Cognitive/Psychomotor Task Performance Measures
eTable 7. Results of Post Hoc Tests for Plasma Cannabinoid Concentrations
eTable 8. Results of Post Hoc Tests for Cardiovascular Measures
eTable 9. Symmetry Analysis of Proportions Impaired and Improved Drivers in Each Treatment Condition, at 2 BAC Thresholds of Impairment
eFigure 1. Perceive Driving Quality
eFigure 2. Additional Outcome Measures for the Digit Symbol Substitution Task (A-B), Divided Attention Task (C), Paced Serial Addition Task (D), and Tower of London (E-F)
eFigure 3. Mean Ratings of Visual Analog Scale (VAS) Items “Strength of Drug Effect” (A), “Liking of Drug Effect” (B), “Stoned” (C), “Sedated” (D), “Relaxed” (E), and “Anxious” (F)
eFigure 4. State Anxiety Inventory (STAI-S) Scores
eFigure 5. Cardiovascular Measures
eFigure 6. Median Concentrations of THC, 11-OH-THC, 11-COOH-THC, and CBD Concentrations (ng/mL) in Plasma Over Time
Data Sharing Statement
References
- 1.Rogeberg O. A meta-analysis of the crash risk of cannabis-positive drivers in culpability studies-Avoiding interpretational bias. Accid Anal Prev. 2019;123:69-78. doi: 10.1016/j.aap.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 2.Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction. 2016;111(8):1348-1359. doi: 10.1111/add.13347 [DOI] [PubMed] [Google Scholar]
- 3.Ramaekers JG. Driving under the influence of cannabis: an increasing public health concern. JAMA. 2018;319(14):1433-1434. doi: 10.1001/jama.2018.1334 [DOI] [PubMed] [Google Scholar]
- 4.Ramaekers JG. Drugs and driving research in medicinal drug development. Trends Pharmacol Sci. 2017;38(4):319-321. doi: 10.1016/j.tips.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Jikomes N, Zoorob M. The cannabinoid content of legal cannabis in washington state varies systematically across testing facilities and popular consumer products. Sci Rep. 2018;8(1):4519. doi: 10.1038/s41598-018-22755-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill KP. Medical use of cannabis in 2019. JAMA. 2019;322(10):974-975. doi: 10.1001/jama.2019.11868 [DOI] [PubMed] [Google Scholar]
- 7.Freeman AM, Petrilli K, Lees R, et al. . How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? Neurosci Biobehav Rev. 2019;107:696-712. doi: 10.1016/j.neubiorev.2019.09.036 [DOI] [PubMed] [Google Scholar]
- 8.Chesney E, Oliver D, Green A, et al. . Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. [published online ahead of print, 2020 Apr 8]. Neuropsychopharmacology. 2020;45(11):1799-1806. doi: 10.1038/s41386-020-0667-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dos Santos RG, Guimarães FS, Crippa JAS, et al. . Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2020;16(6):517-526. doi: 10.1080/17425255.2020.1754793 [DOI] [PubMed] [Google Scholar]
- 10.Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol. 2019;30(30):98-102. doi: 10.1016/j.copsyc.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lintzeris N, Mills L, Suraev A, et al. . Medical cannabis use in the Australian community following introduction of legal access: the 2018-2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18). Harm Reduct J. 2020;17(1):37. doi: 10.1186/s12954-020-00377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arkell TR, Lintzeris N, Kevin RC, et al. . Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology (Berl). 2019;236(9):2713-2724. doi: 10.1007/s00213-019-05246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y). Consulting Psychologists Press; 1983. [Google Scholar]
- 14.O’Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol. 1984;18(S1)(suppl 1):121S-129S. doi: 10.1111/j.1365-2125.1984.tb02590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandrey R, Herrmann ES, Mitchell JM, et al. . Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol. 2017;41(2):83-99. doi: 10.1093/jat/bkx012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006;85(2):114-122. doi: 10.1016/j.drugalcdep.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 17.Mcleod DR, Griffiths RR, Bigelow GE, Yingling J. An Automated version of the Digit Symbol Substitution Test (DSST). Behav Res Meth Instr. 1982;14(5):463-466. doi: 10.3758/BF03203313 [DOI] [Google Scholar]
- 18.Casswell S, Marks D. Cannabis induced impairment of performance of a divided attention task. Nature. 1973;241(5384):60-61. doi: 10.1038/241060b0 [DOI] [PubMed] [Google Scholar]
- 19.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44(2):367-373. doi: 10.2466/pms.1977.44.2.367 [DOI] [PubMed] [Google Scholar]
- 20.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298(1089):199-209. doi: 10.1098/rstb.1982.0082 [DOI] [PubMed] [Google Scholar]
- 21.Richards A, French CC, Johnson W, Naparstek J, Williams J. Effects of mood manipulation and anxiety on performance of an emotional Stroop task. Br J Psychol. 1992;83(Pt 4):479-491. doi: 10.1111/j.2044-8295.1992.tb02454.x [DOI] [PubMed] [Google Scholar]
- 22.Kevin RC, Allsop DJ, Lintzeris N, Dunlop AJ, Booth J, McGregor IS. Urinary cannabinoid levels during nabiximols (Sativex)-medicated inpatient cannabis withdrawal. Forensic Toxicol. 2017;35(1):33-44. doi: 10.1007/s11419-016-0330-029367861 [DOI] [Google Scholar]
- 23.Schwope DM, Scheidweiler KB, Huestis MA. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(4):1273-1283. doi: 10.1007/s00216-011-5197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jongen S, van der Sluiszen NNJJM, Brown D, Vuurman EFPM. Single- and dual-task performance during on-the-road driving at a low and moderate dose of alcohol: a comparison between young novice and more experienced drivers. Hum Psychopharmacol. 2018;33(3):e2661. doi: 10.1002/hup.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veldstra JL, Bosker WM, de Waard D, Ramaekers JG, Brookhuis KA. Comparing treatment effects of oral THC on simulated and on-the-road driving performance: testing the validity of driving simulator drug research. Psychopharmacology (Berl). 2015;232(16):2911-2919. doi: 10.1007/s00213-015-3927-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaekers JG, Robbe HW, O’Hanlon JF. Marijuana, alcohol and actual driving performance. Hum Psychopharmacol. 2000;15(7):551-558. doi: [DOI] [PubMed] [Google Scholar]
- 27.Bosker WM, Kuypers KP, Theunissen EL, et al. . Medicinal Δ(9) -tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in standard field sobriety tests. Addiction. 2012;107(10):1837-1844. doi: 10.1111/j.1360-0443.2012.03928.x [DOI] [PubMed] [Google Scholar]
- 28.Jongen S, Vermeeren A, van der Sluiszen NN, et al. . A pooled analysis of on-the-road highway driving studies in actual traffic measuring standard deviation of lateral position (ie, “weaving”) while driving at a blood alcohol concentration of 0.5 g/L. Psychopharmacology (Berl). 2017;234(5):837-844. doi: 10.1007/s00213-016-4519-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spindle TR, Cone EJ, Goffi E, et al. . Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 2020;211:107937. doi: 10.1016/j.drugalcdep.2020.107937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther. 1976;19(3):300-309. doi: 10.1002/cpt1976193300 [DOI] [PubMed] [Google Scholar]
- 31.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl). 1982;76(3):245-250. doi: 10.1007/BF00432554 [DOI] [PubMed] [Google Scholar]
- 32.Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharm Clin. 1998;13(S2):S70-S78. doi: [DOI] [Google Scholar]
- 33.Hartman RL, Brown TL, Milavetz G, et al. . Cannabis effects on driving lateral control with and without alcohol. Drug Alcohol Depend. 2015;154:25-37. doi: 10.1016/j.drugalcdep.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verster JC, Roth T. Drivers can poorly predict their own driving impairment: a comparison between measurements of subjective and objective driving quality. Psychopharmacology (Berl). 2012;219(3):775-781. doi: 10.1007/s00213-011-2400-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramaekers JG, Mason NL, Theunissen EL. Blunted highs: pharmacodynamic and behavioral models of cannabis tolerance. Eur Neuropsychopharmacol. 2020;36:191-205. doi: 10.1016/j.euroneuro.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 36.Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493. doi: 10.1001/jama.2015.6613 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Cognitive and Psychomotor Performance
eMethods 2. Plasma Cannabinoid Analysis
eTable 1. Test Day Order of Events
eTable 2. Results of Linear Mixed-Effects Model Analyses With Data From Study Completers Only (N=22)
eTable 3. Results of Linear Mixed-Effects Model Analyses With Data From All Participants (N=26)
eTable 4. Results of Post Hoc Tests for Subjective Drug Effect Measures
eTable 5. Results of Post Hoc Tests for Driving Measures
eTable 6. Results of Post Hoc Tests for Cognitive/Psychomotor Task Performance Measures
eTable 7. Results of Post Hoc Tests for Plasma Cannabinoid Concentrations
eTable 8. Results of Post Hoc Tests for Cardiovascular Measures
eTable 9. Symmetry Analysis of Proportions Impaired and Improved Drivers in Each Treatment Condition, at 2 BAC Thresholds of Impairment
eFigure 1. Perceive Driving Quality
eFigure 2. Additional Outcome Measures for the Digit Symbol Substitution Task (A-B), Divided Attention Task (C), Paced Serial Addition Task (D), and Tower of London (E-F)
eFigure 3. Mean Ratings of Visual Analog Scale (VAS) Items “Strength of Drug Effect” (A), “Liking of Drug Effect” (B), “Stoned” (C), “Sedated” (D), “Relaxed” (E), and “Anxious” (F)
eFigure 4. State Anxiety Inventory (STAI-S) Scores
eFigure 5. Cardiovascular Measures
eFigure 6. Median Concentrations of THC, 11-OH-THC, 11-COOH-THC, and CBD Concentrations (ng/mL) in Plasma Over Time
Data Sharing Statement


