Abstract
A transcriptional regulatory nuclear factor kappa B (NF-KB) protein is a modulator of cellular biological activity via binding to a promoter region in the nucleus and transcribing various protein genes. The recent research implicated the intensive role of nuclear factor kappa B (NF-KB) in diseases like autoimmune disorder, inflammatory, cardiovascular and neurodegenerative diseases. Therefore, targeting the nuclear factor kappa B (NF-KB) protein offers a new opportunity as a therapeutic approach. Activation of IKB kinase/NF-KB signaling pathway leads to the development of various pathological conditions in human beings, such as neurodegenerative, inflammatory disorders, autoimmune diseases, and cancer. Therefore, the transcriptional activity of IKB kinase/NF-KB is strongly regulated at various cascade pathways. The nuclear factor NF-kB pathway plays a major role in the expression of pro-inflammatory genes, including cytokines, chemokines, and adhesion molecules. In response to the diverse stimuli, the cytosolic sequestered NF-KB in an inactivated form by binding with an inhibitor molecule protein (IkB) gets phosphorylated and translocated into the nucleus further transcribing various genes necessary for modifying various cellular functions. The various researches confirmed the role of different family member proteins of NF-KB implicated in expressing various genes products and mediating various cellular cascades. MicroRNAs, as regulators of NF- KB microRNAs play important roles in the regulation of the inflammatory process. Therefore, the inhibitor of NF-KB and its family members plays a novel therapeutic target in preventing various diseases. Regulation of NF- KB signaling pathway may be a safe and effective treatment strategy for various disorders.
Keywords: Transcriptional, nuclear factor kappa B (NF-KB), MicroRNAs, pro-inflammatory genes, neurodegenerative, inflammatory disorders, autoimmune diseases
1. Introduction
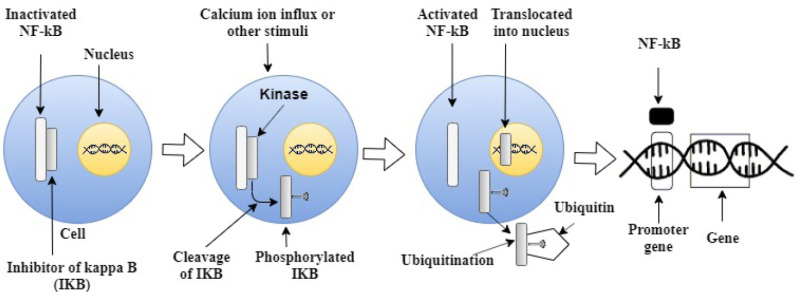
A transcriptional factor NF-KB expresses various genes encoding proteins having a vital role in processes of immunity, inflammation, cell growth, cell survival and apoptosis [1, 2]. Therefore, the NF-KB signaling is involved in vital cellular processes as well the negative regulation of NF-KB by suppression or activation of its target genes seem to be elevated under several pathological conditions [3]. The dysregulation of NF-KB is linked with certain mechanisms underlying the involvement of oxidative stress and inflammation in certain pathological conditions such as ischemic stroke, autoimmune and neurodegenerative diseases [3-5]. The activation of NF-KB by various cellular stress stimuli like the release of cytokines (TNF-alpha, Interleukin-1, growth factors), neurotrophic factors or viral infections undergo the transcription of certain genes resulting in increases in the cellular stress responses [6]. In the absence of stimuli, the NF-KB dimers remain in the inactivated state bounded with an inhibitor molecule protein (IkB), inhibiting the NF-KB translocation into the nucleus or altering the transcription [7]. The IkB protein family consists of (IKBα, IKBβ, IKBε, and Bcl-3) proteins; the IKBα appears to be involved in binding to heterodimer (p50/Rel A) protein complex [8]. The NF-KB dimer includes certain proteins c-Rel, Rel B, p65(Rel A), p105/p50 (NF-KB1), p100/52 (NF-KB2) associated with each other forming an homodimeric complex like Rel A/ p65, p50/p50, p52/p52 or heterodimeric complexes i.e., RelB/P50, RelB/p52 [9-11]. The Rel homology domain (RHD) region has the N- terminal called the DNA binding site specificities to p50 and p65, dimerization and interaction with intracellular inhibitory protein IkB of NF-KB. The N- terminal NF-KB allows the translocation into the nucleus and binds to the GGGRNNYYCC sequence of NF-KB DNA sites, where R is purine, Y is pyrimidine and N is any bases [9, 10]. Whereas, the C terminal has the remaining DNA contacts and controls the dimerization also known as dimerization domain for the nuclear localization signal or sequence (NLS) as the C terminal has an impact on the transcriptional activation properties or the inhibitory properties. On the basis of C-terminal sequence, the Rel/NF-KB proteins are divided into two classes. The class 1 members include NF-KB proteins p105, p100 a precursor for p52 and p50 possessing multiple copies of ankyrin repeat protein found in IkB family member proteins. The second-class members include (Rel proteins) i.e. c-Rel, Rel B and Rel A protein region known as NF-KB mediated gene transactivation domain [11, 12]. In response to diverse stimuli like release of cytokines (TNF- alpha, Interleukin-1, growth factors), neurotrophic factors or viral infections, there is an intracellular activation of the IKB kinase (IKK) complex comprising catalytic kinase enzymes including IKK-α; IKK-β; IKK-γ/NEMO; IKK-β), while the IKK-γ /NEMO is a regulator for sensing which further integrates the downstream activating signals by phosphorylation of the inhibitor (IkB) of NF-KB as shown in Figs. 1 & 2 [11]. The IkB gets cleaved converting the NF-KB in active form, resulting in translocation of NF-KB into the nucleus and binding to the enhancer or promoter region of the target gene, initiating the gene transcription [11-14]. The ubiquitination of IkB protein gets regulated and degraded by proteasomes [15, 16]. The re-synthesization of IKB-α & IKB-ε proteins is a time taking process occurring in the cytoplasm inhibiting the nuclear import by mediating the nuclear export of NF-KB/Rel proteins entering the nucleus and exporting the complex back to the cytoplasm [17]. The IKB-β protein only inhibits the import of NF-KB/Rel proteins into the nucleus avoiding the complex protein nucleus exporting into the cytoplasm [14].
The inactivated heterodimer protein complex NF-KB (RelB/P50) is located in the cytosol bounded with an inhibitor molecule protein (IkB). In response to a stimulus, the intercellular activation of certain catalytic kinases (Ikk) getting cleaved by ubiquitination results in activating NF-KB getting translocated into the nucleus further binding to the enhancer or promoter region of the target gene and initiating the gene transcription.
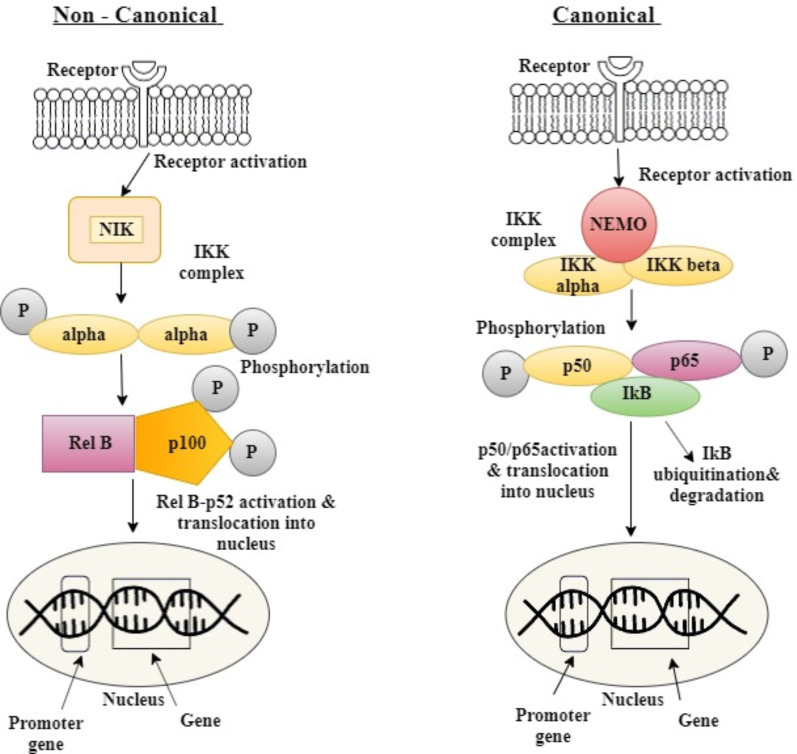
The two signaling pathways, i.e. Canonical/classical and the non-canonical/alternative pathway activate the NF-KB [18]. The Canonical/classical pathway gets activated by extracellular stimuli that include TNF-alpha, RANK (receptor activator of nuclear factor kappa B), TCR (T-cell receptor), CD30, CD40, LPS (bacterial lipopolysaccharides) altering IKK trimetric complex comprising of two catalytic subunits of IKKα and IKKβ, and a regulatory subunit IKKγ (also named NF-KB essential modulator or NEMO) leading to phosphorylation of IKB inhibitor (IKB-α) at Ser 32 and Ser 36 by further ubiquitination of IKBα [19, 20]. The free NF-KB dimer comprising g p50/p65 gets activated and translocated into the nucleus, where it binds to the promoter region to activate the transcription of responsive genes [21, 22]. The non- canonical/ alternative pathway is activated by various extracellular stimuli including BAFF-R (B-cell activator receptor), LTBR (lymphotoxin beta receptor), CD40 activating NIK kinase phosphorylating the IKK complex containing IKBα dimerized complex, at Ser 866 and Ser 870 of IKBα further activating the RelB/p100 to RelB/p52, undergoing transcription [20, 23].
The NF-KB overexpresses various genes which tend to be implicated in various diseases like cancer, ischemia and brain trauma, oxidative stress, neurodegenerative diseases (Huntington disease), Alzheimer’s and Parkinson’s disease, inflammatory diseases, etc. [4, 19, 24]. Therefore, the expression of genes is controlled by NF-KB suggesting an attractive therapeutic approach targeting NF-KB by its selective or non-selective inhibitors or agonists [25, 26].
2. Dual role of NF-KB in neuronal death and neuronal survival in pathological condition
NF-KB regulates the expression of many genes involved in cell death and cell survival by expressing the pro-apoptotic and anti-apoptotic genes seem to be involved in various pathological conditions [49-51] Therefore, the NF-KB serves dual function as protective mechanism for cell survival, promoting the cell death by stimulating the intracellular pathways involved in DNA damage [52]. NF-KB signaling in the central nervous system has a vital role by regulating certain functions like neuronal plasticity, neuronal growth and also regulates certain proteins as defense mechanisms acting in response to the certain cellular stress conditions [53, 54]. The subunits p50, p65, IkB-α and MEKK1 are highly expressed in the neuronal, astrocytes, microglia and oligodenderitic cells. In normal neuronal conditions, the electrical activity and synaptic transmission act as a stimulus to activate the MEKK1, phosphorylating the IkB-α causing the NF-KB translocation into nucleus further transcribing genes for glutamate receptor 2, MnSOD, Bcl2 necessary for the neuronal plasticity and normal physiology [55-57]. Under the normal neuronal development, depolarization of membrane causes the activation of microglial and astrocytes cells producing nerve growth factor (NGF) neurotrophin, IL-6
& nitric oxide; the microglial cells release the cytokines like TNF-α, transforming growth factor (TGF), fibroblast growth factors, nitric oxide, Chemokine (C-C motif) ligand 5 (CCL5) at the site of the cellular stress further acting as an extracellular stimuli for activating NF-KB [56, 57]. Furthermore, the activated NF-KB transcribes genes for certain proteins IL-6, TNF- α causing the inflammation whereas, the IL-10, TGF countering the inflammation by acting as anti-inflammatory [58-60]. The prolonged neuronal stress, i.e. the hyper-activation of the glutaminergic transmission or any other neuronal injury under the chronic pathologies causes the sustained migration of the glial cells which leads to the excitotoxicity stimuli activating the NF-KB to transcribe certain gene of inflammation and regulating the proteins of apoptosis [57, 58, 60]. The pro-apoptotic activity of NF-KB is
by transcribing various genes like p53, c-Myc, cyclin D1, Bcl-Xs, BAX, Fas and NOS which seem to be highly elevated by pathological stimuli i.e. over-stimulation of NMDA receptor causing neuronal excitotoxicity that directly activates NF-KB and causes apoptosis. Under certain pathological conditions like oxidative stress or any other cell stress stimuli causing the cellular damage, the NF-KB levels seem to be elevated with other proteins like p53, c-Myc, cyclin D1, Bcl-Xs, BAX, Fas and NOS [59]. The various research studies concluded the role of NF-KB with proteins like p53, c-Myc, cyclin D1, Bcl-Xs, BAX, Fas and nitric oxide synthase, i.e., by inhibiting the NF-KB, the levels of these elevated proteins causing apoptosis death were decreased in neurodegenerative diseases [1, 59, 61]. On the other side, the NF-KB plays a cell survival role by the activation of a pathway called TNF-alpha/ NF-KB. The Tumor necrosis factor (TNF- α) has a capability to derive apoptosis but also undergoes cell survival pathways in the form of inflammation by cytokine production through NF-KB and avoids the apoptosis process [1]. In apoptosis, the RIP (receptor-interacting protein) and FADDD (Fas-associated protein death domain) molecules are the central regulatory molecules activating the caspase-8 cascade causing the induction of cellular death in the apoptotic pathway [1, 61-63]. In NF-KB pathway, the RIP (receptor-interacting protein) and TRAF2 (TNF receptor-associated factor 2) are the central regulatory molecules regulated by TRADD (TNF receptor-associated receptor- type 1 death domain). Furthermore, the RIP (receptor-interacting protein) and TRAF2 driven NF-KB pathway and apoptosis is inhibited thus, possessing a neuronal survival activity [54, 64]. The cell surface TNFR receptor having a death domain on its intracellular site is attached to the SODD (silencer of death domain) protein keeping TNFR (Tumor necrosis factor receptor) in an activated state prior to external stimuli [1, 64]. On extracellular TNF- α stimuli, the TNF- α binds to the TNFR receptor causing the conformational changes in TNFR receptor, causing the release of SODD (silencer of death domain) from the death domain. The TRADD (TNF receptor-associated receptor- type 1 death domain) protein binds to the death domain of TNFR, further imitating the downstream signal by recruiting another molecule TRAF2 (TNF receptor-associated factor 2) and thus, avoiding the apoptosis pathway [1, 62, 64, 65]. The NF-KB pathway is initiated by the TRADD & TRAF2 complex recruiting IAP protein to bind to the complex. The TRAF2 also recruits the RIP protein further activating the IKK kinase molecule phosphorylating the IKB- α attached to the NF-KB masks NLS on NF-KB, the phosphorylated IKB- α gets degraded. The NF-KB gets disassociated from IKB-α molecule and translocates into the nucleus, further expressing the pro-inflammatory mediators, cytokine production, anti-apoptotic genes like Bcl-2, Bcl- XL, manganese superoxide dismutase (Mn-SOD) and possessing a neuronal survival activity [1, 55]. Therefore, it has been concluded that in normal physiological conditions, the NF-KB acts as an anti-apoptotic function but in pathological conditions, it is involved in apoptosis depending on the stimulus.
3. Involvement of microRNAs in neuronal death and neuronal survival
3.1. Role in Neuronal Death
NF-KB promotes the apoptosis by regulating the expression of many pro-apoptotic genes for proteins like p53, c-Myc, cyclin D1, BcL-Xs, BAX and Fas ligand [66-69]. The NF-KB plays a role in neuronal apoptosis by expressing the genes for p53 protein i.e., due to neuronal excitotoxicity and oxidative stress, p53 was found to be elevated in neurodegenerative diseases [66]. The p53 protein is a tumor suppressor causing the neuronal apoptosis by increasing the expression of BAX and PUMA and suppressing the expression of anti-apoptotic genes like Bcl-2 (which acts as cytoprotective and is anti-apoptotic protein) [70-72]. Hence the NF-KB inhibitor-like aspirin, SN50 reduces the high levels of p53, preventing the apoptotic neuronal death in various neurodegenerative disorders [73, 74]. The expression of various miRNAs including miR- 155, miR- 34a, miR- 23b, miR- 210, miR- 128a, miR- 25, miR- 145 are involved in production of ROS causing neuronal death [75]. The miR- 155 targets SHIP1 by deregulating its level and increasing ROS; the miR- 25 increases the expression of NOX4 responsible for oxidative stress. In neuronal cell, the miR- 145 increases the calcium influx, miR- 128a for target Bim-1, causing production of ROS further activating NF-KB. The miR- 34a enhances the expression of caspase -3, leading to neuronal death [76].
3.2. Role in Neuronal Survival
NF-KB promotes the anti-apoptotic activity by regulating the genes for superoxide dismutase enzymes and proteins (Bcl-2, Bcl- XL). These proteins are anti-apoptotic proteins regulated by NF-KB [77, 78]. Hence, NF-KB acts as anti-apoptotic having a role in cell protection by upregulating the prosurvival genes. Mn-SOD is an important anti-oxidant enzyme essential for the protection of cellular damage [79]. The various studies have reported the increased level of Mn-SOD and SOD1 in the brain while exposures to the neurotoxins or other conditions like excitotoxicity, ischemia, and amyloid-beta toxicity causing neuronal death [80]. The NF-KB mediates the induction of BCL-Xl and Bcl-2 and the expression of these proteins is upregulated by the NF-KB, further preventing the apoptotic cell death [81]. The up-regulation of various miRNAs like miR-27a, miR-153, miR- 27b, miR-181, miR-497, miR-15/16, miR-497, miR-302b, miR-21 are involved in neuronal death by expressing the anti-apoptotic Bcl-2 protein [76, 82-85]. The other miR- 29a decreases the pro-apoptotic PUMA; miR-124 suppresses the neuronal cell apoptosis by decreasing the BAX pro-apoptotic protein and protecting the mitochondrial dysfunction [86].
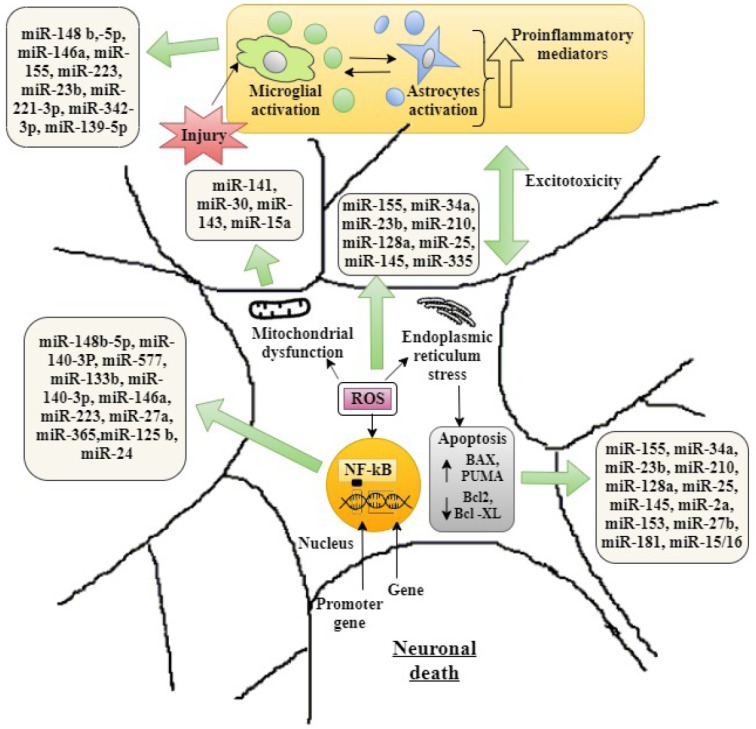
The dysregulation and alteration in the pattern of expression of microRNAs, i.e. either by up-regulation or down-regulation in the expression of miRNAs, further altering the different mechanisms involved in the pathogenesis of neurodegeneration as described in Fig. 3 and Table 3.
Fig. (3).
Involvement of various microRNAs in neuronal death. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 3.
Description of cross-link between NF-KB and altered miRNAs in various pathological conditions.
| Disease | Mutated Gene Involved | Atypical Expressed miRNA | NF-KB Role & Pathological Implications | Refs. |
|---|---|---|---|---|
| Alzheimer disease | BACE1, IRAKI, ERK1, PTBP1, FMR1. | mRNA 3’UTR for (miR-29a/b-1, miR-15a, miR-146 a miR-9, miR-20a, miR-1-5p, miR-106p miR-19b, miR-107). Expansion of CGG in 5’UTR for miR-125 b; miR- 132. |
Increased production of Amyloid beta in anterior temporal cortex, Increased inflammation, Overactivation of tau, Abnormal APP mRNA alternating splicing, Altered synaptic plasticity. |
[227-229] |
| Parkinson’s disease | RRK2, DJ-1, PTEN-induced kinase 1 (PINK1), Parkin, α-synuclein, leucine-rich repeat kinase 2 (LRRK2), Pitx3 |
Down regulation of precrsor-let7a-1, pre-miR7-2, pre-miR-99a, pre-miR7-130, pre-miR-133b, pre-miR-136, pre-miR-224, and pre-miR-143., pre-miR-133b, pre-miR-218-2, pre-miR-15b, pre-miR pre-miR pre-miR-101-1, pre-miR-107, pre-miR-335, pre-miR -345; miR-7 & miR-153; miR-34b and miR-34c. miR-146a, miR-124. |
Loss of dopaminergic neurons in the mid-brain, Pitx3 deficiency results in selective loss of nigrostriatal DA, Increased α-Synuclein undergoes oxidative stress causing dopaminergic degeneration. LRRK2 inhibits let-7 and miR-184 and causes cell death. Depletion of miR-34b and miR-34c causes reduction of Parkin and DJ-1 necessary for mitochondrial homeostasis and cellular redox balance. |
[145, 154-156] |
| Amyotrophic Lateral Sclerosis (ALS) | C90rf72, SOD1, TARDBP, FUS. |
Expansion of a GGGGCC hexanucleotide more than 30 repeats upstream of the C9orf72; GLT1mRNA. |
frontotemporal lobar degeneration by aggregation of TD43 protein and causing endoplasmic stress mediating neuronal death. Mutated SOD1 targets Glial glutamate transporter GLT1 by decreasing its expression and further impairment of motor dysfunction. Impairment of proteasome-mediated protein degradation. |
[230-233] |
| Prion disease | PrP gene. | PrP mRNA, Increased miR-16, miR-14a, expression causes inflammation signalling. |
Missfolding of the prion protein causes endoplasmic stress. |
[234, 235] |
| Depression | BDNF, GPM6A, GPM6B. | BDNF-mRNA, Downregulated GPM6A & GPM6B. |
Decreased neuronal plasticity. | [236, 237] |
| Huntington’s disease (HD) | HTT gene. | Mutated CAG trinucleotide in the exon 1 of htt gene. | Abnormal longer polyglutamine (poly Q) stretch (wild- type htt protein) causing neuronal death. | [120, 121] |
| Spinocerebellar ataxia (SCA) | ATXN1. | Repetition of CAG trinucleotide expanding poly Q in ATXN1 protein. miR-19, miR-101, miR-150, miR-181 and miR-130 |
Downregulation of miR-19, miR 181 increases the expression of NF-KB causing Cerebellum purkinje neurons atrophy and glutaminergic synaptic loss. | [130-133] |
| Cerebral ischemia | BCL-2, BCL-w, SOD2. | miR-320, miR-145, miR -210, miR- 497, miR-181, miR-183, miR-215 and miR-22. |
Downregulation of miR-183, miR-215, miR-22 and upregulation of miR-181 increase the inflammation-causing excitotoxicity. | [160-162] |
| Traumatic brain injury | IL-1, TNF- alpha. | miR-144, miR- 153, miR-340-5p, miR-155, miR-223, miR-124-3p. | Up-regulation of miR-155, miR-223 promotes the inflammation and down-regulation of miR-124-3p promotes the apoptotic neuronal death. | [170-172] |
| Depression | BDNF, NPTX2, TNF-alpha, IL-1 β, IL-6, | let-7e, let-175p, miR -301b, miR -221-3p, miR-21-5p miR-145, miR-223, miR-146a, and miR-155, miR-175p. | up regulating of miR-221-3p, let-7e, miR-223, miR-145, miR-155 and miR-146a increases the inflammation which causes disruption of neurogenesis in depression. | [175-179] |
| Epilepsy | TNF-alpha, IL-1 β, IL-6, HMGB1, | Upregulation of miR-210, miR-30, miR-27a, miR-183, miR- 134, miR-135a, miR-125b, miR-148a, miR- 146a, miR-124 whereas, the downregulation of miR-128, miR-199a, miR-21a, miR-22. | Dysregulation of miR-181a, miR-129-5p, miR-124, miR- 146a, miR-155 increases the neuroinflammation in epilepsy. | [188-197] |
4. N F-KB signaling in Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) is a motor neuron degenerative disease of lower motor and upper motor involving the progressive muscular atrophy, clinically characterized by dyspnea and dysphagia [24]. The mutation of various genes, including (C90rf72, SOD1, TARDBP, FUS) has been diagnosed in individuals with ALS and FALS (familial Amyotrophic Lateral Sclerosis) [87]. The mutation in C90rf72 gene causes motor neurodegeneration leading to an increase in the accumulation of toxins in motor neurons, further altering the immune defense and activating the microglial, which increases inflammatory responses by intracellular NF-KB pathway and regulates the transcription of proinflammatory mediator genes enhancing the neurotoxicity [88]. The SOD1 mutation or misfolding of the superoxide dismutase enzyme increase the reactive oxygen species activating the NF-KB transcribing genes involved in neuroinflammation, causing neurotoxicity in motor neurons [89, 90]. The mutations of FUS and TARDBP genes have a similar function of encoding FUS whereas, the TARDBP gene encodes TDP-43 protein causing the 60 dominant missense mutation in the nucleus of neuron necessary for the synthesis of other proteins by binding to the DNA and regulating transcription [87]. In ALS, the mutation in TARDBP gene causes the aggregation of neurotoxic TDP-43 fragments protein in nucleus to axon by mutated ubiquitin 2 (UBQLN2) and cleaving of TDP-43 forming an aggregation in nerve cell, which increases the inflammatory responses by activation of NF-KB increasing the neuroinflammation leading to oxidative stress causing excitotoxicity in motor neurons [91]. Furthermore, the NF-KB expression seems to be elevated in the ALS during the damaging of the motor neurons in the spinal cord migrating immune cells i.e., astrocytes and microglial [92]. The various studies have concluded that the mutation in the UBQLN2, SOD1 genes activating NF-KB further causes reactive oxygen species generation aggregating TDP-43 in nucleus and axon enhancing the cellular dysfunction including endoplasmic stress mediating neuronal death [24, 89, 90, 93]. Therefore, the overexpression of NF-KB in motor neurons contributing to the pathogenesis of ALS and the Withaferin A, maresin 1 (an inhibitor of NF-KB) tends to possess a potential therapeutic activity as neuroprotective by decreasing the ROS produced due to aggregation of TDP-43 protein further improving the ALS symptoms [92, 94-97]. The dysregulation of various miRNAs like miR-206, miR-140-3p, miR-133, miR-149, miR-338-3p, let-7, miR-148b-5p, miR-577, miR-365 and miR-125b seems to be involved in the pathogenesis of ALS by increasing the expression of NF-KB further initiating the activation of microglia causing neuroinflammation and degeneration of motor neurons in ALS [98, 99].
5. N F-KB signaling in Prion disease
Prion disease is a progressive neurodegenerative disorder or also known as transmissible spongiform encephalopathies (TSE) associated with misfolding of the prion protein (prp) in the brain leading to endoplasmic reticulum stress mediating neuronal death [100]. The abnormality in folding of the prion protein (prp) especially PrPSc isoform in mitochondria causing the neuronal death by proliferation of the microglia in response to the synaptic damage and up-regulation of the pro-inflammatory mediators in the prion brain with enhanced activity of NF-KB signaling has been reported by many studies underlying chronic inflammation in brain [101-104]. There is an over-expression of miR 16, miR 146a, miR 320, miR 328, miR 128, miR -342-3p and down-regulation of miR -338-3-p and miR -337-3p causing neurodegeneration in prion disease [105, 106]. The miR 146a seems to initiate the activation of NF-KB signaling mediating inflammatory mediators and migrating microglial cells, causing neurodegeneration [107]. The activation of transcriptional factor (NF-KB) enhances the apoptotic neuronal death by elevating the expression of many pro-apoptotic genes for certain proteins (p53, BcL-Xs, BAX, Fas ligand), thereby releasing cytochrome C undergoing mitochondrial dysfunction and endoplasmic stress further releasing calcium activating Bad (pro-apoptotic protein). The up-regulation of cytokines IL-1 & TNF-alpha at the site of synaptic damage causes reactive oxygen species such as hydrogen peroxide, resulting in excitotoxicity and neurodegeneration [103, 108-110].
6. N F-KB signaling in Huntington’s disease (HD)
Huntington disease is an inherited autosomal dominant neurodegenerative disease associated with replication of the mutated CAG trinucleotide in the exon 1 of htt gene translating into abnormal longer polyglutamine (poly Q) stretch (wild- type htt protein) causing neuronal death [111-113]. The wild- type htt protein controls the normal neuronal processes by interacting with the transcriptional factor NF-KB; RE-1 silencing transcriptional factor required for the neuronal development including neuronal transcription, synaptic transmission and intracellular transport [114, 115]. The mutated wild- type htt protein binds to dynein which causes mitochondrial dysfunctioning by increased ROS production and interacting with NF-KB to activate from synapse to nucleus in response to the excitatory synaptic input Huntington disease characterized by motor, cognitive and psychiatric disorders [116-119]. The alteration in various miRNA expressions causing stretching of mutant wild-type htt protein includes: miR- 146a, miR-150, miR- 125b, miR-155 and miR- 9. Out of various miRNA, the down-regulation of miR-146, miR-150 and miR-125b and increased expression of NF-KB contribute to neurodegeneration in the Huntington’s disease by aggregation of mutated wild -type htt protein [120, 121].
7. N F-KB signaling in Spinocerebellar ataxia (SCA)
Spinocerebellar ataxia is an inherited neurodegenerative motor deficit disease associated with a mutation in the Ataxi 1 gene by the repetition of CAG trinucleotide expanding poly Q in ATXN1 protein resulting in cerebellum purkinje neurons atrophy and glutaminergic synaptic loss [122, 123]. The migration of astroglail in response to purkinje neurons atrophy like brain insult resulted, in various studies, in the expression of NF-KB increase and activated the glial cells at the early stage of SCA possessing a neuroprotective effect but at the later stage, the glial cells worsen the disease by dysfunction of purkinje neurons characterized by complete loss of movement control [122, 124, 125]. Therefore, the astroglial NF-KB signaling tends to be neuroprotective at an early stage but at a later stage, progresses the neurodegeneration due to the increased production of pro-inflammatory cytokine TNFα. [124, 126]. The various miRNAs have been analyzed for diagnosis in (spinocerebellar ataxia) SCA involved in the pathogenesis of SCA by targeting DNAJB1, ATXN3, and MIDI proteins. The DNAJ protein is involved in neuroprotection and the microRNA including: hsa-miR-370, hsa-miR-543 which controls the expression of DNAJB1 protein; the down-regulation of hsa-miR-370, hsa-miR-543 expression which causes the reduction of DNAJB1 protein [127-129]. The other microRNAs like hsa-miR 9, hsa-miR -32, hsa-miR -92a, hsa-miR -92b, 363, 367, hsa-miR 383, miR 181, hsa-miR 181b, 181c, 181d are analysed for ATXN3 protein. Out of these microRNAs, the over-expression of hsa-miR 9, hsa-miR-32, hsa-miR 383, hsa-miR 181a, 181c is involved in the reduction of ATXN3 protein causing neuronal toxicity as the ATXN3 protein is involved in the ubiquitin-proteasome system [127, 128]. The other microRNAs miR-19, miR-101, miR-150 and miR-130 are involved in regulating the ATXN1 protein found to be mutated in cerebellar purkinje cells and causing the motor dysfunction [128, 130, 131]. The down-regulation of miR-19, miR 181 increases the expression of NF-KB signaling further increasing the production of pro-inflammatory seem to be elevated and causing degeneration of purkinje cells [127, 132, 133].
8. N F-KB signaling in Alzheimer’s disease
Alzheimer’s disease is the most prevalent neurodegenerative disease leading to dementia-like conditions which worsen with time damaging the neurons hippocampus area responsible for memory formation [134]. The hallmark of Alzheimer's disease is due to the formation of abnormal structures of amyloid plaques between the spaces of neurons and neurofibrillary tangles inside the nerve cells made by misfolded proteins in a certain region of the brain initiating the loss in neuronal function causing neuronal death [135]. The abnormal structures of amyloid plaques are caused due to the genetical mutations promoting the production of beta- amyloid peptides i.e., the main component of the amyloid plaques [136]. The peptide is the result of the Amyloid precursor protein (APP) that helps neurons to grow and repair by breaking down and recycling . Normally, the enzyme alpha-secretase cleaves the extracellular domain and gamma-secretase cleaves the APP into discrete fragments which are soluble [137]. In Alzheimer pathological condition, the enzyme called beta-secretase (BACE) or beta–site amyloid precursor protein cleaving enzyme 1 (BACE1) initiates the production of the amyloid beta with another protease called gamma-secretase that cleaves the upper part extracellular domain of the APP which is the N- terminus of the amyloid-beta and the gamma–secretase cleaves the C terminal of beta-amyloid [138]. The left-over fragment is insoluble and creates the monomers called Amyloid beta, which is no longer regulated. These monomers tend to be chemically sticky and form clumps by binding together. These clumps are formed outside the neurons and short 42 amino acid peptides are formed called beta-amyloid plaques [134, 139]. The amyloid-beta plaques are secreted potentially between the synapse of the neurons and disrupt the neuronal signaling which leads to impairment of brain function especially the memory by damaging the surrounding neurons and migrating the glial cells, generating neuroinflammation [140]. The down-regulation of miR-29a, miR-2b-1, miR-9 and increase in the level of miR-107 cause abnormality in BACE1 protein, further increasing the abnormal production of amyloid-beta [141]. The various mRNAs like miR-20a, miR-17-5p and miR-106b have been found to be involved in abnormal APP altering splicing [141, 142]. The studies have concluded the essential role of NF-KB in the regulation of the BACE1 gene expression, which leads to the production of the beta-amyloid and up-regulation of the miR-125b causing neurodegeneration by increasing the levels of pro-inflammatory mediators causing neurodegeneration [143]. The BACE1 expression is mediated by the NF-kB signaling and the inhibition of the NF-KB plays a novel target in Alzheimer's disease therapy.
9. N F-KB signaling in Parkinson’s disease
Parkinson’s disease is mostly seen among older people as characterized by a degeneration of dopaminergic neurons in substantia nigra affecting movement, cognition, etc. [144]. In recent studies, the prominent roles of NF-KB in dopaminergic neurodegeneration have been seen among Parkinson patients [145]. The immune histochemical techniques in the Parkinson patients showed the increased translocation of NF-KB in the nucleus of mesencephalon dopaminergic neurons transcribing the genes of pro-inflammatory mediators (chemokines, cytokines) initiating the production of reactive oxygen species (ROS) by auto-oxidation and enzymatic catabolism of dopamine [146-148]. Furthermore, the excessive production of reactive oxygen species directly damages the neuronal cells by lipid peroxidation and oxidative modification of nucleic acids and proteins or by induction of apoptosis in neurons i.e. sphingomyelin or ceramide dependent pathway leading to neurodegenerative process [149]. The various miRNAs found to be expressed in Parkinson's disease for (RRK2; DJ-1; PTEN-induced kinase 1 (PINK1); Parkin; α-synuclein; leucine-rich repeat kinase 2 (LRRK2); Pitx3) genes i.e., the down-regulation of precrsor-let7a-1, pre-miR7-2, pre-miR−99a, pre-miR7−130, pre-miR−133b, pre-miR−136, pre-miR−224, and pre-miR−143., pre-miR-133b, pre-miR-218-2, pre-miR-15b, pre-miR −101-1, pre-miR −107, pre-miR −335, pre-miR −345; miR-7 & miR-153; miR-34b and miR-34c [150-153]. The study concluded the down-regulation of miR-34b and miR-34c, stimulating the aggregation of alpha-synuclein in substantia nigra causing neurodegeneration [154]. Furthermore, the miR -124 and miR -146a promote the expression of NF-KB, further enhancing the pro-inflammatory levels causing neurodegeneration in Parkinson's disease [145, 155, 156].
10. N F-KB signaling in cerebral ischemia
The cerebral ischemic insult triggers microglia activation; astrocytes cells release inflammatory mediators which further increase cerebral endothelium adhesion causing excitotoxicity with reactive oxygen production [118]. The transcriptional factor NF-KB transcribes various genes for inflammatory mediators like IL-1, IL-6, and TNF- alpha having a prominent role in the activation of intracellular apoptotic pathway causing neuronal death by mitochondrial dysfunction, DNA fragmentation in cerebral ischemic [157]. Therefore, the various medications like Dioscin, Cilostazol as described in Table 2 below for cerebral ischemia act by inhibiting the NF-KB [158]. There are various microRNAs involved in the functioning of the brain; the down-regulation or up-regulation of such microRNAs seem to be implicated in the pathogenesis of cerebral ischemia. The various miRNAs are involved in apoptotic neuronal death which seem to be dysregulated in various pathological conditions like miR-497, miR-181 that unregulate and increase the expression of apoptotic BCL-2, BCL-w genes whereas, the anti-apoptotic miR-320 is down-regulated causing mitochondrial dysfunction. The miR-145, miR -210 expressions are unregulated in cerebral ischemia, further elevating the expression of SOD2 (superoxide dismutase 2) causing oxidative stress [159]. The microRNAs like miR-183, miR-215 and miR-22 regulate the NF-KB expression; the down-regulation of these miRNAs promotes the inflammation, causing excitotoxicity by increasing the tumor necrosis factor-α, interleukin and migrating macrophages and phagocytic neutrophils reactions in
Table 2.
List of various NF-KB inhibitors used in neurodegenerative diseases.
| NF-KB Inhibitors | Diseases | Refs. |
|---|---|---|
| Curcumin, resveratrol, pterostilbene, punicalagin, macranthoin G, salidroside, 4-O-methylhonokiol, lycopene, genistein, obovatol and gallic acid. NSAIDs & Polyphenolic; curcuminoids corticosteroids. |
Alzheimer’s disease | [198-200] |
| Doxycycline, Sulforaphane, Cannabinoids, δ9-tetrahydrocannabinol and cannabidiol, Isobavachalcone | Parkinson’s disease | [201-204] |
| Genistein, Resveratrol, NSAIDs, Withaferin A, maresin Methylprednisolone. | Amyotrophic Lateral Sclerosis (ALS) | [205-208] |
| SN50, Hordeum vulgare | Prion disease | [209, 210] |
| Crocin, Pristimerin, Baicalin, PLX3397, SN50 | Depression | [211-213] |
| Dioscin, Cilostazol, Glycyrrhizin, Diosmetin | Cerebral ischemia | [214-217] |
| Artesunate, Curcumin, Allyl isothiocyanate, Osthole | Traumatic brain injury (TBI) | [218-220] |
| EVP4593, laquinimod, epigallocatechin gallate; Ethyl-EPA: ethyl-eicosapentaenoic acid, Pridopidine. | Huntington’s disease (HD) | [221-225] |
| Morin | Spinocerebellar ataxia (SCA) | [226] |
11. N F-KB signaling in Traumatic brain injury (TBI)
Traumatic brain injury (TBI) is associated with cytotoxic edema due to the complex consequences of immune and inflammatory cascades contributing to the brain injury [163]. The endogenous factors released during the damage in Traumatic brain injury (TBI) are recognized by damage-associated molecular patterns (DAMPs) further initiating the downstream inflammatory signaling and activating the transmembrane toll–like receptors further phosphorylating IKB inhibitor, translocating p65 NF-KB dimer into nucleus and transcribing pro-inflammatory genes leading to oxidative stress [164, 165]. The various studies have concluded that the increased expression of NF-KB in astrocytes and microglial cells migrating at the site of damage neuronal cell generates reactive oxygen species mediating apoptotic neuronal death [166-167]. Therefore, the inhibitor of NF-KB diminishes the Traumatic brain injury by decreasing the levels of IL-1, TNF- alpha and inhibiting the inflammatory cascades causing neurodegeneration [168, 169]. The down-regulation of various miRNA expressions are likely to be involved in TBI including miR-144, miR-153, miR-340-5p which contribute to the cognitive dysfunction by down-regulation of calcium/calmodulin-dependent serine protein kinase (CASK), nuclear factor erythroid 2-related factor 2 (NRF2) and alpha-synuclein (SNCA) target proteins [170]. The other miR-155, miR-223 are upregulated and increase the expression of NF-KB, further promoting the inflammation causing mitochondrial dysfunction which further causes neuronal death and the down-regulation of miR-124-3p promoting apoptotic neuronal death [171, 172].
12. N F-KB signaling in depression
The transcriptional factor NF-KB transcribes Brain-derived neurotrophic factor (BDNF) gene necessary for the regulation of the neuronal plasticity by enhancing the excitatory neurotransmission further modulating the pre and post-synaptic activity [173]. Therefore, suggesting an involvement in the neurobiology of depression depicts a similar function of BDNF and NF-KB in improving the disrupted neuronal circuit [174]. The preclinical studies data of suicidal cases of patients with depression concluded that the decreased expression of the BDNF and the activation of the NF-KB pathway tend to improve the synaptic plasticity by restoring the neurogenesis underlying the therapeutic approach for depression [173]. Whereas, the acute and chronic stress in patients causes the increase in the expression of inflammatory mediators TNF-alpha, IL-1 β & IL-6, causing disruption of neurogenesis in hippocampus cells likely to be increased in stress causing depression [175]. The blockage of the transcriptional factor NF-KB decreases the levels of pro-inflammatory cytokines like TNF-alpha, IL-1 β & IL-6, reversing the depressive behavior in patients [175-177]. The various microRNAs including let-7e, let-175p, miR -301b, miR -221-3p, miR-21-5p miR-145, miR-223, miR-146a, and miR-155, miR-175p tend to be down-regulated or up-regulated in major depressive disorder [178, 179]. The miR -301b targets neuronal pentraxin II (NPTX2) gene involved in depression and increases the expression of NF-KB activating the microglia enhancing the release of inflammatory mediators tumor necrosis factor-α (TNF-α), interleukin-Iβ (IL-Iβ) and cyclooxygenase-2(COX-2), affecting the hippocampus area of brain leading to cognitive dysfunction in depression [179]. The miR-221-3p is up-regulating which increases the expression of IFN-α (Interferon alpha) and activation of NF-KB in mild depressive disorder [179]. The up-regulation of let-7e, miR-223, miR-145, miR-155, and miR-146a regulates the TLR4 expression which further activates NF-KB, causing further disruption of neuronal circuit in depression [178].
13. N F-KB signalling in Epilepsy
Epilepsy is a neurological disease characterized by sudden recurrent long-lasting episodic seizures promoting abnormal brain activity due to the excessive glutaminergic neurotransmission resulting in excitotoxicity causing the brain damage [180]. Therefore, the excessive neuronal excitotoxicity triggers the activation of neuroglial cells releasing the inflammatory mediators and eliciting intracellular cascades of inflammatory events resulting in oxidative stress provoking apoptotic neuronal death in epilepsy [181]. Furthermore, there is an activation of astrocytes, glial cells releasing cytokines and chemokines migrating at the site of brain lesions contributing to neuronal degeneration by mitochondrial dysfunction and activation of the apoptotic caspase -3 protein mediating neuronal death seen in the status epilepticus or prolonged repetitive seizures [182, 183]. The various studies have demonstrated the elevated level of transcriptional factor NF-KB in epileptic brain transcribing genes for pro-inflammatory mediators such as IL-1 beta, IL-6, TNF- alpha; high-mobility-group box 1 (HMGB1) tends to be released from astrocytes and microglia promoting the seizures threshold [184-187]. The dysregulation of various miRNAs is linked with increased epileptic seizures analysed in epileptic models. The up-regulation of miR-210, miR-30, miR-27a, miR-183, miR-134, miR-135a, miR-125b, miR-148a, miR- 146a, miR-124 whereas, the down-regulation of miR-128, miR-199a, miR-21a, miR-22 lead to increases in the epileptic seizures [188-192]. The dysregulation of miR-181a, miR-129-5p, miR-124, miR- 146a, miR-155 increases the expression of NF-KB further transcribing genes for pro-inflammatory mediators causing excitotoxicity [193-197].
As mentioned above, the role of NF-KB in different neurodegenerative diseases is related to inflammation and immune responses. In response to various extracellular or intracellular stimuli (Table 1), the transcriptional factor NF-KB gets activated and transcribes genes for inflammatory mediators seem to be elevated and causing oxidative stress involved in neurodegenerative diseases.
Table 1.
Describes the NF-KB inducer with variant stimuli activating NF-KB complex by IKK enzyme and transcribing particular genes involved in various pathological conditions.
| NF-KB Inducers |
Stimuli
(Intracellular /Extracellular) |
Receptor Activation | Multiple Adaptors | IKK Enzyme Type | NF-KB Complex | Target Gene | Disease | Refs. |
|---|---|---|---|---|---|---|---|---|
| Bacteria, Microbes, and viruses. | Pathogen-associated molecular pattern (PAMPs), NOD2 (Nucleotide Binding Oligomerization Domain Containing 2) | Pattern recognition receptors (PRRs), Toll –like receptors. Cytokine receptors (interleukin-1 receptors (IL-1Rs), TNF receptors (TNFRs), NLRs (NOD-like receptors) |
TNFR-associated factors (TRAFs), Myeloid differentiation primary response protein 88 (MYD88), TIR domain containing adaptor protein (TIRAP), IL-1 R associated kinases (RIPK3), NIK (NF-KB inducing kinases) or MAP3K14. | IkB α, TBK1, IkB β |
p52 RelB, p100 RelB, p50 RelA, p105 RelB |
BAFF, BLIMP1, CCL17, CCL19, CCL5, CCL5, IL-1 a, IL-b, IL-2, 6, 8, 9, 10, 11, TNF-α, TNF- β, NOD2 | Alzheimer’s, Parkinson’s, Epilepsy, CNS lymphoma. |
[27-38] |
| Physical stress (UV and gamma radiations), Stress metal | Cell stress | Sensory receptor or non-receptor | P38 /MAPK, CkK II (casein kinase). ASK1, SEK1, MEK2, JNK1, β -TrcP |
IKBα | p50 RelA | BAX, Bim, Bcl2 |
Alzheimer’s, Parkinson’s, Huntington, Ischemia. | [39-42] |
| Oxidative stress (Glutathione, H2O2) | TNF-alpha, Reactive oxygen species (ROS), NOD2 (Nucleotide Binding Oligomerization Domain Containing 2) |
Toll–like receptors. Cytokine receptors (interleukin-1 receptors (IL-1Rs), TNF receptors (TNFRs), NLRs (NOD-like receptors) |
ERKs, JNKs, or p38 MAPKs, tyrosine kinase | MEKK1 (mitogen-activated protein kinase kinase kinase), IKBα, IkB β | p50 RelA) | BAX, Bim, Bcl2, Cu/Zn SOD, SOD1, MAP4K1, CCL17, CCL19, CCL5, CCL5, IL-1 a, IL-b, IL-2, 6, 8, 9, 10, 11, TNF-α, TNF- β, NOD2. |
Alzheimer’s, Parkinson, Huntington’s, Ischemia, Epilepsy. | [43-45] |
| Apoptotic mediators | TNF-alpha, Reactive oxygen species (ROS), Interleukin (IK) | Toll–like receptors. Cytokine receptors (interleukin-1 receptors (IL-1Rs), TNF receptors (TNFRs), |
(Anti-fas/ Apo-1, Ploy(ADP) Ribose polymerase (PARP), TRAIL | IkB α, TBK1, IkB β |
p52 RelB, p100 RelB, p50 RelA, p105 RelB |
BAX, Bim, Bcl2 |
Alzheimer’s, Parkinson’s, Huntington’s, Ischemia, Epilepsy. | [31-34, 46-48] |
CONCLUSION
The current review categorized the involvement of nuclear transcriptional factor (NF-KB) in neurodegenerative conditions as a prominent modulator, transcribing target genes involved in elevating the stress causing apoptosis in neuronal cells. In addition, the various research studies have concluded the evidenced-based data depicting the increased expression of NF-KB and dysregulation of various miRNAs either by up-regulation or down-regulation of miRNAs targeting genes further exacerbating apoptotic neuronal death in neurodegenerative diseases. Therefore, targeting NF-KB has provided a successful pharmacotherapy or adjuvant drug therapy for various diseases and also indicates a future direction for the development of new molecules directly targeting miRNAs.
Fig. (1).
Mechanisms of activation of NF-KB. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
Canonical/ Classical pathway and the Non- Canonical/ Alternative pathway of NF-KB. In the canonical pathway, the toll-like receptor gets activated by large stimuli and binding of ligand and recruiting the activation of IKB complex (IKK) complex, and then phosphorylates inhibitory enzyme (IKB) getting degraded by ubiquination. Further, the activated NF-KB complex (p50/p65) translocates into the nucleus to transcribe genes. In Non-canonical/alternative pathway, the activation of NF-KB inducing kinase (NIK) in response to very small stimuli (lymphotoxin B, B cell-activating factor) carries the intracellular signaling. The NIK phosphorylates the 2 IKK –alpha subunits phosphorylating p100, further activating heterodimer p52/RelB complex to translocate into the nucleus. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
The authors are grateful to Chitkara University, Punjab, India for support and institutional facilities.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Qin Z.H., Tao L.Y., Chen X. Dual roles of NF-kappaB in cell survival and implications of NF-kappaB inhibitors in neuroprotective therapy. Acta Pharmacol. Sin. 2007;28(12):1859–1872. doi: 10.1111/j.1745-7254.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen C.H., Zhou W., Liu S., Deng Y., Cai F., Tone M., Tone Y., Tong Y., Song W. Increased NF-KB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012;15(1):77–90. doi: 10.1017/S1461145711000149. [DOI] [PubMed] [Google Scholar]
- 3.Camandola S., Mattson M.P. NF-κ B as a therapeutic target in neurodegenerative diseases. Expert Opin. Ther. Targets. 2007;11(2):123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- 4.Kaltschmidt B., Baeuerle P.A., Kaltschmidt C. Potential involvement of the transcription factor NF-κ B in neurological disorders. Mol. Aspects Med. 1993;14(3):171–190. doi: 10.1016/0098-2997(93)90004-W. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill L.A., Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20(6):252–258. doi: 10.1016/S0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 6.Thanos D., Maniatis T. NF-κ B: a lesson in family values. Cell. 1995;80(4):529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18(49):6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi P., Aggarwal A. NF-kB transcription factor: a key player in the generation of immune response. Curr. Sci. 2006;90(4):519. [Google Scholar]
- 9.Cornwell W.D., Kirkpatrick R.B. Cactus-independent nuclear translocation of Drosophila RELISH. J. Cell. Biochem. 2001;82(1):22–37. doi: 10.1002/jcb.1144. [DOI] [PubMed] [Google Scholar]
- 10.Heissmeyer V., Krappmann D., Hatada E.N., Scheidereit C. Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol. Cell. Biol. 2001;21(4):1024–1035. doi: 10.1128/MCB.21.4.1024-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Z., Chiao P., Zhang X., Zhang X., Lazar M.A., Seto E., Young H.A., Ye J. Coactivators and corepressors of NF-kappaB in IkappaB alpha gene promoter. J. Biol. Chem. 2005;280(22):21091–21098. doi: 10.1074/jbc.M500754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Z., He Q., Peng B., Chiao P., Ye J. Regulation of nuclear translocation of HDAC3 by IkBalpha is required for TNF-inhibition of PPARgamma function. J. Biochem. 2006;281(7):4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barré B., Perkins N.D. A cell cycle regulatory network controlling NF-kappaB subunit activity and function. EMBO J. 2007;26(23):4841–4855. doi: 10.1038/sj.emboj.7601899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Courtois G., Fauvarque M.O. The many roles of ubiquitin in NF-KB signaling. Biomedicines. 2018;6(2):43. doi: 10.3390/biomedicines6020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Kang J., Zhang L., Liang Z., Tang X., Yan Y., Qian H., Zhang X., Xu W., Mao F. Ubiquitination regulation of inflammatory responses through NF-KB pathway. Am. J. Transl. Res. 2018;10(3):881–891. [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 18.Perkins N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 19.Shih R.H., Wang C.Y., Yang C.M. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front. Mol. Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendellen M.F., Bradford J.W., Lawrence C.L., Clark K.S., Baldwin A.S. Canonical and non-canonical NF-KB signaling promotes breast cancer tumor-initiating cells. Oncogene. 2014;33(10):1297–1305. doi: 10.1038/onc.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstrepen L., Bekaert T., Chau T.L., Tavernier J., Chariot A., Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell. Mol. Life Sci. 2008;65(19):2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israël A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010;2(3):a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma S., De Jesus P., Chanda S.K., Verma I.M. SNW1, a Novel transcriptional regulator of the NF-KB pathway. Mol. Cell. Biol. 2019;39(3):e00415–e00418. doi: 10.1128/MCB.00415-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaltschmidt B., Sparna T., Kaltschmidt C. Activation of NF-κ B by reactive oxygen intermediates in the nervous system. Antioxid. Redox Signal. 1999;1(2):129–144. doi: 10.1089/ars.1999.1.2-129. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A., Roy A., Liu X., Kordower J.H., Mufson E.J., Hartley D.M., Ghosh S., Mosley R.L., Gendelman H.E., Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2007;104(47):18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedmann-Morvinski D., Narasimamurthy R., Xia Y., Myskiw C., Soda Y., Verma I.M. Targeting NF-KB in glioblastoma: A therapeutic approach. Sci. Adv. 2016;2(1):e1501292. doi: 10.1126/sciadv.1501292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negroni A., Pierdomenico M., Cucchiara S., Stronati L. NOD2 and inflammation: current insights. J. Inflamm. Res. 2018;11:49–60. doi: 10.2147/JIR.S137606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed A.U., Williams B.R., Hannigan G.E. Transcriptional activation of inflammatory genes: mechanistic insight into selectivity and diversity. Biomolecules. 2015;5(4):3087–3111. doi: 10.3390/biom5043087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman M.M., McFadden G. Modulation of NF-KB signalling by microbial pathogens. Nat. Rev. Microbiol. 2011;9(4):291–306. doi: 10.1038/nrmicro2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F., Demers L.M., Shi X. Upstream signal transduction of NF-kappaB activation. Curr. Drug Targets Inflamm. Allergy. 2002;1(2):137–149. doi: 10.2174/1568010023344706. [DOI] [PubMed] [Google Scholar]
- 31.Shaftel S.S., Griffin W.S.T., O’Banion M.K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation. 2008;5(1):7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stojakovic A., Paz-Filho G., Arcos-Burgos M., Licinio J., Wong M.L., Mastronardi C.A. Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol. Neurobiol. 2017;54(6):4486–4495. doi: 10.1007/s12035-016-9988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia J.M., Stillings S.A., Leclerc J.L., Phillips H., Edwards N.J., Robicsek S.A., Hoh B.L., Blackburn S., Doré S. Role of interleukin-10 in acute brain injuries. Front. Neurol. 2017;8:244. doi: 10.3389/fneur.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantini E., D’Angelo C., Reale M. The role of immunosenescence in neurodegenerative diseases. Mediators Inflamm. 2018;2018:6039171. doi: 10.1155/2018/6039171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung Y.J., Tweedie D., Scerba M.T., Greig N.H. Neuroinflammation as a factor of neurodegenerative disease: Thalidomide analogs as treatments. Front. Cell Dev. Biol. 2019;7:313. doi: 10.3389/fcell.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krumbholz M., Theil D., Derfuss T., Rosenwald A., Schrader F., Monoranu C.M., Kalled S.L., Hess D.M., Serafini B., Aloisi F., Wekerle H., Hohlfeld R., Meinl E. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 2005;201(2):195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L., Li R., Huang H., Yuan J., Ou S., Xu T., Yu X., Liu X., Chen Y. Up-regulated BAFF and BAFF receptor expression in patients with intractable temporal lobe epilepsy and a pilocarpine-induced epilepsy rat model. Seizure. 2017;48:79–88. doi: 10.1016/j.seizure.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 39.O’Dea E.L., Kearns J.D., Hoffmann A. UV as an amplifier rather than inducer of NF-kappaB activity. Mol. Cell. 2008;30(5):632–641. doi: 10.1016/j.molcel.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma A., Kushwaha H.N., Srivastava A.K., Srivastava S., Jamal N., Srivastava K., Ray R.S. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: An application for photoprotection. J. Photochem. Photobiol. B. 2017;172:139–148. doi: 10.1016/j.jphotobiol.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Liu L., Hui L., Zhang Z.Z. 2011. [Google Scholar]
- 42.Begum N., Wang B., Mori M., Vares G. Does ionizing radiation influence Alzheimer’s disease risk? J. Radiat. Res. (Tokyo) 2012;53(6):815–822. doi: 10.1093/jrr/rrs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Jiao Q., Xu H., Du X., Shi L., Jia F., Jiang H. Biometal dyshomeostasis and toxic metal accumulations in the development of Alzheimer’s disease. Front. Mol. Neurosci. 2017;10:339. doi: 10.3389/fnmol.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J., Nan G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target. Int. J. Mol. Med. 2017;39(6):1338–1346. doi: 10.3892/ijmm.2017.2962. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.K., Kim N.J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules. 2017;22(8):1287. doi: 10.3390/molecules22081287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi C., Park J.Y., Lee J., Lim J.H., Shin E.C., Ahn Y.S., Kim C.H., Kim S.J., Kim J.D., Choi I.S., Choi I.H. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-α, or IFN-γ. J. Immunol. 1999;162(4):1889–1895. [PubMed] [Google Scholar]
- 48.Vuong B., Hogan-Cann A.D., Alano C.C., Stevenson M., Chan W.Y., Anderson C.M., Swanson R.A., Kauppinen T.M. NF-KB transcriptional activation by TNFα requires phospholipase C, extracellular signal-regulated kinase 2 and poly(ADP-ribose) polymerase-1. J. Neuroinflammation. 2015;12(1):229. doi: 10.1186/s12974-015-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin D., Woodruff M., Zhang Y., Whaley S., Miao J., Ferslew K., Zhao J., Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J. Neuroimmunol. 2006;174(1-2):101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Yang L., Tao L.Y., Chen X.P. Roles of NF-kappaB in central nervous system damage and repair. Neurosci. Bull. 2007;23(5):307–313. doi: 10.1007/s12264-007-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinaci M.K., Erkasap N., Kucuk A., Koken T., Tosun M. Effects of quercetin on apoptosis, NF-KB and NOS gene expression in renal ischemia/reperfusion injury. Exp. Ther. Med. 2012;3(2):249–254. doi: 10.3892/etm.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia G., Zhang Y., Li W., Dai H. Neuroprotective role of icariin in experimental spinal cord injury via its antioxidant, anti-neuroinflammatory and anti-apoptotic properties. Mol. Med. Rep. 2019;20(4):3433–3439. doi: 10.3892/mmr.2019.10537. [DOI] [PubMed] [Google Scholar]
- 53.Gutierrez H., Hale V.A., Dolcet X., Davies A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132(7):1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- 54.Gutierrez H., Davies A.M. Regulation of neural process growth, elaboration and structural plasticity by NF-KB. Trends Neurosci. 2011;34(6):316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattson M.P., Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 2001;107(3):247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mincheva-Tasheva S., Soler R.M. NF-KB signaling pathways: role in nervous system physiology and pathology. Neuroscientist. 2013;19(2):175–194. doi: 10.1177/1073858412444007. [DOI] [PubMed] [Google Scholar]
- 57.Snow W.M., Albensi B.C. Neuronal gene targets of NF-KB and their dysregulation in Alzheimer’s disease. Front. Mol. Neurosci. 2016;9:118. doi: 10.3389/fnmol.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engelmann C., Weih F., Haenold R. Role of nuclear factor kappa B in central nervous system regeneration. Neural Regen. Res. 2014;9(7):707–711. doi: 10.4103/1673-5374.131572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattson M.P., Meffert M.K. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 60.Dresselhaus E.C., Meffert M.K. Cellular specificity of NF-kappaB function in the nervous system. Front. Immunol. 2019;10:1043. doi: 10.3389/fimmu.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motyl J., Strosznajder J.B. Sphingosine kinase 1/sphingosine-1-phosphate receptors dependent signalling in neurodegenerative diseases. The promising target for neuroprotection in Parkinson’s disease. Pharmacol. Rep. 2018;70(5):1010–1014. doi: 10.1016/j.pharep.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Strozyk E., Kulms D. NFKB: cell survival or cell death? Signal Transduct. 2005;5(6):334–349. doi: 10.1002/sita.200500070. [DOI] [Google Scholar]
- 63.Pozniak P.D., White M.K., Khalili K. TNF-α/NF-KB signaling in the CNS: possible connection to EPHB2. J. Neuroimmune Pharmacol. 2014;9(2):133–141. doi: 10.1007/s11481-013-9517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sedger L.M., McDermott M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25(4):453–472. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 65.Karin M., Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 66.Yi J., Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta. 2010;1804(8):1684–1689. doi: 10.1016/j.bbapap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nkpaa K.W., Adedara I.A., Amadi B.A., Wegwu M.O., Farombi E.O. Ethanol via Regulation of NF-KB/p53 Signaling Pathway Increases Manganese-Induced Inflammation and Apoptosis in Hypothalamus of Rats. Biol. Trace Elem. Res. 2019;190(1):101–108. doi: 10.1007/s12011-018-1535-3. [DOI] [PubMed] [Google Scholar]
- 68.Park A., Koh H.C. NF-KB/mTOR-mediated autophagy can regulate diquat-induced apoptosis. Arch. Toxicol. 2019;93(5):1239–1253. doi: 10.1007/s00204-019-02424-7. [DOI] [PubMed] [Google Scholar]
- 69.Pourhanifeh M.H., Shafabakhsh R., Reiter R.J., Asemi Z. The Effect of resveratrol on neurodegenerative disorders: possible protective actions against autophagy, apoptosis, inflammation and oxidative stress. Curr. Pharm. Des. 2019;25(19):2178–2191. doi: 10.2174/1381612825666190717110932. [DOI] [PubMed] [Google Scholar]
- 70.Culmsee C., Mattson M.P. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 2005;331(3):761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 71.Grilli M., Memo M. Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Death Differ. 1999;6(1):22–27. doi: 10.1038/sj.cdd.4400463. [DOI] [PubMed] [Google Scholar]
- 72.Karova K., Wainwright J.V., Machova-Urdzikova L., Pisal R.V., Schmidt M., Jendelova P., Jhanwar-Uniyal M. Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-KB pathway inhibition. J. Neuroinflammation. 2019;16(1):12. doi: 10.1186/s12974-019-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swarbrick S., Wragg N., Ghosh S., Stolzing A. Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol. Neurobiol. 2019;56(9):6156–6167. doi: 10.1007/s12035-019-1500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muhammad T., Ikram M., Ullah R., Rehman S.U., Kim M.O. Hesperetin, a citrus flavonoid, attenuates lps-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-KB signaling. Nutrients. 2019;11(3):648. doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu K., Xie Y.Y., Zhang C., Ouyang D.S., Long H.Y., Sun D.N., Long L.L., Feng L., Li Y., Xiao B. MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci. 2012;13(1):115. doi: 10.1186/1471-2202-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Hu Y., Deng H., Xu S., Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 2015;16(10):24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savari F., Badavi M., Rezaie A., Gharib-Naseri M.K., Mard S.A. Evaluation of the therapeutic potential effect of Fas receptor gene knockdown in experimental model of non-alcoholic steatohepatitis. Free Radic. Res. 2019;53(5):486–496. doi: 10.1080/10715762.2019.1608982. [DOI] [PubMed] [Google Scholar]
- 78.Kempuraj D., Thangavel R., Natteru P.A., Selvakumar G.P., Saeed D., Zahoor H., Zaheer S., Iyer S.S., Zaheer A. Neuroinflammation induces neurodegeneration. J. Neurol Neurosurg. Spine. 2016;1(1):1003. [PMC free article] [PubMed] [Google Scholar]
- 79.Tan D.X., Reiter R.J. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019;2(1):44–66. doi: 10.32794/mr11250011. [DOI] [Google Scholar]
- 80.Borg J., London J. Copper/zinc superoxide dismutase overexpression promotes survival of cortical neurons exposed to neurotoxins in vitro. J. Neurosci. Res. 2002;70(2):180–189. doi: 10.1002/jnr.10404. [DOI] [PubMed] [Google Scholar]
- 81.Sun L., Guo Y., He P., Xu X., Zhang X., Wang H., Tang T., Zhou W., Xu P., Xie P. Genome-wide profiling of long noncoding RNA expression patterns and CeRNA analysis in mouse cortical neurons infected with different strains of borna disease virus. Genes Dis. 2019;6(2):147–158. doi: 10.1016/j.gendis.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M., Rassenti L., Alder H., Volinia S., Liu C.G., Kipps T.J., Negrini M., Croce C.M. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102(39):13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Liu P., Zhu H., Xu Y., Ma C., Dai X., Huang L., Liu Y., Zhang L., Qin C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res. Bull. 2009;80(4-5):268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Ouyang Y.B., Lu Y., Yue S., Giffard R.G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12(2):213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Q., Xu J., Li L., Li H., Mao S., Zhang F., Zen K., Zhang C.Y., Zhang Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014;5(3):e1132. doi: 10.1038/cddis.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu Z., Zhang K., Wang Q., Zheng Y. MicroRNA-124 improves functional recovery and suppresses Bax-dependent apoptosis in rats following spinal cord injury. Mol. Med. Rep. 2019;19(4):2551–2560. doi: 10.3892/mmr.2019.9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blokhuis A.M., Groen E.J., Koppers M., van den Berg L.H., Pasterkamp R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125(6):777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fomin V., Richard P., Hoque M., Li C., Gu Z., Fissore-O’Leary M., Tian B., Prives C., Manley J.L. The C9ORF72 gene, implicated in ALS/FTD, encodes a protein that functions in control of endothelin and glutamate signaling. Mol. Cell. Biol. 2018;38(22):e00155–e18. doi: 10.1128/MCB.00155-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Casciati A., Ferri A., Cozzolino M., Celsi F., Nencini M., Rotilio G., Carrì M.T. Oxidative modulation of nuclear factor-kappaB in human cells expressing mutant fALS-typical superoxide dismutases. J. Neurochem. 2002;83(5):1019–1029. doi: 10.1046/j.1471-4159.2002.01232.x. [DOI] [PubMed] [Google Scholar]
- 90.Prell T., Lautenschläger J., Weidemann L., Ruhmer J., Witte O.W., Grosskreutz J. Endoplasmic reticulum stress is accompanied by activation of NF-KB in amyotrophic lateral sclerosis. J. Neuroimmunol. 2014;270(1-2):29–36. doi: 10.1016/j.jneuroim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Dong Y., Chen Y. The role of ubiquitinated TDP-43 in amyotrophic lateral sclerosis. Neuroimmunol. Neuroinflamm. 2018;5:5. doi: 10.20517/2347-8659.2017.47. [DOI] [Google Scholar]
- 92.Frakes A.E., Ferraiuolo L., Haidet-Phillips A.M., Schmelzer L., Braun L., Miranda C.J., Ladner K.J., Bevan A.K., Foust K.D., Godbout J.P., Popovich P.G., Guttridge D.C., Kaspar B.K. Microglia induce motor neuron death via the classical NF-KB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81(5):1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cereda C., Gagliardi S., Cova E., Diamanti L., Ceroni M. The role of TNF-Alpha in ALS: new hypotheses for future therapeutic approaches. Amyotroph. Lateral Scler. 2012;•••:413–436. [Google Scholar]
- 94.Swarup V., Phaneuf D., Dupré N., Petri S., Strong M., Kriz J., Julien J.P. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor KB-mediated pathogenic pathways. J. Exp. Med. 2011;208(12):2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ajmone-Cat M.A., Onori A., Toselli C., Stronati E., Morlando M., Bozzoni I., Monni E., Kokaia Z., Lupo G., Minghetti L., Biagioni S., Cacci E. Increased FUS levels in astrocytes leads to astrocyte and microglia activation and neuronal death. Sci. Rep. 2019;9(1):4572. doi: 10.1038/s41598-019-41040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohta Y., Tremblay C., Schneider J.A., Bennett D.A., Calon F., Julien J.P. Interaction of transactive response DNA binding protein 43 with nuclear factor KB in mild cognitive impairment with episodic memory deficits. Acta Neuropathol. Commun. 2014;2(1):37. doi: 10.1186/2051-5960-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohuchi K., Ono Y., Joho M., Tsuruma K., Ogami S., Yamane S., Funato M., Kaneko H., Nakamura S., Hara H., Shimazawa M. A docosahexaenoic acid-derived pro-resolving agent, Maresin 1, protects motor neuron cells death. Neurochem. Res. 2018;43(7):1413–1423. doi: 10.1007/s11064-018-2556-1. [DOI] [PubMed] [Google Scholar]
- 98.Rinchetti P., Rizzuti M., Faravelli I., Corti S. 2018. [DOI] [PubMed]
- 99.Dardiotis E., Aloizou A.M., Siokas V., Patrinos G.P., Deretzi G., Mitsias P., Aschner M., Tsatsakis A. The role of microRNAs in patients with amyotrophic lateral sclerosis. J. Mol. Neurosci. 2018;66(4):617–628. doi: 10.1007/s12031-018-1204-1. [DOI] [PubMed] [Google Scholar]
- 100.Shah S.Z.A., Zhao D., Hussain T., Yang L. The role of unfolded protein response and mitogen-activated protein kinase signaling in neurodegenerative diseases with special focus on prion diseases. Front. Aging Neurosci. 2017;9:120. doi: 10.3389/fnagi.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Julius C., Heikenwalder M., Schwarz P., Marcel A., Karin M., Prinz M., Pasparakis M., Aguzzi A. Prion propagation in mice lacking central nervous system NF-kappaB signalling. J. Gen. Virol. 2008;89(Pt 6):1545–1550. doi: 10.1099/vir.0.83622-0. [DOI] [PubMed] [Google Scholar]
- 102.Aguzzi A., Nuvolone M., Zhu C. The immunobiology of prion diseases. Nat. Rev. Immunol. 2013;13(12):888–902. doi: 10.1038/nri3553. [DOI] [PubMed] [Google Scholar]
- 103.Prasad K.N., Bondy S.C. Oxidative and inflammatory events in prion diseases: can they be therapeutic targets? Curr. Aging Sci. 2019;11(4):216–225. doi: 10.2174/1874609812666190111100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carroll J.A., Chesebro B. Neuroinflammation, microglia, and cell-association during prion disease. Viruses. 2019;11(1):65. doi: 10.3390/v11010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saba R., Goodman C.D., Huzarewich R.L., Robertson C., Booth S.A. A miRNA signature of prion induced neurodegeneration. PLoS One. 2008;3(11):e3652. doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanata E., Thüne K., Xanthopoulos K., Ferrer I., Dafou D., Zerr I., Sklaviadis T., Llorens F. MicroRNA alterations in the brain and body fluids of humans and animal prion disease models: current status and perspectives. Front. Aging Neurosci. 2018;10:220. doi: 10.3389/fnagi.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saba R., Gushue S., Huzarewich R.L., Manguiat K., Medina S., Robertson C., Booth S.A. MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS One. 2012;7(2):e30832. doi: 10.1371/journal.pone.0030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bacot S.M., Lenz P., Frazier-Jessen M.R., Feldman G.M. Activation by prion peptide PrP106-126 induces a NF-kappaB-driven proinflammatory response in human monocyte-derived dendritic cells. J. Leukoc. Biol. 2003;74(1):118–125. doi: 10.1189/jlb.1102521. [DOI] [PubMed] [Google Scholar]
- 109.Bourteele S., Oesterle K., Weinzierl A.O., Paxian S., Riemann M., Schmid R.M., Planz O. Alteration of NF-kappaB activity leads to mitochondrial apoptosis after infection with pathological prion protein. Cell. Microbiol. 2007;9(9):2202–2217. doi: 10.1111/j.1462-5822.2007.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu G.R., Mu T.C., Gao Z.X., Wang J., Sy M.S., Li C.Y. Prion protein is required for tumor necrosis factor α (TNFα)-triggered nuclear factor KB (NF-KB) signaling and cytokine production. J. Biol. Chem. 2017;292(46):18747–18759. doi: 10.1074/jbc.M117.787283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sawa A., Wiegand G.W., Cooper J., Margolis R.L., Sharp A.H., Lawler J.F., Jr, Greenamyre J.T., Snyder S.H., Ross C.A. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 1999;5(10):1194–1198. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- 112.Marcora E., Kennedy M.B. The Huntington’s disease mutation impairs Huntingtin’s role in the transport of NF-KB from the synapse to the nucleus. Hum. Mol. Genet. 2010;19(22):4373–4384. doi: 10.1093/hmg/ddq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin D.D.O., Hayden M.R. Neurodegeneration: Role of repeats in protein clearance. Nature. 2017;545(7652):33–34. doi: 10.1038/nature22489. [DOI] [PubMed] [Google Scholar]
- 114.Alexandrov A.I., Serpionov G.V., Kushnirov V.V., Ter-Avanesyan M.D. Wild type huntingtin toxicity in yeast: Implications for the role of amyloid cross-seeding in polyQ diseases. Prion. 2016;10(3):221–227. doi: 10.1080/19336896.2016.1176659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatters D.M. Proteome Aggregation patterns under proteostasis stress as signatures for understanding Huntington’s Disease. Biophys. J. 2019;116(3):5a. doi: 10.1016/j.bpj.2018.11.054. [DOI] [Google Scholar]
- 116.Napolitano M., Zei D., Centonze D., Palermo R., Bernardi G., Vacca A., Calabresi P., Gulino A. NF-kB/NOS cross-talk induced by mitochondrial complex II inhibition: implications for Huntington’s disease. Neurosci. Lett. 2008;434(3):241–246. doi: 10.1016/j.neulet.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 117.Turillazzi E., Neri M., Cerretani D., Cantatore S., Frati P., Moltoni L., Busardò F.P., Pomara C., Riezzo I., Fineschi V. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J. Cell. Mol. Med. 2016;20(4):601–612. doi: 10.1111/jcmm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou B., Zuo Y.X., Jiang R.T. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci. Ther. 2019;25(6):665–673. doi: 10.1111/cns.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou P., Du S., Zhou L., Sun Z., Zhuo L.H., He G., Zhao Y., Wu Y., Zhang X. Tetramethylpyrazine-2'O-sodium ferulate provides neuroprotection against neuroinflammation and brain injury in MCAO/R rats by suppressing TLR-4/NF-KB signaling pathway. Pharmacol. Biochem. Behav. 2019;176:33–42. doi: 10.1016/j.pbb.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 120.Ghose J., Sinha M., Das E., Jana N.R., Bhattacharyya N.P. Regulation of miR-146a by RelA/NFkB and p53 in STHdh(Q111)/Hdh(Q111) cells, a cell model of Huntington’s disease. PLoS One. 2011;6(8):e23837. doi: 10.1371/journal.pone.0023837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang K.H., Wu Y.R., Chen C.M. Down-regulation of miR-9* in the peripheral leukocytes of Huntington’s disease patients. Orphanet J. Rare Dis. 2017;12(1):185. doi: 10.1186/s13023-017-0742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paulson H.L., Shakkottai V.G., Clark H.B., Orr H.T. Polyglutamine spinocerebellar ataxias - from genes to potential treatments. Nat. Rev. Neurosci. 2017;18(10):613–626. doi: 10.1038/nrn.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J., Gu X., Ma Y., Calicchio M.L., Kong D., Teng Y.D., Yu L., Crain A.M., Vartanian T.K., Pasqualini R., Arap W., Libermann T.A., Snyder E.Y., Sidman R.L. Nna1 mediates Purkinje cell dendritic development via lysyl oxidase propeptide and NF-KB signaling. Neuron. 2010;68(1):45–60. doi: 10.1016/j.neuron.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim J.H., Lukowicz A., Qu W., Johnson A., Cvetanovic M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 (SCA1) in a biphasic, stage-of-disease specific manner. Glia. 2018;66(9):1972–1987. doi: 10.1002/glia.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mellesmoen A., Sheeler C., Ferro A., Rainwater O., Cvetanovic M. Brain derived neurotrophic factor (BDNF) delays onset of pathogenesis in transgenic mouse model of spinocerebellar ataxia type 1 (SCA1). Front. Cell. Neurosci. 2019;12:509. doi: 10.3389/fncel.2018.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferro A., Qu W., Lukowicz A., Svedberg D., Johnson A., Cvetanovic M. Inhibition of NF-KB signaling in IKKβF/F;LysM Cre mice causes motor deficits but does not alter pathogenesis of Spinocerebellar ataxia type 1. PLoS One. 2018;13(7):e0200013. doi: 10.1371/journal.pone.0200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koscianska E., Krzyzosiak W.J. Current understanding of the role of microRNAs in spinocerebellar ataxias. Cerebellum Ataxias. 2014;1(1):7. doi: 10.1186/2053-8871-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van der Stijl R., Withoff S., Verbeek D.S. Spinocerebellar ataxia: miRNAs expose biological pathways underlying pervasive Purkinje cell degeneration. Neurobiol. Dis. 2017;108:148–158. doi: 10.1016/j.nbd.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 129.Zarouchlioti C., Parfitt D.A., Li W., Gittings L.M., Cheetham M.E. DNAJ Proteins in neurodegeneration: essential and protective factors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1738;2018(373):20160534. doi: 10.1098/rstb.2016.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee Y., Samaco R.C., Gatchel J.R., Thaller C., Orr H.T., Zoghbi H.Y. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat. Neurosci. 2008;11(10):1137–1139. doi: 10.1038/nn.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodriguez-Lebron E., Liu G., Keiser M., Behlke M.A., Davidson B.L. Altered Purkinje cell miRNA expression and SCA1 pathogenesis. Neurobiol. Dis. 2013;54:456–463. doi: 10.1016/j.nbd.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gantier M.P., Stunden H.J., McCoy C.E., Behlke M.A., Wang D., Kaparakis-Liaskos M., Sarvestani S.T., Yang Y.H., Xu D., Corr S.C., Morand E.F., Williams B.R. A miR-19 regulon that controls NF-KB signaling. Nucleic Acids Res. 2012;40(16):8048–8058. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hutchison E.R., Kawamoto E.M., Taub D.D., Lal A., Abdelmohsen K., Zhang Y., Wood W.H., III, Lehrmann E., Camandola S., Becker K.G., Gorospe M., Mattson M.P. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61(7):1018–1028. doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen X.Q., Mobley W.C. Alzheimer disease pathogenesis: insights from molecular and cellular biology studies of oligomeric Aβ and tau species. Front. Neurosci. 2019;13:659. doi: 10.3389/fnins.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun J., Roy S. The physical approximation of APP and BACE-1: A key event in alzheimer’s disease pathogenesis. Dev. Neurobiol. 2018;78(3):340–347. doi: 10.1002/dneu.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oh S.B., Kim M.S., Park S., Son H., Kim S.Y., Kim M.S., Jo D.G., Tak E., Lee J.Y. Clusterin contributes to early stage of Alzheimer’s disease pathogenesis. Brain Pathol. 2019;29(2):217–231. doi: 10.1111/bpa.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pung L., Wang X., Li M., Xue L. The role of APP in Alzheimer’s disease. Adv. Alzheimer Dis. 2013;2(02):60. doi: 10.4236/aad.2013.22008. [DOI] [Google Scholar]
- 139.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19(10):610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 141.Hébert S.S., Horré K., Nicolaï L., Bergmans B., Papadopoulou A.S., Delacourte A., De Strooper B. MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol. Dis. 2009;33(3):422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 142.Sujeetha P., Cheerian J., Basavaraju P., Moorthi P.V., Anand A.V. The role of epigenetics in Alzheimer’s disease. J Geri Ment Health. 2018;5(2):94. doi: 10.4103/jgmh.jgmh_33_17. [DOI] [Google Scholar]
- 143.Zhao Y., Bhattacharjee S., Jones B.M., Hill J., Dua P., Lukiw W.J. Regulation of neurotropic signaling by the inducible, NF-kB-sensitive miRNA-125b in Alzheimer’s disease (AD) and in primary human neuronal-glial (HNG) cells. Mol. Neurobiol. 2014;50(1):97–106. doi: 10.1007/s12035-013-8595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walker L., Stefanis L., Attems J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies - current issues and future directions. J. Neurochem. 2019;150(5):467–474. doi: 10.1111/jnc.14698. [DOI] [PubMed] [Google Scholar]
- 145.Gan L., Li Z., Lv Q., Huang W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int. J. Pharm. 2019;567:118449. doi: 10.1016/j.ijpharm.2019.118449. [DOI] [PubMed] [Google Scholar]
- 146.Goes A.T.R., Jesse C.R., Antunes M.S., Lobo Ladd F.V., Lobo Ladd A.A.B., Luchese C., Paroul N., Boeira S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 2018;279:111–120. doi: 10.1016/j.cbi.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 147.Jiang X., Wang X., Tuo M., Ma J., Xie A. RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci. Lett. 2018;672:65–69. doi: 10.1016/j.neulet.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 148.Hassanzadeh K., Rahimmi A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: Could targeting these pathways write a good ending? J. Cell. Physiol. 2018;234(1):23–32. doi: 10.1002/jcp.26865. [DOI] [PubMed] [Google Scholar]
- 149.Asanuma M., Miyazaki I., Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox. Res. 2003;5(3):165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- 150.Miñones-Moyano E., Porta S., Escaramís G., Rabionet R., Iraola S., Kagerbauer B., Espinosa-Parrilla Y., Ferrer I., Estivill X., Martí E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011;20(15):3067–3078. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- 151.Harraz M.M., Dawson T.M., Dawson V.L. MicroRNAs in Parkinson’s disease. J. Chem. Neuroanat. 2011;42(2):127–130. doi: 10.1016/j.jchemneu.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Correddu D., Leung I.K.H. Targeting mRNA translation in Parkinson’s disease. Drug Discov. Today. 2019;24(6):1295–1303. doi: 10.1016/j.drudis.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 153.Martín-Nieto J., Uribe M.L., Esteve-Rudd J., Herrero M.T., Campello L. A role for DJ-1 against oxidative stress in the mammalian retina. Neurosci. Lett. 2019;708:134361. doi: 10.1016/j.neulet.2019.134361. [DOI] [PubMed] [Google Scholar]
- 154.Kabaria S., Choi D.C., Chaudhuri A.D., Mouradian M.M., Junn E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett. 2015;589(3):319–325. doi: 10.1016/j.febslet.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yao L., Zhu Z., Wu J., Zhang Y., Zhang H., Sun X., Qian C., Wang B., Xie L., Zhang S., Lu G. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 2019;33(7):8648–8665. doi: 10.1096/fj.201900363R. [DOI] [PubMed] [Google Scholar]
- 156.Wu S.P., Zhang J.W., Ma J.J., Li X., Qi Y.W., Yang H.Q. The role of miR-146a in MPTP treated mice with Parkinson’s disease. Int. J. Clin. Exp. Med. 2019;12(4):3668–3676. [Google Scholar]
- 157.Du S., Deng Y., Yuan H., Sun Y. 2019.
- 158.Ansari M.N., Ganaie M.A., Rehman N.U., Alharthy K.M., Khan T.H., Imam F., Ansari M.A., Al-Harbi N.O., Jan B.L., Sheikh I.A., Hamad A.M. Protective role of Roflumilast against cadmium-induced cardiotoxicity through inhibition of oxidative stress and NF-KB signaling in rats. Saudi Pharm. J. 2019;27(5):673–681. doi: 10.1016/j.jsps.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Y., Wang Y., Yang G.Y. MicroRNAs in cerebral ischemia. Stroke Res. Treat. 2013;2013:276540. doi: 10.1155/2013/276540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang S.W., Liu Z., Shi Z.S. Non-Coding RNA in acute ischemic stroke: mechanisms, biomarkers and therapeutic targets. Cell Transplant. 2018;27(12):1763–1777. doi: 10.1177/0963689718806818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiang B., Zhong P., Fang L., Wu X., Song Y., Yuan H. miR-183 inhibits microglia activation and expression of inflammatory factors in rats with cerebral ischemia reperfusion via NF-KB signaling pathway. Exp. Ther. Med. 2019;18(4):2540–2546. doi: 10.3892/etm.2019.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dong H., Cui B., Hao X. MicroRNA-22 alleviates inflammation in ischemic stroke via p38 MAPK pathways. Mol. Med. Rep. 2019;20(1):735–744. doi: 10.3892/mmr.2019.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Feng Y., Cui C., Liu X., Wu Q., Hu F., Zhang H., Ma Z., Wang L. Protective role of apocynin via suppression of neuronal autophagy and TLR4/NF-KB signaling pathway in a rat model of traumatic brain injury. Neurochem. Res. 2017;42(11):3296–3309. doi: 10.1007/s11064-017-2372-z. [DOI] [PubMed] [Google Scholar]
- 164.Rahimifard M., Maqbool F., Moeini-Nodeh S., Niaz K., Abdollahi M., Braidy N., Nabavi S.M., Nabavi S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017;36:11–19. doi: 10.1016/j.arr.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 165.Shi H., Hua X., Kong D., Stein D., Hua F. Role of Toll-like receptor mediated signaling in traumatic brain injury. 2019. [DOI] [PubMed]
- 166.Jayakumar A.R., Tong X.Y., Ruiz-Cordero R., Bregy A., Bethea J.R., Bramlett H.M., Norenberg M.D. Activation of NF-KB mediates astrocyte swelling and brain edema in traumatic brain injury. J. Neurotrauma. 2014;31(14):1249–1257. doi: 10.1089/neu.2013.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiong Y., Mahmood A., Chopp M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018;21(3):137–151. doi: 10.1016/j.cjtee.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feng Y., Cui Y., Gao J.L., Li M.H., Li R., Jiang X.H., Tian Y.X., Wang K.J., Cui C.M., Cui J.Z. Resveratrol attenuates neuronal autophagy and inflammatory injury by inhibiting the TLR4/NF-KB signaling pathway in experimental traumatic brain injury. Int. J. Mol. Med. 2016;37(4):921–930. doi: 10.3892/ijmm.2016.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kong L., Yao Y., Xia Y., Liang X., Ni Y., Yang J. Osthole alleviates inflammation by down-regulating NF-KB signaling pathway in traumatic brain injury. Immunopharmacol. Immunotoxicol. 2019;41(2):349–360. doi: 10.1080/08923973.2019.1608560. [DOI] [PubMed] [Google Scholar]
- 170.Liu L., Sun T., Liu Z., Chen X., Zhao L., Qu G., Li Q. Traumatic brain injury dysregulates microRNAs to modulate cell signaling in rat hippocampus. PLoS One. 2014;9(8):e103948. doi: 10.1371/journal.pone.0103948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang W.X., Visavadiya N.P., Pandya J.D., Nelson P.T., Sullivan P.G., Springer J.E. Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp. Neurol. 2015;265:84–93. doi: 10.1016/j.expneurol.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Su X., Ye Y., Yang Y., Zhang K., Bai W., Chen H., Kang E., Kong C., He X. The Effect of SPTLC2 on promoting neuronal apoptosis is alleviated by MiR-124-3p through TLR4 signalling pathway. Neurochem. Res. 2019;44(9):2113–2122. doi: 10.1007/s11064-019-02849-7. [DOI] [PubMed] [Google Scholar]
- 173.Lee B.H., Kim Y.K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7(4):231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Caviedes A., Lafourcade C., Soto C., Wyneken U. BDNF/NF-KB signaling in the neurobiology of depression. Curr. Pharm. Des. 2017;23(21):3154–3163. doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- 175.Liu C.H., Zhang G.Z., Li B., Li M., Woelfer M., Walter M., Wang L. Role of inflammation in depression relapse. J. Neuroinflammation. 2019;16(1):90. doi: 10.1186/s12974-019-1475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Koo J.W., Russo S.J., Ferguson D., Nestler E.J., Duman R.S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. USA. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tang C.Z., Zhang D.F., Yang J.T., Liu Q.H., Wang Y.R., Wang W.S. Overexpression of microRNA-301b accelerates hippocampal microglia activation and cognitive impairment in mice with depressive-like behavior through the NF-KB signaling pathway. Cell Death Dis. 2019;10(4):316. doi: 10.1038/s41419-019-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hung Y.Y., Wu M.K., Tsai M.C., Huang Y.L., Kang H.Y. Aberrant Expression of Intracellular let-7e, miR-146a, and miR-155 correlates with severity of depression in patients with major depressive disorder and is ameliorated after antidepressant treatment. Cells. 2019;8(7):647. doi: 10.3390/cells8070647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Feng J., Wang M., Li M., Yang J., Jia J., Liu L., Zhou J., Zhang C., Wang X. Serum miR-221-3p as a new potential biomarker for depressed mood in perioperative patients. Brain Res. 2019;1720:146296. doi: 10.1016/j.brainres.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 180.Vezzani A., Aronica E., Mazarati A., Pittman Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 181.Rana A., Musto A.E. The role of inflammation in the development of epilepsy. J. Neuroinflammation. 2018;15(1):144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li G., Bauer S., Nowak M., Norwood B., Tackenberg B., Rosenow F., Knake S., Oertel W.H., Hamer H.M. Cytokines and epilepsy. Seizure. 2011;20(3):249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 183.Rowley S., Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic. Biol. Med. 2013;62:121–131. doi: 10.1016/j.freeradbiomed.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vezzani A., French J., Bartfai T., Baram T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Devinsky O., Vezzani A., Najjar S., De Lanerolle N.C., Rogawski M.A. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36(3):174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 186.Shimada T., Takemiya T., Sugiura H., Yamagata K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediators Inflamm. 2014;2014:901902. doi: 10.1155/2014/901902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Somade O.T., Ajayi B.O., Adeyi O.E., Aina B.O., David B. O., Sodiya I.D. 2019.
- 188.Aronica E., Fluiter K., Iyer A., Zurolo E., Vreijling J., van Vliet E.A., Baayen J.C., Gorter J.A. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 2010;31(6):1100–1107. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 189.Li M.M., Li X.M., Zheng X.P., Yu J.T., Tan L. MicroRNAs dysregulation in epilepsy. Brain Res. 2014;1584:94–104. doi: 10.1016/j.brainres.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 190.Alsharafi W., Xiao B. Dynamic expression of microRNAs (183, 135a, 125b, 128, 30c and 27a) in the rat pilocarpine model and temporal lobe epilepsy patients. CNS Neurol. Disord. Drug Targets. 2015;14(8):1096–1102. doi: 10.2174/1871527314666150317225945. [DOI] [PubMed] [Google Scholar]
- 191.Henshall D.C., Hamer H.M., Pasterkamp R.J., Goldstein D.B., Kjems J., Prehn J.H.M., Schorge S., Lamottke K., Rosenow F. MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol. 2016;15(13):1368–1376. doi: 10.1016/S1474-4422(16)30246-0. [DOI] [PubMed] [Google Scholar]
- 192.Huang W.S., Zhu L. MiR-134 expression and changes in inflammatory cytokines of rats with epileptic seizures. Eur. Rev. Med. Pharmacol. Sci. 2018;22(11):3479–3484. doi: 10.26355/eurrev_201806_15174. [DOI] [PubMed] [Google Scholar]
- 193.Sonkoly E., Pivarcsi A. microRNAs in inflammation. Int. Rev. Immunol. 2009;28(6):535–561. doi: 10.3109/08830180903208303. [DOI] [PubMed] [Google Scholar]
- 194.Rippo M.R., Olivieri F., Monsurrò V., Prattichizzo F., Albertini M.C., Procopio A.D. MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014;56:154–163. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 195.Ping H. P.; Feng Bo, T.; Li, L.; Nan, H. Y.; Hong, Z. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch. Biochem. Biophys. 2016;604:20–26. doi: 10.1016/j.abb.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 196.Srivastava A., Dixit A.B., Banerjee J., Tripathi M., Sarat Chandra P. Role of inflammation and its miRNA based regulation in epilepsy: Implications for therapy. Clin. Chim. Acta. 2016;452:1–9. doi: 10.1016/j.cca.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 197.Liu A.H., Wu Y.T., Wang Y.P. MicroRNA-129-5p inhibits the development of autoimmune encephalomyelitis-related epilepsy by targeting HMGB1 through the TLR4/NF-kB signaling pathway. Brain Res. Bull. 2017;132:139–149. doi: 10.1016/j.brainresbull.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 198.Srinivasan M., Lahiri D.K. Significance of NF-KB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer’s disease and multiple sclerosis. Expert Opin. Ther. Targets. 2015;19(4):471–487. doi: 10.1517/14728222.2014.989834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Karunaweera N., Raju R., Gyengesi E., Münch G. Plant polyphenols as inhibitors of NF-KB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015;8:24. doi: 10.3389/fnmol.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Seo E.J., Fischer N., Efferth T. Phytochemicals as inhibitors of NF-KB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018;129:262–273. doi: 10.1016/j.phrs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 201.Santa-Cecília F.V., Socias B., Ouidja M.O., Sepulveda-Diaz J.E., Acuña L., Silva R.L., Michel P.P., Del-Bel E., Cunha T.M., Raisman-Vozari R. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox. Res. 2016;29(4):447–459. doi: 10.1007/s12640-015-9592-2. [DOI] [PubMed] [Google Scholar]
- 202.Jing H., Wang S., Wang M., Fu W., Zhang C., Xu D. Isobavachalcone attenuates mptp-induced Parkinson’s Disease in mice by inhibition of microglial activation through NF-KB pathway. PLoS One. 2017;12(1):e0169560. doi: 10.1371/journal.pone.0169560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Subedi L., Lee J.H., Yumnam S., Ji E., Kim S.Y. Anti-inflammatory effect of sulforaphane on lps-activated microglia potentially through JNK/AP-1/NF-KB inhibition and Nrf2/HO-1 Activation. Cells. 2019;8(2):194. doi: 10.3390/cells8020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Majdi F., Taheri F., Salehi P., Motaghinejad M., Safari S. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol may be effective against methamphetamine induced mitochondrial dysfunction and inflammation by modulation of Toll-like type-4(Toll-like 4) receptors and NF-KB signaling. Med. Hypotheses. 2019;133:109371. doi: 10.1016/j.mehy.2019.109371. [DOI] [PubMed] [Google Scholar]
- 205.Lu H., Le W.D., Xie Y.Y., Wang X.P. Current therapy of drugs in amyotrophic lateral sclerosis. Curr. Neuropharmacol. 2016;14(4):314–321. doi: 10.2174/1570159X14666160120152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Crisafulli S.G., Brajkovic S., Cipolat Mis M.S., Parente V., Corti S. Therapeutic strategies under development targeting inflammatory mechanisms in amyotrophic lateral sclerosis. Mol. Neurobiol. 2018;55(4):2789–2813. doi: 10.1007/s12035-017-0532-4. [DOI] [PubMed] [Google Scholar]
- 207.Zhao Z., Fu J., Li S., Li Z. Neuroprotective effects of genistein in a sod1-g93a transgenic mouse model of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2019;14(4):688–696. doi: 10.1007/s11481-019-09866-x. [DOI] [PubMed] [Google Scholar]
- 208.Yun Y.C., Jeong S.G., Kim S.H., Cho G.W. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J. Tissue Eng. Regen. Med. 2019;13(1):110–115. doi: 10.1002/term.2776. [DOI] [PubMed] [Google Scholar]
- 209.Bai Y., Li Q., Yang J., Zhou X., Yin X., Zhao D. p75(NTR) activation of NF-kappaB is involved in PrP106-126-induced apoptosis in mouse neuroblastoma cells. Neurosci. Res. 2008;62(1):9–14. doi: 10.1016/j.neures.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 210.Choi J., Kim J., Min D.Y., Jung E., Lim Y., Shin S.Y., Lee Y.H. Inhibition of TNFα-induced interleukin-6 gene expression by barley (Hordeum vulgare) ethanol extract in BV-2 microglia. Genes Genomics. 2019;41(5):557–566. doi: 10.1007/s13258-018-00781-8. [DOI] [PubMed] [Google Scholar]
- 211.Zhang L., Previn R., Lu L., Liao R.F., Jin Y., Wang R.K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018;142:352–359. doi: 10.1016/j.brainresbull.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 212.Yu H., Zhang F., Guan X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signaling pathway in olfactory bulbectomized rats. Phytother. Res. 2019;33(5):1480–1489. doi: 10.1002/ptr.6340. [DOI] [PubMed] [Google Scholar]
- 213.El-Agamy D.S., El-Harbi K.M., Khoshhal S., Ahmed N., Elkablawy M.A., Shaaban A.A., Abo-Haded H.M. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag. Res. 2018;11:47–61. doi: 10.2147/CMAR.S186696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhu S., Tang S., Su F. Dioscin inhibits ischemic stroke-induced inflammation through inhibition of the TLR4/MyD88/NF-KB signaling pathway in a rat model. Mol. Med. Rep. 2018;17(1):660–666. doi: 10.3892/mmr.2017.7900. [DOI] [PubMed] [Google Scholar]
- 215.Sun X., Zeng H., Wang Q., Yu Q., Wu J., Feng Y., Deng P., Zhang H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp. Cell Res. 2018;369(1):112–119. doi: 10.1016/j.yexcr.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 216.Luan L., Cao L., Zhu L., Sun J. Diosmetin ameliorates cerebral ischemia/reperfusion injury in pc12 cells through nuclear factor-kB (NF-kB) and nuclear factor erythroid 2-related factor/heme oxygenase-1 (Nrf 2/HO-1) Pathway. Curr. Top. Nutraceutical Res. 2019;17(3):322–328. doi: 10.37290/ctnr2641-452X.17:322-328. [DOI] [Google Scholar]
- 217.Nan D., Jin H., Deng J., Yu W., Liu R., Sun W., Huang Y. Cilostazol ameliorates ischemia/reperfusion-induced tight junction disruption in brain endothelial cells by inhibiting endoplasmic reticulum stress. FASEB J. 2019;33(9):10152–10164. doi: 10.1096/fj.201900326R. [DOI] [PubMed] [Google Scholar]
- 218.Gugliandolo E., D’Amico R., Cordaro M., Fusco R., Siracusa R., Crupi R., Impellizzeri D., Cuzzocrea S., Di Paola R. Neuroprotective effect of artesunate in experimental model of traumatic brain injury. Front. Neurol. 2018;9:590. doi: 10.3389/fneur.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dai W., Wang H., Fang J., Zhu Y., Zhou J., Wang X., Zhou Y., Zhou M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018;140:65–71. doi: 10.1016/j.brainresbull.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 220.Caglayan B., Kilic E., Dalay A., Altunay S., Tuzcu M., Erten F., Orhan C., Gunal M.Y., Yulug B., Juturu V., Sahin K. Allyl isothiocyanate attenuates oxidative stress and inflammation by modulating Nrf2/HO-1 and NF-KB pathways in traumatic brain injury in mice. Mol. Biol. Rep. 2019;46(1):241–250. doi: 10.1007/s11033-018-4465-4. [DOI] [PubMed] [Google Scholar]
- 221.Vigont V., Gusev K., Kaznacheyeva E. EVP4593 Compound Decreases abnormal store-operated calcium entry in ipscs-based model of Huntington’s Disease. Biophysical. 2018;114(3):285a–286a. doi: 10.1016/j.bpj.2017.11.1638. [DOI] [Google Scholar]
- 222.Wickenberg A.K., Hagai E.L., Eyal E. 2018.
- 223.Pandey M., Rajamma U. Huntington’s disease: the coming of age. J. Genet. 2018;97(3):649–664. doi: 10.1007/s12041-018-0957-1. [DOI] [PubMed] [Google Scholar]
- 224.Mestre T.A. Recent advances in the therapeutic development for Huntington disease. Parkinsonism Relat. Disord. 2019;59:125–130. doi: 10.1016/j.parkreldis.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 225.Denis H.L., Lauruol F., Cicchetti F. Are immunotherapies for Huntington’s disease a realistic option? Mol. Psychiatry. 2019;24(3):364–377. doi: 10.1038/s41380-018-0021-9. [DOI] [PubMed] [Google Scholar]
- 226.Kataria R., Sobarzo-Sanchez E., Khatkar A. Role of morin in neurodegenerative diseases: a review. Curr. Top. Med. Chem. 2018;18(11):901–907. doi: 10.2174/1568026618666180711153416. [DOI] [PubMed] [Google Scholar]
- 227.Provost P. MicroRNAs as a molecular basis for mental retardation, Alzheimer’s and prion diseases. Brain Res. 2010;1338:58–66. doi: 10.1016/j.brainres.2010.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Satoh J. Molecular network of microRNA targets in Alzheimer’s disease brains. Exp. Neurol. 2012;235(2):436–446. doi: 10.1016/j.expneurol.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 229.da Silva F.C., Iop R.D., Vietta G.G., Kair D.A., Gutierres Filho P.J., de Alvarenga J.G., da Silva R. microRNAs involved in Parkinson’s disease: A systematic review. Mol. Med. Rep. 2016;14(5):4015–4022. doi: 10.3892/mmr.2016.5759. [DOI] [PubMed] [Google Scholar]
- 230.Nagai M., Abe K., Okamoto K., Itoyama Y. Identification of alternative splicing forms of GLT-1 mRNA in the spinal cord of amyotrophic lateral sclerosis patients. Neurosci. Lett. 1998;244(3):165–168. doi: 10.1016/S0304-3940(98)00158-X. [DOI] [PubMed] [Google Scholar]
- 231.Trotti D., Rolfs A., Danbolt N.C., Brown R.H., Jr, Hediger M.A. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat. Neurosci. 1999;2(5):427–433. doi: 10.1038/8091. [DOI] [PubMed] [Google Scholar]
- 232.Chen S., Sayana P., Zhang X., Le W. Genetics of amyotrophic lateral sclerosis: an update. Mol. Neurodegener. 2013;8(1):28. doi: 10.1186/1750-1326-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Souza P.V., Pinto W.B., Oliveira A.S. C9orf72-related disorders: expanding the clinical and genetic spectrum of neurodegenerative diseases. Arq. Neuropsiquiatr. 2015;73(3):246–256. doi: 10.1590/0004-282X20140229. [DOI] [PubMed] [Google Scholar]
- 234.Krasemann S., Zerr I., Weber T., Poser S., Kretzschmar H., Hunsmann G., Bodemer W. Prion disease associated with a novel nine octapeptide repeat insertion in the PRNP gene. Brain Res. Mol. Brain Res. 1995;34(1):173–176. doi: 10.1016/0169-328X(95)00175-R. [DOI] [PubMed] [Google Scholar]
- 235.Baybutt H., Manson J. Characterisation of two promoters for prion protein (PrP) gene expression in neuronal cells. Gene. 1997;184(1):125–131. doi: 10.1016/S0378-1119(96)00600-2. [DOI] [PubMed] [Google Scholar]
- 236.Boakye P.A., Olechowski C., Rashiq S., Verrier M.J., Kerr B., Witmans M., Baker G., Joyce A., Dick B.D. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin. J. Pain. 2016;32(4):327–336. doi: 10.1097/AJP.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 237.Burak K., Lamoureux L., Boese A., Majer A., Saba R., Niu Y., Frost K., Booth S.A. MicroRNA-16 targets mRNA involved in neurite extension and branching in hippocampal neurons during presymptomatic prion disease. Neurobiol. Dis. 2018;112:1–13. doi: 10.1016/j.nbd.2017.12.011. [DOI] [PubMed] [Google Scholar]