Abstract
Modern times have seen depression and cardiovascular disease (CVD) become notorious public health concerns, corresponding to alarming proportions of morbidity, mortality, decreased quality of life, and economic costs. Expanding comprehension of the pathogenesis of depression as an immunometabolic disorder has identified numerous pathophysiologic phenomena in common with CVD, including chronic inflammation, insulin resistance, and oxidative stress. These shared components could be exploited to offer improved alternatives in the joint management of these conditions. Abundant preclinical and clinical data on the impact of established treatments for CVD in the management of depression have allowed for potential candidates to be proposed for the joint management of depression and CVD as immunometabolic disorders. However, a large proportion of the clinical investigation currently available exhibits marked methodological flaws which preclude the formulation of concrete recommendations in many cases. This situation may be a reflection of pervasive problems present in clinical research in psychiatry, especially pertaining to study homogeneity. Therefore, further high-quality research is essential in the future in this regard.
Keywords: Depression, cardiovascular disease, chronic inflammation, insulin resistance, oxidative stress, metabolism
1. INTRODUCTION
In recent years, depression has become one of the most prominent conditions in daily clinical practice and is currently recognized as the leading cause of disability globally, amounting to extremely high direct and indirect financial costs, as well as representing a severe detriment to the quality of life [1]. Interestingly, depression stands alongside cardiovascular disease (CVD) as some of the most prominent problems in public health at present, with CVD being the first cause of mortality and morbidity worldwide [2]. The parallels in the epidemiology of these conditions have sparked abundant research on their interrelated pathophysiology and clinical management.
Although depression is notorious for frequently co-occurring with a myriad of medical comorbidities [3], the link with CVD appears to be particularly powerful, with these entities sharing various risk factors such as chronic stress, physical inactivity, westernized dietary patterns and various metabolic alterations [4] and depression increasing CVD-related mortality by up to 60% [5]. Moreover, they share several pathophysiologic components, including chronic low-grade inflammation, insulin resistance (IR), and dysthrombogenesis [6]. The presence of these shared elements blurs the traditional distinction between mental and physical illness, and could significantly change the management standards of depression and CVD by posing the question: How can the treatment of these conditions be integrated on the basis of their common pathophysiologic components? This review aims to summarize current views on depression as an immunometabolic disorder and its link with CVD, as well as potential novel pharmacological options for their joint management. A literature search was performed on PubMed, EMBASE, Scopus, ISI Web of Science, and Google Scholar databases, from inception to January 2020.
2. REVISITING DEPRESSION AS AN IMMUNO-METABOLIC DISORDER
2.1. From the Sparks to the Flame: Emphasis on Chronic Inflammation
Depression was historically conceived as an illness limited to the brain-mind. However, in recent decades, accumulating evidence has propelled a paradigm shift, where depression is now understood as a systemic disease, with the brain-mind and the body sharing a bidirectional relationship [7]. Chronic inflammation (CI) has been identified as a key common component in depression and multiple medical conditions, including CVD, endocrine-metabolic disorders, autoimmune disorders, cancer, and many others [8-11]. Although inflammation is a key physiologic mechanism that aims to preserve homeostasis in the face of injury, it comes at the cost of profound disturbances in the functionality of target tissues [12]. Classic examples of these include vascular changes to allow exudate formation, such as increased endothelial adhesiveness and permeability in response to cytokine signaling [13]. Nonetheless, all tissues are vulnerable to inflammation-induced changes, with each displaying distinct patterns of dysfunction. Thus, systemic CI entails the dysregulation of multiple organ systems [14].
The term “neuroinflammation” has been coined to describe CI in the central nervous system (CNS), which involves activation of microglia, astrocytes and oligodendrocytes, with the release of cytokines, chemokines, acute-phase reactants, and other mediators [15]. Although neuroinflammation may be beneficial in the acute setting, for example, in the limitation of CNS infections; its persistence results in hyperactivation of microglia and neurotoxicity [16, 17]. As with all forms of CI, it is hypothesized to stem from the conflation of extrinsic and intrinsic proinflammatory factors (Fig. 1) [18, 19].
Fig. (1).
Intrinsic and extrinsic etiologic factors of neuroinflammation. The additive and synergic effects of various intrinsic and extrinsic factors results in chronic inflammation. Neuroinflammation in particular is associated with depression and other neuropsychiatric disorders. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A great body of research has documented the presence of elevated circulating biomarkers of inflammation in participants with depression, including IL-1β, IL-6, IFNγ, TNFα and acute-phase reactants, especially high-sensitivity C-Reactive Protein (hs-CRP), among others [20, 21]. Neuroinflammation and chronic stress are both powerful inducers of the neuroendocrine changes typical of depression, especially sustained activation of sympathetic autonomous signaling and the hypothalamus-pituitary-adrenal axis (HPAA) [22]. Notably, in non-depressed participants, acute and chronic stress, as well as increased inflammatory biomarkers have been associated with “sickness behavior”, which features many depressive characteristics, such as low mood, anhedonia, fatigue, and feeding and sleep disorders [18,23]. Indeed, neuroinflammation can significantly disrupt the metabolism and signaling of monoamines serotonin, norepinephrine and dopamine the central neurotransmitters involved in the neurobiology of depression [24].
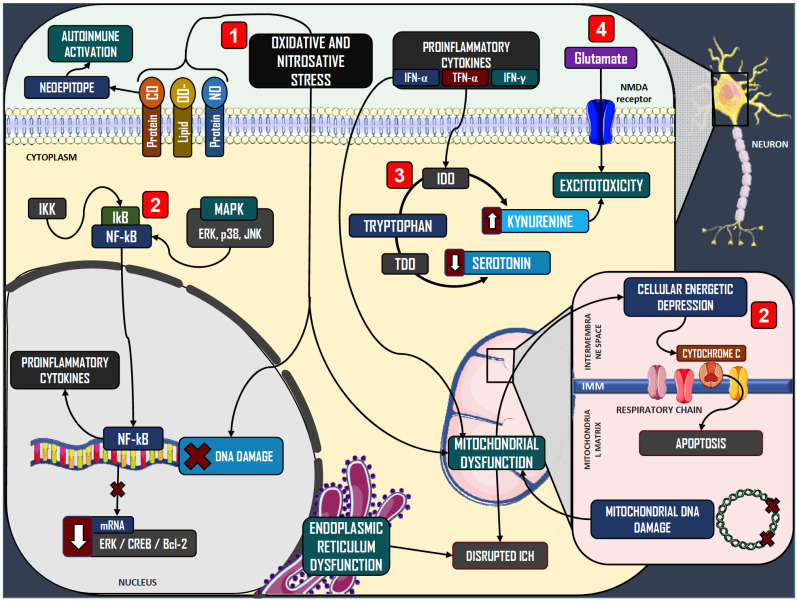
At the molecular level, oxidative stress (OS), alterations in intraneuronal signaling, disruptions in monoamine metabolism and excitotoxicity are the major pathophysiologic phenomena induced by CI in the context of depression (Fig. 2). Oxidation of inflammatory mediators such as arachidonic acid and its precursor, linoleic acid, entails increased production of reactive oxygen species (ROS) [25-27], which can cause membrane lipid peroxidation, DNA damage and protein carbonylation in neurons [28]. Patients with depression may be especially vulnerable to OS, with lower levels of antioxidant molecules such as glutathione, coenzyme Q10, and possibly zinc, vitamin A and vitamin D; as well as decreased expression of superoxide dismutase and glutathione peroxidase [29, 30]. CI also involves hyperactivity of inducible nitric oxide synthase (iNOS) with increased production of nitric oxide and nitrosative stress (NS) by nitrosylation of proteins, membrane lipids and DNA [29, 31].
Fig. (2).
Molecular events associated with chronic inflammation in neurons. In neurons, chronic inflammation is associated with four major pathophysiologic components: 1) Increased oxidative and nitrosative stress, which is associated with protein carbonylation and nitrosylation as well as lipid peroxidation, which results in formation of neoepitopes and favors autoimmunity. Oxidative stress and nitrosative stress also damages nuclear DNA and induces mitochondrial dysfunction. 2) Activation of stress-related intracellular signaling pathways, which promote apoptosis and disruptions in intracellular calcium homeostasis, worsen inflammation and oxidative stress, and impair neurotrophic signals. 3) Disruptions in monoamine metabolism, through activation of IDO, leading to kynurenine synthesis, which decreases serotonin availability and promotes excitotoxicity. 4) Excitotoxicity, promoted by kynurenine, disrupted calcium metabolism, and increased glutamate signaling from glial cells. Abbreviations: Protein-CO: Protein carbonylation. Protein-NO: Protein nitrosylation. Lipid-OO-: Lipid peroxidation. IKK: I kappa B Kinase. IkB: inhibitor kB protein. NFkB: Nuclear factor kappa B. MAPK: Mitogen-activated protein kinases. ERK: Extracellular signal-regulated kinase. JNK: Jun N-terminal kinase. CREB: Cyclic AMP responsive element binding protein. Bcl-2: B-cell lymphoma-2. IDO: Indoleamine 2,3-dioxygenase. TDO: Tryptophan 2,3-dioxygenase. ICH: Intracellular calcium homeostasis. IMM: Internal mitochondrial membrane. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Proinflammatory cytokines, OS and NS act as alarm signals and can activate cellular stress-related kinases such as JNK, p38 and IKK-kinase. These promote the nuclear translocation of NF-κB, a potent proinflammatory transcription factor, thus worsening CI in a positive feedback loop [32-34]. Furthermore, patients with depression have been described to exhibit significantly higher levels of IgM antibodies against neoepitopes produced from CI, OS and NS [35]. Neuronal stress also potentiates signaling of the ERK/CREB/Bcl-2 pathway, which promotes apoptosis, alterations in intracellular calcium traffic, and release of cytochrome C [36-38]. Neurons in the prefrontal cortex, anterior cingulate cortex, amygdala and hippocampus may be particularly susceptible to these changes in depression [38-42]. Guan et al. reported prenatally stressed offspring rats to display the decreased expression of these proteins in the prefrontal cortex and hippocampus, in association with depression-like behavior [38]. Conversely, patients with depression may have impaired activity of Nrf-2, a transcription factor that promotes the expression of cytoprotective enzymes such as thioredoxin reductase, glutathione peroxidase, glutathione-S-transferase, haeme oxygenases, and others [43-45]. Indeed, imaging and postmortem studies have identified neuronal and glial modifications, as well as volumetric changes in the hippocampus, amygdala, basal nuclei, the prefrontal cortex, and the anterior cingulate cortex, in association with cognitive impairment [46-48]. This structural neurodegeneration is thought to be due to decreased signaling by neuroprotective mediators, such as the brain-derived neurotrophic factor (BDNF) and fibroblast growth factor (FGF) [49-51]; which in turn are disrupted by the damaging environment promoted by CI and OS [52-54]. These alterations in neurotrophic signaling may be reversible by antidepressant treatment [55, 56]; and BDNF levels have been observed to rise in parallel with the improvement of depressive symptoms in a clinical study by Piccinni et al. [57].
Finally, in neurons, IFN-α, IFN-γ and TNF-α can activate indoleamine 2,3-dioxygenase (IDO), which synthesizes kynurenine (KYN) from tryptophan, the precursor to serotonin, thus implicating decreased production of this monoamine. In addition, the metabolism of kynurenine yields quinolinic acid and kynurenic acid (KA), both of which promote excitotoxicity by binding to NMDA receptors and promoting glutamate release in glial cells [58]. Furthermore, KA may impair dopamine release [59,60]. In patients with hepatitis C undergoing therapy with IFN-α for 24 weeks, this treatment was associated not only with increased depressive symptoms, but also increased KYN/tryptophan ratios, reflecting higher IDO activity, as well as increased KYN/KA ratios, corresponding to the degree of neurotoxicity involved [61].
2.2. Feeding the Fire: Proinflammatory Neuroendocrine Signaling
Certainly, the impact of CI on depression is hardly limited to changes in the brain; it is widely recognized as a pivotal pathophysiologic component in atherosclerosis, by potentiating vascular chemotaxis, release of growth factors, and proliferation of vascular smooth muscle cells, among other mechanisms. This underlines the shared mechanisms underlying the pathogenesis of depression and CVD [62]. Participants with depression also appear to have increased expression of VCAM-1 and other vascular adhesion and thrombogenic molecules in endothelial cells [63-67]. Hyperactivation of the HPAA and hypercortisolemia have been related to the downregulation of endothelial nitric oxide synthase (eNOS), impairing relaxation of vascular walls [68, 69]. Other possible alterations of vascular tone in depression include decreased vagal tone with sympathetic hyperactivation, with increased non-selective α-adrenergic and β-adrenergic activity in the cardiovascular system [70, 71]. Platelet dysfunction has also been described in depression, including augmented intraplatelet traffic of calcium and other disruptions in signaling, upregulation of α-adrenergic and 5HT2A receptors, P-selectin, glycoprotein IIb/IIIa and β-thromboglobulin, and downregulation of serotonin transporters [72-74]. Indicators of endothelial dysfunction in depressed patients may improve with antidepressant therapy. López-Vílchez et al. found participants with depression to display higher levels of circulating endothelial cells, VCAM-1 and soluble von Willebrand factor, which decreased gradually along 24 weeks in treatment with escitalopram [63].
In addition, IR is a pivotal mediator between CI, CVD and depression. IR, defined as decreased peripheral tissue responsivity to insulin signaling [75], is promoted by proinflammatory mediators, particularly by inducing serine phosphorylation of IRS-1 [76], as well as ectopic fat deposition in the liver and muscle tissue [77]. Typical hormonal changes of depression, such as increased catecholamine and glucocorticoid signaling, can also promote IR. This impact may be most marked regarding the cognitive symptoms of depression, as described by Austin et al. in a cohort of 328 patients [78]. This reduced sensitivity entails hyperinsulinemia, which in turn yields deleterious effects on all organ systems, and predisposes to numerous cardiometabolic disturbances such as obesity, hyperglycemia, dyslipidemia and hypertension, among others [79]. In turn, these are all promoters of CI, thus constituting a vicious cycle involving depression, CI, and IR (Fig. 3) [80].
Fig. (3).
Relationship between chronic inflammation, insulin resistance and depression. Chronic inflammation, insulin resistance and depression constitute a positive feedback loop, each worsening each other through diverse disruptions in peripheral tissues and various cardiometabolic disturbances. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Obesity is a powerful enhancer of IR and all its associated disturbances. Adipose tissue has been recognized as an immunologically active organ, through the secretion of proinflammatory cytokines and adipokines: leptin, resistin and adiponectin [81]. Leptin plays a physiological role, where it promotes satiety in accordance with increasing adipose tissue deposits. However, in obesity, leptin resistance is a frequent finding, favoring an energetic imbalance towards excess [82]. Leptin also intervenes in the pathogenesis of depression by potentiating HHPA activation [83] and promoting the expression of IL-6 and TNFα [84]. Resistin and adiponectin display opposite effects regarding CI and energetic homeostasis, with the former being proinflammatory and upregulated in obesity, and the latter being anti-inflammatory and upregulated by weight loss, with decreased expression in obesity [85]. Although the role of resistin in depression remains obscure; adiponectin expression has been found to be downregulated by glucocorticoid signaling, which could further favor obesity and CI in depression [86]. Altered adipokine levels have been widely reported in depressed patients, especially increased leptin and decreased adiponectin [87]. These mediators have been proposed as putative biomarkers for depression, though variables such as the severity of depression and obesity may be important confounders in this context as determined in a systematic review and meta-analysis by Carvalho et al. [88]. Adipokines may be predictors of antidepressant therapy outcomes, although similar concerns remain [89]. Because IR is a natural stepping stone in the development of Type 2 Diabetes Mellitus (DM2), and owing to the added psychosocial challenges by the disease [90], it is unsurprising that the prevalence of depression is two to three times greater in diabetic patients [91]. DM2 majorly enhances all pathophysiologic components related to IR, CI, and obesity [92], which leads to potentiated neuroinflammation through the increased OS and deleterious microvascular and macrovascular changes [93]. Notoriously, brain structures involved in circuits related to suicidal behavior may be especially susceptible to damage in DM2 [91, 94, 95]. Hypertension is also closely related to IR: Angiotensin II, a key mediator in the renin-angiotensin-aldosterone system (RAAS) has been reported to modulate neuroprotection via AT2 receptors in neurons [96-100]. In addition, activation of AT1 receptor favors CI and OS by triggering the release of TNFα and other cytokines, activation of the NADPH-oxidase complex and NF-κB, and expression of iNOS and cyclooxygenase-2 (COX-2) [101-103].
The sum of these CI- and IR-related risk factors results in endothelial dysfunction [104], which has also been associated with depression. Measures of endothelial dysfunction such as intima-media thickness and flow-mediated dilation have been inversely correlated with the severity of depression [105, 106]; highlighting the progressive impact of the immunometabolic disturbances in the evolution of depression.
3. IMPACT OF ESTABLISHED TREATMENTS FOR CARDIOVASCULAR DISEASE IN THE MANAGE-MENT OF DEPRESSION
There have been significant advances in the elucidation of the mechanistic interplay between depression and CVD [4], and the effect of antidepressants on discrete cardiometabolic variables has been outlined [107]. Nevertheless, the effects of antidepressants on specific cardiovascular outcomes remain undetermined [108]. The same is true for the
effects of cardiometabolic treatments on depression [109, 110], remaining an equally provoking, yet uncertain field of research (Table 1).
Table 1.
Summary of key evidence regarding established treatments for cardiovascular disease in the management of depression.
| Class | Compounds (REF) | Methodology | Relevant Results | |||
|---|---|---|---|---|---|---|
| Non-steroidal anti-inflammatory drugs | NSAIDs, cytokine inhibitors (113) | Systematic review and meta-analysis on 14 trials (6262 participants), 10 with NSAIDs and 4 with cytokine inhibitors assessing their use for depression and depressive symptoms. | Anti-inflammatory treatment was associated with reduced depressive symptoms (SMD, -0.34; 95% CI, -0.57 to -0.11; I2=90%). This was most prominent for celecoxib (SMD, -0.29; 95% CI, -0.49 to -0.08; I2=73%) on remission (OR, 7.89; 95% CI, 2.94 to 21.17; I2=0%) and response (OR, 6.59; 95% CI, 2.24 to 19.42; I2=0%). | |||
| ASA + SSRI (117) | Pilot open-label trial which included 24 patients with major depression who had not responded to treatment during at least 4 weeks with an SSRI, and received add-on ASA 160 mg/day during 4 weeks. | Of the 21 patients who completed the study, 52.4% showed a significant response to the ASA + SSRI combination; and 82% achieved remission by the end of the study. Significant changes were observed in the HDRS ratings, with a baseline mean of 29.3±4.5 points, which decreased to 14.0±4.1 points by day 7 (P<0.0001). This trend persisted until the end of the study on day 28. | ||||
| Statins | Lovastatin + Fluoxetine (124) |
Randomized, placebo-controlled trial which included 68 patients with major depressive disorder who received up to 40 mg/day of fluoxetine + lovastatin 30 mg/day or fluoxetine + placebo for 6 weeks. | Both groups obtained a significant reduction in HDRS scores, although this was greater in the fluoxetine + lovastatin group. The fluoxetine + lovastatin group had a baseline mean HDRS score of 28.9±6.86 points, which decreased to 16.3±5.03 by week 6 (P<0.05). | |||
| Simvastatin + Fluoxetine (125) |
Double-blind, placebo-controlled trial which included 48 patients with moderate-severe depression which received fluoxetine 20-40 mg/day + simvastatin 20 mg/day or fluoxetine + placebo for 6 weeks. | Patients treated with fluoxetine + simvastatin had a significantly greater reduction in HDRS scores in comparison with the fluoxetine + placebo group. The reductions in HDRS scores for the former were of 8.04±4.09 by week 2 (P<0.01), 13.45±4.58 by week 4 (P<0.02), and 18.5±7.1 by week 6 (P<0.02). No adverse effects were reported during the study. | ||||
| Antidiabetic drugs | Various (128) |
Systematic review and meta-analysis on 19 trials (3369 participants), 9 with thiazoldinediones, 5 with metformin, 2 with thiazolidenediones against metformin, 2 with incretin-based therapies and 1 with insulin, assessing their impact on depressive symptoms. | Pioglitazone was associated with reduced depressive symptoms compared to controls (pooled effect size = -0.68 (95% C.I. -1.12 to -0.24), p = .003, Nstudies = 8, I2 = 83.2%); while metformin was compared to controls. Female sex was a predictor for improvement of depressive symptoms with pioglitazone. | |||
| Pioglitazone (131) |
Meta-analysis with 4 randomized controlled trials comprising 161 patients with a major depressive episode. | In comparison with controls, pioglitazone was associated with increased remission rates (27% versus 10%, I2=17.3%, fixed-effect model: [OR] =3.3, 95% confidence interval [95% CI; 1.4; 7.8], P=0.008). | ||||
| Metformin (138) |
Double-blind, randomized, placebo-controlled trial which included 58 patients with depression and DM2 who received metformin 1-2 g/day or placebo for 24 weeks. | Administration of metformin was associated with a decrease in MADRS (F1,112 = 26.43, p < 0.001) and HDRS-17 (F1,112 = 27.61, p < 0.001) scores compared to baseline. In addition, at week 24, patients on metformin showed a significant improvement in cognitive function; with improved WMS-R scores in the verbal memory index (F1,112 = 22.19, p < 0.001), visual memory index (F1,112 = 10.53, p < 0.01), general memory index (F1,112 = 4.27, p <0.05), attention and concentration index (F1,112 = 12.62, p < 0.01), and delayed memory index (F1,112 = 19.84, p < 0.001). | ||||
| Antihypertensive drugs | Irbesartan + Fluoxetine (145) |
Preclinical study on rats subjected to an unpredictable mild stress protocol which were treated with irbesartan 40 mg/kg and/or fluoxetine 25 mg/kg in monotherapy or combination. Behavioral responses were assessed with MFST and TST at week 6. | Treatment with Irbesartan + Fluoxetine decreased immobility time (166s, p<0.001) in the TST, whereas it increased swimming (184.16s, p<0.001) and climbing times (184.16s, p<0.001) and decreased immobility time (8.5s, p<0.001) in the MFST. | |||
| Class | Compounds (REF) | Methodology | Relevant Results | |||
| Polyunsaturated fatty acids | Omega-3 Fatty Acids (161) |
Meta-analysis which included 13 randomized, placebo-controlled trials with a total of 1233 adults with major depressive disorder who received supplemental omega-3 fatty acids. A meta-regression was performed to evaluate the effects of the supplement according to several variables. | Omega-3 fatty acids appear to ameliorate depressive symptoms in patients with MDD, especially at high doses, and in patients who receive treatment with antidepressants. The overall SMD was 0.172 (95% CI 0.018, 0.325; P=0.028) when compared with placebo. Studies on participants with MDD employing ⩾60% EPA yielded a highly significant SMD of 0.892 (95% CI 0.543, 1.241; P<0.001), compared to those with <60% EPA, which showed no effect. | |||
Abbreviations: NSAIDs: Non-steroidal anti-inflammatory drugs; ASA: Acetylsalicylic acid; SSRI: Selective serotonin reuptake inhibitor; HDRS: Hamilton depression rating scale; DM2: Type 2 diabetes mellitus; MADRS: Montgomery-Asberg depression rating scale; HDRS: Hamilton depression rating scale-17 items; WMS-R: Wechsler memory scale-revised; MFST: Modified forced swim test; TST: Tail suspension test; MDD: Major depressive disorder; EPA: Eicosapentaenoic acid.
Non-steroidal anti-inflammatory drugs (NSAIDs) have been posited as potentially useful modulators of CI in depression due to their relatively selective pharmacodynamics [111, 112]. Selective COX-2 inhibitors may be the most promising in this regard. In a systematic review and meta-analysis, celecoxib appeared to significantly decrease depressive symptoms without notable adverse effects, in contrast with other NSAIDs and cytokine inhibitors [113]. Similar findings have been supported by multiple trials [114-116]. However, different NSAIDs appear to yield different results in depression. In a pilot study on patients with treatment-resistant depression (TRD), 52.4% of participants responded positively to the coadministration of acetylsalicylic acid with a selective serotonin reuptake inhibitor (SSRI) [117]. In contrast, other studies with differing combinations of non-selective NSAIDs and SSRIs have failed to obtain similar results [118-121]. At any rate, these findings should be interpreted with caution, as the available trials were short and executed on younger participants. Indeed, the need for optimization and uniformity of trial methodology is a recurring theme in the assessment of NSAIDs and several other treatments for depression.
Empirical evidence shows that the use of statins is associated with a decreased risk of depression in adults [122]. This effect has been hypothesized to be mediated by the reduction of excitotoxicity and OS through antagonism of NMDA receptors and IDO [123]. Several small, short, placebo-controlled trials have reported improved antidepressant responses in participants treated with fluoxetine + lovastatin [124], fluoxetine + simvastatin [125], and citalopram + simvastatin [126]. Yet, again, future trials require larger samples and longer duration to better ascertain the efficacy of statins as antidepressant adjuvants. Clinical research on other hypolipidemic drugs in depression is scarce, and preclinical findings seem discouraging [127].
A variety of antidiabetic drugs have also been evaluated in depression [128]. Most research has focused on thiazolidinediones, which have powerful anti-inflammatory activity via activation of PPAR-γ and downregulation of eNOS [129] and have shown antidepressant activity in rat and mouse models [130]. In a meta-analysis, these drugs displayed a pooled effect size of −0.68 (95% C.I. −1.12 to −0.24) for symptom amelioration in depression [128]; and another supports the role of pioglitazone in improving the probability of remission [131]. Indeed, numerous studies have reported favorable results for the use of pioglitazone as an adjuvant to antidepressants [132-134]. Indeed, there is evidence that the antidepressant effect of pioglitazone is more perdurable when compared with other similar adjuvants, with trials as long as 24 weeks returning positive results [135]. However, it should be noted that these studies mostly included individuals with obesity, DM2 and other established metabolic disorders. Thus, the effects of pioglitazone in depression in more metabolically healthy participants remain to be ascertained.
Metformin also has notorious anti-inflammatory activity, by decreasing expression of NF-κB via AMPK-dependent and independent pathways, as well as improving energetic balance irrespective of the presence of DM2 and other metabolic disturbances [136, 137]. In a 24-week double-blind, placebo-controlled, randomized clinical trial of patients with DM2, the administration of metformin significantly improved depressive symptoms in comparison to placebo [138]. However, these results are not consistent across trials [139]; and pioglitazone may be a superior alternative: In a 6-week double-blind study on obese patients with depression and polycystic ovary syndrome and depression, monotherapy with pioglitazone granted greater improvement in depressive symptoms than monotherapy with metformin [140]. Similarly to pioglitazone, the antidepressant potential of metformin in metabolically healthy participants remains rather unexplored. Research on other antidiabetic drugs including glibenclamide [141], liraglutide [142], and sitagliptin [143] for depression remains chiefly in preclinical stages.
Concerning antihypertensive drugs, amounting preclinical and clinical evidence suggests a link between modulation of the RAAS to intervene in the pathophysiology of depression [144-150]. Angiotensin-converting enzyme inhibitors (ACEI), and angiotensin-receptor blockers (ARB) may impact depression by reducing CI and OS, and promoting neurogenesis [148]. Out of all classes of antihypertensive drugs, only ACEI and ARB were associated with decreased risk for hospitalization related to a mood disorder in a large retrospective study by Boal et al. [149]. Likewise, in the HUNT study from Norway, hypertensive patients treated with ACEIs had lower odds of displaying symptoms of depression [150]. However, future studies accounting for confounders such as disease severity, comorbidities and polypharmacy should clarify the true role of antihypertensive drugs as antidepressant adjuvants.
Finally, in recent decades, omega-3 fatty acids received widespread acceptance as augmenting agents for antidepressant therapy [151-154]. These molecules have been recognized due to their direct anti-inflammatory and antioxidant properties [155]. They may also participate in the neurobiology of depression by modulating the expression and functionality of serotonin and dopamine receptors [156]. Nevertheless, more recent meta-analyses have reframed the role of these molecules for depression, with reports of small, non-significant effect sizes [157-159]. The variable concentrations of eicosapentaenoic acid (EPA) in omega-3 preparations may be an important intervening factor in this scenario [160]. A meta-analysis by Martins et al. [161] found omega-3 fatty acids to enhance antidepressant response, yet with great variability depending on EPA contents: Only studies with EPA contents ≥60% showed significant antidepressant effects, in contrast with studies using EPA contents <60%. This highlights the importance of continuous evaluation of novel antidepressant alternatives in clinical settings.
4. POTENTIAL PHARMACOLOGICAL CANDIDATES FOR THE JOINT MANAGEMENT OF DEPRESSION AND CARDIOVASCULAR DISEASE
In addition to the use of antidepressants for CVD and the use of cardiometabolic treatments for depression, other pharmacological options have been studied in an effort to attack both problems simultaneously. CI remains a prime therapeutic target in this context, with numerous other forms of anti-inflammatory agents being studied in these circumstances (Table 2) [119]. Immunotherapy may be a frontrunner in this regard, as it has been ascertained to diminish cardiovascular risk in patients with rheumatoid arthritis and other similar conditions [162, 163]. Immunotherapy may also be useful in depression: in a randomized, double-blind, placebo-controlled, 24-week trial carried out on 1230 patients with moderate-severe psoriasis, treatment with ustekinumab, an IL-12 and IL-23 antagonist, was associated with significant improvement of anxious and depressive symptoms [164]. In a similar study on 380 patients with severe atopic dermatitis, the administration of dupilumab, an IL-4 antagonist, was also associated with a significant reduction of anxious and depressive symptoms [165]. Indeed, to date, improvement of depression is a secondary outcome in most trials assessing immunotherapeutics. Nevertheless, a small randomized, double-blind, placebo-controlled, 12-week trial by Raison et al. [166] evaluating the use of TNFα antagonist infliximab for TRD reported more promising results. In this study, participants in the control group with initial hs-CRP levels >5 mg/L showed >50% improvement of depressive symptoms. Future studies should explore more in-depth the utility of immunotherapy in populations with depression without other inflammatory comorbidities.
Table 2.
Summary of key evidence regarding new pharmacological candidates for the joint management of depression and cardiovascular disease.
| Class | Compounds (REF) | Methodology | Relevant results |
|---|---|---|---|
| Interleukin antagonists | Ustekinumab (164) | Multicentric, double-blind, randomized, placebo-controlled trial where 1230 patients with psoriasis who received ustekinumab 45 mg, ustekinumab 90 mg, or placebo for 24 weeks, and had their depressive and anxious symptoms evaluated. | At week 12, treatment with ustekinumab was associated with significant reductions in HADS scores both in patients who received 45 mg (-1.7 ± 3.1) and 90 mg (-2.1 ± 3.4); P<0.001. |
| Dupilumab (165) | Double-blind, randomized, placebo-controlled trial with 380 patients with atopic dermatitis who were treated with dupilumab 100 mg, 200 mg or 300 mg, or placebo for 16 weeks, and had their depressive and anxious symptoms evaluated. | A significant reduction in depressive and anxious symptoms was observed at 16 weeks in patients treated with dupilumab (P<0.001), with 66.7-75% reductions in the treated groups vs 22.2% in the placebo groups. | |
| Infliximab (166) | Double-blind, randomized, placebo-controlled, 12-week trial with 60 patients with major depression who received three infusions of infliximab (5 mg/kg, at baseline and weeks 2 and 6) or placebo. | Of patients with high-sensitivity C-reactive protein levels >5 mg/L, 62% showed an improvement of ≥50% in depressive symptoms as assessed with the HDRS. | |
| Antioxidants | NAC (171) | Systematic review including 65 studies on the use of NAC for various neuropsychiatric disorders, of which 2 were on depressive disorder. | The grade of recommendation for depressive disorder was B. Authors highlight the need for further controlled studies and longer follow-up for assessing consistent improvement. |
| Vitamins | Various (174) | Systematic review and meta-analysis with 40 studies on various nutraceuticals, including 9 on folate, folinic acid, methylfolate, or a combination of folic acid with vitamins B6 and B12. | The pooled effect size was 0.49 inconsequential, with a non-significant difference between folic acid and placebo (p=50.23; z=51.19, 95% confidence interval [CI], –0.31 to 1.29). Similarly, isolated analysis of methylfolate yielded a non-signifcant effect (p=50.25; z=51.15, 95% CI, –0.22 to 0.83). |
| L-Methylfolate (175) | Naturalistic clinical trial with 554 patients, of which 502 received L-methylfolate as adjunctive therapy, and 52 as monotherapy. | A mean reduction of 8.5 points (58.2% decrease) was found in patients’ PHQ-9 score (mean baseline PHQ-9 score= 14.6, mean follow-up PHQ-9 score= 6.1; P = .000). In addition, 376 patients (67.9%) showed treatment response, while 253 (45.7%) achieved remission after an average of 95 days in treatment. | |
| Nutritional supplements | SAMe (185) | Double-blind, randomized, placebo-controlled, 12-week trial on 189 patients with MDD who were treated with SAMe 1600-3200 mg/d, escitalopram 10-20 mg/d or placebo. | All treatment arms showed a significant reduction in HDRS scores (p<0,001); with a reduction of mean scores from 18.98 ± 5.09 to 12.79 ± 7.38 (p < 0.001) in the group treated with SAMe. Remission rates were 28% for SAMe, 28% for escitalopram, and 17% for placebo. |
Abbreviations: HADS: Hospital anxiety and depression scale; HDRS: Hamilton depression rating scale; NAC: N-acetylcysteine; PHQ-9: Patient Health Questionnaire-9; SAMe: S-adenosylmethionine; MDD: Major depressive disorder.
Various nutritional supplements have also been studied in the management of depression. N-acetylcysteine (NAC) has particularly ignited research interest given its role as an antioxidant by replenishing glutathione levels, as well as being an immunomodulator, and regulator of glutamate and dopamine neurotransmission [167, 168]. Its antioxidant properties have proved useful in the management of CVD [169, 170]. Current clinical evidence on NAC for depression is considered only preliminary, with further confirmatory research required, especially on the exploration of optimal dosing schemes and candidate selection, as determined in a systematic review by Deepmala et al. [171]. Indeed, clinical outcomes remain equivocal, with trials reporting improvement of depressive symptoms without changes in inflammatory biomarkers [172]; or major uncertainty in regards to sufficient and optimal duration of administration [173].
Finally, folate has also been studied substantially in the context of depression. A large systematic review and meta-analysis concluded that available data assessing folate, folinic acid and methylfolate on this matter are contradictory, without any determinant evidence in favor of folate, and relatively more positive results for methylfolate [174]. Interestingly, in isolated clinical trials methylfolate appears to be beneficial both alone and as adjunctive therapy [175, 176]. This should warrant further investigation, as in the National Health and Nutrition Examination Survey (NHANES), Americans with low serum folate were found to be at increased risk for depression [177]. Future continued investigation is essential, as folic acid derivates may aid in the prevention of CVD by intervening in the metabolism of homocysteine, a known biomarker for cardiovascular risk [178].
Research on the use of other supplements with joint effects on depression and CVD, such as zinc and various vitamins, is currently underway [179, 180]. In this setting, S-adenosylmethionine (SAMe) represents a peculiar case, as it has raised concerns of increased cardiovascular risk, due to being a precursor of homocysteine [181]. In animal models, SAMe has been shown to increase the synthesis of monoamines, modulate neurotransmission and improve membrane fluidity [182]. Although current findings suggest SAMe to be innocuous regarding cardiovascular risk [183], evidence regarding its efficacy for depression is inconsistent, and numerous studies have failed to show significant benefits to its use [184, 185].
CONCLUSION
The integration of the management of depression and CVD on the basis of their shared pathophysiologic components is an attractive prospect. However, great gaps in currently available preclinical and clinical knowledge preclude the introduction of novel alternatives in this regard at this time. CI is undoubtedly the most appealing target in this context. Although the need for further clinical investigation is indisputable, researchers should mind the common research design problems frequently seen in clinical psychiatry. Indeed, beyond the necessity for more homogenized methodology and clear study outcomes, a wide spectrum of questions must be addressed earnestly, ranging from the practical, in population selection and follow-up duration; to the conceptual, including the very definition of TRD, remission and relapse [186, 187].
The resolution of these conundrums is necessary to improve the quality of research in clinical psychiatry, and consequently facilitate the introduction of revolutionizing therapeutic measures in depression, CVD, and other associated conditions. In the meantime, lifestyle recommendations, in the form of sufficient physical activity and dietary modifications, may be invaluable, safe and useful tools in the treatment of depression, CVD, and many related immunometabolic disorders.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Friedrich M.J. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017;317(15):1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 2.Joseph P., Leong D., McKee M., Anand S.S., Schwalm J-D., Teo K., Mente A., Yusuf S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017;121(6):677–694. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 3.Kang H-J., Kim S-Y., Bae K-Y., Kim S-W., Shin I-S., Yoon J-S., Kim J.M. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med. J. 2015;51(1):8–18. doi: 10.4068/cmj.2015.51.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhar A.K., Barton D.A. Depression and the Link with Cardiovascular Disease. Front. Psychiatry. 2016;7:33. doi: 10.3389/fpsyt.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiedorowicz J.G. Depression and cardiovascular disease: an update on how course of illness may influence risk. Curr. Psychiatry Rep. 2014;16(10):492. doi: 10.1007/s11920-014-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chávez-Castillo M., Martínez M.S., Calvo M.J., Rojas M., Núñez V., Lameda V. A Chronic State of Systemic Stress: The Link between Depression and Cardiovascular Disease? SM J Clin Med. 2017;3(2):1025. [Google Scholar]
- 7.Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015;2015:581976. doi: 10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S., Allen N.B., Stuart A.L., Hayley A.C., Byrne M.L., Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11(1):200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Candales A., Hernández Burgos P.M., Hernandez-Suarez D.F., Harris D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017;3(4):e341. [PMC free article] [PubMed] [Google Scholar]
- 10.Coope A., Torsoni A.S., Velloso L.A. MECHANISMS IN ENDOCRINOLOGY: Metabolic and inflammatory pathways on the pathogenesis of type 2 diabetes. Eur. J. Endocrinol. 2016;174(5):R175–R187. doi: 10.1530/EJE-15-1065. [DOI] [PubMed] [Google Scholar]
- 11.Hunter P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012;13(11):968–970. doi: 10.1038/embor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okin D., Medzhitov R. Evolution of inflammatory diseases. Curr. Biol. 2012;22(17):R733–R740. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claesson-Welsh L. Vascular permeability--the essentials. Ups. J. Med. Sci. 2015;120(3):135–143. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garn H., Bahn S., Baune B.T., Binder E.B., Bisgaard H., Chatila T.A., Chavakis T., Culmsee C., Dannlowski U., Gay S., Gern J., Haahtela T., Kircher T., Müller-Ladner U., Neurath M.F., Preissner K.T., Reinhardt C., Rook G., Russell S., Schmeck B., Stappenbeck T., Steinhoff U., van Os J., Weiss S., Zemlin M., Renz H. Current concepts in chronic inflammatory diseases: Interactions between microbes, cellular metabolism, and inflammation. J. Allergy Clin. Immunol. 2016;138(1):47–56. doi: 10.1016/j.jaci.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 15.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: the devil is in the details. J. Neurochem. 2016;139(Suppl. 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polazzi E., Contestabile A. Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev. Neurosci. 2002;13(3):221–242. doi: 10.1515/REVNEURO.2002.13.3.221. [DOI] [PubMed] [Google Scholar]
- 17.Ling Z., Zhu Y., Tong Cw., Snyder J.A., Lipton J.W., Carvey P.M. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp. Neurol. 2006;199(2):499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Leonard B.E. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30(1):1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- 19.Buckwalter M.S., Wyss-Coray T. Modelling neuroinflammatory phenotypes in vivo. J. Neuroinflammation. 2004;1(1):10. doi: 10.1186/1742-2094-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasco J.A., Nicholson G.C., Williams L.J., Jacka F.N., Henry M.J., Kotowicz M.A., Schneider H.G., Leonard B.E., Berk M. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry. 2010;197(5):372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 21.Yang C., Tiemessen K.M., Bosker F.J., Wardenaar K.J., Lie J., Schoevers R.A. Interleukin, tumor necrosis factor-α and C-reactive protein profiles in melancholic and non-melancholic depression: A systematic review. J. Psychosom. Res. 2018;111:58–68. doi: 10.1016/j.jpsychores.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Hannibal K.E., Bishop M.D. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014;94(12):1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capuron L., Miller A.H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanescu C., Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J. Affect. Disord. 2012;143(1-3):34–38. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Selley M.L. Increased (E)-4-hydroxy-2-nonenal and asymmetric dimethylarginine concentrations and decreased nitric oxide concentrations in the plasma of patients with major depression. J. Affect. Disord. 2004;80(2-3):249–256. doi: 10.1016/S0165-0327(03)00135-6. [DOI] [PubMed] [Google Scholar]
- 27.Romano A., Serviddio G., Calcagnini S., Villani R., Giudetti A.M., Cassano T., Gaetani S. Linking lipid peroxidation and neuropsychiatric disorders: focus on 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2017;111:281–293. doi: 10.1016/j.freeradbiomed.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Leonard B., Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Moylan S., Berk M., Dean O.M., Samuni Y., Williams L.J., O’Neil A., Hayley A.C., Pasco J.A., Anderson G., Jacka F.N., Maes M. Oxidative & nitrosative stress in depression: why so much stress? Neurosci. Biobehav. Rev. 2014;45:46–62. doi: 10.1016/j.neubiorev.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Scapagnini G., Davinelli S., Drago F., De Lorenzo A., Oriani G. Antioxidants as antidepressants: fact or fiction? CNS Drugs. 2012;26(6):477–490. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Morris G., Berk M., Klein H., Walder K., Galecki P., Maes M. Nitrosative Stress, Hypernitrosylation, and Autoimmune Responses to Nitrosylated Proteins: New Pathways in Neuroprogressive Disorders Including Depression and Chronic Fatigue Syndrome. Mol. Neurobiol. 2017;54(6):4271–4291. doi: 10.1007/s12035-016-9975-2. [DOI] [PubMed] [Google Scholar]
- 32.Rawdin B.J., Mellon S.H., Dhabhar F.S., Epel E.S., Puterman E., Su Y., Burke H.M., Reus V.I., Rosser R., Hamilton S.P., Nelson J.C., Wolkowitz O.M. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013;31:143–152. doi: 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gloire G., Legrand-Poels S., Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72(11):1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Siomek A. NF-κB signaling pathway and free radical impact. Acta Biochim. Pol. 2012;59(3):323–331. doi: 10.18388/abp.2012_2116. [DOI] [PubMed] [Google Scholar]
- 35.Maes M., Kubera M., Mihaylova I., Geffard M., Galecki P., Leunis J-C., Berk M. Increased autoimmune responses against auto-epitopes modified by oxidative and nitrosative damage in depression: implications for the pathways to chronic depression and neuroprogression. J. Affect. Disord. 2013;149(1-3):23–29. doi: 10.1016/j.jad.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Wojda U., Salinska E., Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60(9):575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- 37.Uemura T., Green M., Corson T.W., Perova T., Li P.P., Warsh J.J. Bcl-2 SNP rs956572 associates with disrupted intracellular calcium homeostasis in bipolar I disorder. Bipolar Disord. 2011;13(1):41–51. doi: 10.1111/j.1399-5618.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- 38.Guan L., Jia N., Zhao X., Zhang X., Tang G., Yang L., Sun H., Wang D., Su Q., Song Q., Cai D., Cai Q., Li H., Zhu Z. The involvement of ERK/CREB/Bcl-2 in depression-like behavior in prenatally stressed offspring rats. Brain Res. Bull. 2013;99:1–8. doi: 10.1016/j.brainresbull.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Morris G., Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13:68. doi: 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Shachar D., Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One. 2008;3(11):e3676. doi: 10.1371/journal.pone.0003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao L., Martin M.V., Watson S.J., Schatzberg A., Akil H., Myers R.M., Jones E.G., Bunney W.E., Vawter M.P. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008;40(4):281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karry R., Klein E., Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol. Psychiatry. 2004;55(7):676–684. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas M.R., Johnson D.A., Sirkis D.W., Messing A., Johnson J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008;28(50):13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 46.Stockmeier C.A., Mahajan G.J., Konick L.C., Overholser J.C., Jurjus G.J., Meltzer H.Y., Uylings H.B., Friedman L., Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry. 2004;56(9):640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karege F., Vaudan G., Schwald M., Perroud N., La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005;136(1-2):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 48.Campbell S., MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr. Opin. Psychiatry. 2006;19(1):25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- 49.Turner C.A., Akil H., Watson S.J., Evans S.J. The fibroblast growth factor system and mood disorders. Biol. Psychiatry. 2006;59(12):1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Angelucci F., Brenè S., Mathé A.A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry. 2005;10(4):345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 51.Molendijk M.L., Bus B.A., Spinhoven P., Penninx B.W., Kenis G., Prickaerts J., Voshaar R.C., Elzinga B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol. Psychiatry. 2011;16(11):1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gałecki P., Talarowska M., Anderson G., Berk M., Maes M. Mechanisms underlying neurocognitive dysfunctions in recurrent major depression. Med. Sci. Monit. 2015;21:1535–1547. doi: 10.12659/MSM.893176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaváková M., Ďuračková Z., Trebatická J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell. Longev. 2015;2015:898393. doi: 10.1155/2015/898393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes M., Kubera M., Obuchowiczwa E., Goehler L., Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol. Lett. 2011;32(1):7–24. [PubMed] [Google Scholar]
- 55.Aydemir O., Deveci A., Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(2):261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Piccinni A., Marazziti D., Catena M., Domenici L., Del Debbio A., Bianchi C., Mannari C., Martini C., Da Pozzo E., Schiavi E., Mariotti A., Roncaglia I., Palla A., Consoli G., Giovannini L., Massimetti G., Dell’Osso L. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J. Affect. Disord. 2008;105(1-3):279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Gervasoni N., Aubry J-M., Bondolfi G., Osiek C., Schwald M., Bertschy G., Karege F. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51(4):234–238. doi: 10.1159/000085725. [DOI] [PubMed] [Google Scholar]
- 58.Lugo-Huitrón R., Ugalde Muñiz P., Pineda B., Pedraza-Chaverrí J., Ríos C., Pérez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013;2013:104024. doi: 10.1155/2013/104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maes M., Mihaylova I., Ruyter M.D., Kubera M., Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation. Neuroendocrinol. Lett. 2007;28(6):826–831. [PubMed] [Google Scholar]
- 60.Maes M., Leonard B.E., Myint A.M., Kubera M., Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Wichers M.C., Koek G.H., Robaeys G., Verkerk R., Scharpé S., Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry. 2005;10(6):538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 62.Paz-Filho G., Licinio J., Wong M.L. Pathophysiological basis of cardiovascular disease and depression: a chicken-and-egg dilemma. Br. J. Psychiatry. 2010;32(2):181–191. doi: 10.1590/S1516-44462010000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez-Vilchez I., Diaz-Ricart M., Navarro V., Torramade S., Zamorano-Leon J. Lopez-Farre, A Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl. Psychiatry. 2016;066(9):e886. doi: 10.1038/tp.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felice F., Di Stefano R., Pini S., Mazzotta G., Bovenzi F.M., Bertoli D., Abelli M., Borelli L., Cardini A., Lari L., Gesi C., Michi P., Morrone D., Gnudi L., Balbarini A. Influence of depression and anxiety on circulating endothelial progenitor cells in patients with acute coronary syndromes. Hum. Psychopharmacol. 2015;30(3):183–188. doi: 10.1002/hup.2470. [DOI] [PubMed] [Google Scholar]
- 65.van Sloten T.T., Schram M.T., Adriaanse M.C., Dekker J.M., Nijpels G., Teerlink T., Scheffer P.G., Pouwer F., Schalkwijk C.G., Stehouwer C.D., Henry R.M. Endothelial dysfunction is associated with a greater depressive symptom score in a general elderly population: the Hoorn Study. Psychol. Med. 2014;44(7):1403–1416. doi: 10.1017/S0033291713002043. [DOI] [PubMed] [Google Scholar]
- 66.Dimopoulos N., Piperi C., Salonicioti A., Mitsonis C., Liappas I., Lea R.W., Kalofoutis A. Elevation of plasma concentration of adhesion molecules in late-life depression. Int. J. Geriatr. Psychiatry. 2006;21(10):965–971. doi: 10.1002/gps.1592. [DOI] [PubMed] [Google Scholar]
- 67.van Dooren F.E.P., Schram M.T., Schalkwijk C.G., Stehouwer C.D.A., Henry R.M.A., Dagnelie P.C., Schaper N.C., van der Kallen C.J., Koster A., Sep S.J., Denollet J., Verhey F.R., Pouwer F. Associations of low grade inflammation and endothelial dysfunction with depression - The Maastricht Study. Brain Behav. Immun. 2016;56:390–396. doi: 10.1016/j.bbi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Wallerath T., Witte K., Schäfer S.C., Schwarz P.M., Prellwitz W., Wohlfart P., Kleinert H., Lehr H.A., Lemmer B., Förstermann U. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc. Natl. Acad. Sci. USA. 1999;96(23):13357–13362. doi: 10.1073/pnas.96.23.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilbert-Lampen U., Straube F., Trapp A., Deutschmann A., Plasse A., Steinbeck G. Effects of corticotropin-releasing hormone (CRH) on monocyte function, mediated by CRH-receptor subtype R1 and R2: a potential link between mood disorders and endothelial dysfunction? J. Cardiovasc. Pharmacol. 2006;47(1):110–116. doi: 10.1097/01.fjc.0000196240.58641.d3. [DOI] [PubMed] [Google Scholar]
- 70.Bruno R.L., Myers S.J., Glassman A.H. A correlational study of cardiovascular autonomic functioning and unipolar depression. Biol. Psychiatry. 1983;18(2):227–235. [PubMed] [Google Scholar]
- 71.Scalco A.Z., Scalco M.Z., Azul J.B.S., Lotufo Neto F. Hypertension and depression. Clinics (São Paulo) 2005;60(3):241–250. doi: 10.1590/S1807-59322005000300010. [DOI] [PubMed] [Google Scholar]
- 72.Halperin D., Reber G. Influence of antidepressants on hemostasis. Dialogues Clin. Neurosci. 2007;9(1):47–59. doi: 10.31887/DCNS.2007.9.1/dhalperin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serebruany V.L., Glassman A.H., Malinin A.I., Sane D.C., Finkel M.S., Krishnan R.R., Atar D., Lekht V., O’Connor C.M. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagul. Fibrinolysis. 2003;14(6):563–567. doi: 10.1097/00001721-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Gehi A., Musselman D., Otte C., Bruce Royster E., Ali S., Whooley M.A. Depression and platelet activation in outpatients with stable coronary heart disease: findings from the Heart and Soul Study. Psychiatry Res. 2010;175(3):200–204. doi: 10.1016/j.psychres.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 75.Bloomgarden Z.T. World congress on insulin resistance, diabetes, and cardiovascular disease: Part 1. Diabetes Care. 2011;34(7):e115–e120. doi: 10.2337/dc11-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes. 2006;55(8):2392–2397. doi: 10.2337/db06-0391. [DOI] [PubMed] [Google Scholar]
- 77.Snel M., Jonker J.T., Schoones J., Lamb H., de Roos A., Pijl H., Smit J.W., Meinders A.E., Jazet I.M. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012;2012:983814. doi: 10.1155/2012/983814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Austin A.W., Gordon J.L., Lavoie K.L., Arsenault A., Dasgupta K., Bacon S.L. Differential association of insulin resistance with cognitive and somatic symptoms of depression. Diabet. Med. 2014;31(8):994–1000. doi: 10.1111/dme.12465. [DOI] [PubMed] [Google Scholar]
- 79.McCracken E., Monaghan M., Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018;36(1):14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Webb M., Davies M., Ashra N., Bodicoat D., Brady E., Webb D., Moulton C., Ismail K., Khunti K. The association between depressive symptoms and insulin resistance, inflammation and adiposity in men and women. PLoS One. 2017;12(11):e0187448. doi: 10.1371/journal.pone.0187448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mancuso P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sáinz N., Barrenetxe J., Moreno-Aliaga M.J., Martínez J.A. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 83.Cimmino M.A., Andraghetti G., Briatore L., Salani B., Parodi M., Cutolo M., Cordera R. Changes in adiponectin and leptin concentrations during glucocorticoid treatment: a pilot study in patients with polymyalgia rheumatica. Ann. N. Y. Acad. Sci. 2010;1193:160–163. doi: 10.1111/j.1749-6632.2009.05364.x. [DOI] [PubMed] [Google Scholar]
- 84.Agrawal S., Gollapudi S., Su H., Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011;31(3):472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor V.H., Macqueen G.M. The Role of Adipokines in Understanding the Associations between Obesity and Depression. J. Obes. 2010;2010:1–6. doi: 10.1155/2010/748048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sukumaran S., Dubois D.C., Jusko W.J., Almon R.R. Glucocorticoid effects on adiponectin expression. Vitam. Horm. 2012;90:163–186. doi: 10.1016/B978-0-12-398313-8.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Milaneschi Y., Lamers F., Bot M., Drent M.L. Penninx, BWJH Leptin Dysregulation Is Specifically Associated With Major Depression With Atypical Features: Evidence for a Mechanism Connecting Obesity and Depression. Biol. Psychiatry. 2017;0181(9):807–814. doi: 10.1016/j.biopsych.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 88.Carvalho A.F., Rocha D.Q.C., McIntyre R.S., Mesquita L.M., Köhler C.A., Hyphantis T.N., Sales P.M., Machado-Vieira R., Berk M. Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. J. Psychiatr. Res. 2014;59:28–37. doi: 10.1016/j.jpsychires.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Machado-Vieira R., Gold P.W., Luckenbaugh D.A., Ballard E.D., Richards E.M., Henter I.D., De Sousa R.T., Niciu M.J., Yuan P., Zarate C.A., Jr The role of adipokines in the rapid antidepressant effects of ketamine. Mol. Psychiatry. 2017;22(1):127–133. doi: 10.1038/mp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu R. Y-Hua, L.; Hong, L. Depression in newly diagnosed type 2 diabetes. Int. J. Diabetes Dev. Ctries. 2010;30(2):102–104. doi: 10.4103/0973-3930.62601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bădescu S.V., Tătaru C., Kobylinska L., Georgescu E.L., Zahiu D.M., Zăgrean A.M., Zăgrean L. The association between Diabetes mellitus and Depression. J. Med. Life. 2016;9(2):120–125. [PMC free article] [PubMed] [Google Scholar]
- 92.Pereira S.S., Alvarez-Leite J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014;3(4):422–431. doi: 10.1007/s13679-014-0124-9. [DOI] [PubMed] [Google Scholar]
- 93.Muriach M., Flores-Bellver M., Romero F.J., Barcia J.M. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid. Med. Cell. Longev. 2014;2014:102158. doi: 10.1155/2014/102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korgaonkar M.S., Fornito A., Williams L.M., Grieve S.M. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol. Psychiatry. 2014;76(7):567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Rentería M.E., Schmaal L., Hibar D.P., Couvy-Duchesne B., Strike L.T., Mills N.T., de Zubicaray G.I., McMahon K.L., Medland S.E., Gillespie N.A., Hatton S.N., Lagopoulos J., Veltman D.J., van der Wee N., van Erp T.G.M., Wittfeld K., Grabe H.J., Block A., Hegenscheid K., Völzke H., Veer I.M., Walter H., Schnell K., Schramm E., Normann C., Schoepf D., Konrad C., Zurowski B., Godlewska B.R., Cowen P.J., Penninx B.W.J.H., Jahanshad N., Thompson P.M., Wright M.J., Martin N.G., Christensen H., Hickie I.B. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl. Psychiatry. 2017;7(5):e1116. doi: 10.1038/tp.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J-M., Mogi M., Tsukuda K., Tomochika H., Iwanami J., Min L-J., Nahmias C., Iwai M., Horiuchi M. Angiotensin II-induced neural differentiation via angiotensin II type 2 (AT2) receptor-MMS2 cascade involving interaction between AT2 receptor-interacting protein and Src homology 2 domain-containing protein-tyrosine phosphatase 1. Mol. Endocrinol. 2007;21(2):499–511. doi: 10.1210/me.2006-0005. [DOI] [PubMed] [Google Scholar]
- 97.Stroth U., Meffert S., Gallinat S., Unger T. Angiotensin II and NGF differentially influence microtubule proteins in PC12W cells: role of the AT2 receptor. Brain Res. Mol. Brain Res. 1998;53(1-2):187–195. doi: 10.1016/S0169-328X(97)00298-2. [DOI] [PubMed] [Google Scholar]
- 98.Gendron L., Laflamme L., Rivard N., Asselin C., Payet M.D., Gallo-Payet N. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol. Endocrinol. 1999;13(9):1615–1626. doi: 10.1210/mend.13.9.0344. [DOI] [PubMed] [Google Scholar]
- 99.McCarthy C.A., Vinh A., Miller A.A., Hallberg A., Alterman M., Callaway J.K. 2014. [Google Scholar]
- 100.Fouda A.Y., Pillai B., Dhandapani K.M., Ergul A., Fagan S.C. Role of interleukin-10 in the neuroprotective effect of the Angiotensin Type 2 Receptor agonist, compound 21, after ischemia/reperfusion injury. Eur. J. Pharmacol. 2017;799:128–134. doi: 10.1016/j.ejphar.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saavedra J.M., Sánchez-Lemus E., Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zawada W.M., Banninger G.P., Thornton J., Marriott B., Cantu D., Rachubinski A.L., Das M., Griffin W.S., Jones S.M. Generation of reactive oxygen species in 1-methyl-4-phenylpyridinium (MPP+) treated dopaminergic neurons occurs as an NADPH oxidase-dependent two-wave cascade. J. Neuroinflammation. 2011;8(1):129. doi: 10.1186/1742-2094-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borrajo A., Rodriguez-Perez A.I., Diaz-Ruiz C., Guerra M.J., Labandeira-Garcia J.L. Microglial TNF-α mediates enhancement of dopaminergic degeneration by brain angiotensin. Glia. 2014;62(1):145–157. doi: 10.1002/glia.22595. [DOI] [PubMed] [Google Scholar]
- 104.Pizzi C., Santarella L., Costa M.G., Manfrini O., Flacco M.E., Capasso L., Chiarini S., Di Baldassarre A., Manzoli L. Pathophysiological mechanisms linking depression and atherosclerosis: an overview. J. Biol. Regul. Homeost. Agents. 2012;26(4):775–782. [PubMed] [Google Scholar]
- 105.Cooper D.C., Tomfohr L.M., Milic M.S., Natarajan L., Bardwell W.A., Ziegler M.G., Dimsdale J.E. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom. Med. 2011;73(5):360–369. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pizzi C., Costa G.M., Santarella L., Flacco M.E., Capasso L., Bert F., Manzoli L. Depression symptoms and the progression of carotid intima-media thickness: a 5-year follow-up study. Atherosclerosis. 2014;233(2):530–536. doi: 10.1016/j.atherosclerosis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 107.Chávez-Castillo M., Ortega Á., Nava M., Fuenmayor J., Lameda V., Velasco M. Metabolic risk in depression and treatment with selective serotonin reuptake inhibitors: are the metabolic syndrome and an increase in cardiovascular risk unavoidable? Vessel Plus. 2018;2(4):6. doi: 10.20517/2574-1209.2018.02. [DOI] [Google Scholar]
- 108.Coupland C., Hill T., Morriss R., Moore M., Arthur A., Hippisley-Cox J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ. 2016;352:i1350. doi: 10.1136/bmj.i1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh D, Lippmann S. Can Statins Diminish Depression? 2018. [DOI] [PubMed]
- 110.Hare D.L., Toukhsati S.R., Johansson P., Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur. Heart J. 2014;35(21):1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 111.Baune B.T. Are Non-steroidal Anti-Inflammatory Drugs Clinically Suitable for the Treatment of Symptoms in Depression-Associated Inflammation? Curr. Top. Behav. Neurosci. 2017;31:303–319. doi: 10.1007/7854_2016_19. [DOI] [PubMed] [Google Scholar]
- 112.Eyre H.A., Air T., Proctor S., Rositano S., Baune B.T. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;57:11–16. doi: 10.1016/j.pnpbp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 113.Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 114.Abbasi S-H., Hosseini F., Modabbernia A., Ashrafi M., Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J. Affect. Disord. 2012;141(2-3):308–314. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 115.Müller N., Schwarz M.J., Dehning S., Douhe A., Cerovecki A., Goldstein-Müller B., Spellmann I., Hetzel G., Maino K., Kleindienst N., Möller H.J., Arolt V., Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry. 2006;11(7):680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 116.Akhondzadeh S., Jafari S., Raisi F., Nasehi A.A., Ghoreishi A., Salehi B., Mohebbi-Rasa S., Raznahan M., Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress. Anxiety. 2009;26(7):607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 117.Mendlewicz J., Kriwin P., Oswald P., Souery D., Alboni S., Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int. Clin. Psychopharmacol. 2006;21(4):227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 118.Uher R., Carver S., Power R.A., Mors O., Maier W., Rietschel M., Hauser J., Dernovsek M.Z., Henigsberg N., Souery D., Placentino A., Farmer A., McGuffin P. Non-steroidal anti-inflammatory drugs and efficacy of antidepressants in major depressive disorder. Psychol. Med. 2012;42(10):2027–2035. doi: 10.1017/S0033291712000190. [DOI] [PubMed] [Google Scholar]
- 119.Pasco J.A., Jacka F.N., Williams L.J., Henry M.J., Nicholson G.C., Kotowicz M.A., Berk M. Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother. Psychosom. 2010;79(5):323–325. doi: 10.1159/000319530. [DOI] [PubMed] [Google Scholar]
- 120.Almeida O.P., Flicker L., Yeap B.B., Alfonso H., McCaul K., Hankey G.J. Aspirin decreases the risk of depression in older men with high plasma homocysteine. Transl. Psychiatry. 2012;2:e151. doi: 10.1038/tp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallagher P.J., Castro V., Fava M., Weilburg J.B., Murphy S.N., Gainer V.S., Churchill S.E., Kohane I.S., Iosifescu D.V., Smoller J.W., Perlis R.H. Antidepressant response in patients with major depression exposed to NSAIDs: a pharmacovigilance study. Am. J. Psychiatry. 2012;169(10):1065–1072. doi: 10.1176/appi.ajp.2012.11091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Redlich C., Berk M., Williams L.J., Sundquist J., Sundquist K., Li X. Statin use and risk of depression: a Swedish national cohort study. BMC Psychiatry. 2014;14:348. doi: 10.1186/s12888-014-0348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kilic F.S., Ozatik Y., Kaygisiz B., Baydemir C., Erol K. Acute antidepressant and anxiolytic effects of simvastatin and its mechanisms in rats. Neurosciences (Riyadh) 2012;17(1):39–43. [PubMed] [Google Scholar]
- 124.Ghanizadeh A., Hedayati A. Augmentation of fluoxetine with lovastatin for treating major depressive disorder, a randomized double-blind placebo controlled-clinical trial. Depress. Anxiety. 2013;30(11):1084–1088. doi: 10.1002/da.22195. [DOI] [PubMed] [Google Scholar]
- 125.Gougol A., Zareh-Mohammadi N., Raheb S., Farokhnia M., Salimi S., Iranpour N., Yekehtaz H., Akhondzadeh S. Simvastatin as an adjuvant therapy to fluoxetine in patients with moderate to severe major depression: A double-blind placebo-controlled trial. J. Psychopharmacol. (Oxford) 2015;29(5):575–581. doi: 10.1177/0269881115578160. [DOI] [PubMed] [Google Scholar]
- 126.Köhler O., Gasse C., Petersen L., Ingstrup K.G., Nierenberg A.A., Mors O., Østergaard S.D. The Effect of Concomitant Treatment With SSRIs and Statins: A Population-Based Study. Am. J. Psychiatry. 2016;173(8):807–815. doi: 10.1176/appi.ajp.2016.15040463. [DOI] [PubMed] [Google Scholar]
- 127.Fakhraei N., Javedan R., Nikoui V., Bakhtiarian A., Pournaghash Tehrani S.S. Effect of Clofibrate, A PPAR-A Receptors Agonist, On Behavioral Despair Associated With Exposure to Forced Swim in Rats. Adv. J. Toxicol. Curr. Res. 2017;1(2):107–115. [Google Scholar]
- 128.Moulton C.D., Hopkins C.W.P., Ismail K., Stahl D. Repositioning of diabetes treatments for depressive symptoms: A systematic review and meta-analysis of clinical trials. Psychoneuroendocrinology. 2018;94:91–103. doi: 10.1016/j.psyneuen.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 129.Sadaghiani M.S., Javadi-Paydar M., Gharedaghi M.H., Fard Y.Y., Dehpour A.R. Antidepressant-like effect of pioglitazone in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Behav. Brain Res. 2011;224(2):336–343. doi: 10.1016/j.bbr.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 130.Eissa Ahmed A.A., Al-Rasheed N.M., Al-Rasheed N.M. Antidepressant-like effects of rosiglitazone, a PPARγ agonist, in the rat forced swim and mouse tail suspension tests. Behav. Pharmacol. 2009;20(7):635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- 131.Colle R., de Larminat D., Rotenberg S., Hozer F., Hardy P., Verstuyft C., Fève B., Corruble E. Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr. Dis. Treat. 2016;13:9–16. doi: 10.2147/NDT.S121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kemp D.E., Ismail-Beigi F., Ganocy S.J., Conroy C., Gao K., Obral S., Fein E., Findling R.L., Calabrese J.R. Use of insulin sensitizers for the treatment of major depressive disorder: a pilot study of pioglitazone for major depression accompanied by abdominal obesity. J. Affect. Disord. 2012;136(3):1164–1173. doi: 10.1016/j.jad.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu Y., Xing H., Dong X., Lu W., Xiao X., Gao L., Cui M., Chen J. Pioglitazone is an effective treatment for patients with post-stroke depression combined with type 2 diabetes mellitus. Exp. Ther. Med. 2015;10(3):1109–1114. doi: 10.3892/etm.2015.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeinoddini A., Sorayani M., Hassanzadeh E., Arbabi M., Farokhnia M., Salimi S., Ghaleiha A., Akhondzadeh S. Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial. Depress. Anxiety. 2015;32(3):167–173. doi: 10.1002/da.22340. [DOI] [PubMed] [Google Scholar]
- 135.Roohafza H., Shokouh P., Sadeghi M., Alikhassy Z., Sarrafzadegan N. A Possible Role for Pioglitazone in the Management of Depressive Symptoms in Metabolic Syndrome Patients (EPICAMP Study): A Double Blind, Randomized Clinical Trial. Int. Sch. Res. Notices. 2014;2014:697617. doi: 10.1155/2014/697617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cameron A.R., Morrison V.L., Levin D., Mohan M., Forteath C., Beall C., McNeilly A.D., Balfour D.J., Savinko T., Wong A.K., Viollet B., Sakamoto K., Fagerholm S.C., Foretz M., Lang C.C., Rena G. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016;119(5):652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saisho Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr. Metab. Immune Disord. Drug Targets. 2015;15(3):196–205. doi: 10.2174/1871530315666150316124019. [DOI] [PubMed] [Google Scholar]
- 138.Guo M., Mi J., Jiang Q-M., Xu J-M., Tang Y-Y., Tian G., Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014;41(9):650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- 139.Ackermann R.T., Edelstein S.L., Narayan K.M.V., Zhang P., Engelgau M.M., Herman W.H., Marrero D.G., Diabetes Prevention Program Research Group Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity (Silver Spring) 2009;17(12):2176–2181. doi: 10.1038/oby.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kashani L., Omidvar T., Farazmand B., Modabbernia A., Ramzanzadeh F., Tehraninejad E.S., Ashrafi M., Tabrizi M., Akhondzadeh S. Does pioglitazone improve depression through insulin-sensitization? Results of a randomized double-blind metformin-controlled trial in patients with polycystic ovarian syndrome and comorbid depression. Psychoneuroendocrinology. 2013;38(6):767–776. doi: 10.1016/j.psyneuen.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 141.Su W-J., Peng W., Gong H., Liu Y-Z., Zhang Y., Lian Y-J., Cao Z.Y., Wu R., Liu L.L., Wang B., Wang Y.X., Jiang C.L. Antidiabetic drug glyburide modulates depressive-like behavior comorbid with insulin resistance. J. Neuroinflammation. 2017;14(1):210. doi: 10.1186/s12974-017-0985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weina H., Yuhu N., Christian H., Birong L., Feiyu S., Le W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Res. 2018;1694:55–62. doi: 10.1016/j.brainres.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 143.Kamble M., Gupta R., Rehan H.S., Gupta L.K. Neurobehavioral effects of liraglutide and sitagliptin in experimental models. Eur. J. Pharmacol. 2016;774:64–70. doi: 10.1016/j.ejphar.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 144.Ping G., Qian W., Song G., Zhaochun S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol. Biochem. Behav. 2014;124:5–12. doi: 10.1016/j.pbb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 145.Ayyub M., Najmi A.K., Akhtar M. Protective Effect of Irbesartan an Angiotensin (AT1) Receptor Antagonist in Unpredictable Chronic Mild Stress Induced Depression in Mice. Drug Res. (Stuttg.) 2017;67(1):59–64. doi: 10.1055/s-0042-118172. [DOI] [PubMed] [Google Scholar]
- 146.Torika N., Asraf K., Danon A., Apte R.N., Fleisher-Berkovich S. Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies. PLoS One. 2016;11(5):e0155823. doi: 10.1371/journal.pone.0155823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benicky J., Sánchez-Lemus E., Honda M., Pang T., Orecna M., Wang J., Leng Y., Chuang D.M., Saavedra J.M. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36(4):857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vian J., Pereira C., Chavarria V., Köhler C., Stubbs B., Quevedo J., Kim S.W., Carvalho A.F., Berk M., Fernandes B.S. The renin-angiotensin system: a possible new target for depression. BMC Med. 2017;15(1):144. doi: 10.1186/s12916-017-0916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boal A.H., Smith D.J., McCallum L., Muir S., Touyz R.M., Dominiczak A.F., Padmanabhan S. Monotherapy With Major Antihypertensive Drug Classes and Risk of Hospital Admissions for Mood Disorders. Hypertension. 2016;68(5):1132–1138. doi: 10.1161/HYPERTENSIONAHA.116.08188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johansen A., Holmen J., Stewart R., Bjerkeset O. Anxiety and depression symptoms in arterial hypertension: the influence of antihypertensive treatment. the HUNT study, Norway. Eur. J. Epidemiol. 2012;27(1):63–72. doi: 10.1007/s10654-011-9641-y. [DOI] [PubMed] [Google Scholar]
- 151.Kraguljac N.V., Montori V.M., Pavuluri M., Chai H.S., Wilson B.S., Unal S.S. Efficacy of omega-3 fatty acids in mood disorders - a systematic review and metaanalysis. Psychopharmacol. Bull. 2009;42(3):39–54. [PubMed] [Google Scholar]
- 152.Appleton K.M., Hayward R.C., Gunnell D., Peters T.J., Rogers P.J., Kessler D., Ness A.R. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am. J. Clin. Nutr. 2006;84(6):1308–1316. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 153.Appleton K.M., Rogers P.J., Ness A.R. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr. 2010;91(3):757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 154.Lin P-Y., Su K-P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry. 2007;68(7):1056–1061. doi: 10.4088/JCP.v68n0712. [DOI] [PubMed] [Google Scholar]
- 155.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 156.Grosso G., Galvano F., Marventano S., Malaguarnera M., Bucolo C., Drago F., Caraci F. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014;2014:313570. doi: 10.1155/2014/313570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bloch M.H., Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol. Psychiatry. 2012;17(12):1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bai Z-G., Bo A., Wu S-J., Gai Q-Y., Chi I. Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: A systematic review and meta-analysis. J. Affect. Disord. 2018;241:241–248. doi: 10.1016/j.jad.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 159.Appleton K.M., Sallis H.M., Perry R., Ness A.R., Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev. 2015;(11):CD004692. doi: 10.1002/14651858.CD004692.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mocking R.J.T., Harmsen I., Assies J., Koeter M.W.J., Ruhé H.G., Schene A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry. 2016;6:e756. doi: 10.1038/tp.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martins J.G., Bentsen H., Puri B.K. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol. Psychiatry. 2012;17(12):1144–1149. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- 162.Puig L. Cardiovascular Risk and Psoriasis: the Role of Biologic Therapy. Actas Dermosifiliogr. 2012;103(10):853–862. doi: 10.1016/j.ad.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 163.Roubille C., Martel-Pelletier J., Haraoui B., Tardif J-C., Pelletier J-P. Biologics and the cardiovascular system: a double-edged sword. Antiinflamm. Antiallergy Agents Med. Chem. 2013;12(1):68–82. doi: 10.2174/1871523011312010009. [DOI] [PubMed] [Google Scholar]
- 164.Langley R.G., Feldman S.R., Han C., Schenkel B., Szapary P., Hsu M-C., Ortonne J.P., Gordon K.B., Kimball A.B. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J. Am. Acad. Dermatol. 2010;63(3):457–465. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 165.Simpson E., Worm M., Soong W., Blauvelt A., Eckert L., Wu R. Dupilumab Improves Patient-Reported Outcomes (PROs) in a Phase 2 Study in Adults with Moderate-to-Severe Atopic Dermatitis. J. Allergy Clin. Immunol. 2015;135(2):AB167. doi: 10.1016/j.jaci.2014.12.1484. [DOI] [Google Scholar]
- 166.Raison C.L., Rutherford R.E., Woolwine B.J., Shuo C., Schettler P., Drake D.F., Haroon E., Miller A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dean O., Giorlando F., Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J. Psychiatry Neurosci. 2011;36(2):78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Minarini A., Ferrari S., Galletti M., Giambalvo N., Perrone D., Rioli G., Galeazzi G.M. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2017;13(3):279–292. doi: 10.1080/17425255.2017.1251580. [DOI] [PubMed] [Google Scholar]
- 169.Liu C., Lu X-Z., Shen M-Z., Xing C-Y., Ma J., Duan Y-Y., Yuan L.J. N-Acetyl Cysteine improves the diabetic cardiac function: possible role of fibrosis inhibition. BMC Cardiovasc. Disord. 2015;15(1):84. doi: 10.1186/s12872-015-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reyes D.R.A., Gomes M.J., Rosa C.M., Pagan L.U., Damatto F.C., Damatto R.L., Depra I., Campos D.H.S., Fernandez A.A.H., Martinez P.F., Okoshi K., Okoshi M.P. N-Acetylcysteine Influence on Oxidative Stress and Cardiac Remodeling in Rats During Transition from Compensated Left Ventricular Hypertrophy to Heart Failure. Cell. Physiol. Biochem. 2017;44(6):2310–2321. doi: 10.1159/000486115. [DOI] [PubMed] [Google Scholar]
- 171.Deepmala, null; Slattery, J; Kumar, N Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 172.Hasebe K., Gray L., Bortolasci C., Panizzutti B., Mohebbi M., Kidnapillai S., Spolding B., Walder K., Berk M., Malhi G., Dodd S., Dean O.M. Adjunctive N-acetylcysteine in depression: exploration of interleukin-6, C-reactive protein and brain-derived neurotrophic factor. Acta Neuropsychiatr. 2017;29(6):337–346. doi: 10.1017/neu.2017.2. [DOI] [PubMed] [Google Scholar]
- 173.Berk M., Dean O.M., Cotton S.M., Jeavons S., Tanious M., Kohlmann K., Hewitt K., Moss K., Allwang C., Schapkaitz I., Robbins J., Cobb H., Ng F., Dodd S., Bush A.I., Malhi G.S. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry. 2014;75(6):628–636. doi: 10.4088/JCP.13m08454. [DOI] [PubMed] [Google Scholar]
- 174.Sarris J., Murphy J., Mischoulon D., Papakostas G.I., Fava M., Berk M., Ng C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry. 2016;173(6):575–587. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- 175.Shelton RC, Sloan Manning J, Barrentine LW. 2013. [DOI] [PMC free article] [PubMed]
- 176.Papakostas G.I., Shelton R.C., Zajecka J.M., Etemad B., Rickels K., Clain A., Baer L., Dalton E.D., Sacco G.R., Schoenfeld D., Pencina M., Meisner A., Bottiglieri T., Nelson E., Mischoulon D., Alpert J.E., Barbee J.G., Zisook S., Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry. 2012;169(12):1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- 177.Nguyen B., Weiss P., Beydoun H., Kancherla V. Association between blood folate concentrations and depression in reproductive aged U.S. women, NHANES (2011-2012). J. Affect. Disord. 2017;223:209–217. doi: 10.1016/j.jad.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 178.Bazzano L.A. Folic acid supplementation and cardiovascular disease: the state of the art. Am. J. Med. Sci. 2009;338(1):48–49. doi: 10.1097/MAJ.0b013e3181aaefd6. [DOI] [PubMed] [Google Scholar]
- 179.Sosnowska B., Penson P., Banach M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017;7(Suppl. 1):S21–S31. doi: 10.21037/cdt.2017.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiao Y., Su X., Huang W., Zhang J., Peng C., Huang H., Wu X., Huang H., Xia M., Ling W. Role of S-adenosylhomocysteine in cardiovascular disease and its potential epigenetic mechanism. Int. J. Biochem. Cell Biol. 2015;67:158–166. doi: 10.1016/j.biocel.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 181.Gao J., Cahill C.M., Huang X., Roffman J.L., Lamon-Fava S., Fava M., Mischoulon D., Rogers J.T. S-Adenosyl Methionine and Transmethylation Pathways in Neuropsychiatric Diseases Throughout Life. Neurotherapeutics. 2018;15(1):156–175. doi: 10.1007/s13311-017-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sharma A., Gerbarg P., Bottiglieri T., Massoumi L., Carpenter L.L., Lavretsky H., Muskin P.R., Brown R.P., Mischoulon D. as Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry. 2017;78(6):e656–e667. doi: 10.4088/JCP.16r11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thompson M.A., Bauer B.A., Loehrer L.L., Cha S.S., Mandrekar J.N., Sood A., Wahner-Roedler D.L. Dietary supplement S-adenosyl-L-methionine (AdoMet) effects on plasma homocysteine levels in healthy human subjects: a double-blind, placebo-controlled, randomized clinical trial. J. Altern. Complement. Med. 2009;15(5):523–529. doi: 10.1089/acm.2008.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mischoulon D., Price L.H., Carpenter L.L., Tyrka A.R., Papakostas G.I., Baer L., Dording C.M., Clain A.J., Durham K., Walker R., Ludington E., Fava M. A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J. Clin. Psychiatry. 2014;75(4):370–376. doi: 10.4088/JCP.13m08591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sarris J, Price L, Carpenter L, Tyrka A, Ng C. 2015.
- 186.Trevino K., McClintock S.M., McDonald Fischer N., Vora A., Husain M.M. Defining treatment-resistant depression: a comprehensive review of the literature. Ann. Clin. Psychiatry. 2014;26(3):222–232. [PubMed] [Google Scholar]
- 187.McIntyre R.S., Filteau M-J., Martin L., Patry S., Carvalho A., Cha D.S., Barakat M., Miguelez M. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]