Abstract
Biliary atresia (BA) is a destructive inflammatory obliterative cholangiopathy of the neonate that affects various parts of the bile duct. If early diagnosis followed by Kasai portoenterostomy is not performed, progressive liver cirrhosis frequently leads to liver transplantation in the early stage of life. Therefore, prompt diagnosis is necessary for the rescue of BA patients. However, the prompt diagnosis of BA remains challenging because specific and reliable biomarkers for BA are currently unavailable. In this study, we discovered potential biomarkers for BA using deep proteome analysis by data-independent acquisition mass spectrometry (DIA–MS). Four patients with BA and three patients with neonatal cholestasis of other etiologies (non-BA) were recruited for stool proteome analysis. Among the 2110 host-derived proteins detected in their stools, 49 proteins were significantly higher in patients with BA and 54 proteins were significantly lower. These varying stool protein levels in infants with BA can provide potential biomarkers for BA. As demonstrated in this study, the deep proteome analysis of stools has great potential not only in detecting new stool biomarkers for BA but also in elucidating the pathophysiology of BA and other pediatric diseases, especially in the field of pediatric gastroenterology.
Keywords: biliary atresia, proteome analysis, DIA–MS, stool biomarker
1. Introduction
Biliary atresia (BA) is a destructive inflammatory obliterative cholangiopathy of neonates that affects various parts of the intra- and extrahepatic bile duct and causes cholestasis, which manifests as jaundice with hyperbilirubinemia. BA is a pediatric emergency because progression frequently leads to cirrhosis and liver transplantation [1]. As there are many other rare diseases that present with cholestasis in infancy and specific biomarkers for BA have not yet been identified, the prompt diagnosis of BA remains challenging for pediatric surgeons and pediatric gastroenterologists. Operative cholangiogram, an invasive diagnostic procedure, is the gold standard for the definitive diagnosis of BA, as it distinguishes BA from other causes of neonatal cholestasis (non-BA) [1]. Therefore, a simple and noninvasive examination with sufficient accuracy and reliability using biomarkers for BA is urgently required.
Plasma and serum are used as samples for biomarker discovery because they can be collected with low invasiveness. However, plasma/serum collection sometimes becomes a burden, especially in small children. On the other hand, stool is an ideal sample for discovering new biomarkers for pediatric diseases because it can be obtained noninvasively from patients. As an analysis that uses stool samples, the Sudan III stain of stool fat is available for the early diagnosis of BA, but its specificity is not sufficient [2]. In addition, gut microbiota analysis by 16S rRNA sequencing has been attracting attention in recent years and has been used for biomarker discovery in various diseases. The relevant microbiota, which is a potential diagnostic marker for BA, has also been reported [3,4]. On the other hand, the clinical application of these biomarkers has problems, such as large individual differences in gut microbiota [5] and high costs of the analysis. Thus, we focused on host proteins rather than bacteria in stools. Stools are formed via the gastrointestinal tract, which contains many host proteins that originate from gastrointestinal tissues [6]. Therefore, stool is a feasible clinical sample for exploring biomarkers of gastrointestinal diseases such as BA. This expectation also originated from one of the clinical symptoms of cholestasis, such as BA, in which the stool color gradually changed from normal (yellowish, brown, and greenish) to abnormal (clay-colored, pale yellowish, and light yellowish), followed by a gradual reduction in bile supply from the liver and gallbladder [7]. In actuality, host proteins in the stool of patients with colorectal cancer have been examined using proteomics, leading to the discovery of candidate colon biomarkers [8,9,10]. For proteomic analysis of stools, optimized methods have not been established, and up to one thousand host proteins are detectable in stools [6]. Therefore, high-depth proteome analysis is required to detect more host-derived proteins in stools.
Regarding proteomics technologies, data-dependent acquisition mass spectrometry (DDA–MS)-based proteomics is the mainstream; however, in recent years, data-independent acquisition mass spectrometry (DIA–MS)-based proteomics has attracted attention. Single-shot analysis by DIA–MS enables deep proteome analysis with higher reproducibility than DDA–MS and is suitable for biomarker discovery [11,12,13]. In particular, overlapping-window DIA–MS reduces the complexity of the MS2 spectrum, allowing deeper proteome analysis than normal DIA–MS [14,15]. In addition, the Prosit web tool enables the creation of high-quality spectral libraries for the identification of MS2 spectra in DIA–MS from protein sequence databases, which eliminates the need to create spectral libraries by DDA–MS [16]. In this way, the DIA–MS-based proteome analysis technologies have been refined, and it has become possible to easily perform deep proteome analysis.
In this study, we focused on host-derived proteins in stools and established an overlapping window DIA-based proteome analysis using noninvasive stool samples for the discovery of new stool biomarkers for BA. We successfully detected over 2000 host-derived proteins in stool samples. This is the first research article that attempts to discover new biomarkers for BA using deep proteome analysis of stools.
2. Methods
2.1. Patients
This was a retrospective observational study that analyzed stool proteins of BA and non-BA patients. Four BA patients before Kasai portoenterostomy and three non-BA patients were recruited for stool proteome analysis. Naturally defecated stools were preserved at −80 °C. All patients with BA were classified as type III (obstruction of the most proximal part of the extrahepatic biliary tract at the porta hepatis), whereas three non-BA patients had neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD), cholestasis after repair of gastroschisis, and veno-occlusive disease (VOD). The average ages of BA patients and non-BA patients at the time of stool collection were 56 and 80 days, respectively (Table 1).
Table 1.
Clinical features of subjects in this study.
| Disease | Sample | Type of BA | Sex | Age | AST | ALT | T-Bil | D-Bil | GGTP | |
|---|---|---|---|---|---|---|---|---|---|---|
| BA | RR | 13–30 | 1.0–42 | 0.4–1.5 | 0–0.3 | 13–64 | ||||
| BA.1 | III-c1-ν | M | 65 | 261 | 238 | 13 | 8.9 | 813 | ||
| BA.2 | III-a1-ν | M | 69 | 102 | 80 | 7.7 | 5.4 | 536 | ||
| BA.3 | III-b1-ν | M | 63 | 186 | 120 | 9.1 | 6.0 | 276 | ||
| BA.4 | III-b1-ν | F | 25 | 99 | 50 | 4.1 | 2.8 | 195 | ||
| Ave | 56 | 162 | 122 | 8.5 | 5.8 | 455 | ||||
| Disease | Sample | Type of non-BA | Sex | Age | AST | ALT | T-Bil | D-Bil | GGTP | |
| non-BA | RR | 13–30 | 1.0–42 | 0.4–1.5 | 0–0.3 | 13–64 | ||||
| non-BA.1 | NICCD | M | 90 | 241 | 48 | 9.4 | 5.7 | 172 | ||
| non-BA.2 | GS | F | 38 | 143 | 110 | 5.4 | 3.2 | 194 | ||
| non-BA.3 | VOD | F | 112 | 116 | 150 | 25.7 | 17.0 | 37 | ||
| Ave | 80 | 167 | 103 | 14 | 8.6 | 134 |
Abbreviations: Ave, avellage; BA, biliary atresia; NICCD, intrahepatic cholestasis caused by citrin deficiency; GS, gastroschisis; VOD, veno-occlusive disease; AST, aspartate amino transferase (U/L); ALT, alanine aminotransferase (U/L); T-Bil, total bilirubin (mg/dL); D-Bil, direct bilirubin (mg/dL); GGTP, gamma-glutamyl transpeptidase (U/L); RR, reference range. Non-BA: cholestasis other than BA. Age: days after birth.
2.2. Proteome Analysis
Soluble proteins in stools prepared in PBS with protease inhibitors were extracted by pipetting and inverting after incubating for 30 min on ice. After centrifugation at 15,000× g for 15 min at 4 °C to remove insoluble matter, the supernatants were transferred to new tubes and subjected to trichloroacetic acid precipitation (final concentration 12.5% v/v), followed by acetone washing and drying via an opened lid. The dried samples were redissolved in 0.5% sodium dodecanoate and 100 mM Tris-HCl (pH 8.5) using a water-bath-type sonicator (Bioruptor UCD-200, SonicBio Corporation, Kanagawa, Japan). The pretreatment for shotgun proteome analysis was performed as reported previously [15].
Peptides were directly injected onto a 75 μm × 20 cm PicoFrit emitter (New Objective, Woburn, MA, USA) packed in-house with C18 core-shell particles (CAPCELL CORE MP 2.7 μm, 160 Å material; Osaka Soda Co., Ltd., Osaka, Japan) at 45 °C and then separated with an 80-min gradient at a flow rate of 100 nL/min using an UltiMate 3000 RSLCnano LC system (Thermo Fisher Scientific, Waltham, MA, USA). Peptides that eluted from the column were analyzed on a Q Exactive HF-X (Thermo Fisher Scientific) for overlapping window DIA–MS [15]. MS1 spectra were collected in the range of 495–785 m/z at 30,000 resolutions to set an automatic gain control target of 3e6 and maximum injection time of 55. MS2 spectra were collected in the range of more than 200 m/z at 30,000 resolutions to set an automatic gain control target of 3e6, maximum injection time of “auto”, and stepped normalized collision energies of 22, 26, and 30%. The isolation width for MS2 was set to 4 m/z, and overlapping window patterns in 500–780 m/z using window placements were optimized using Skyline.
MS files were searched for a human spectral library using Scaffold DIA (Proteome Software, Inc., Portland, OR, USA). The human spectral library was generated from the human protein sequence database (UniProt id UP000005640, reviewed, canonical) using Prosit [16]. The Scaffold DIA search parameters were as follows: experimental data search enzyme, trypsin; maximum missed cleavage sites, 1; precursor mass tolerance, 8 ppm; fragment mass tolerance, 8 ppm; and static modification, cysteine carbamidomethylation. The protein identification threshold was set for both peptide and protein false discovery rates of less than 1%. Peptide quantification was calculated using the EncyclopeDIA algorithm [17] in Scaffold DIA. For each peptide, the four highest-quality fragment ions were selected for quantitation. Protein quantification was estimated from the summed peptide quantification.
2.3. Data Analysis
The Gene Ontology (GO) enrichment analysis tool (Enrichr) was used to retrieve functional annotation [18]. To determine differential proteins between BA and non-BA, the statistical p-value (Mann–Whitney U test, p < 0.05) was used in data analysis. Elevated proteins in plasma and liver tissues were referred to the Human Protein Atlas (HPA; https://www.proteinatlas.org/) [19]. A heatmap was drawn based on Z-scores calculated from the DIA protein quantification using the R (version 3.5.1) function “heatmap2”.
2.4. Ethical Approval and Consent to Participate
This study was approved by the institutional review board (IRB) of the Faculty of Medicine, University of Tokyo (IRB No. 2019010NI), and informed consent was obtained from all subjects.
3. Results and Discussion
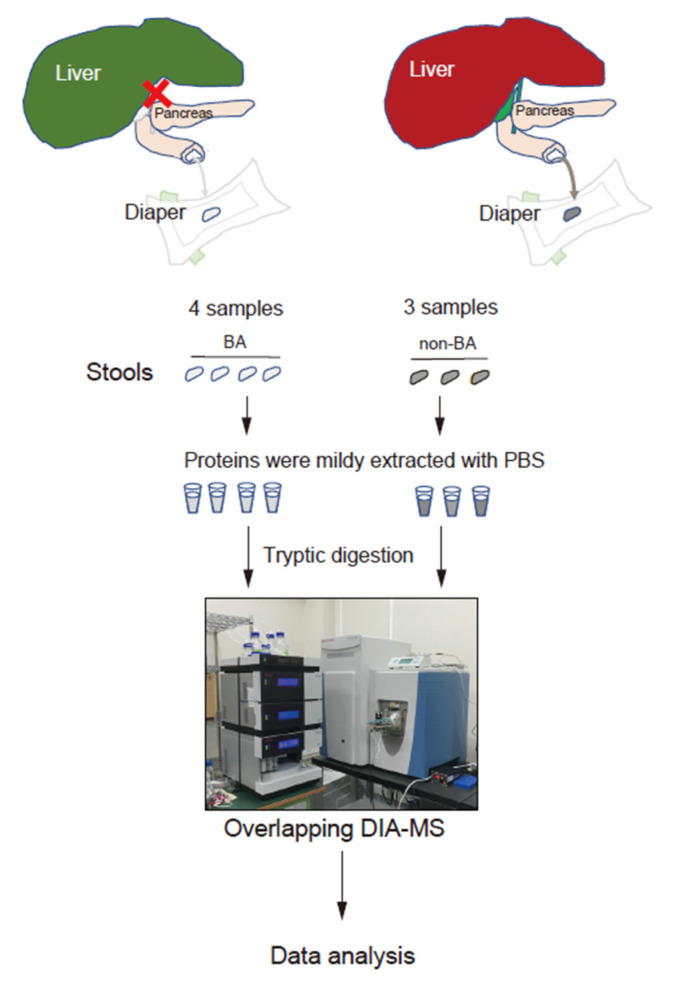
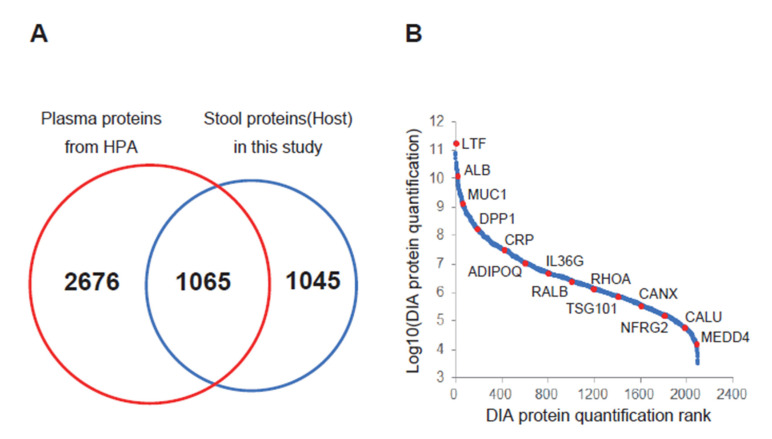
Figure 1 shows the stool proteome analysis workflow. In this study, to prevent entry of proteins other than those derived from the host as much as possible, proteins were mildly extracted with PBS so as not to break the bacteria and food debris in the stool, and proteins were purified by TCA precipitation. Then, the proteins were digested and measured using overlapping DIA–MS. Stool proteins were identified and quantified from the seven MS data (four samples from patients with BA and three from non-BA individuals). In our proteome analysis, 2110 host-derived proteins were identified in stool samples. The host stool proteins overlapped only approximately 50% with the plasma proteins, and the plasma and stools had different protein profiles (Figure 2A). Thus, unique stool biomarkers may be identified. In addition, a wide range of proteome analysis was performed with dynamic ranges of 107 or greater, and the number of identified proteins was approximately 2000 (Figure 2B). When measuring HEK293 digests in the same analysis, more than 8000 proteins were observed. Based on these facts, the host stool protein concentrations presented a wide dynamic range, and high-depth analyses such as DIA–MS have great value for discovering biomarkers from stool samples.
Figure 1.
Scheme of stool proteome analysis targeting host-derived proteins. Abbreviations: BA, biliary atresia.
Figure 2.
Proteome analysis of stool samples by overlapping data-independent acquisition mass spectrometry (DIA–MS). (A) Venn diagram of the number of stool proteins identified from our result and the number of plasma proteins registered in the Human Protein Atlas (HPA; https://www.proteinatlas.org/). (B) Dynamic range of our stool proteome analysis. The average protein quantitative values of seven samples were plotted. Representative proteins are indicated by red dots. Abbreviations: ALB, serum albumin; ADIPOQ, adiponectin; CALU, calumenin; CANX, calnexin; CRP, C-reactive protein; DPP7, dipeptidyl peptidase 2; IL36G, interleukin-36 gamma; LTF, lactotransferrin; MUC1, mucin-1; NEDD4, E3 ubiquitin-protein ligase NEDD4; NDRG2, protein NDRG2; RALB, Ras-related protein Ral-B; RHOA, transforming protein RhoA; TSG101, tumor susceptibility gene 101 protein.
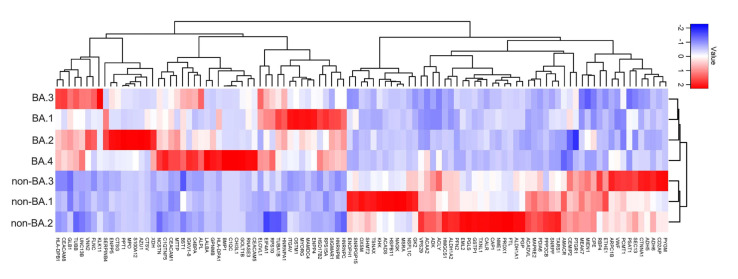
Among the identified proteins, 103 were significantly different (p < 0.05) between the two groups (BA vs. non-BA) (Figure 3). Of these 103 proteins, 49 proteins were significantly higher in patients with BA (BA-dominant: Table 2), whereas 54 proteins were significantly lower in patients with BA (non-BA-dominant: Table 3).
Figure 3.
Heatmap showing the relative abundance of proteins in stools between patients with BA versus non-BA patients. Each column represents a single patient. Abbreviations: BA, biliary atresia.
Table 2.
Summary of proteins that are significantly high in stools of BA patients.
| Uniprot ID | Protein Name | Gene Symbol | BA/Non-BA |
|---|---|---|---|
| P13497 | Bone morphogenetic protein 1 | BMP1 | ∞ |
| P36222 | Chitinase-3-like protein 1 | CHI3L1 | 347.3 |
| Q9BW60 | Elongation of very long chain fatty acids protein 1 | ELOVL1 | 142.3 |
| P80511 | Protein S100-A12 | S100A12 | 72.5 |
| Q14956 | Transmembrane glycoprotein NMB | GPNMB | 64.4 |
| P50897 | Palmitoyl-protein thioesterase 1 | PPT1 | 37.9 |
| P02747 | Complement C1q subcomponent subunit C | C1QC | 32.4 |
| Q9Y3E0 | Vesicle transport protein GOT1B | GOLT1B | 25.3 |
| P20160 | Azurocidin | AZU1 | 19.6 |
| P47989 | Xanthine dehydrogenase/oxidase | XDH | 18.3 |
| O60911 | Cathepsin L2 | CTSV | 17.8 |
| P00709 | Alpha-lactalbumin | LALBA | 17.0 |
| P20036 | HLA class II histocompatibility antigen, DP alpha 1 chain | HLA-DPA1 | 14.5 |
| P05164 | Myeloperoxidase | MPO | 11.4 |
| P12724 | Eosinophil cationic protein | RNASE3 | 10.5 |
| P04003 | C4b-binding protein alpha chain | C4BPA | 9.2 |
| Q9UBX7 | Kallikrein-11 | KLK11 | 8.6 |
| P55157 | Microsomal triglyceride transfer protein large subunit | MTTP | 7.9 |
| P37059 | Estradiol 17-beta-dehydrogenase 2 | HSD17B2 | 7.9 |
| P08311 | Cathepsin G | CTSG | 7.7 |
| Q9UJA9 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 5 | ENPP5 | 7.5 |
| P48594 | Serpin B4 | SERPINB4 | 6.8 |
| P04440 | HLA class II histocompatibility antigen, DP beta 1 chain | HLA-DPB1 | 6.3 |
| Q6UXC1 | Apical endosomal glycoprotein | MAMDC4 | 5.6 |
| P31997 | Carcinoembryonic antigen-related cell adhesion molecule 8 | CEACAM8 | 5.6 |
| O14795 | Protein unc-13 homolog B | UNC13B | 5.4 |
| A0A0C4DH67 | Immunoglobulin kappa variable 1–8 | IGKV1–8 | 5.4 |
| Q6NSJ0 | Myogenesis-regulating glycosidase | MYORG | 4.9 |
| P16278 | Beta-galactosidase | GLB1 | 4.9 |
| P06731 | Carcinoembryonic antigen-related cell adhesion molecule 5 | CEACAM5 | 4.7 |
| Q86WC4 | Osteopetrosis-associated transmembrane protein 1 | OSTM1 | 4.7 |
| P13688 | Carcinoembryonic antigen-related cell adhesion molecule 1 | CEACAM1 | 4.6 |
| P05186 | Alkaline phosphatase, tissue-nonspecific isozyme | ALPL | 4.3 |
| O95498 | Vascular non-inflammatory molecule 2 | VNN2 | 4.0 |
| P62244 | 40S ribosomal protein S15a | RPS15A | 4.0 |
| Q9Y6 × 5 | Bis(5′-adenosyl)-triphosphatase ENPP4 | ENPP4 | 3.8 |
| P09651 | Heterogeneous nuclear ribonucleoprotein A1 | HNRNPA1 | 3.4 |
| P52272 | Heterogeneous nuclear ribonucleoprotein M | HNRNPM | 3.2 |
| Q14315 | Filamin-C | FLNC | 3.0 |
| Q9BXJ0 | Complement C1q tumor necrosis factor-related protein 5 | C1QTNF5 | 2.9 |
| Q92542 | Nicastrin | NCSTN | 2.8 |
| P06756 | Integrin alpha-V | ITGAV | 2.6 |
| Q10588 | ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 2 | BST1 | 2.2 |
| Q99720 | Sigma non-opioid intracellular receptor 1 | SIGMAR1 | 2.1 |
| P07910 | Heterogeneous nuclear ribonucleoproteins C1/C2 | HNRNPC | 2.1 |
| P46783 | 40S ribosomal protein S10 | RPS10 | 1.9 |
| P60842 | Eukaryotic initiation factor 4A-I | EIF4A1 | 1.8 |
| P07437 | Tubulin beta chain | TUBB | 1.8 |
| P68363 | Tubulin alpha-1B chain | TUBA1B | 1.7 |
Abbreviations: BA, biliary atresia.
Table 3.
Summary of proteins that are significantly high in stools of non-BA patients.
| Uniprot ID | Protein Name | Gene Symbol | Non-BA/BA |
|---|---|---|---|
| P11217 | Glycogen phosphorylase, muscle form | PYGM | ∞ |
| Q15555 | Microtubule-associated protein RP/EB family member 2 | MAPRE2 | ∞ |
| Q9Y547 | Intraflagellar transport protein 25 homolog | HSPB11 | 48.6 |
| P02792 | Ferritin light chain | FTL | 26.7 |
| P02753 | Retinol-binding protein 4 | RBP4 | 11.8 |
| O43396 | Thioredoxin-like protein 1 | TXNL1 | 11.1 |
| O95336 | 6-phosphogluconolactonase | PGLS | 10.6 |
| P34897 | Serine hydroxymethyltransferase, mitochondrial | SHMT2 | 10.3 |
| Q9UNZ2 | NSFL1 cofactor p47 | NSFL1C | 10.1 |
| Q01581 | Hydroxymethylglutaryl-CoA synthase, cytoplasmic | HMGCS1 | 9.7 |
| Q9NWU1 | 3-oxoacyl-(acyl-carrier-protein) synthase, mitochondrial | OXSM | 9.5 |
| P28332 | Alcohol dehydrogenase 6 | ADH6 | 9.5 |
| Q9UJ68 | Mitochondrial peptide methionine sulfoxide reductase | MSRA | 9.1 |
| P62306 | Small nuclear ribonucleoprotein F | SNRPF | 8.9 |
| P00352 | Retinal dehydrogenase 1 | ALDH1A1 | 7.5 |
| Q15084 | Protein disulfide-isomerase A6 | PDIA6 | 6.8 |
| Q9UJ70 | N-acetyl-D-glucosamine kinase | NAGK | 6.3 |
| O94788 | Retinal dehydrogenase 2 | ALDH1A2 | 6.2 |
| P16219 | Short-chain specific acyl-CoA dehydrogenase, mitochondrial | ACADS | 6.1 |
| O15143 | Actin-related protein 2/3 complex subunit 1B | ARPC1B | 6.1 |
| O95571 | Persulfide dioxygenase ETHE1, mitochondrial | ETHE1 | 5.9 |
| P15531 | Nucleoside diphosphate kinase A | NME1 | 5.8 |
| P27797 | Calreticulin | CALR | 5.7 |
| Q06830 | Peroxiredoxin-1 | PRDX1 | 5.5 |
| P49748 | Very long-chain specific acyl-CoA dehydrogenase, mitochondrial | ACADVL | 5.5 |
| Q99598 | Translin-associated protein X | TSNAX | 5.3 |
| P55263 | Adenosine kinase | ADK | 5.3 |
| Q01518 | Adenylyl cyclase-associated protein 1 | CAP1 | 5.2 |
| Q9Y5K6 | CD2-associated protein | CD2AP | 5.1 |
| P50053 | Ketohexokinase | KHK | 5.0 |
| Q6P9B6 | MTOR-associated protein MEAK7 | MEAK7 | 5.0 |
| P11766 | Alcohol dehydrogenase class-3 | ADH5 | 4.8 |
| P42765 | 3-ketoacyl-CoA thiolase, mitochondrial | ACAA2 | 4.4 |
| A8MWD9 | Putative small nuclear ribonucleoprotein G-like protein 15 | SNRPGP15 | 4.3 |
| P62714 | Serine/threonine-protein phosphatase 2A catalytic subunit beta isoform | PPP2CB | 4.3 |
| P35221 | Catenin alpha-1 | CTNNA1 | 4.2 |
| P26639 | Threonine--tRNA ligase 1, cytoplasmic | TARS1 | 3.8 |
| O95834 | Echinoderm microtubule-associated protein-like 2 | EML2 | 3.6 |
| A6NDG6 | Glycerol-3-phosphate phosphatase | PGP | 3.4 |
| P55735 | Protein SEC13 homolog | SEC13 | 3.3 |
| Q14410 | Glycerol kinase 2 | GK2 | 3.3 |
| P09211 | Glutathione S-transferase P | GSTP1 | 3.3 |
| Q9UBQ0 | Vacuolar protein sorting-associated protein 29 | VPS29 | 3.2 |
| P35080 | Profilin-2 | PFN2 | 3.1 |
| P53396 | ATP-citrate synthase | ACLY | 2.9 |
| P16870 | Carboxypeptidase E | CPE | 2.7 |
| Q9Y617 | Phosphoserine aminotransferase | PSAT1 | 2.5 |
| Q9UHN6 | Cell surface hyaluronidase | CEMIP2 | 2.5 |
| Q9BRT3 | Migration and invasion enhancer 1 | MIEN1 | 2.4 |
| Q9UHK6 | Alpha-methylacyl-CoA racemase | AMACR | 2.3 |
| P22061 | Protein-L-isoaspartate(D-aspartate) O-methyltransferase | PCMT1 | 2.3 |
| P04275 | von Willebrand factor | VWF | 2.2 |
| Q9UHY7 | Enolase-phosphatase E1 | ENOPH1 | 2.0 |
| Q14914 | Prostaglandin reductase 1 | PTGR1 | 1.7 |
Abbreviations: BA, biliary atresia.
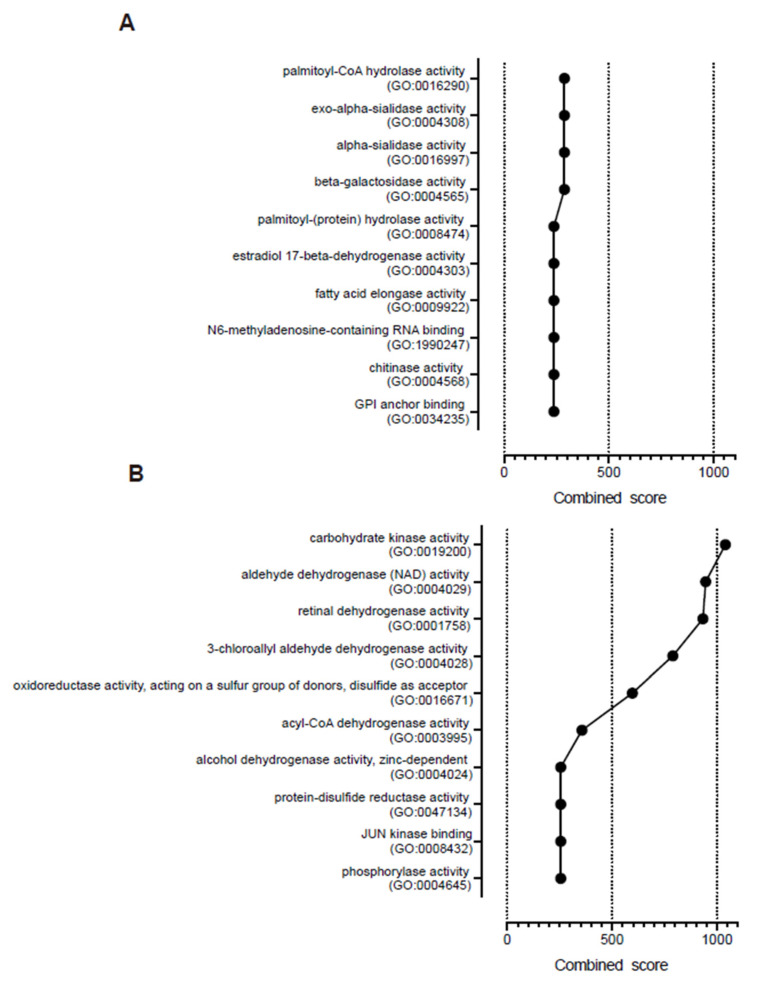
GO enrichment analysis demonstrated that palmitoyl-CoA hydrolase activity (GO:0016290), exo-alpha-sialidase activity (GO:0004308), alpha-sialidase activity (GO:0016997), beta-galactosidase activity (GO:0004565), and palmitoyl-(protein) hydrolase activity (GO:0008474) were the top five combined molecular function scores in the BA-dominant stools (Figure 4A). Furthermore, carcinoembryonic antigens such as carcinoembryonic antigen-related cell adhesion molecule (CEACAM) 1, CEACAM5, and CEACAM8 were included in the 49 dominant proteins in the stools of the BA group. In addition to CEACAM1, proteins that were elevated in liver tissues, such as chitinase-3-like protein 1(CHI3L1), xanthine dehydrogenase/oxidase (XDH), C4b-binding protein alpha chain (C4BPA), microsomal triglyceride transfer protein large subunit (MTTP), estradiol 17-beta-dehydrogenase 2 (HSD17B2), apical endosomal glycoprotein (MAMDC4), alkaline phosphatase, tissue-nonspecific isozyme (ALPL), and sigma non-opioid intracellular receptor 1(SIGMAR1) were also included in the 49 dominant proteins in the stools of the BA group [19].
Figure 4.
Molecular functions determined by the gene ontology (GO) enrichment analysis. The GO enrichment was analyzed using Enrichr. (A) The top ten combined scores of molecular function in the BA-dominant stools. (B) The top ten combined scores of molecular function in the non-BA-dominant stools.
GO enrichment analysis also revealed that carbohydrate kinase activity (GO:0019200), aldehyde dehydrogenase (NAD) activity (GO:0004029), retinal dehydrogenase activity (GO:0001758), 3-chloroallyl aldehyde dehydrogenase activity (GO:0004028), and oxidoreductase activity, acting on a sulfur group of donors, with disulfide as acceptor (GO:0016671), were the top five combined molecular function scores in the non-BA-dominant stools (Figure 4B). In addition, proteins that were elevated in liver tissues, such as retinol-binding protein 4 (RBP4), serine hydroxymethyltransferase, mitochondrial (SHMT2), hydroxymethylglutaryl-CoA synthase, cytoplasmic (HMGCS1), alcohol dehydrogenase 6 (ADH6), retinal dehydrogenase 1 (ALDH1A1), short-chain specific acyl-CoA dehydrogenase, mitochondrial (ACADS), adenosine kinase (ADK), ketohexokinase (KHK), 3-ketoacyl-CoA thiolase, mitochondrial (ACAA2), phosphoserine aminotransferase (PSAT1), alpha-methylacyl-CoA racemase (AMACR), and prostaglandin reductase 1 (PTGR1) were included in the 54 dominant proteins in the stools of the non-BA group [19].
BA is a disorder that occurs during infancy with unknown etiology, which may lead to liver cirrhosis [1]. BA requires prompt and accurate diagnosis because late Kasai portoenterostomy is one of the risk factors of inappropriate bile drainage, which is an early indication for liver transplantation [20]. However, neonatal cholestasis has many causes (other than BA); thus, the accurate diagnosis of BA is challenging. Although many examinations are available for diagnosing BA, including Sudan III staining of stool fat [2], measurement of duodenal bile acid [21], and hepatobiliary scintigraphy [22], more reliable examinations or biomarkers for BA are needed. Unfortunately, invasive procedures, such as operative cholangiogram [1], are eventually required to distinguish BA from non-BA causes of neonatal cholestasis, as in non-BA.1 patient in this study, who underwent an operative cholangiogram in order to distinguish the condition from BA.
Many studies have attempted to determine the etiology of BA and discover new specific BA biomarkers. Recently, a serum proteome analysis of patients with BA found that serum levels of matrix metalloprotease-7 (MMP-7) are high in patients with BA, and this has been considered a feasible BA biomarker [23,24,25]. These studies suggested that serum MMP-7 may help diagnose BA, but the diagnostic range of enzyme-linked immunosorbent assay (ELISA) kits for MMP-7 is inconsistent. Therefore, the role of MMP-7 as a feasible biomarker remains controversial [26].
We hypothesized that stools of patients with BA contained fewer proteins produced in the biliary tract, secondary to the obstruction to the normal route of the bile juice, which is the pathophysiological mechanism of BA. Therefore, certain specific proteins that originate in the biliary tract are possibly absent or dramatically reduced in the stools of patients with BA, compared to the stools of non-BA patients.
We found that specific proteins that are elevated in the liver tissues, such as RBP4, SHMT2, HMGCS1, ADH6, ALDH1A1, ACADS, ADK, KHK, ACAA2, PSAT1, AMACR, and PTGR1, presented significantly lower abundances in stool samples from patients with BA. This finding supported our hypothesis that stools in patients with BA contained fewer proteins produced in the biliary tract. Measurement of these specific proteins or a combination of these proteins may assist in the early diagnosis of BA.
In addition, among the 49 dominant proteins in the stools of the BA group, we found that specific proteins such as CEACAM1, CEACAM5, and CEACAM8 were significantly higher in patients with BA than in non-BA patients. CEACAM1, known as biliary glycoprotein (BGP-I) is considered a cell adhesion molecule that is also distributed in the biliary tract [19,27,28]. Furthermore, soluble CEACAM1 is shed into human bile, where it can serve as an indicator of obstructive and inflammatory liver diseases [28]. Regarding CEACAM5, components of human bile from patients with biliary obstruction exhibited cross-reactivity with CEACAM5 antisera in the absence of gastrointestinal malignancies [29]. Moreover, CHI3L1 is correlated with worse outcomes of BA [30], and XDH plays a role in oxidative stress and hepatic disease pathogenesis [31]. Therefore, these dominant proteins in the stools of the BA group may contribute to the pathogenesis of BA, and measurement of these proteins in stool may also assist in the early diagnosis of BA.
This study has several limitations. First, the sample size of the preliminary study was small because BA is a rare disease, and the prevalence of BA ranges from 1 in 5000 to 25,000 live births [1]. Second, the incidence of non-BA patients is also very small. Third, although our data were statistically analyzed, a study with larger cohorts may show different results, and it is possible that these results are not generalizable to larger, more diverse populations.
However, deep proteomic analysis of BA patient stools is a new method that has the potential to detect biomarkers and elucidate the unknown etiology of BA according to stool proteins. The stool biomarker candidates of BA that were found in this study can be clinically applied after they are validated by studies that use target-based high-throughput methods, such as ELISA and selected reaction monitoring (SRM). Therefore, it is quite important to validate our preliminary results with larger cohorts.
4. Conclusions
Our study is the first to establish deep proteome analysis of stools and apply it to infants with cholestasis, including both BA and non-BA cohorts. Our new method of deep proteome analysis by DIA–MS can detect over 2000 host-derived proteins in stools and provides a method for discovering new BA biomarkers. Further large-scale studies are needed to validate our results regarding the varying levels of host-derived stool proteins in BA as potential specific and reliable biomarkers for diagnosing this disease. Moreover, deep proteome analysis of stools has great potential to elucidate the pathophysiology of BA and other pediatric diseases, especially in the field of pediatric gastroenterology.
Mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the jPOST partner repository (http://jpostdb.org) with the dataset identifier PXD022391.
Abbreviation
| ACAA2 | 3-ketoacyl-CoA thiolase, mitochondrial |
| ACADS | short-chain specific acyl-CoA dehydrogenase, mitochondrial |
| ADH6 | alcohol dehydrogenase 6 |
| ADK | adenosine kinase |
| ALDH1A1 | retinal dehydrogenase 1 |
| ALPL | alkaline phosphatase, tissue-nonspecific isozyme |
| AMACR | alpha-methylacyl-CoA racemase |
| BA | biliary atresia |
| BGP-I | biliary glycoprotein |
| CEACAM | carcinoembryonic antigen-related cell adhesion molecule |
| CHI3L1 | chitinase-3-like protein 1 |
| C4BPA | C4b-binding protein alpha chain |
| DDA | data-dependent acquisition |
| DIA | data-independent acquisition |
| ELISA | enzyme-linked immunosorbent assay |
| GO | gene ontology |
| HMGCS1 | hydroxymethylglutaryl-CoA synthase, cytoplasmic |
| HSD17B2 | estradiol 17-beta-dehydrogenase 2 |
| IRB | institutional review board |
| KHK | ketohexokinase |
| MAMDC4 | apical endosomal glycoprotein |
| MMP-7 | matrix metalloprotease-7 |
| MTTP | microsomal triglyceride transfer protein large subunit |
| NICCD | neonatal intrahepatic cholestasis caused by citrin deficiency |
| PSAT1 | phosphoserine aminotransferase |
| PTGR1 | prostaglandin reductase 1 |
| RBP4 | retinol-binding protein 4 |
| SHMT2 | serine hydroxymethyltransferase, mitochondrial |
| SIGMAR1 | sigma non-opioid intracellular receptor |
| SRM | selected reaction monitoring |
| VOD | veno-occlusive disease |
| XDH | xanthine dehydrogenase/oxidase |
Author Contributions
Conceptualization, E.W.; methodology, E.W. and Y.K.; software, E.W., Y.K., and W.S.; validation, E.W., and Y.K.; formal analysis, E.W., Y.K., and W.S.; investigation, E.W., Y.K., D.N., and R.N.; resources, E.W., T.K., S.T., A.N., K.S., and J.F.; data curation, E.W., Y.K., and T.K.; writing—original draft preparation, E.W. and Y.K.; writing—review and editing, O.O. and J.F.; visualization, E.W., Y.K., and W.S.; supervision, O.O. and J.F.; project administration, J.F.; funding acquisition, E.W. and J.F. All authors consent to the publication of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant Numbers 19H05561 and 19K24007).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hartley J.L., Davenport M., Kelly D.A. Biliary atresia. Lancet. 2009;374:1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 2.Okajima K., Nagaya K., Azuma H., Suzuki T. Biliary atresia and stool: Its consistency and fat content, another potentially useful clinical information. Eur. J. Gastroenterol. Hepatol. 2016;28:118. doi: 10.1097/MEG.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Qian T., Jiang J., Yang Y., Shen Z., Huang Y., Chen G., Zheng S., Dong R. Gut microbial profile in biliary atresia: A case-control study. J. Gastroenterol. Hepatol. 2019;35:334–342. doi: 10.1111/jgh.14777. [DOI] [PubMed] [Google Scholar]
- 4.Tessier M.E.M., Cavallo L., Yeh J., Harpavat S., Hoffman K.L., Petrosino J.F., Shneider B.L. The Fecal Microbiome in Infants With Biliary Atresia Associates With Bile Flow After Kasai Portoenterostomy. J. Pediatr. Gastroenterol. Nutr. 2020;70:789–795. doi: 10.1097/MPG.0000000000002686. [DOI] [PubMed] [Google Scholar]
- 5.Healey G., Murphy R., Brough L., Butts C.A., Coad J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017;75:1059–1080. doi: 10.1093/nutrit/nux062. [DOI] [PubMed] [Google Scholar]
- 6.Lichtman J., Alsentzer E., Jaffe M., Sprockett D., Masutani E., Ikwa E., Fragiadakis G.K., Clifford D., Huang B.E., Sonnenburg J.L., et al. The effect of microbial colonization on the host proteome varies by gastrointestinal location. ISME J. 2015;10:1170–1181. doi: 10.1038/ismej.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiao C.-H., Chang M.-H., Chen H.-L., Lee H.-C., Wu T.-C., Lin C.-C., Yang Y.-J., Chen A.-C., Tiao M.-M., Lau B.-H., et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. 2007;47:1233–1240. doi: 10.1002/hep.22182. [DOI] [PubMed] [Google Scholar]
- 8.Ang C.-S., Baker M.S., Nice E. Mass spectrometry-based analysis for the discovery and validation of potential colorectal cancer stool biomarkers. Methods Enzymol. 2017;586:247–274. doi: 10.1016/bs.mie.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Jin P., Wang K., Huang C., Nice E. Mining the fecal proteome: From biomarkers to personalised medicine. Expert Rev. Proteom. 2017;14:445–459. doi: 10.1080/14789450.2017.1314786. [DOI] [PubMed] [Google Scholar]
- 10.Lin H., He Q.-Y., Shi L., Sleeman M., Baker M.S., Nice E. Proteomics and the microbiome: Pitfalls and potential. Expert Rev. Proteom. 2018;16:501–511. doi: 10.1080/14789450.2018.1523724. [DOI] [PubMed] [Google Scholar]
- 11.Shao S., Guo T., Aebersold R. Mass spectrometry-based proteomic quest for diabetes biomarkers. Biochim. Biophys. Acta Proteins Proteom. 2015;1854:519–527. doi: 10.1016/j.bbapap.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Ting Y.S., Egertson J.D., Bollinger J.G., Searle B.C., Payne S.H., Noble W.S., MacCoss M.J. PECAN: Library-free peptide detection for data-independent acquisition tandem mass spectrometry data. Nat. Methods. 2017;14:903–908. doi: 10.1038/nmeth.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima D., Kawashima Y., Shibata H., Yasumi T., Isa M., Izawa K., Nishikomori R., Heike T., Ohara O. Simple and sensitive analysis for dried blood spot proteins by sodium carbonate precipitation for clinical proteomics. J. Proteome Res. 2020;19:2821–2827. doi: 10.1021/acs.jproteome.0c00271. [DOI] [PubMed] [Google Scholar]
- 14.Amodei D., Egertson J., MacLean B.X., Johnson R., Merrihew G.E., Keller A., Marsh D., Vitek O., Mallick P., MacCoss M.J. Improving precursor selectivity in data-independent acquisition using overlapping windows. J. Am. Soc. Mass Spectrom. 2019;30:669–684. doi: 10.1007/s13361-018-2122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawashima Y., Watanabe E., Umeyama T., Nakajima D., Hattori M., Honda K., Ohara O. Optimization of data-independent acquisition mass spectrometry for deep and highly sensitive proteomic analysis. Int. J. Mol. Sci. 2019;20:5932. doi: 10.3390/ijms20235932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessulat S., Schmidt T., Zolg D.P., Samaras P., Schnatbaum K., Zerweck J., Knaute T., Rechenberger J., Delanghe B., Huhmer A., et al. Prosit: Proteome-wide prediction of peptide tandem mass spectra by deep learning. Nat. Methods. 2019;16:509–518. doi: 10.1038/s41592-019-0426-7. [DOI] [PubMed] [Google Scholar]
- 17.Searle B.C., Pino L.K., Egertson J.D., Ting Y.S., Lawrence R.T., MacLean B.X., Villén J., MacCoss M.J. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-07454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A., et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thul P.J., Lindskog C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018;27:233–244. doi: 10.1002/pro.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber R.A., Barker C.C., Roberts E.A., Martin S.R., Alvarez F., Smith L., Butzner J.D., Wrobel I., Mack D., Moroz S., et al. Biliary atresia: The canadian experience. J. Pediatr. 2007;151:659–665. doi: 10.1016/j.jpeds.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 21.Larrosa–Haro A., Caro–López A.M., Coello–Ramírez P., Zavala–Ocampo J., Vázquez–Camacho G. Duodenal tube test in the diagnosis of biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2001;32:311–315. doi: 10.1097/00005176-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Yoshii D., Inomata Y., Yamamoto H., Irie T., Kadohisa M., Okumura K., Isono K., Honda M., Hayashida S., Oya Y., et al. The duodenal tube test is more specific than hepatobiliary scintigraphy for identifying bile excretion in the differential diagnosis of biliary atresia. Surg. Today. 2020;50:1–8. doi: 10.1007/s00595-020-02010-w. [DOI] [PubMed] [Google Scholar]
- 23.Huang C.-C., Chou M.-H., Wu C.-L., Chen C.-M., Wang C.-C., Chen Y.-S., Chen C.-L., Tai M.-H. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod. Pathol. 2005;18:941–950. doi: 10.1038/modpathol.3800374. [DOI] [PubMed] [Google Scholar]
- 24.Lertudomphonwanit C., Mourya R., Fei L., Zhang Y., Gutta S., Yang L., Bove K.E., Shivakumar P., Bezerra J.A. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci. Transl. Med. 2017;9:eaan8462. doi: 10.1126/scitranslmed.aan8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L., Zhou Y., Xu P.-P., Mourya R., Lei H.-Y., Cao G.-Q., Xiong X.-L., Xu H., Duan X.-F., Wang N., et al. Diagnostic accuracy of serum matrix metalloproteinase-7 for biliary atresia. Hepatology. 2018;68:2069–2077. doi: 10.1002/hep.30234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harpavat S. MMP-7: The next best serum biomarker for biliary atresia? J. Pediatr. 2019;208:8–9. doi: 10.1016/j.jpeds.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Hammarström S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 28.Horst A.K., Najjar S.M., Wagener C., Tiegs G. CEACAM1 in liver injury, metabolic and immune regulation. Int. J. Mol. Sci. 2018;19:3110. doi: 10.3390/ijms19103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int. J. Cancer. 1976;17:588–596. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y., Brigham D., Bednarek J., Torres R., Wang D., Ahmad S., Mack C.L. Unique cholangiocyte-targeted IgM autoantibodies correlate with poor outcome in biliary atresia. Hepatology. 2020 doi: 10.1002/hep.31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehér J., Lengyel G., Blázovics A. Oxidative stress in the liver and biliary tract diseases. Scand. J. Gastroenterol. 1998;228:38–46. doi: 10.1080/003655298750026543. [DOI] [PubMed] [Google Scholar]