Abstract
Background
Atherosclerosis is a chronic inflammatory condition that affects different arteries in the human body and often leads to severe neurological complications, such as stroke and its sequelae. Affected blood vessels develop atherosclerotic lesions in the form of focal thickening of the intimal layer, so called atherosclerotic plaques.
Objectives
Despite the high priority of atherosclerosis research for global health and the numerous preclinical and clinical studies conducted, currently, there is no effective pharmacological treatment that directly impacts atherosclerotic plaques. Many knowledge gaps exist in our understanding of the mechanisms of plaque formation. In this review, we discuss the role of mitochondria in different cell types involved in atherogenesis and provide information about mtDNA mutations associated with the disease.
Results
Mitochondria of blood and arterial wall cells appear to be one of the important factors in disease initiation and development. Significant experimental evidence connects oxidative stress associated with mitochondrial dysfunction and vascular disease. Moreover, mitochondrial DNA (mtDNA) deletions and mutations are being considered as potential disease markers. Further study of mtDNA damage and associated dysfunction may open new perspectives for atherosclerosis treatment.
Conclusion
Mitochondria can be considered as important disease-modifying factors in several chronic pathologies. Deletions and mutations of mtDNA may be used as potential disease markers. Mitochondria-targeting antioxidant therapies appear to be promising for the development of treatment of atherosclerosis and other diseases associated with oxidative stress and chronic inflammation.
Keywords: Atherosclerosis, mitochondrion, inflammation, mtDNA, oxidative stress, LDL metabolism
1. Introduction
Atherosclerosis and associated pathologies remain the leading cause of mortality in industrialized countries, and their incidence is increasing alongside the ageing of the population. One of the main consequences of atherosclerosis is ischemic heart disease (IHD), which remains the leading cause of death around the globe. For example, in 2013, stroke along with IHD caused 247.9 deaths/100,000 persons which represent 84.5% of cardiovascular deaths and 28.2% of all-cause mortality [1]. The atherosclerotic process causes different human diseases depending on the location and severity of developing lesions that can impact large arteries. If brain vessels are affected, the pathology may result in neurological consequences, such as stroke, dementia, and disability [2]. Atherosclerosis is a chronic process that remains asymptomatic for a long time and is often subclinically present in relatively young people [3]. It is a multifactorial disease that involves changes in lipid metabolism, oxidative stress, immune factors, and impaired tissue response to damage.
To date, there is no effective pharmacological treatment that allows curing the disease. Available therapies mostly focus on ameliorating the symptoms or reducing the known risk factors of disease development [4]. Despite the significant progress made in studying the mechanisms of atherogenesis and certain success reached in developing methods for the disease treatment and prevention, some key aspects of the onset and development of atherosclerotic lesions remain unclear. In particular, it is not well understood why atherosclerosis occurs locally, or even focally. Microscopic inspection of the affected arteries reveals that in some areas of the arterial wall, the normal pro-inflammatory response of the innate immunity is resolved by rapid repair, while in other adjacent areas, chronic inflammation leads to atherosclerotic lesion formation [5, 6]. The reason for such focal development of the pathology remains unclear and may have important consequences for the development of future anti-atherosclerotic therapies.
Mitochondrion is a semi-autonomous organelle of endosymbiotic origin that plays the role of a cellular powerhouse. Mitochondrial DNA (mtDNA) is a relatively small circular molecule that is much more susceptible for mutagenesis than nuclear DNA, due to its primitive repair mechanisms, frequent replication, lack of histone packing, and proximity to reactive oxygen species (ROS) [7]. Deletions in mtDNA caused by oxidative stress can lead to compensatory overproliferation of mtDNA and can potentially be used as markers of oxidative stress and mitochondrial damage in atherosclerosis and other diseases [8]. During recent years, mitochondria received increasing attention as possible disease-modifying players in several chronic human pathologies. Mitochondrial dysfunction may explain the phenotype heterogeneity between different individuals and, potentially, between different tissues and cells within the same organism [9, 10]. Recent advances in studying the mitochondrial genome allowed revealing and classifying mtDNA mutations associated with human pathologies. The development of cytoplasmic hybrid (cybrid) technology allowed studying the functional significance of these mutations and mitochondrial dysfunction in detail [11, 12].
Atherosclerosis is currently considered a chronic inflammatory condition [13]. It would be important to understand the mechanisms of the transition from the transient pro-inflammatory response of the innate immunity to chronic inflammation. This knowledge will allow creating effective approaches for the prevention and treatment of atherosclerotic diseases.
2. Role of mitochondrion in the endothelial cells
Endothelial cells (ECs) cover all elements of the cardiovascular system, from the heart to the smallest capillary. The main function of cells of this type is to maintain the vessel homeostasis and to control transport between the cells of the vessel wall and the bloodstream [14]. In atherosclerosis development, the ECs appear to be the site of several key events of the disease initiation: activation in response to external stimuli, recruitment of circulating inflammatory cells, and increase of vascular wall permeability that allows penetration of immune cells and lipoprotein particles [15].
ECs differ phenotypically and morphologically between tissues where vessels are located. Moreover, there is a diversity between mitochondrial content in ECs according to specific features of surrounding tissues. For instance, brain ECs contain more mitochondria than ECs in other tissues. Mitochondria commonly play the role of a metabolic regulator that is capable not only of energy production but of controlling cell proliferation and apoptosis [16]. In the ECs, mitochondrial content is relatively low and takes merely 2-6% of cytoplasm volume, while in cardiomyocytes, this proportion can be as high as 32% [14]. Therefore, EC mitochondria have mostly regulatory functions and only generate about 20% of all cellular ATP, while the other 80% are covered by glycolysis [17]. However, the respiratory activity of EC mitochondria can increase in response to stress factors, for example, oxidative stress and glucose deprivation.
Importantly, ECs serve as mediators of oxygen transport from the blood to perivascular tissues underlying the endothelium. Own oxygen consumption of ECs is relatively low, which makes these cells effective agents of oxygen transfer that also protect perivascular tissues from damage associated with oxidative stress. It is now under discussion whether a drastic drop in oxygen levels across the arteriolar vessel wall reflects fast diffusion through the endothelium or whether there is another mechanism behind this observation since the available experimental data are inconsistent [18].
Mitochondria of ECs play part in the response to hypoxia via the angiogenic cascade initiation. Mitochondrial biogenesis is increased by the VEGF level that is enhanced by the hypoxic environment. This process involves AKT-depending signaling and results in increased vascular branching. By contrast, sirtuin 1 (SIRT1) silencing can inhibit mitochondrial biogenesis in the ECs, which leads to decreased vessel branching. A recent study reported that in a pulmonary hypertension model induced by intermittent hypoxia, the inner mitochondrial membrane protein UCP-2 (uncoupling protein 2) stimulated mitophagy, increased apoptosis of lung ECs, and contributed to inadequate mitochondrial biogenesis [19].
In relation to cardiovascular pathology, of particular interest are the sources and ways of regulation of mitochondrial ROS in the ECs. Cytochrome C oxidase (complex IV) is responsible for the majority of oxygen consumption within the mitochondria, but the role of complexes I and III was also figured out. Superoxide anion formation at these complexes was estimated as 0.1-2% of the total amount in isolated mitochondria, while the percentage attributable to ROS production in endothelial cells in vivo remained uncertain [20]. However, sources of mitochondrial ROS are not limited to complexes I and III. NADPH oxidase 4 (NOX4) is involved in ROS signaling, inflammation, oxidative stress, and adaptive response to hypoxia. NOX4 also promotes endothelial cell senescence, migration, and angiogenesis. The expression level of this enzyme is relatively high in the ECs, but, despite the fact that mitochondrial localization of NOX4 was shown for a range of tissues, its localization in the ECs remains uncertain [21].
Enzymes from monoamine oxidase family (MAO), which consists of two subfamilies, MAO-A and MAO-B, produce ROS as a by-product of catecholamine catabolism. MAOs can be found on the outer membrane of the mitochondria. ROS produced by MAO was reported to contribute to adverse cardiac remodeling and heart failure in response to pressure overload in mice, while hydrogen peroxide derived from MAO-A is associated with serotonin-induced vasoconstriction.
3. The role of mitochondria in macrophages
Macrophages play a prominent role in inflammation and pathogen-triggered immune response. These cells actively participate in lipid accumulation in the arterial wall. They can be found in large numbers in growing atherosclerotic plaques, where they can internalize lipoprotein particles by means of uncontrolled phagocytosis leading to foam cell formation [22]. The initiation of inflammatory processes includes the detection of pathogens via pattern-recognition receptors (PRRs) that are expressed by macrophages and other immune cells. PRRs control classic activation of macrophages by sensing pathogen-associated molecular patterns (PAMPs), such as compounds of the bacterial cell wall, nucleic acids, and proteins. PRRs consist of several protein families: Toll-like receptors (TLRs), nucleotide oligomerization domain-like receptors (NLRs), C-type lectin receptors, and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) [23]. Upon binding to ligands of PRRs, multiple signaling pathways activate for producing pro-inflammatory cytokines, chemokines, type I interferons (IFNs), and costimulatory molecules that support the immune response. Besides sensing microbial PAMPs, PRRs also detect danger-associated molecular patterns (DAMPs) that are released during cellular stress and damage, such as ROS, mtDNA, ATP, or uric acid crystals [24].
Certain downstream effectors and adaptors of PRRs are located in the proximity to the mitochondrial membrane. For instance, conserved signaling intermediate in Toll pathways (ECSIT) that interacts with TRAF6 after the stimulation of TLRs, is involved in the mitochondrial respiratory complex I assembly promoting mitochondrial ROS (mtROS) production for antibacterial responses [25]. Furthermore, mtDNA released into the cytoplasm from damaged mitochondria can trigger the activation of the NFκ-B signaling pathway and activate the expression TNFα, IL-6, and other pro-inflammatory genes [26].
Several other proteins are implicated into the mitochondrial signaling and functioning, among which the main fields of interest are mitochondrial antiviral signaling protein (MAVS). MAVS works as an adaptor protein that transfers the inductive signal for the production of type I IFNs and pro-inflammatory cytokines and type I interferon [25]. While the molecular mechanisms of these interactions have been identified, it remains unclear whether these regulations can be controlled by metabolic alterations in mitochondria.
Activation of NLRP3 inflammasomes that play an important role in sterile inflammation was shown to be triggered by mitochondrial ROS production. However, NF-kB-induced mitophagy can restrain NLRP3- induced inflammatory responses in macrophages [27]. Moreover, NLRP3 inflammasomes were reported to modulate glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages [28].
Two main types of macrophage polarization are classically-activated pro-inflammatory M1 macrophages and alternatively activated M2 macrophages that maintain tissue homeostasis. Macrophage polarization is tightly linked to changes in cellular metabolism and bioenergetic profile [29]. Pro-inflammatory M1 macrophages are dependent on glycolysis for ATP production [30]. Macrophages polarization to M1 phenotype is accompanied by increased aerobic glycolysis and glucose uptake, deficiency of isocitrate dehydrogenase 1 (IDH1), and accumulation of succinate. Moreover, M1 macrophages produce relatively large amounts of ROS partly due to the up-regulation of the pentose phosphate pathway, that is utilized for pathogens killing but can also influence cellular processes. Production of NO by inducible nitric oxide synthase (iNOS) is another feature of M1 macrophages. Glycolysis is also important for M2 polarization of macrophages, in which glucose oxidation contributes to the synthesis of fatty acids [31]. This macrophage subset is also characterized by increased arginase activity and production of ornithine from arginine to facilitate tissue repair [30]. Apart from classically and alternatively activated macrophages, other macrophage phenotypes have been identified in humans and mice that play important roles in atherosclerosis [32]. Differentiated in response to oxidized phospholipids, Mox macrophages play a role in controlling the redox status, while Mhem macrophages regulate iron metabolism at the sites of intraplaque hemorrhage. Improved understanding of these profound changes in macrophage metabolism gave rise to the concept of immunometabolism that plays an important role in macrophage polarization and for the large part is directed by the mitochondria [29]. Metabolic pathways modulation has been already proposed as a new therapeutic approach for educating macrophages in different disease contexts.
4. Role of mitochondrion in pericytes and vascular smooth muscle cells
The cellular population of the subendothelial intima of large blood vessels consists mainly of smooth muscle cells (about 50-60%) and pericytes (about 30%) [33, 34]. These cell types have common features such as expression of smooth muscle α-actin and subendothelial localization; however, their functions differ significantly. Vascular smooth muscle cells (VSMCs) are crucially involved in the regulation of vascular tone and blood pressure. These cells can undergo reversible phenotype changes in response to various environmental signals. After differentiation, VSMCs acquire a contractile phenotype, which is characterized by the low rate of proliferation, decreased production of extracellular matrix and expression of specific contractile proteins, such as smooth muscle myosin heavy chain, smooth muscle α-actin, and calponin. However, in response to vessel injury, VSMCs can acquire synthetic phenotype, gaining the ability to proliferation and migration towards the injury site. Such cells also produce extracellular matrix components and express vimentin and tropomyosin-4 [35].
Pericytes are multifunctional cells that have a characteristic star-like shape with long cellular protrusions and can be found in the subendothelial layer of small and large blood vessels. These cells are responsible for maintaining tissue homeostasis and supporting the endothelium. They also play a key role in atherogenesis [36]. Pericytes can have both contractile and synthetic activity. Like macrophages, pericytes are capable of phagocytes and accumulation of lipid inclusions in atherosclerotic lesions. Like dendritic cells, pericytes can participate in antigen presentation and are actively involved in the innate immune response. By forming a continuous three-dimensional network through cellular contacts between themselves and the ECs, pericytes create a second line of defense under the endothelium.
Rapid respiration generating superoxide, the ROS precursor, can cause pericyte death. Respiration is controlled by carbonic anhydrases that are expressed in the mitochondria. This phenomenon is crucial for the development of diabetes, due to the high glucose level in the blood that accompanies the free influx of glucose into insulin-insensitive tissues [37].
The relationship between mitochondrial dynamics and the proliferative phenotype of VSMCs is currently under discussion [38, 39]. It was shown that PDGF can modify the VSMCs metabolic profile by regulating the oxidative processes: increase fatty acid oxidation and decrease glucose oxidation and promote mitochondrial fragmentation. Further studies involving the use of mdivi-1, a pharmacological inhibitor of a fundamental component of mitochondrial fission called dynamin-related protein 1 (DRP1), revealed that PDGF stimulation of VSMCs prevented proliferation and altered glucose and fatty acid oxidation, suggesting that mitochondrial fission is required for these processes [38, 40].
5. Oxidative stress and mitochondrial dysfunction
The modern concept of oxidative stress determines this process as “the lack of balance between the occurrence of reactive oxygen/nitrogen species (ROS/RNS) and the capacity of an organism to counteract their action by the antioxidative protection systems” [41]. Among different ROS are superoxide (O2∙), hydrogen peroxide (H2O2), and hydroxyl radical (∙OH). Reactive nitrogen species (RNS) also play a prominent role in oxidative stress. Under physiological conditions, both ROS and RNS are involved in cell signaling, modulation of transcriptional factors, apoptosis, and other important cell processes [42]. However, in many pathologies, excessive ROS generation or insufficient functioning of antioxidant systems leads to the development of mitochondrial oxidative stress. Oxidative damage incurred by ROS and RNS contributes to the development of cancer, neurodegenerative disorders, chronic inflammatory, and cardiovascular diseases. Oxidative stress is one of the key events in atherosclerosis initiation and development. Most of the known risk factors of atherosclerosis, such as hypertension, diabetes, and dyslipidemia, are also accompanied by enhanced ROS generation in the vessel wall. The main enzyme responsible for oxidative stress in the vasculature is considered to be NADPH oxidase, but there are also a variety of other enzymatic systems that are involved in ROS overproduction, such as xanthine oxidase, enzymes of the mitochondrial respiratory chain, and a dysfunctional, uncoupled endothelial NO synthase [43]. Progressive impairment of mitochondrial function associated with oxidative stress is observed in aging and in such chronic diseases as atherosclerosis and Alzheimer’s disease. Aliev with colleagues demonstrated that atherosclerotic lesions and mitochondrial DNA (mtDNA) deletions in brain microvessels affected by chronic hypoperfusion are crucially involved in the pathogenesis of Alzheimer's disease [44, 45].
Mitochondrial dysfunction may result from the impaired turnover of mitochondria, inability to provide necessary substrates, or a deficiency in the electron transport and ATP synthesis machinery [46]. Mitochondrial volume and functional status are regulated by turnover mechanisms of mitochondrial fission and fusion and mitophagy, through which dysfunctional mitochondria are degraded [47, 48]. Oxidative stress and mitochondrial dysfunction are tightly linked: most of the ROS generating systems are associated with mitochondria, while mtDNA is a ready target for oxidative damage due to the lack of histones and poor capacity for repair. Mutations and deletions in mtDNA, in turn, stimulate the new cycles of ROS generation forming a vicious circle. Ballinger with colleagues reported that enhanced ROS production is capable to damage mtDNA, stimulate proliferation of VSMCs, and trigger atherosclerosis development in apoE-/- mice deficient for antioxidant enzyme superoxide dismutase-2 (SOD-2) [49]. Another group conducted a study on people who underwent intravascular ultrasound characterization of coronary artery plaques that revealed that mtDNA damage in leukocytes is associated with the existence of vulnerable plaques but not with plaque burden [50].
6. The role of modified LDL in mitochondrial damage
LDL particles undergo multiple modifications while circulating in blood flow, which involves many different physical and chemical changes at the level of their lipid, protein, and carbohydrate moieties. In brief, modification cascade is likely to start with the desialylation of LDL, which leads to the formation of small dense LDL particles and facilitates oxidation and other chemical modifications. Such modified particles are prone to self-association, which further increases their atherogenicity [4, 51]. Modified LDL accumulates in the loci of the arterial wall predisposed to atherosclerosis, where it is taken up by both recruited and resident cells of the arterial wall that transform to lipid-rich foam cells. Inflammatory cells are attracted to the lesion by the cytokines and adhesion molecules, that are released by foam cells. Pro-inflammatory mediators that are produced by foam cells trigger the formation of neointima through hyperplasia, migration, and proliferation of pericytes [52] and partially VSMCs [53].
Activation of mitochondrial apoptotic pathways by modified LDL has been demonstrated in. human aortic ECs [54]. Mechanisms of such activation include the increase of cytosolic Ca2+ influx followed by mitochondrial mPTP opening and the release of cytochrome c into the cytoplasm [55]. All these data indicate that mitochondrial dysfunction contributes not only to the initiation of atherosclerosis, but also its development, which makes it a promising target for atherosclerosis treatment.
7. Mitophagy as a special case of autophagy
Autophagy is an evolutionarily conserved subcellular process ensuring the degradation of proteins and damaged organelles [56]. Mitophagy is a specific type of autophagy responsible for the inactivation and degradation of damaged and dysfunctional mitochondria. The initiation of mitophagy is dependent on P-TEN-induced kinase (PINK1) and Parkin activation. PINK1 accumulates at the outer membrane in response to the reduction of mitochondrial membrane potential and then recruits Parkin assisting the autophagic degradation of dysfunctional mitochondria. Excessive induction of autophagy may result in autophagic cell death, as the potential result of the degradation of vital cellular components [57]. Oxidative stress has a destructive effect on lysosomal membrane integrity, causing the release of lysosomal hydrolases and leading to inadequate mitophagy with prolonged exposure. That promotes the release of cytochrome c potentially leading to apoptosis. Cholesterol crystals accumulating in advanced atherosclerotic plaque are also capable of disrupting the lysosomal membrane [58, 59].
Inhibition of autophagy in macrophages promotes atherosclerosis in mice receiving a high-fat diet or ldlr−/− mice through conditional deletion of Atg5 in Lyz2 (lysozyme 2)-expressing cells or via specific small interfering RNAs (siRNAs). That was shown to be mediated by local inflammation, decreased autophagic competence, reduced efferocytosis, and exacerbated necrosis [60]. The depletion of PINK1 or PARK2 by siRNA seems to enhance the cytotoxic response of human VSMCs challenged with modified LDL in vitro. By contrast, overexpression of PINK1 or PARK2 had a cytoprotective effect and possibly due to mitophagic responses that counteract atherosclerosis progression [58, 61]. The etiologic association between atherosclerosis, inflammation, and loss of autophagic or mitophagic responses is further supported by the observation that a supramolecular complex consisting of mtDNA and cathelicidin promotes atherosclerosis in ldlr−/− mice [62]. Although it is currently established that mitophagy is highly involved in cell functioning, its role in atherosclerosis needs to be further investigated further to determine the exact mechanisms and identify potential therapeutic targets.
8. Mitochondrial mutations and their link to mitochondrial dysfunction and inflammation
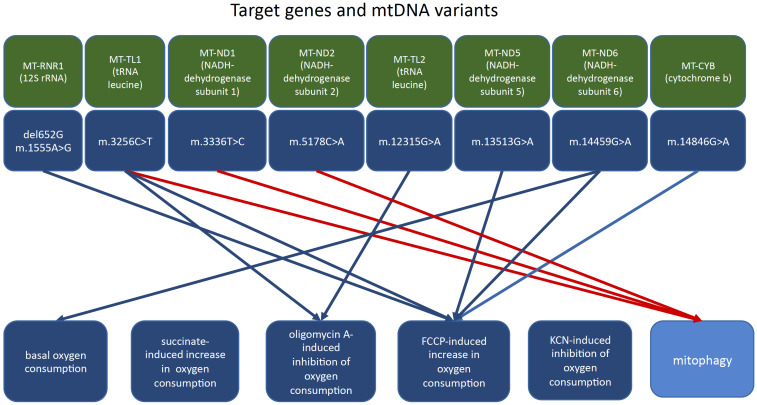
Studies of mtDNA from the leukocytes of atherosclerotic patients revealed a correlation between certain mtDNA mutations and atherosclerosis [63-65]. Such mutations could be homoplasmic (absence or presence of the mutation) or heteroplasmic (different proportions of mutant allele). Monocytes were isolated from the blood of subjects with asymptomatic atherosclerosis to evaluate their pro-inflammatory activation in primary culture and atherosclerosis-associated mitochondrial mutations [66]. It was found that two homoplasmic mutations, A1811G and G9477A, correlated with the degree of monocyte activation. At least three heteroplasmic mutations of mtDNA (G14459A, A1555G, G12315A), also correlated with pro-inflammatory activation of circulating human monocytes. It was concluded that some mutations may alter monocyte-derived macrophage activation in atherosclerosis through mitochondrial dysfunction. The relationship between mitochondrial functions and atherosclerosis-associated mitochondrial mutations was further investigated on cybrid cell lines bearing various variants of the mitochondrial genome obtained from atherosclerotic patients (Fig. 1). The experiments were conducted in accordance with the Helsinki declaration and were approved by the local Ethics Committee. The study identified variants of mtDNA that uncoupled oxidative phosphorylation, as assessed by the rate of FCCP-induced increase in oxygen consumption and variants of mtDNA that, as assessed by the oligomycin A-induced inhibition of oxygen consumption (Table 1). The mtDNA variant m.14459G>A was associated with a higher basal rate of oxygen consumption in the MiR05 medium and a lower basal rate of oxygen consumption in the Hanks medium and by the coefficient of change in the rate of FCCP-induced oxygen consumption. None of the mtDNA variants affected the rate of oxygen consumption upon the adding of succinate and KCN. The mtDNA variants m.1555A>G and m.14846G>A were associated with the opposite effect; therefore, they may bear protective function.
Fig. (1).
Relationship between the levels of mtDNA heteroplasmy and markers of mitochondrial function. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Mitochondrial DNA variants associated with inflammation and mitochondrial dysfunction.
| Function | mtDNA Variant | r | p |
|---|---|---|---|
| Oxygen phosphorylation uncoupling | del562G | 0.634 | 0.020 |
| m.1555A>G | 0.714 | 0.006 | |
| m.14459G>A | -0.564 | 0.045 | |
| m.14846G>A | 0.613 | 0.026 | |
| sustained ATP synthase activity | m.3256C>T | 0.543 | 0.055 |
| m.12315G>A | 0.602 | 0.030 | |
| m.13513G>A | 0.496 | 0.084 | |
| Increased basal oxygen consumption | m.14459G>A | -0.513 | 0.073 |
| Reduced basal oxygen consumption | m.1555A>G | - | - |
| m.14846G>A | - | - | |
| Increased mitophagy | m.3336T>C | 0.560 | 0.046 |
| m.3256C>T | 0.515 | 0.072 | |
| m.5178C>A | 0.719 | 0.006 | |
| m.3336T>C | - | - | |
| m.3256C>T | - | - | |
| m.5178C>A | - | - |
Several identified mtDNA variants were associated with increased mitophagy, as assessed by LAMP gene expression (Table 1). For the variant m.15059G>A, no associations with oxygen consumption and activity of mitophagy were revealed. Therefore, under the conditions of uncoupling of oxidative phosphorylation in vitro, the mtDNA variants del562G, m.3256C>T, m.12315G>A, m.13513G>A, and m.14459G>A were associated with increased proton leakage and oxygen consumption, thus promoting the production of excessive amounts of ROS and further development of mitochondrial dysfunction.
To determine the oxygen consumption rate, 5 million cells were used in 500 μl of special MiR05 medium (0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, 0.1% bovine serum albumin). Subsequently, succinate was introduced at a concentration of 1 mM, which is a substrate of the Krebs cycle and succinate dehydrogenase, a component of the mitochondrial enzyme complex II, then the following oxidative phosphorylation uncouplers: (1) oligomycin A at a concentration of 1 μm (Sigma-Aldrich, USA), which blocks the proton channel (F0 in complex V) and prevents protons to return to the matrix, as a result of which ATP synthase F1 loses its ability to synthesize ATP; (2) FCCP (4- (trifluoromethoxy) phenylhydrazone carbonyl cyanide) at a concentration of 1 μm (Sigma-Aldrich, USA), which is able to intercept protons and transfer them through the inner mitochondrial membrane, bypassing the proton channel of complex V, and (3) potassium cyanide in concentration 1 M, which completely inhibits cytochrome c oxidase, thereby blocking electron transfer in the respiratory chain. The rate of oxygen consumption by cells was expressed in ng oxygen atoms/min.
To determine the rate of oxygen consumption in the alternative type of experiments, 5 million cells in 500 μl of Hanks medium were used; the following oxidative phosphorylation uncouplers were sequentially used: (1) oligomycin A at a concentration of 1 μm, and (2) FCCP at a concentration of 1 μM.
The relative change in the rate of oxygen consumption was calculated at each stage of the experiment. The increase of the coefficient of oxygen consumption rate under the addition of succinate was regarded as a positive effect; the increase in the rate of oxygen consumption under the addition of oligomycin was regarded as a positive effect - maintaining the activity of ATP synthase; the increase in oxygen consumption rate coefficient with the addition of FCCP was regarded as the efficiency of dissociation of oxidative phosphorylation; the increase in the rate of oxygen consumption rate with the addition of KCN was regarded as a positive effect - the inhibition of cytochrome c oxidase).
The efficiency of mitophagy in intact cells was assessed by the relative expression of the mRNA of the mitophagy marker Lysosomal-associated membrane protein 1 (LAMP1), which is involved in the fusion of late endosomes and autophagosomes or phagosomes. The evaluation was performed by qPCR [67]; expression of the housekeeping genes GAPDH and CAP1 was used as a control. Higher LAMP1 expression values were regarded as more efficient mitophagy.
Blue arrows, effects on oxygen consumption.
Red arrows, effects on mitophagy.
The role of mitophagy in the response of innate immunity was studied on human monocyte-derived macrophages. Mitophagy was inhibited by 3-methyladenine (3-MA) or 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR). The inflammatory response was stimulated by lipopolysaccharides (LPS). The degree of the pro-inflammatory response was estimated by the expression of the pro-inflammatory cytokine IL-1beta gene determined by RT-PCR (Table 2).
Table 2.
The effects of 3-methyladenine and 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside on the formation of tolerance by human macrophages.
| IL-1beta Gene Expression | |||
|---|---|---|---|
| Day 1 | Day 6 | ||
| - | + LPS | + LPS | |
| control | 1.0±0.3 | 5.8±0.6 | 1.9±0.1 |
| + 3-MA, 2.5 mM | 3.5±0.2 (p=0.012) | 35.4±1.0 (p=0.001) | 5.5±0.03 (p=0.001) |
| + AICAR, 0.8 mM | 0.7±0.1 (p=0.176) | 12.7±1.2 (p=0.008) | 15.2±1.3 (p=0.010) |
The significance of differences from control is indicated in brackets.
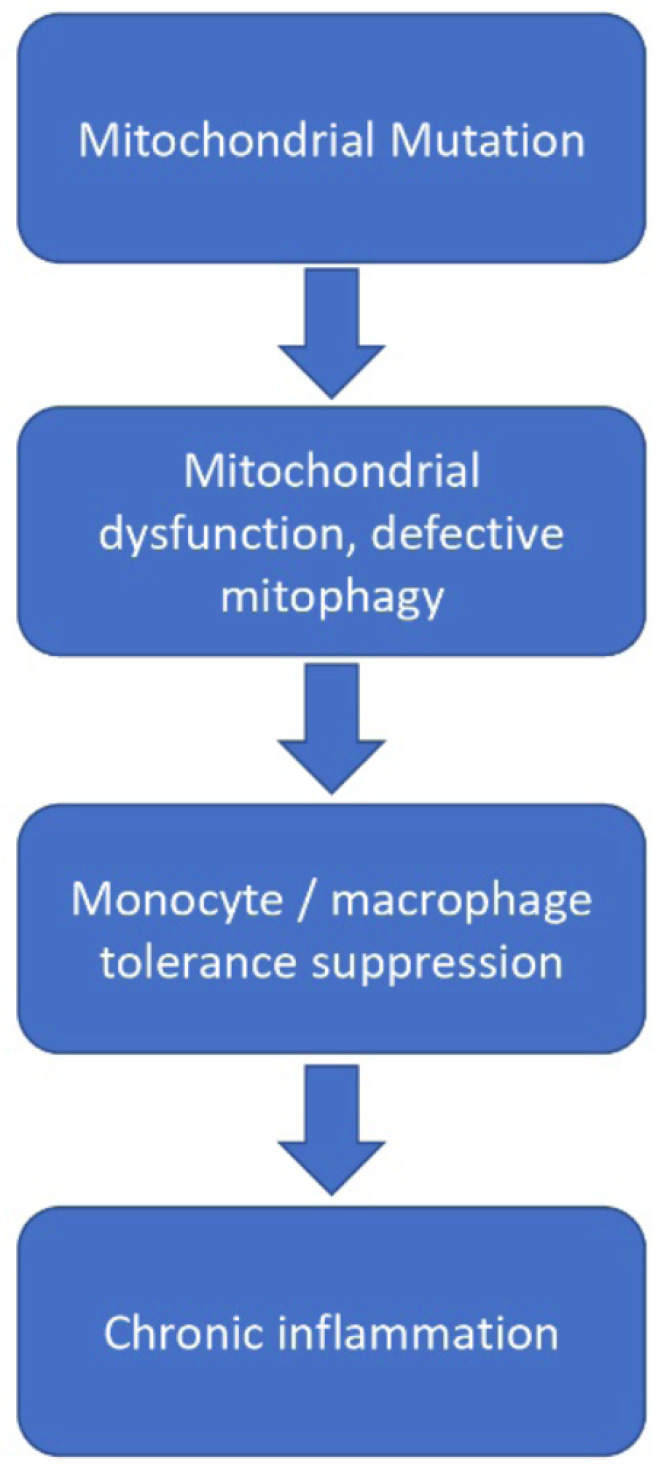
The addition of LPS on the first day resulted in a pro-inflammatory response in the form of up-regulation of the IL-1β gene both in control cells and in the presence of mitophagy inhibitors. Repeated addition of LPS on the sixth day caused a much smaller pro-inflammatory response in control cells, indicative of the presence of immune tolerance. Upon suppression of mitophagy, immune tolerance did not occur and the cells continued to demonstrate a pro-inflammatory response. These results highlight the important role of mitochondrial mutations in the impairment of the innate immune response and the occurrence of chronic inflammation (Fig. 2).
Fig. (2).
Schematic representation of events linking mtDNA mutations with chronic inflammation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
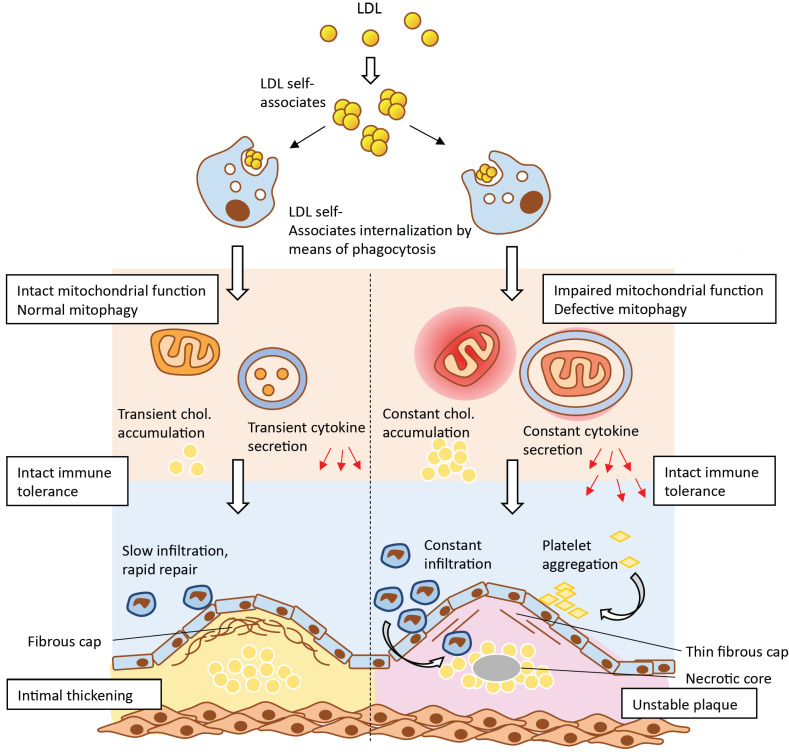
Based on the abovementioned results, a hypothesis was proposed to explain the important role of mitochondrial mutations in the occurrence and development of atherosclerotic lesions (Fig. 3). According to this hypothesis, atherogenic modified LDL circulating in the blood of atherosclerotic patients [68] induces lipid accumulation in the arterial wall cells. Modified LDL particles form self-associates that penetrate the cell by nonspecific phagocytosis [69]. Stimulation of phagocytosis by LDL associates activates the pro-inflammatory response of macrophages [70], leading to increased accumulation of intracellular lipids. With a normally functioning innate immunity, the pro-inflammatory reaction resolves rather quickly and further lipid accumulation does not occur. However, when macrophages contain mtDNA mutations associated with defective mitophagy, the pro-inflammatory response of macrophages does not arrest, but rather intensifies with each repeated pro-inflammatory stimulation. Local inflammation in the vascular wall becomes chronic and accompanied by uncontrolled lipid accumulation giving rise to an atherosclerotic lesion. Another intriguing possibility is that cells may recognize the dysfunctional mitochondrion as a pathogen that presents foreign epitopes, therefore triggering the immune response. This may be a consequence of the bacterial origin of mitochondria, due to which defective mitochondria could be recognized by immune cells as pathogens triggering the innate immunity response [71].
Fig. (3).
Impaired mitochondrial function and deficient mitophagy promote atherosclerotic lesion formation. Multiply modified LDL particles being accumulated and then internalized by macrophages are capable to alter mitochondrial function which ultimately leads to the formation of atherosclerotic plaques. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
9. Mitochondrion as a potential therapeutic target
Currently, mitochondria appear a promising target for developing novel treatments for several chronic human disorders. A variety of drugs, including some that have already been on the market to treat other conditions, have antioxidant properties, while others work as modifiers of mitochondrial dynamics. The list of currently tested therapeutic strategies is constantly growing. Despite the increasing number of potential pharmaceutical agents potentially effective against mitochondria-related diseases, the development and, more importantly, proper testing of new therapeutic strategies is a very slow process because of the complicity and poor understanding of the mechanisms of these disorders [72].
The use of antioxidants has long been regarded as a promising and affordable strategy. Vitamins C and E are well-known for their anti-oxidant properties, which makes these molecules potentially effective against a large group of chronic diseases [73]. However, large clinical trials investigating the efficacy of vitamins against mitochondrial dysfunction failed to demonstrate convincing results. That could possibly be explained by the genetic variability between patients and by the fact that mitochondria absorb only a small percent of these anti-oxidants. Another possible reason is that there are still no suitable methods to measure the level of oxidative stress in the body [74]. Coenzyme Q10 was shown to be a safe treatment option for mitochondrial dysfunction [75].
MitoQ represents an antioxidant that is capable to target mitochondria selectively because of the conjugation with lipophilic molecules [76]. Other examples of such molecules are EUK-8 and EUK-134 [77, 78]. Finally, the SkQ family of mitochondria-targeted antioxidants has shown neuroprotective properties through lowering of trauma-induced neurological deficit in animals and the prevention of amyloid-β-induced impairment of long-term potentiation in rat hippocampal slices [79].
Photo-theranostics is a novel approach for diagnostics and therapy of various pathological conditions based on photo-sensibilization [80]. This approach allows inducing apoptotic cell death selectively in cells and tissues affected by pathological changes. Selective accumulation of the photo-sensitizing agent in cellular organelles allows targeting the pathologies at the sub-cellular level. For mitochondria, several molecules that selectively accumulate in the organelle are already known and can be harnessed for therapeutic purposes. One of them is protoporphyrin IX (PpIX), which is already used in clinical practice [81]. The molecule is produced from a prodrug 5-aminolevulinic acid (5-ALC) in the mitochondria. The addition of exogenous 5-ALC leads to excessive formation and accumulation of PpIX, which depends on cellular metabolic activity. A study conducted on cybrid cell lines bearing pro-atherogenic mtDNA variants evaluated the fluorescence intensity of mitochondrial dye MitoTracker™ Orange CMTMRos [82]. Accumulation of PpIX varied significantly between cells, and cybrid lines accumulated more of PpIX as compared to control monocytes. Accumulation of PpIX correlated with mitochondrial membrane potential. It is likely that by careful adjustment of laser intensity it is possible to induce photodynamic destruction of defective mitochondria accumulating PpIX while keeping functional mitochondria intact. More studies are needed, however, to explore this possibility.
Conclusion
Mitochondrion emerges as a potent disease modifier and potential therapeutic target in several chronic human diseases, including atherosclerosis. One challenge in leveraging the diagnostic and therapeutic potential of mitochondria is the dynamism of the mitochondrial genome. Advances made in sequencing techniques and the development of hybrid technology allowed identifying and studying a panel of mtDNA mutations associated with the disease. These mutations, together with mtDNA deletions, can possibly be used as markers of the pathology. Another challenge is developing therapeutic approaches directly targeting oxidative stress at the level of individual organelles. Several mitochondria-targeting antioxidants have been developed to date, and are being tested in preclinical studies.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
This work was supported by the Russian Science Foundation (Grant # 19-15-00010).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 2.Marulanda-Londoño E., Chaturvedi S. Stroke due to large vessel atherosclerosis: Five new things. Neurol. Clin. Pract. 2016;6(3):252–258. doi: 10.1212/CPJ.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dima-Cozma C. Atherosclerosis in the young adult: Fewer hypotheses, more facts. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2016;120(4):768–776. [PubMed] [Google Scholar]
- 4.Orekhov A.N., Ivanova E.A. Introduction of the special issue “Atherosclerosis and related diseases”. Vessel Plus. 2017;1:163–165. doi: 10.20517/2574-1209.2017.33. [DOI] [Google Scholar]
- 5.Katsouras C.S., Baltogiannis G.G., Naka K.K., Roukos D.H., Michalis L.K. Decoding coronary artery disease: somatic mosaicism and genomics for personal and population risk prediction. Biomarkers Med. 2013;7(2):189–192. doi: 10.2217/bmm.13.4. [DOI] [PubMed] [Google Scholar]
- 6.Sazonova M.A., Sinyov V.V., Barinova V.A., Ryzhkova A.I., Zhelankin A.V., Postnov A.Y., Sobenin I.A., Bobryshev Y.V., Orekhov A.N. Mosaicism of mitochondrial genetic variation in atherosclerotic lesions of the human aorta. BioMed Res. Int. 2015;2015:825468. doi: 10.1155/2015/825468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Bliek A.M., Sedensky M.M., Morgan P.G. Cell Biology of the Mitochondrion. Genetics. 2017;207(3):843–871. doi: 10.1534/genetics.117.300262. [Review. Erratum in: Genetics. 2018, 208. ]. [4]. [:1673. PubMed PMID: 29097398]. [. http://dx.doi.org/10.1534/genetics.117.300262 PMID: 29097398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aliev G., Obrenovich M.E., Tabrez S., Jabir N.R., Reddy V.P., Li Y., Burnstock G., Cacabelos R., Kamal M.A. Link between cancer and Alzheimer disease via oxidative stress induced by nitric oxide-dependent mitochondrial DNA over proliferation and deletion. Oxid. Med. Cell. Longev. 2013;••• doi: 10.1155/2013/962984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truban D., Hou X., Caulfield T.R., Fiesel F.C., Springer W. PINK1, Parkin, and Mitochondrial Quality Control: What can we Learn about Parkinson’s Disease pathobiology? J. Parkinsons Dis. 2017;7(1):13–29. doi: 10.3233/JPD-160989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gowdar S., Syal S., Chhabra L. Probable protective role of diabetes mellitus in takotsubo cardiomyopathy: a review. Vessel Plus. 2017;1:129–136. doi: 10.20517/2574-1209.2017.12. [DOI] [Google Scholar]
- 11.Stefano G.B., Bjenning C., Wang F., Wang N., Kream R.M. mitochondrial heteroplasmy. Adv. Exp. Med. Biol. 2017;982:577–594. doi: 10.1007/978-3-319-55330-6_30. [DOI] [PubMed] [Google Scholar]
- 12.Sazonova M.A., Sinyov V.V., Ryzhkova A.I., Galitsyna E.V., Melnichenko AA, Postnov AY, Orekhov A.N., Sobenin I.A. Cybrid models of pathological cell processes in different diseases. 2018. [DOI] [PMC free article] [PubMed]
- 13.Ruparelia N., Chai J.T., Fisher E.A., Choudhury R.P. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat. Rev. Cardiol. 2017;14(3):133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caja S., Enríquez J.A. Mitochondria in endothelial cells: Sensors and integrators of environmental cues. Redox Biol. 2017;•••:821–827. doi: 10.1016/j.redox.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimbrone M.A. Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 17.De Bock K., Georgiadou M., Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18(5):634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Golub A.S., Song B.K., Pittman R.N. The rate of O2 loss from mesenteric arterioles is not unusually high. Am. J. Physiol. Heart Circ. Physiol. 2011;301(3):H737–H745. doi: 10.1152/ajpheart.00353.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslip M., Dostanic I., Huang Y., Zhang Y., Russell K.S., Jurczak M.J., Mannam P., Giordano F., Erzurum S.C., Lee P.J. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol. 2015;35(5):1166–1178. doi: 10.1161/ATVBAHA.114.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widlansky M.E., Gutterman D.D. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid. Redox Signal. 2011;15(6):1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F., Haigh S., Barman S., Fulton D.J. From form to function: the role of Nox4 in the cardiovascular system. Front. Physiol. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chistiakov D.A., Melnichenko A.A., Myasoedova V.A., Grechko A.V., Orekhov A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. (Berl.) 2017;95(11):1153–1165. doi: 10.1007/s00109-017-1575-8. [DOI] [PubMed] [Google Scholar]
- 23.Kieser K.J., Kagan J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017;17(6):376–390. doi: 10.1038/nri.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tur J., Vico T., Lloberas J., Zorzano A., Celada A. Macrophages and mitochondria: A critical interplay between metabolism, signaling, and the functional activity. Adv. Immunol. 2017;•••:1–36. doi: 10.1016/bs.ai.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Liu P.S., Ho P.C. Mitochondria: A master regulator in macrophage and T cell immunity. Mitochondrion. 2018;••• doi: 10.1016/j.mito.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Sinyov V.V., Sazonova M.A., Ryzhkova A.I., Galitsyna E.V., Melnichenko A.A., Postnov A.Y., Orekhov A.N., Grechko A.V., Sobenin I.A. Potential use of buccal epithelium for genetic diagnosis of atherosclerosis using mtDNA mutations. Vessel Plus. 2017;1:145–150. doi: 10.20517/2574-1209.2016.04. [DOI] [Google Scholar]
- 27.Zhong Z., Umemura A., Sanchez-Lopez E., Liang S., Shalapour S., Wong J., He F., Boassa D., Perkins G., Ali S.R., McGeough M.D., Ellisman M.H., Seki E., Gustafsson A.B., Hoffman H.M., Diaz-Meco M.T., Moscat J., Karin M. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finucane O.M., Sugrue J., Rubio-Araiz A., Guillot-Sestier M.V., Lynch M.A. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci. Rep. 2019;9(1):4034. doi: 10.1038/s41598-019-40619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill L.A., Pearce E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nonnenmacher Y., Hiller K. Biochemistry of proinflammatory macrophage activation. Cell. Mol. Life Sci. 2018;75(12):2093–2109. doi: 10.1007/s00018-018-2784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S.C., Smith A.M., Everts B., Colonna M., Pearce E.L., Schilling J.D., Pearce E.J. Metabolic reprogramming mediated by the mtorc2-irf4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45(4):817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bories G.F.P., Leitinger N. Macrophage metabolism in atherosclerosis. FEBS Lett. 2017;591(19):3042–3060. doi: 10.1002/1873-3468.12786. [DOI] [PubMed] [Google Scholar]
- 33.Andreeva E.R., Pugach I.M., Gordon D., Orekhov A.N. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30(1):127–135. doi: 10.1016/S0040-8166(98)80014-1. [DOI] [PubMed] [Google Scholar]
- 34.Orekhov A.N., Bobryshev Y.V., Chistiakov D.A. The complexity of cell composition of the intima of large arteries: focus on pericyte-like cells. Cardiovasc. Res. 2014;103(4):438–451. doi: 10.1093/cvr/cvu168. [DOI] [PubMed] [Google Scholar]
- 35.Vásquez-Trincado C., García-Carvajal I., Pennanen C., Parra V., Hill J.A., Rothermel B.A., Lavandero S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016;594(3):509–525. doi: 10.1113/JP271301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summerhill V., Orekhov A. Pericytes in Atherosclerosis. Adv. Exp. Med. Biol. 2019;1147:279–297. doi: 10.1007/978-3-030-16908-4_13. [DOI] [PubMed] [Google Scholar]
- 37.Price T.O., Sheibani N., Shah G.N. Regulation of high glucose-induced apoptosis of brain pericytes by mitochondrial CA VA: A specific target for prevention of diabetic cerebrovascular pathology. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863(4):929–935. doi: 10.1016/j.bbadis.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalmers S., Saunter C.D., Girkin J.M., McCarron J.G. Age decreases mitochondrial motility and increases mitochondrial size in vascular smooth muscle. J. Physiol. 2016;594(15):4283–4295. doi: 10.1113/JP271942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsboom G., Toth P.T., Ryan J.J., Hong Z., Wu X., Fang Y.H., Thenappan T., Piao L., Zhang H.J., Pogoriler J., Chen Y., Morrow E., Weir E.K., Rehman J., Archer S.L. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012;110(11):1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salabei J.K., Hill B.G. Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol. 2013;1(1):542–551. doi: 10.1016/j.redox.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;•••:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 42.Kattoor A.J., Pothineni N.V.K., Palagiri D., Mehta J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017;19(11):42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 43.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120(4) doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 44.Aliev G., Gasimov E., Obrenovich M.E., Fischbach K., Shenk J.C., Smith M.A., Perry G. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels: implication in the pathogenesis of Alzheimer’s disease. Vasc. Health Risk Manag. 2008;4(3):721–730. doi: 10.2147/VHRM.S2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aliev G., Smith M.A., de la Torre J.C., Perry G. Discuss how the oxidative stress indices and initiates mtDNA overproliferation that when become as a de compensatory stages mtDNA deletion is occurs. Mitochondrion. 2004;4(5-6):649–663. doi: 10.1016/j.mito.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Nicolson G.L. Mitochondrial Dysfunction and chronic disease: treatment with natural supplements. Integr. Med. (Encinitas) 2014;13(4):35–43. [PMC free article] [PubMed] [Google Scholar]
- 47.Twig G., Shirihai O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011;14(10):1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chistiakov D.A., Shkurat T.P., Melnichenko A.A., Grechko A.V., Orekhov A.N. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann. Med. 2018;20(2):121–127. doi: 10.1080/07853890.2017.1417631. [DOI] [PubMed] [Google Scholar]
- 49.Ballinger S.W., Patterson C., Knight-Lozano C.A., Burow D.L., Conklin C.A., Hu Z., Reuf J., Horaist C., Lebovitz R., Hunter G.C., McIntyre K., Runge M.S. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106(5):544–549. doi: 10.1161/01.CIR.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 50.Yu E., Calvert P.A., Mercer J.R., Harrison J., Baker L., Figg N.L., Kumar S., Wang J.C., Hurst L.A., Obaid D.R., Logan A., West N.E., Clarke M.C., Vidal-Puig A., Murphy M.P., Bennett M.R. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128(7):702–712. doi: 10.1161/CIRCULATIONAHA.113.002271. [DOI] [PubMed] [Google Scholar]
- 51.Alipov V.I., Sukhorukov V.N., Karagodin V.P., Grechko A.V., Orekhov A.N. Chemical composition of circulating native and desialylated low density lipoprotein: what is the difference? Vessel Plus. 2017;1:107–115. doi: 10.20517/2574-1209.2017.20. [DOI] [Google Scholar]
- 52.Chistiakov D.A. The complexity of cell composition of the intima of large arteries: focus on pericyte-like cells. Cardiovasc. Res. 2014;103(4):438–451. doi: 10.1093/cvr/cvu168. [DOI] [PubMed] [Google Scholar]
- 53.Xu Q., Yuan F., Shen X., Wen H., Li W., Cheng B., Wu J. Polymorphisms of C242T and A640G in CYBA gene and the risk of coronary artery disease: a meta-analysis. PLoS One. 2014 doi: 10.1371/journal.pone.0084251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimoto H., Taguchi J., Imai Y., Ayabe S., Hashimoto H., Kobayashi H., Ogasawara K., Aizawa T., Yamakado M., Nagai R., Ohno M. Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoprotein-induced apoptosis of macrophages and coronary artery disease. Eur. Heart J. 2008;29(10):1267–1274. doi: 10.1093/eurheartj/ehm500. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J.X., Wang Z.M., Zhang J.J., Zhu L.L., Gao X.F., Chen S.L. Association of glutathione peroxidase-1 (GPx-1) rs1050450 Pro198Leu and Pro197Leu polymorphisms with cardiovascular risk: a meta-analysis of observational studies. J. Geriatr. Cardiol. 2014;11(2):141–150. doi: 10.3969/j.issn.1671-5411.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boya P., Reggiori F., Codogno P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013;15(7):713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swiader A., Nahapetyan H., Faccini J., D’Angelo R., Mucher E., Elbaz M., Boya P., Vindis C. Mitophagy acts as a safeguard mechanism against human vascular smooth muscle cell apoptosis induced by atherogenic lipids. Oncotarget. 2016;7(20):28821–28835. doi: 10.18632/oncotarget.8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grootaert M.O.J., Roth L., Schrijvers D.M., De Meyer G.R.Y., Martinet W. Defective autophagy in atherosclerosis: To die or to senesce? Oxid. Med. Cell. Longev. 2018;2018:7687083. doi: 10.1155/2018/7687083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao X., Sluimer J.C., Wang Y., Subramanian M., Brown K., Pattison J.S., Robbins J., Martinez J., Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15(4):545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng W., Cai G., Xia Y., Chen J., Wu P., Wang Z., Li G., Wei D. Mitochondrial Dysfunction in Atherosclerosis. DNA Cell Biol. 2019;38(7):597–606. doi: 10.1089/dna.2018.4552. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z., Meng P., Han Y., Shen C., Li B., Hakim M.A., Zhang X., Lu Q., Rong M., Lai R. Mitochondrial DNA-LL-37 Complex promotes atherosclerosis by escaping from autophagic recognition. Immunity. 2015;43(6):1137–1147. doi: 10.1016/j.immuni.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Sazonova M.A., Sinyov V.V., Ryzhkova A.I., Galitsyna E.V., Khasanova Z.B., Postnov A.Y., Yarygina E.I., Orekhov A.N., Sobenin I.A. Role of mitochondrial genome mutations in pathogenesis of carotid atherosclerosis. Oxid. Med. Cell. Longev. 2017;2017:6934394. doi: 10.1155/2017/6934394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sobenin I.A., Zhelankin A.V., Mitrofanov K.Y., Sinyov V.V., Sazonova M.A., Postnov A.Y., Orekhov A.N. Mutations of mitochondrial DNA in atherosclerosis and atherosclerosis-related diseases. Curr. Pharm. Des. 2015;21(9):1158–1163. doi: 10.2174/1381612820666141013133000. [DOI] [PubMed] [Google Scholar]
- 65.Sobenin I.A., Sazonova M.A., Postnov A.Y., Salonen J.T., Bobryshev Y.V., Orekhov A.N. Association of mitochondrial genetic variation with carotid atherosclerosis. PLoS One. 2013;8(7):e68070. doi: 10.1371/journal.pone.0068070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orekhov A.N., Zhelankin A.V., Kolmychkova K.I., Mitrofanov K.Y., Kubekina M.V., Ivanova E.A., Sobenin I.A. Susceptibility of monocytes to activation correlates with atherogenic mitochondrial DNA mutations. Exp. Mol. Pathol. 2015;99(3):672–676. doi: 10.1016/j.yexmp.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Warren L., Bryder D., Weissman I.L., Quake S.R. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc. Natl. Acad. Sci. USA. 2006;103(47):17807–17812. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tertov V.V., Sobenin I.A., Gabbasov Z.A., Popov E.G., Jaakkola O., Solakivi T., Nikkari T., Smirnov V.N., Orekhov A.N. Multiple-modified desialylated low density lipoproteins that cause intracellular lipid accumulation. Isolation, fractionation and characterization. Lab. Invest. 1992;67(5):665–675. [PubMed] [Google Scholar]
- 69.Tertov V.V., Sobenin I.A., Gabbasov Z.A., Popov E.G., Orekhov A.N. Lipoprotein aggregation as an essential condition of intracellular lipid accumulation caused by modified low density lipoproteins. Biochem. Biophys. Res. Commun. 1989;163(1):489–494. doi: 10.1016/0006-291X(89)92163-3. [DOI] [PubMed] [Google Scholar]
- 70.Orekhov A.N., Nikiforov N.G., Elizova N.V., Korobov G.A., Aladinskaya A.V., Sobenin I.A., Bobryshev Y.V. Tumor necrosis factor-α and c-c motif chemokine ligand 18 associate with atherosclerotic lipid accumulation In situ and In vitro. Curr. Pharm. Des. 2018;24(24):2883–2889. doi: 10.2174/1381612824666180911120726. [DOI] [PubMed] [Google Scholar]
- 71.Meyer A., Laverny G., Bernardi L., Charles A.L., Alsaleh G., Pottecher J., Sibilia J., Geny B. Mitochondria: An organelle of bacterial origin controlling inflammation. Front. Immunol. 2018;9:536. doi: 10.3389/fimmu.2018.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dominic E.A., Ramezani A., Anker S.D., Verma M., Mehta N., Rao M. Mitochondrial cytopathies and cardiovascular disease. Heart. 2014;100(8):611–618. doi: 10.1136/heartjnl-2013-304657. [DOI] [PubMed] [Google Scholar]
- 73.Tousoulis D., Antoniades C., Vasiliadou C., Kourtellaris P., Koniari K., Marinou K., Charakida M., Ntarladimas I., Siasos G., Stefanadis C. Effects of atorvastatin and vitamin C on forearm hyperaemic blood flow, asymmentrical dimethylarginine levels and the inflammatory process in patients with type 2 diabetes mellitus. Heart. 2007;93(2):244–246. doi: 10.1136/hrt.2006.093112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michels A.J., Frei B. Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients. 2013;5(12):5161–5192. doi: 10.3390/nu5125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pepe S., Marasco S.F., Haas S.J., Sheeran F.L., Krum H., Rosenfeldt F.L. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;••• doi: 10.1016/j.mito.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Chen S., Wang Y., Zhang H., Chen R., Lv F., Li Z., Jiang T., Lin D., Zhang H., Yang L., Kong X. The antioxidant mitoq protects against cse-induced endothelial barrier injury and inflammation by inhibiting ros and autophagy in human umbilical vein endothelial cells. Int. J. Biol. Sci. 2019;15(7):1440–1451. doi: 10.7150/ijbs.30193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cochemé H.M., Murphy M.P., Dominiczak A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54(2):322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 78.Siasos G., Tsigkou V., Kosmopoulos M., Theodosiadis D., Simantiris S., Tagkou N.M., Tsimpiktsioglou A., Stampouloglou P.K., Oikonomou E., Mourouzis K., Philippou A., Vavuranakis M., Stefanadis C., Tousoulis D., Papavassiliou A.G. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018;6(12):256. doi: 10.21037/atm.2018.06.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isaev N.K., Stelmashook E.V., Genrikhs E.E., Korshunova G.A., Sumbatyan N.V. Kapkaeva –M.R.; Skulachev. V.P. Neuroprotective properties of mitochondria-targeted antioxidants of the SkQ-type. Rev. Neurosci. 2016;27(8):849–855. doi: 10.1515/revneuro-2016-0036. [DOI] [PubMed] [Google Scholar]
- 80.Ng K.K., Zheng G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem. Rev. 2015;115(19):11012–11042. doi: 10.1021/acs.chemrev.5b00140. [DOI] [PubMed] [Google Scholar]
- 81.Maytin E.V., Anand S., Riha M., Lohser S., Tellez A., Ishak R., Karpinski L., Sot J., Hu B., Denisyuk A., Davis S.C., Kyei A., Vidimos A. 5-Fluoruracil enhances protoporphyrin IX accumulation and lesion clearance during photodynamic therapy of actinic keratoses: A mechanism-based clinical trial. Clin. Cancer Res. 2018;24(13):3026–3035. doi: 10.1158/1078-0432.CCR-17-2020. [DOI] [PubMed] [Google Scholar]
- 82.Ryabova A.V., Romanishkin I.D., Skobeltsin A.S., Moskalev A.S., Makarov V.I., Loschenov V.B., Nikiforov N.G., Sobenin I.A., Orekhov A.N. Subcellular anti-atherosclerotic therapy. Vessel Plus. 2019;3:17. [Google Scholar]