Abstract
This study uses IBM MarketScan database data to describe trends in zolpidem and low-dose trazodone dispensing among adults with employer-sponsored insurance or Medicare supplemental plans between 2011 and 2018, before and after a 2017 clinical practice guideline discouraged trazodone use for insomnia.
In 2012, 19% of US adults reported regular insomnia or sleeping problems.1 Medications most commonly used for insomnia are zolpidem (a benzodiazepine receptor agonist) and trazodone (a sedating antidepressant used in low doses for insomnia).2 Prescriptions for these drugs increased during the 1990s and early 2000s,2,3 but recent trends have not been adequately described. Understanding contemporary trends is important given that zolpidem is approved by the US Food and Drug Administration (FDA) for insomnia and backed by abundant efficacy data,4 but documented safety concerns exist.5,6 In contrast, trazodone is not FDA approved for insomnia and has limited efficacy data and undocumented safety for this indication.4 We measured the dispensing of these drugs from 2011 to 2018 among commercially insured US adults.
Methods
Using the 2011 to 2018 IBM MarketScan Research Databases, we measured trends in dispensing of zolpidem and low-dose trazodone among adults (aged ≥18 years) with employer-sponsored insurance or Medicare supplemental plans. The MarketScan databases contain health care and pharmacy claims from approximately 350 payers across the US and are considered generally representative of individuals with employer-provided insurance.
We calculated the annual percentage of adults with at least 1 dispensing of zolpidem or low-dose trazodone among all adults who contacted the health care system in a given year and had at least 12 months of prior continuous medical and drug insurance. We used the dosage to identify whether trazodone was dispensed for insomnia because an insomnia diagnosis was not a reliable marker of treatment indication (in the MarketScan data, only 41% of adults dispensed zolpidem had an insomnia diagnosis, although zolpidem is only known to be used for insomnia). Low-dose trazodone was defined as less than 150 mg/d, which is commonly used for insomnia and below the starting dosage of 150 mg/d for depression. Individuals with trazodone dispensed multiple times in a given year were counted only if all prescriptions dispensed were for low dosages. We used multivariable binomial regression to estimate the annual percentage change in dispensing, adjusted for age, sex, and depression diagnosis. We conducted sensitivity analyses with higher specificity for identifying prescriptions dispensed for insomnia that (1) analyzed trends for trazodone at dosages less than or equal to 50 mg/d and (2) considered only adults with a diagnosis code for insomnia in the past year. All models used generalized estimating equations to account for within-individual correlation in the data across years. Analyses were conducted using SAS version 9.4 (SAS Institute Inc). The study was approved by the Harvard Pilgrim Health Care Institutional Review Board with a waiver of informed consent.
Results
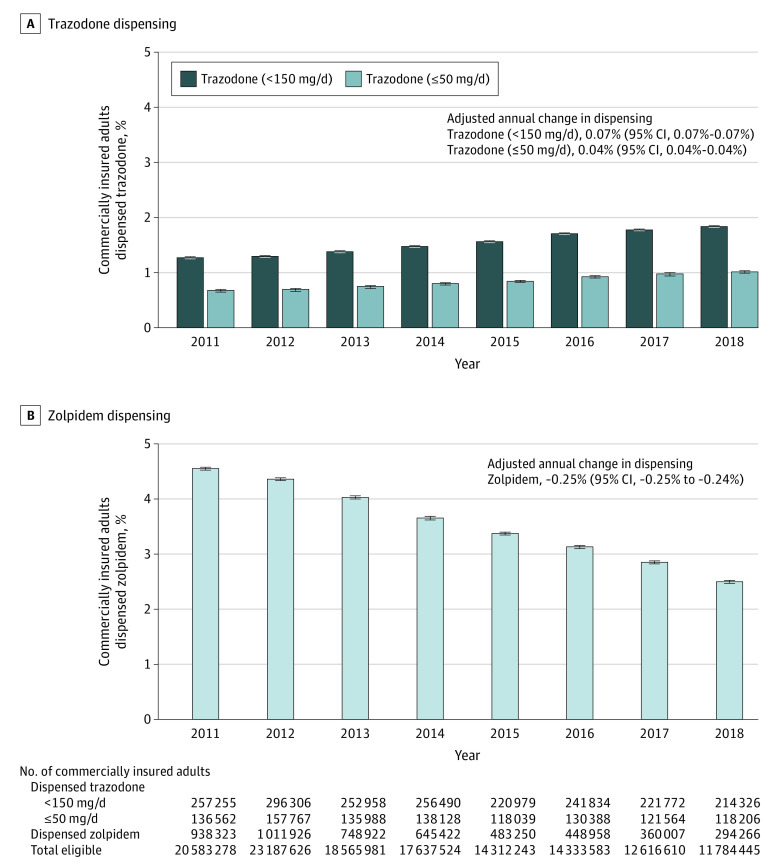
From 2011 to 2018, among an annual mean of 16.6 million adults in MarketScan, the percentage of adults who were dispensed low-dose trazodone (<150 mg/d) increased from 1.25% (95% CI, 1.25%-1.25%) (257 255 of 20 583 278) to 1.82% (95% CI, 1.81%-1.83%) (214 326 of 11 784 445), while the percentage of adults dispensed zolpidem decreased from 4.56% (95% CI, 4.55%-4.57%) (938 323 of 20 583 278) to 2.50% (95% CI, 2.49%-2.51%) (294 266 of 11 784 445). The adjusted annual change was 0.07% (95% CI, 0.07%-0.07%) for trazodone and −0.25% (95% CI, −0.25% to −0.24%) for zolpidem. Results for trazodone were similar for dosages less than or equal to 50 mg/d (Figure 1).
Figure 1. Percentage of Commercially Insured Adults Dispensed Zolpidem or Low-Dose Trazodone, 2011-2018.
The 2011 to 2018 IBM MarketScan Research Databases were used to measure trends in the dispensing of zolpidem and low-dose trazodone among US adults (aged ≥18 years) with employer-sponsored insurance or Medicare supplemental plans. For each year, the percentage of adults who received at least 1 dispensing of low-dose trazodone (<150 mg/d or ≤50 mg/d) or any dosage of zolpidem was calculated among all adults who contacted the health care system in the year and had at least 12 months of prior continuous medical and drug insurance (mean of 16.6 million adults per year in the MarketScan databases). Individuals who had trazodone dispensed multiple times in a given year were counted only if all prescriptions dispensed were below the dose threshold to avoid counting individuals tapering up to or down from higher dosages (more likely indicated for depression). Multivariable binomial regression models were used to estimate the annual percentage change in the dispensing of each drug in 2011 to 2018, adjusted for age, sex, and depression diagnosis, in which depression diagnosis was defined as having at least 2 outpatient codes or 1 inpatient code for depression in the past year. The short black dashes above the vertical bars indicate the 95% CIs around the annual percentages.
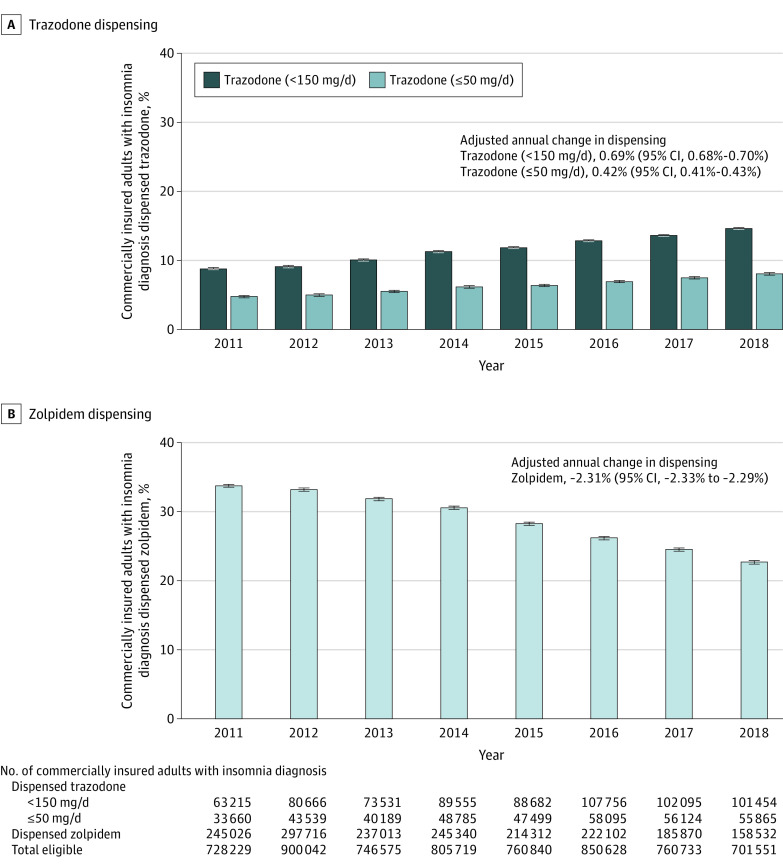
Among adults with a diagnosis of insomnia (annual mean of 781 790 adults), the percentage of adults dispensed low-dose trazodone (<150 mg/d) increased from 8.68% (95% CI, 8.62%-8.75%) (63 215 of 728 229) to 14.46% (95% CI, 14.38%-14.54%) (101 454 of 701 551) from 2011 to 2018, while the percentage of adults dispensed zolpidem decreased from 33.65% (95% CI, 33.54%-33.76%) (245 026 of 728 229) to 22.60% (95% CI, 22.50%-22.70%) (158 532 of 701 551). The adjusted annual change was 0.69% (95% CI, 0.68%-0.70%) for trazodone and −2.31% (95% CI, −2.33% to −2.29%) for zolpidem. Results for trazodone were also similar for dosages less than or equal to 50 mg/d (Figure 2).
Figure 2. Percentage of Commercially Insured Adults With an Insomnia Diagnosis Dispensed Zolpidem or Low-Dose Trazodone, 2011-2018.
The 2011 to 2018 IBM MarketScan Research Databases were used to measure trends in the dispensing of zolpidem and low-dose trazodone among US adults (aged ≥18 years) with employer-sponsored insurance or Medicare supplemental plans who had a diagnosis code for insomnia. For each year, the percentage of adults who received at least 1 dispensing of low-dose trazodone (<150 mg/d or ≤50 mg/d) or any dosage of zolpidem was calculated among all adults who contacted the health care system in the year, had at least 12 months of prior continuous medical and drug insurance, and had a diagnosis code for insomnia recorded in the past year (mean of 781 790 adults per year in the MarketScan databases). Individuals who had trazodone dispensed multiple times in a given year were counted only if all prescriptions dispensed were below the dose threshold to avoid counting individuals tapering up to or down from higher dosages (more likely indicated for depression). Multivariable binomial regression models were used to estimate the annual percentage change in the dispensing of each drug in 2011 to 2018, adjusted for age, sex, and depression diagnosis, in which depression diagnosis was defined as having at least 2 outpatient codes or 1 inpatient code for depression in the past year. The short black dashes above the vertical bars indicate the 95% CIs around the annual percentages.
Discussion
Low-dose trazodone was dispensed with increased frequency during the period before to nearly 2 years after a US guideline in early 20174 suggested not using trazodone for insomnia, although these increases were modest. Dispensing of zolpidem was already decreasing before 2 FDA safety announcements in 20135 and a black box warning in 2019.6 In 2018, zolpidem was still being dispensed more frequently than low-dose trazodone, but the gap between these drugs has narrowed since 2011, suggesting an increasing preference toward off-label use of trazodone for insomnia. Study limitations include that the MarketScan databases lacked explicitly documented treatment indications and captured only commercially insured individuals. More studies evaluating the efficacy and safety of trazodone for insomnia are warranted.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015;16(3):372-378. doi: 10.1016/j.sleep.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37(2):343-349. doi: 10.5665/sleep.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh JK, Schweitzer PK. Ten-year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371-375. doi: 10.1093/sleep/22.3.371 [DOI] [PubMed] [Google Scholar]
- 4.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration FDA drug safety communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. Published May 14, 2013. Accessed June 30, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-new-label-changes-and-dosing-zolpidem-products-and
- 6.US Food and Drug Administration FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines: FDA drug safety communication. Published April 30, 2019. Accessed June 30, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia