Abstract
Accumulative evidence has shown that mitochondrial dysfunction plays a pivotal role in the pathogenesis of Alzheimer's disease (AD). Mitochondrial impairment actively contributes to the synaptic and cognitive failure that characterizes AD. The presence of soluble pathological forms of tau like hyperphosphorylated at Ser396 and Ser404 and cleaved at Asp421 by caspase 3, negatively impacts mitochondrial bioenergetics, transport, and morphology in neurons. These adverse effects against mitochondria health will contribute to the synaptic impairment and cognitive decline in AD. Current studies suggest that mitochondrial failure induced by pathological tau forms is likely the result of the opening of the mitochondrial permeability transition pore (mPTP). mPTP is a mitochondrial mega-channel that is activated by increases in calcium and is associated with mitochondrial stress and apoptosis. This structure is composed of different proteins, where Ciclophilin D (CypD) is considered to be the primary mediator of mPTP activation. Also, new studies suggest that mPTP contributes to Aβ pathology and oxidative stress in AD.
Further, inhibition of mPTP through the reduction of CypD expression prevents cognitive and synaptic impairment in AD mouse models. More importantly, tau protein contributes to the physiological regulation of mitochondria through the opening/interaction with mPTP in hippocampal neurons. Therefore, in this paper, we will discuss evidence that suggests an important role of pathological forms of tau against mitochondrial health. Also, we will discuss the possible role of mPTP in the mitochondrial impairment produced by the presence of tau pathology and its impact on synaptic function present in AD.
Keywords: Tau, Mitochondria, oxidative stress, Alzheimer´s disease, mitochondrial permeability transition pore, calcium
1. Introduction
AD is a progressive neurodegenerative disease with a high worldwide prevalence [1, 2]. AD represents around 80% of all dementia cases present today [3]. AD is considered a world health problem because it is estimated that by the year 2050, approximately 150 million people will manifest this disease [2]. Despite the vast body of evidence generated in AD research, there is no clear definition of the mechanisms involved in the pathogenesis of this disorder. However, there is scientific consensus about two distinctive hallmarks present in AD; the presence of extracellular senile plaques, formed by Aβ peptide accumulation [4, 5], and the generation of intra-neuronal neuro-fibrillary tangles (NFTs), formed by the accumulation of pathological forms of tau protein [4, 5].
Tau belongs to the family of microtubule-associated proteins (MAPs) [6] and confers stability to micro tubules [6, 7], allowing the correct transport of molecules and organelles essentials for neuronal function [6, 7]. Tau is mainly distributed in the axon; however, under pathological conditions, tau changes its localization and its affinity for microtubules decays, causing the destabilization of these structures [7]. At the same time, tau is prone to undergo several post-translational modifications, including ubiquitination, nitrosylation, glycosylation, hyperphosphorylation, and cleavage [7, 8]. From this group, hyperphosphorylation and cleaved of tau have been suggested as an essential contributor to the genesis and progression of AD [9-13]. Tau cleaved by caspase 3 has been indicated as an important factor in the formation of NFTs [14], and current evidence suggests that this tau form impairs both pre and post-synaptic structures [15]. These deleterious effects on the synaptic process have also been documented for hyperphosphorylated tau [16]. Despite this evidence, the underlying mechanisms of how these pathological forms of tau contribute to synaptic deterioration in AD are not fully understood.
In this context, several studies have shown that mitochondrial impairment is an essential player in the neurodegenerative changes present in AD [17]. More important, several groups have suggested that the presence of pathological forms of tau (principally hyperphosphorylated or cleaved) significantly affects mitochondrial function and bioenergetics [18-23]. This evidence suggests that mitochondria could be the initial target of neuronal damage and synaptic alteration in AD and that these changes could be vital for the cognitive and memory impairment observed in the early stages of this disease.
Here, we review an interesting body of work related to the effects of pathological forms of tau against mitochondrial function. We will focus on discussing the actions of hyperphosphorylated and cleaved/truncated tau against mitochondrial health, and its contribution to synaptic dysfunction showed in AD. Also, we proposed that these pathological forms of tau can especially modify mitochondrial function, promoting oxidative damage, and affecting bioenergetics. These negative changes could be achieved through specific mechanisms that include the impairment of mitochondrial dynamics, a process that controls mitochondrial morphology, and the opening of mitochondrial permeability transition pore (mPTP), a mitochondrial structure involved in the process of cellular death induced by apoptosis.
2. Tau protein and AD
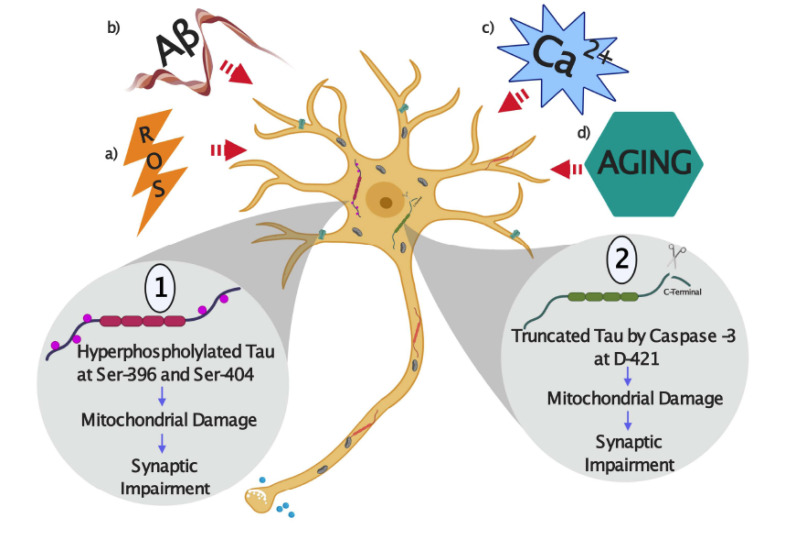
AD is a neurodegenerative disorder that is clinically characterized by a progressive cognitive decline and deterioration in the ability of the individuals to care for their basic needs [1, 2]. Along with the clinical manifestations of cognitive and memory impairment, there are defining neuropathological hallmarks in the AD brain, primarily in the hippocampus and cortex [1, 2, 24]. These pathological features include the extracellular senile plaques with amyloid deposits, which are formed with aggregates of the Aβ peptide, and the intraneuronal accumulations of NFTs composed of the tau protein, which is abnormally phosphorylated and cleaved [1-5]. The growing interest in tau studies increased after the realization that abnormally modified tau is forming the paired helical filaments (PHFs), which are part of the NFTs found in the AD brain [4, 8]. Pathological tau modifications present in these filaments include hyperphosphorylation and truncation which both affects the ability of tau to stabilize microtubules [3, 14]. One of the current hypothesis that explains neurodegeneration present in AD suggests that these changes are the consequence of several events in where an increase in Aβ production subsequently facilitates tau hyperphosphorylation and cleavage, which converts this protein into a toxic species that negatively impact neuronal function [25] (Fig. 1). Studies based on tau gene mutations responsible for rare autosomal dominant neurodegenerative diseases (known as frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17), exhibited a pronounced tau pathology, but no amyloid aggregates, indicating that the alterations in this protein are sufficient to cause neurodegeneration [26, 27]. Also, other studies showed that primary neurons from tau knockout mice (-/-) are resistant to neurodegeneration, mitochondrial impairment, and calcium stress induced by Aβ challenging, compared to neurons that expressed normal tau levels [28-30]. These findings indicate that alterations in tau could directly result in neurodegeneration and remarks on the importance of tau in the pathogenic processes present in AD (Fig. 1).
Fig. (1).
Schematic representation of how tau protein can be pathologically modified by the action of different stressors in Alzheimer´s disease (AD). (a) Reactive oxygen species (ROS), (b) Amyloid-β peptide (Aβ), (c) Calcium (Ca2+) stress, and, (d) Aging. 1) As a result of several stressors, tau can be abnormally hyperphosphorylated at residues Ser396 and Ser404 and cleaved by the action of caspase-3 at D421 residue. These modifications can also affect mitochondrial health, and these actions could impact neuronal communication through synaptic impairment. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
In this context, several studies showed that non-aggregated forms of tau but not NFTs, play a critical role in the pathogenesis of AD [31, 32]. For example, in an inducible murine tauopathy model, the reduction in the mutant tau (FTDP-17) expression prevented behavioral deficits and neuronal loss, without affecting NFT numbers [32]. Also, complementary studies have shown that suppressing tau expression reduced Aβ-induced cognitive deficits but did not affect Aβ levels in an APP mouse model of AD [33] and that the deficiency in axonal mitochondria transport does not occur in neurons from tau (–/–) mice [34]. These findings have contributed to a re-evaluation of the role of tau in neurodegeneration, including the exploration of different targets that could be affected by the action of pathological forms of tau.
2.1. Pathological Modifications of Tau in AD
In humans, tau protein is encoded by a single gene located on the long arm of chromosome 17 [6, 35] and belongs to a family of microtubule-associated proteins (MAPs). Under physiological conditions, tau is located predominantly in the axon; meanwhile, under pathological circumstances, modified tau migrates towards other neuronal compartments, including the somatodendritic area [36]. A hallmark in the AD brain is the presence of pathological forms of tau [7, 35, 36]. Studies on AD brains have shown different post-translational modifications of tau, including phosphorylation and truncation [4, 14]. Given that abnormal hyperphosphorylation and tau truncation seem likely to play a crucial role in the genesis and progression of AD [7, 14] (Fig. 1), we will discuss these two modifications a bit further.
2.2. Role of Pathological Tau Phosphorylation in AD
Tau is a phosphoprotein, and its phosphorylation plays a prominent role in regulating its physiological function [6]. Anomalous tau phosphorylation is present in the AD brain, and its presence has been associated with progression and neurodegeneration related to this disease [4, 8, 36]. Pathological phosphorylation of tau at specific sites can significantly increase its tendency to aggregate and disassemble from microtubules [71]. For example, hyperphosphorylation at Ser396 and Ser404 (PHF-1 epitope), makes tau more fibrillogenic [7 ]. Also, tau phosphorylated at Ser-396 and Ser-404 is a significant component of PHFs and its presence induced toxicity in neurons by affecting microtubules’ stability [37, 38]. Glycogen synthase kinase 3β (GSK3β) is often considered to be a “tau kinase” as it efficiently phosphorylates tau at Ser396 and Ser404, as well as other AD-relevant tau epitopes in vitro and neuronal cells [39]. Increased expression of GSK3β results in an augment in tau phosphorylation levels at pathological sites, including the PHF-1 epitope (Ser-396/404), contributing to neuronal toxicity [40, 41]. Also, exposure of primary rat cultured neurons to Aβ fibrils induces tau hyperphosphorylation at Ser-396/Ser-404, increasing its accumulation and blocking its ability to bind with microtubules [41].
Pathological phosphorylation of tau has been related to synaptic dysfunction observed in AD [42]. These synaptic alterations have been strongly related to the clinical symptomatology of memory loss, and cognitive decline shown in patients with AD [43]. Memory loss is distinctive since the
beginning of AD, where patients start forgetting trivial things and then show a significant cognitive failure and loss of essential memories [43].
Importantly, it has been determined that in AD, there is a significant decrease in enzymes responsible for producing and synthesizing acetylcholine [44, 45], which correlate with the accumulation of one the AD hallmarks (amyloid plaques and neurofibrillary tangles) [35]. In turn, synaptic protein markers such as synaptophysin, a protein associated with the transport and functionality of presynaptic vesicles, suffer from a significant decline in the brains of patients with AD as the disease progresses [46]. It has also been shown that in AD, the shape and number of dendritic spines are strongly reduced [47, 48], which are highly essential structures involved in memory and learning processes [49].
Although it is known that under physiological conditions, tau is mostly distributed in the axon, it has been established that the tau’s presence at the postsynaptic level could play an essential role in neuronal communication [50]. In this context, studies have [51] shown that the presence of tau could be observed in the pre and postsynaptic compartments of brains from both AD and non-AD patients, although in the case of AD brains, an accumulation of these pathological forms may occur in the synaptosomes fraction of neurons [51]. Also, other studies showed that hyperphosphorylated tau (phosphorylated at both serine 202 and threonine 205) is present in the presynaptic terminals of the AD brain [51]. However, in vitro experiments showed that hyperphosphorylated tau is capable of binding to synaptic vesicles, which affects their correct movement [52]. Similar to the above, new studies reported that synaptogyrin-3 (a synaptic vesicle membrane protein) is responsible for the abnormal tau-vesicle association [52]. These effects are consistent with some studies showing that hyperphosphorylated tau could be released by exosomes [53] and that this tau form can be transported in the brain through neuronal connections (synapses), extending hyperphosphorylated tau to other regions [54]. Together, these antecedents allow us to assume active participation of this aberrant tau form in the synaptic impairment in AD.
Phosphorylated tau could also affect the synapses process, altering the neuronal electrophysiology [55]. Neurons from the CA1 hippocampal zone expressing hyperphosphorylated tau showed a migration of the initial segment of the axon, which significantly reduced the neuronal excitability [55]. Also, other studies suggested that hyperphosphorylated tau favors the altered post-synaptic excitability due to the interaction between tau, Fyn, and PSD95-NMDA [56]. This interaction between tau, Fyn, and PSD95 is also consistent with other studies showing that decreasing the expression of hyperphosphorylated tau does not reduce the levels of Aβ in the hAPP transgenic mice, but significantly decreases the behavioral alterations in this murine model [57]. This tripartite relationship between tau, Fyn, and PSD95 has been corroborated by studies of whole-cell electrophysiological recordings in hippocampal slices of hAPP mice expressing and not expressing hyperphosphorylated tau [58]. These studies correlate with evidence that showed that the Fyn protein expression is significantly higher in the brain samples obtained from AD patients [59]. Despite these indications, the exact cascade of events of how this non-physiological tau phosphorylation affects neuronal function remains unsolved.
2.3. Role of the Truncated tau in the Pathogenesis of AD
During the progression of tau pathology, this protein appears to undergo several cleavage events [60]. Caspases, the expression and activity of which are elevated in the AD brain [61, 62], are likely to be involved in the proteolytic processing of tau. Previously it was shown that tau is cleaved by caspase 3 at aspartic acid 421 (D421) in the AD brain and appeared to be generated in the early stages of the pathogenic process [14, 63, 64] with an increase in neuronal death [63, 64]. More importantly, pioneer studies using multiphoton microscopy showed a transient increase in caspase 3 activity in neurons present in a tauopathies mouse model, where the formation of NFTs followed this process without affecting neuronal viability [14]. Additionally, the expression of tau truncated at D421 in wild-type mouse brain also resulted in the formation of similar tau aggregates, indicating that caspase 3 activation and tau cleavage precede the formation of tau fibrils [65]. In AD, increases in tau cleavage may compromise neuronal function. For example, inducible expression of tau truncated at D421 in a cortical neuronal cell line results in significant defects in mitochondrial function and oxidative damage [66]. More importantly, both the cleaved and hyperphosphorylated tau were colocalized in the temporal cortex of the AD brain, suggesting that caspase-3 may contribute to the cleavage of hyperphosphorylated tau (at Ser306/404) and neurodegeneration [65]. Therefore, truncated tau and phosphorylated tau could be actively present at the same time, indicating that the presence of both pathological forms could mediate tau-mediated toxicity.
Interestingly, some studies showed that tau truncated at D421 negatively influences synaptic processes causing memory deficits and cognitive disabilities [67]. This was demonstrated in young BALB / c mice (2 months old) who expressed truncated tau at a developmental level in the brain [67]. These studies indicated that mice who expressed truncated tau show a significant deficit in memory and learning tests [67]. At the same time, there was a significant loss of the dendritic spines and a decrease in the levels of the synaptic proteins: postsynaptic density 95 (PSD-95) and the integral protein of presynaptic vesicular membrane, Synaptophysin [67]. In turn, a recent study based on progressive supranuclear palsy showed a close relationship between Appoptosin (a mitochondrial protein), caspase 3 activation, and truncated tau generation [68]. The increase in truncated tau levels due to the activation of caspase-3 generated a reduction in the expression of the PSD-95 protein associated with the postsynaptic compartment, in addition to a decrease in the expression and localization of NMDA type glutamate receptors, which have active participation in the synaptic process [68].
Interestingly, appoptosin is a protein with pro-apoptotic traits that have been found to be accumulated in the brains of patients with AD [69] and is associated with the internal mitochondrial membrane [69]. Therefore, changes in the activity and location of Appoptosin could modify mitochondrial bioenergetics and ROS production, generating a highly toxic environment in neurons (Fig. 3). In general, the presence of a highly oxidative environment is characteristic of several
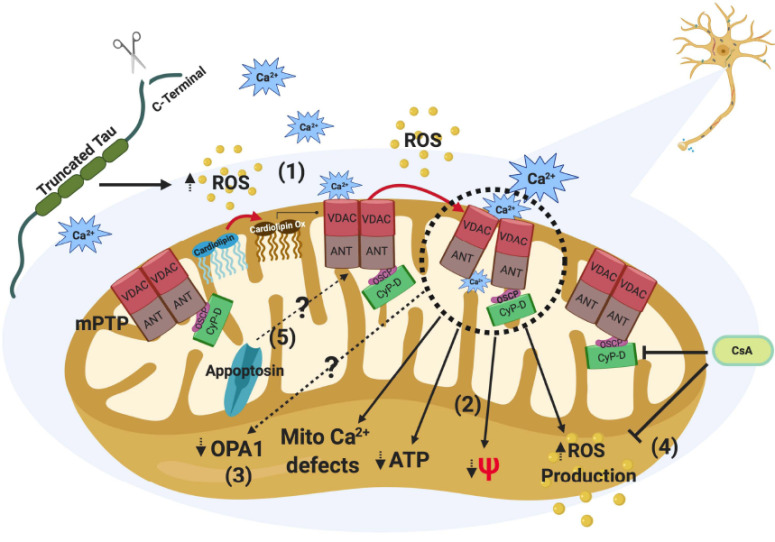
Fig. (3).
Effects of truncated tau by caspase 3 (TauC3) against mitochondria health. The presence of TauC3 through the stressors agents could increase ROS levels affecting cardiolipin function and then sensitizes the mPTP opening to calcium. High ROS levels can also induced a sustained opening of the mPTP (1). mPTP opening will impair different aspects of mitochondrial health, including ROS production, mitochondrial membrane potential (MMP), ATP production, and mitochondrial calcium regulation (2). We showed that truncated tau expression induces mitochondrial fragmentation through the reduction in Opa1 levels in neuronal cells [19]. However, it is not clear if activation of mPTP would be involved in the reduction of mitochondrial length induced by truncated tau (3). Defects in mitochondrial bioenergetics produced by TauC3 can be prevented using cyclosporine A (CsA), an immunosuppressive drug that inhibits mPTP opening (4). Interestingly, other mitochondrial components like Appoptosin, a mitochondrial carrier protein for glycine, which expression increases TauC3, can also be involved in mitochondrial dysfunction and mPTP activation induced by TauC3 in the brain (5). (A higher resolution/colour version of this figure is available in the electronic copy of the article).
neurodegenerative diseases, especially in AD [70, 71]. Therefore, it is possible that pathological forms of tau, such as truncated tau at D421, affect synaptic function through the modification of mitochondrial function and oxidative damage in the context of AD (Fig. 3).
3. Mitochondrial impairment in Alzheimer´s disease
Mitochondria is an organelle of unquestionable evolutionary transcendence, known by its function in the production of ATP and heat in cells [72]. Mitochondria contributes to calcium regulation, ROS production, cell death, detoxification, and plays an essential role in synaptic communication [72, 73, 74]. Evidence from cellular and AD mice models indicates that mitochondria could be affected through the impairment of mitochondrial dynamics, bioenergetics failure, and mitochondrial transport defects [17-23, 74-76].
3.1. Defects in Mitochondrial Dynamics During AD
Mitochondria are organelles that form an active intracellular network that undergoes continuous fission and fusion processes called mitochondrial dynamics [77]. These actions play a crucial role in the control of mitochondrial shape and number, contributing to bioenergetics and mitochondria quality control [77]. Importantly, regulation of mitochondrial dynamics is essential for cellular function and plays a pivotal role in neuronal communication through the synapse [78]. Impairment of mitochondrial dynamics and incorrect fission regulation have been identified in sporadic and familial AD [75] as well as in AD mouse models [76]. These defects are mediated in part by altered function of dynamin-like protein 1 (Drp1), a regulator of mitochondrial fission and distribution, disturbing the balance between fission and fusion of mitochondria followed by mitochondrial depletion from axons and dendrites [76].
At the same time, defects in mitochondrial fusion and proteins involved in its regulation have been reported in AD [79]. The actions of three GTPases proteins mainly control mitochondrial fusion: optic atrophy protein (Opa1) and mitofusins 1 and 2 (Mfn1 and Mfn2) [77]. Opa1 contributes to the fusion of the inner mitochondrial membranes, and the process is dependent on appropriate proteolytic processing of this protein [77, 80]. Also, relevant evidence suggests that Opa1 contributes to the synaptic process in the hippocampus and other brain areas [81]. Previous studies from our group have suggested an interesting relationship between neuronal dysfunction and the impairment of mitochondrial fusion induced by the presence of truncated tau at 421D [18-20]. We showed that the expression of this tau form induced mitochondrial fragmentation in immortalized cortical neurons and hippocampal neurons from rats [20], mice [18, 19], and tau knock out (-/-) mice [19]. In these models, mitochondrial fragmentation induced by truncated tau contributed to mitochondrial depolarization and oxidative stress induced by calcium stress [20] and Aβ treatment [19]. Importantly, the presence of truncated tau at 421D induced a significant decrease in Opa1 expression, which could contribute to the mitochondrial fragmentation observed in these neurons [19].
Mitochondrial dynamics defects have been observed in other neurodegenerative disorders that significantly present the accumulation of pathological forms of tau, including Huntington’s disease (HD) [82] and Traumatic Brain Injury [83]. HD is an autosomal dominant inherited disease caused by an abnormal expansion of CAG repeats in exon 1 of the Huntingtin (Htt) gene located on chromosome 4p16.3, resulting in a pathological elongation of polyglutamine in the Htt protein [84]. Regularly, HD has not been indicated as a tauopathy; however, further analysis of HD brain samples has shown the presence of NFTs [85-87]. More importantly, these observations have corroborated in cell and animal models of HD [88, 89]. Bioenergetic defects mediated by mitochondria are a characteristic of the HD brain [84]. Early ultrastructural studies of cortical biopsies from HD patients showed abnormal mitochondria [90], and mitochondrial functional abnormalities were observed as well [91]. Also, defects in mitochondrial dynamics in HD have been extensively reported [92]. Mutant striatal cells (STHdhQ111/ Q111) expressing mutant huntingtin (mHtt) showed mitochondrial fragmentation and impairment of mitochondrial fusion measured with the mitochondrial photo-switchable Dendra [93]. More importantly, these changes were accompanied by alterations in the expression levels of Drp1 and Opa1, critical regulators of mitochondrial fission and fusion, respectively [93]. Also, studies by Kim et al., [94] and the Reedy’s lab have shown an increase in the expression of Drp1, Fis1, and a decrease in the levels of Mnf1/2 and Opa1 in HD late-stage patients brain [95-97]. Further studies from this group showed increased mRNA levels of Drp1 and Fis1 and decreased levels of fusion genes Mnf1/2 in 2-month-old BACHD mice [96] (a mouse model that expresses the full-length human Htt gene with 97 CAA and CAG) [98], which suggests that the impairment of mitochondrial dynamics could be an early event in HD. Also, additional studies by Costa et al., showed that clonal striatal cells that express wild type and mHtt (STHdhQ7/Q7 and STHdhQ111/Q111) showed an increase in calcineurin activity which induces dephosphorylation of Drp1, increasing its mitochondrial translocation and leading to the fragmentation of the organelle [99].
Traumatic brain injury (TBI) is considered a public health concern associated with mid-term and long-term disabilities and neurodegeneration [100]. These neuropathological changes resemble AD brains, and they have been found in postmortem brains of patients suffering from TBI and chronic traumatic encephalopathy (CTE) [101]. These pathological signs include an increase in hyperphosphorylated tau levels and, in some cases, Aβ deposits [102, 103]. Indeed, recent consensus criteria consider TBI as a tauopathy [101]. Interestingly, mitochondrial impairment and oxidative stress are both considered essential contributors to neuropathological changes showed in TBI [104]. More important, changes in mitochondrial dynamics have been reported in different study models of TBI [105, 106]. For example, TBI animals showed an increase in Drp1 levels in hippocampal mitochondria extracts as compared to untreated animals [106]. Interestingly, electronic microscopy analysis of hippocampal tissue from TBI animals showed a significant decrease in mitochondrial length at 72 h post-TBI [106]. Also, treatment with the mitochondrial division inhibitor-1 (Mdivi-1), a pharmacological inhibitor of Drp1, prevented this decrease in mitochondria length in TBI animals [106].
Complementary studies by Wu et al., showed that the inhibition of Drp1 could attenuate TBI-induced neuropathological changes by inhibiting the fragmentation of mitochondria and activation of apoptosis [107]. Also, the same group using a cell model for TBI showed that treatment with Mdivi-1 reduced scratch injury-induced cell death, mitochondrial depolarization, ROS production, and ATP reduction in primary cortical neurons (PCNs) [108]. These findings suggest that changes in mitochondrial dynamics could represent a common mechanism in neurodegenerative disorders that show an evident tau pathology. Although in HD and TBI, the contribution of tau pathology to mitochondrial dynamics deficiency is unknown and requires to be investigated.
3.2. Mitochondrial Bioenergetics Impairment in AD
Excessive generation of ROS and reactive nitrogen species (RNS), including superoxide anion (O 2 -) and NO, contribute to neuronal dysfunction and mitochondrial bioenergetics failure in AD [109]. Increased nitrosative stress can result in defects in mitochondrial function. For example, S-nitrosylation affects mitochondrial respiration by inhibiting complexes I and IV [110, 111]. Also, in AD transgenic models, like APPSw transgenic strain Tg2576, the up-regulation of genes related to mitochondrial metabolism was observed at two months of age [112]. Also, decreased expression of mitochondrial respiratory chain complexes (I and III), and impairment of mitochondrial respiration are detected at around six months of age before Aβ plaque formation [113-114], indicating that mitochondrial dysfunction and oxidative stress may play a role in early stages of AD.
3.3. Defects in Mitochondrial Transport in AD
Axonal mitochondrial transport is crucial for neuronal maintenance and synaptic function, and its dysregulation can contribute to AD [115]. Mitochondrial transport is regulated by a series of molecular adaptors that mediate the union of mitochondria to molecular motors [116-119]. In Drosophila, mitochondrial transport is facilitated by two proteins: Milton and Miro, which regulate mitochondrial attachment to microtubules via kinesin heavy chain [116]. In mammals, two isoforms of Milton (OIP106 and GRIF1) and Miro (Miro1 and Miro2) have been identified and are proposed to act similarly [118]. In Drosophila, in the absence of Milton or Miro, synaptic terminals and axons lack mitochondria, although mitochondria are numerous in the neuronal cell body [119]. Interestingly, studies showed a reduction in the number of mitochondria in the axon in the brains of AD patients [120], and we and others reported that Aβ treatment reduced the number of mitochondria in the axons of hippocampal neurons [121, 122].
Importantly, several reports have suggested an essential role of tau in regulating mitochondrial transport in neuronal cells. For example, overexpression of the human tau gene in wild type mice reduced mitochondrial movement through the axon [123, 124]. Also, the expression of hyperphosphorylated tau at AT8 epitopes (Ser199, Ser202, and Thr205) reduced mitochondrial movement in cultured neurons [125]. More importantly, we previously showed that the expression of caspase-cleaved tau impaired mitochondrial anterograde/retrograde transport, reducing the number of moving organelles through the neuronal processes [20, 122]. Interestingly, these adverse effects against mitochondrial transport induced by hyperphosphorylated and truncated tau presence could be enhanced by a failure in tau-Microtubules (MT) physiological interaction, induced by these pathological tau modifications present in the axon [126].
Even though mitochondrial impairment is a multifactorial event, the bioenergetics function of mitochondria is vital to produce ATP and provide with calcium regulation for synapse. In this context, recent studies have suggested the participation of specific mitochondria structures in the neurodegenerative changes in AD.
3.4. Contribution of Mitochondrial Permeability Transition Pore on Neurodegeneration Present in AD
The mitochondrial permeability transition pore (mPTP) is a non-specific channel located on the inner mitochondrial membrane, which is formed by cyclophilin D (CypD), the voltage-dependent anion channel (VDAC) and the ATP synthase oligomycin sensitivity conferring protein (OSCP) subunit [127, 128]. This structure participates in the mitochondrial permeability process that results in the release of mitochondrial content and apoptosis induced by several stressors [128, 129]. It has been suggested that in some neurological disorders, the impairment of calcium homeostasis and oxidative stress induces mPTP opening, which results in increased permeability to different molecules affecting ROS production, mitochondrial membrane potential, and ATP production [130-132]. Current evidence has suggested that CypD is a structural protein that participates in the formation of the mPTP [131, 132]. Under normal conditions, CypD is confined to the mitochondrial matrix. However, in the presence of calcium overload or an increase in ROS, CypD binds to VDAC and ATP synthase (OSCP) inducing mitochondrial permeability [132]. This process drives to mitochondrial depolarization, increases ROS levels, and later affects ATP production [127, 128]. Notably, several studies have described an interesting association between mitochondrial failure and mPTP in the AD context [133, 134]. For example, studies on the postmortem brains of AD patients and AD animal models showed increased levels of CypD [27, 29]. These studies are critical because an increase in CypD expression has been suggested as a principal factor in the activation of mPTP [27, 29].
Moreover, the crossing of the amyloid precursor protein (mAPP) transgenic mice, which is a genetic model of AD that generates Aβ plaques, with a CypD knock out (-/-) mice, resulted in a complete restoration of mitochondrial dysfunction and cognitive impairment observed in mAPP mice expressing normal levels of CypD [130, 131]. Complementary evidence of hippocampal neurons from the mAPPxCypD(-/-) mice showed a significant reduction in mitochondrial dysfunction and an increase in ATPase activity compared to mature hippocampal neurons obtained from mAPP mice [134]. More importantly, we studied mitochondrial dysfunction in fibroblasts from AD patients as a way to explore new biomarkers for AD [135]. Fibroblasts share a common developmental origin with neurons, and they can present similar metabolic changes experimented by neurons in the AD brain [135]. Interestingly, we observed that AD fibroblasts showed mitochondrial depolarization, calcium dysregulation, and reduction in ATP levels compared with aged-match control fibroblasts [135, 136]. More importantly, these negative changes showed in mitochondria were prevented using cyclosporine A (CsA), an immunosuppressive drug that inhibits mPTP opening, indicating an essential role of this structure in the pathogenesis of AD [136].
4. Pathological forms of tau affect mitochondrial function in AD
Accumulative evidence suggests that mitochondrial impairment is an active contributor to the pathogenesis of several neurodegenerative diseases [137] and AD [17-23]. Mitochondrial failure has been widely associated with the development and progression of several neurodegenerative diseases where the presence of pathological tau has been shown to affect mitochondrial bioenergetics [reviewed in 138]. For example, in triple transgenic mice (3xTg-AD), used as a murine model for AD studies, a significant decrease in pyruvate dehydrogenase (PDH) and cytochrome c oxidase (COX) levels was found compared to non-transgenic mice [139]. These changes suggest a decrease in mitochondrial bioenergetics, demonstrated through the mitochondrial respiration ratio and the measurement of the rate of the free-radical leakage [139]. At the same time, isolated brain mitochondria from 3xTg-AD female mice showed elevated levels of ROS, with an increase in the production of hydrogen peroxide and lipid oxidation, compared to the control group [139]. Previously studies showed that Post-Morten brain tissue of AD patients, presented a significant increase in mitochondrial DNA and cytochrome oxidase, accompanied by oxidative damage; expressed in high levels of 8-OHG and nitrotyrosine in hippocampal neurons and cerebral neocortex [140].
In the same context, defects in mitochondrial dynamics have been reported in different AD mice models expressing tau pathology. For example, neuronal cells obtained from mice that overexpress human tau (hTau) showed enhanced mitochondrial fusion accompanied by an increase in Mnf1/2 and Opa1 expression [141]. Also, the expression of P301L mutant tau modified mitochondrial dynamics, reducing the expression of both fission and fusion protein regulators [142]. The brain tissue obtained from APP, APP/PS1, and 3Xtg AD mice showed an increase in the Drp1 binding with hyperphosphorylated tau, the interaction of which enhanced mitochondrial dysfunction and synaptic impairment in these AD mice models [143]. Finally, transgenic mice overexpressing P301L mutant tau showed a significant modification of mitochondrial and antioxidant protein levels, including a decrease in the expression of complex V and the reduction of mitochondrial respiratory capacity and ATP production [144].
Also, it has been shown that the reduction of tau expression has positive effects on mitochondrial health of the brain [34, 145, 146]. Tau reduction improved mitochondrial transport in the axons of hippocampal neurons obtained from APP transgenic mice [34]. Also, the genetic ablation of tau prevented dendritic spine loss and cognitive decline induced by chronic stress in wild type mice [145]. Interestingly, this study also showed that synaptic-related tissue from tau knock out (-/-) mice submitted to chronic stress presented an increase in mitochondrial transport and antioxidant proteins compared with wild type animals exposed to the same stressor [145]. Finally, we determined that tau young tau (-/-) mice presented an enhanced mitochondrial function, increased activation of antioxidant pathways (Nrf-2 and PGC1-α), reduced oxidative damage, and increased ATP production in the hippocampus compared to wild type animals [146]. More important, young tau (-/-) mice showed better cognitive and memory performance compared to wild type animals [146]. All these findings suggest an essential role of tau protein in the regulation of mitochondrial function and its potential impact on cognitive and memory performance.
4.1. ROS as a Mediator of Mitochondrial Impairment Induced by Tau Pathology
It has been shown that pathological forms of tau facilitate ROS production and jointly affect several parameters of mitochondrial health [147]. For example, the transgenic expression of truncated human tau protein, specifically in rat neurons, alters the mitochondrial distribution and decreases neuronal viability under exogenous oxidative stress conditions [147]. Also, it has been established that the expression of mutated tau at P301L in SY5Y cells affects mitochondrial respiratory activity, decreases baseline ATP levels and mitochondrial membrane potential, parameters that worsen when cells are subjected to ROS stress by external addition of hydrogen peroxide [142]. Additionally, a change in mitochondrial structure occurs, and mitochondrial dynamics regulation is affected in neuronal cells expressing tau P301L [142].
Interestingly, other studies suggest that the lack of antioxidant “counter-attack” actions facilitates the production and accumulation of pathological forms of tau in the brain. For example, knockout SOD2 mice (SOD2-/-) exhibited significantly higher levels of hyperphosphorylated tau accumulation, when animals were treated with low doses of an antioxidant catalyst, administered through intraperitoneal injection, compared to those with SOD2 mice (-/-) treated with high doses of the same antioxidant [148]. In this same study, they also crossed SOD2 (-/-) with Tg2576 transgenic mice expressing the human APP (amyloid precursor protein), thus obtaining mice lacking SOD2 and prone to Aβ accumulation. These experiments showed an increased accumulation of Aβ peptide and elevated levels of hyperphosphorylated tau in the brain [148].
On the other hand, several studies have indicated the age-dependent nature of the deleterious effects produced by the synergy between tau and Aβ. For example, in the early stages of life, triple transgenic-AD mice (8 months old) showed a reduction in mitochondrial membrane potential levels, while middle-age (12 months-old) animals exhibited a significant compromise on ATP production and excessive ROS levels [149]. In the same context, previous studies have studied the harmful effects of P301L mutant tau expression on mitochondrial health [144]. Mitochondria isolated from the brain of 24-month-old transgenic mice showed a deficit in ATP levels and an increase in ROS levels, which was not observed in samples obtained from wild type 12-month-old mice [1-44]. Interestingly, in a study conducted by our group, the protective nature of the deletion of tau against mitochondria function was determined [146]. We showed that the litter of young tau knock out (-/-) mice exhibited lower oxidative damage, improvements in recognition memory and attention capacity and higher ATP levels compared to wild type-age match animals [146].
It is noteworthy that, under oxidative stress conditions, neurons have the natural capacity to generate antioxidant enzymes in order to counteract the damage [150]. The inducible expression of genes that encode for these detoxifying enzymes is commanded by Nrf-2 [151], the master regulator of redox homeostasis [152, 153]. In this regard, the pharmacological use of sulforaphane, a potent activator of the Nrf-2 pathway, significantly reduced the presence of abnormal tau in both hippocampal neuronal cultures and immortalized cortical cell (CN1.4) [154]. In the same context, complementary studies showed that treatment with sulforaphane reduced memory impairment and increased synaptophysin levels affected by treatment with oral doses of Fe2+ [155].
Whereas mitochondrial impairment is accompanied by oxidative damage in AD and Parkinson's disease [156-159], the use of the mitochondria-targeted antioxidant MitoQ (mitoquinone mesylate: [10- (4, 5-dimethoxy-2-methyl- 3, 6-dioxo-1, 4-cyclohexadienyl) decyl triphenylphosphonium methanesulfonate]) has been proposed to ameliorate oxidative damage produced by the presence of abnormal tau [160]. For example, the treatment with MitoQ of cortical neurons from a murine model for AD prevented mitochondrial depolarization, increased ROS, and reduced Aβ-induced neurotoxicity [160].
4.2. Pathological forms of Tau Interact with Mitochondria and Induce Neurodegeneration and Bioenergetics Failure
Previously, we observed that the genetic reduction of tau could have positive effects on mitochondrial function and ATP production [146]. In the same context, our group studied the effects of caspase-cleaved tau at D421 on mitochondrial and calcium regulation in immortalized cortical neurons [18]. We induced the expression of full-length tau (T4) or D421-truncated tau in immortalized cortical neurons (CN 1.4), observing that cells expressing truncated tau showed a significant decrease in the mitochondrial length and mitochondrial membrane potential compared to cells expressing full-length tau [18] (Fig. 3). Also, truncated tau expressing cells showed an increase in the ROS levels compared to full-length tau [18]. Then, we showed that in primary cortical neurons, the expression of truncated tau induced mitochondrial fragmentation [20]. In contrast, when this tau form was expressed and challenged with sublethal doses of peptide amyloid-beta (Aβ), we observed an increase in the ROS levels and mitochondrial depolarization [20]. Interestingly, these results were corroborated in primary hippocampal cultures from tau (-/-) mice where the expression of truncated tau induced mitochondrial fragmentation and deregulation of mitochondrial dynamics evidenced by a decrease in Opa-1 expression [19] (Fig. 3).
4.3. A Possible Role of mPTP on Mitochondrial Dysfunction Induced by Tau Pathology
News studies have suggested that the effects of tau pathology against neurons could be mediated by the mPTP activation in AD [18, 23, 161, 162]. For example, immunoprecipitation studies from Reddy´s group using AD brains, and brain tissue from APP/PS1 and 3xTg.AD mice showed a significant binding of VDAC with aggregates of hyperphosphorylated tau obtained from the brain extracts of AD patients, APP/PS1, and 3xTg. AD transgenic mice [23] (Fig. 2). These changes were accompanied by a significant mitochondrial dysfunction in all AD-related brain samples compared to age-matched control samples [23]. Also, further studies demonstrated that a cleaved N-terminal fragment of tau could interact with some components of the mPTP leading to mitochondrial failure and calcium handling defects [161, 162]. More importantly, we have been studying the possible role of tau protein in the regulation of mitochondrial function through the opening of the mPTP [146]. Our studies showed that the genetic ablation of tau improves mitochondrial function and cognitive performance in the hippocampus of tau (-/-) mice [146]. Interestingly, we observed that tau (-/-) mice showed decreased levels of CypD (protein and mRNA) compared to WT mice. Also, hippocampal tissues from tau (-/-) mice showed a reduction in oxidative damage and an increase in ATP production compared to wild type mice with regular tau expression [146]. These are significant findings because a reduction in CypD expression is considered the most efficient way to prevent mPTP opening [163-165].
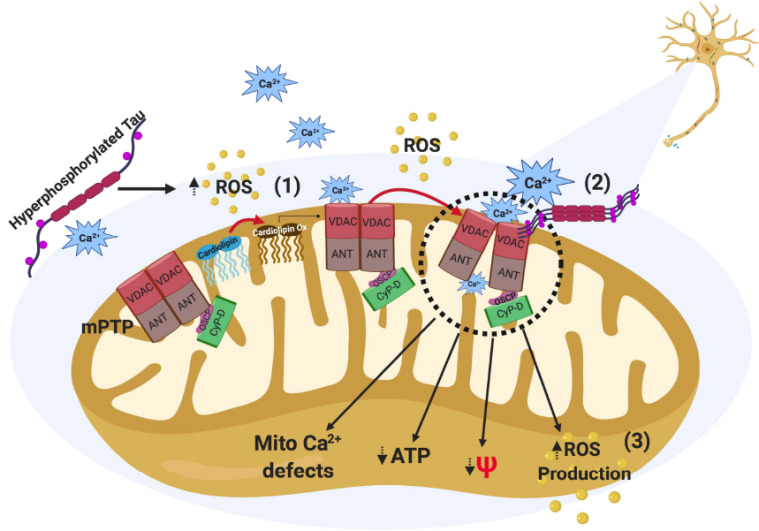
Fig. (2).
Effects of hyperphosphorylated tau against mitochondria function. The presence of tau modifications like hyperphosphorylation (p-Tau) increases ROS levels, which could affect cardiolipin, a mitochondrial membrane phospholipid, increasing its oxidation. This modification may contribute to increasing the sensitivity of mitochondrial permeability transition pore (mPTP) to calcium and then produces its opening (1). Interestingly, it has been proposed that the presence of p-Tau in neurofibrillary tangles from AD patients interacts with voltage-dependent anion channel (VDAC), which is an important protein component of mPTP, producing a sustained opening state of the mPTP (2). The opening of mPTP impairs mitochondrial health inducing ROS production, mitochondrial membrane potential (MMP) loss, ATP reduction, and mitochondrial calcium handling defects (3). (A higher resolution/colour version of this figure is available in the electronic copy of the article).
Several studies indicate that mPTP is a structure highly sensitive to the increase of calcium levels in the mitochondrial matrix, suggesting that its transient opening would participate in the regulation of calcium levels in the cell [163-165]. A sustained mPTP opening allows the intra-mitochondrial calcium release toward the cytosol. An increase in cytosolic calcium will disrupt the signaling pathways dependent on this element because of the over activation of the calcium-dependent enzymes [165]. Furthermore, the deacetylation of CypD, due to the decrease in the Sirt3 enzyme, could facilitate the interaction of CypD with ANT, which increases the affinity of CypD with calcium, resulting in the mPTP opening [165]. Mitochondrial calcium handling defects produced by mPTP activation were observed in cortical neurons that natively expressed cleaved tau at D421 [19]. Cells expressing this tau form exposed to a minimal concentration of thapsigargin (1 μm) showed a significant decrease in mitochondrial calcium uptake and mitochondrial potential loss compared with cells expressing full-length tau [19]. Interestingly, these adverse effects were entirely prevented by CsA (0.5 μM 2h), a pharmacological inhibitor of mPTP, in cells expressing caspase-cleaved tau [19]. These observations indicate that caspase-cleaved tau affects mitochondrial function through the activation of mPTP, which has negative consequences for calcium regulation and ATP synthesis in the mitochondria.
In the same context, current studies have linked aberrant modifications of Cardiolipin, a phospholipid located at the mitochondrial membrane, due to incubation with soluble oligomeric tau forms [166]. Mitochondrial extracts exposed to these tau forms showed loss of mitochondrial integrity, swelling, and mitochondrial depolarization [166]. However, mitochondrial dysfunction was prevented with 10-N-nonyl acridine orange (NAO), a compound that specifically binds to cardiolipin at the mitochondria [166]. These positive effects on mitochondria are produced because of NAO capacity to block the binding of tau oligomeric forms to the mitochondria [166]. These findings suggest that tau pathology could affect mitochondria function through an enhanced association between these organelle and tau forms.
Finally, our previous studies showed that the expression of truncated D421 and hyperphosphorylated tau forms affect mitochondrial function, inducing fragmentation, depolarization, and increasing the ROS production in response to different stressors [18, 20]. Additionally, we have shown that the harmful effects on mitochondrial health, specifically mitochondrial fragmentation, depolarization, and mitochondrial calcium handling defects induced by the constitutive expression of truncated tau, can be prevented using the drug Cyclosporine A (CsA) [18], an immunosuppressive drug that prevents opening of the mPTP through the inhibition of the union of CypD with other elements that form part of mPTP [163-165] (Fig. 3). Also, it has been shown that the genetic deletion of CypD acts favorably on mitochondrial impairment and synaptic deterioration in different AD mice models [132-134]. For example, the crossing of knock out mice for CypD [164] with APP/PS1 AD model, which generates a high accumulation of Aβ peptide [132], prevented mitochondrial failure and induced a significant improvement in learning and memory tests, compared to APP/PS1 mice that express CypD in its endogenous form [130].
In the same context, antecedents published by Manczak and Reddy showed that the expression levels of VDAC, another protein component of mPTP, were significantly higher in brains of patients with AD compared to age-matched control samples [23]. Importantly, they found an increased co-localization and interaction between hyperphosphorylated tau and VDAC in AD brain samples, which was similarly observed in the brain tissue of 3xTgAD triple transgenic mice, a murine model for the study of AD [23] (Fig. 2). Therefore, these findings suggest that the accumulation of hyperphosphorylated tau interacts with VDAC, which would produce a mitochondrial deficit, expressed through parameters such as an increase in superoxide production, oxidative damage, and low ATP production induced by mPTP activation.
Conclusion
In this paper, we discussed evidence that suggests a vital role of pathological forms of tau in mitochondrial failure in AD. Mitochondria is essential for neuronal function, and their bioenergetics properties are vital for synapsis. Pathological forms of tau affect mitochondria health, modifying dynamics, transport, and ATP production. Evidence analyzed here suggests that these anomalous tau forms can be pathologically associated with mitochondria through different proteins that form the mPTP (see Figs. 2 and 3). mPTP is a mitochondrial structure in which opening responds to calcium increase, and high ROS levels, which are regular signs of neurodegeneration in AD. Previously, we showed that tau regulates mitochondrial function at physiological levels, and in this process, CypD contributes, a vital protein in the activation of mPTP. Therefore, it is possible that pathological modifications of tau facilitate the opening of the mPTP (see Figs. 2 and 3), causing mitochondrial failure, which leads to neurodegeneration and synaptic failure observed in AD.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
This work was supported by Fondo de Ciencia y Tecnología (FONDECYT), Chile (Grant No. 1170441, 1200178) (RAQ), and Ph.D. fellowship from Universidad Autónoma de Chile (CTM).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461(7266):895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 3.Kosik K.S., Joachim C.L., Selkoe D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1986;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ihara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J. Biochem. 1986;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- 5.Mandelkow E-M., Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8(11):425–427. doi: 10.1016/S0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 6.Tapia-Rojas C., Cabezas-Opazo F., Deaton C.A., Vergara E.H., Johnson G.V.W., Quintanilla R.A. It’s all about tau. Prog. Neurobiol. 2019;175:54–76. doi: 10.1016/j.pneurobio.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritchard S.M., Dolan P.J., Vitkus A., Johnson G.V.W. The toxicity of tau in Alzheimer disease: turnover, targets and potential therapeutics. J. Cell. Mol. Med. 2011;15(8):1621–1635. doi: 10.1111/j.1582-4934.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polanco J.C., Li C., Bodea L-G., Martinez-Marmol R., Meunier F.A., Götz J. Amyloid-β and tau complexity — towards improved biomarkers and targeted therapies. Nat. Rev. Neurol. 2017;•••:1–18. doi: 10.1038/nrneurol.2017.162. [DOI] [PubMed] [Google Scholar]
- 9.Dorval V., Fraser P.E. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J. Biol. Chem. 2006;281(15):9919–9924. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 10.Min S.W., Cho S.H., Zhou Y., Schroeder S., Haroutunian V., Seeley W.W., Huang E.J., Shen Y., Masliah E., Mukherjee C., Meyers D., Cole P.A., Ott M., Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledesma M.D., Pérez M., Colaco C., Avila J. Tau glycation is involved in aggregation of the protein but not in the formation of filaments. Cell. Mol. Biol. 1998;44(7):1111–1116. [PubMed] [Google Scholar]
- 12.Takahashi M., Tsujioka Y., Yamada T., Tsuboi Y., Okada H., Yamamoto T., Liposits Z. Glycosylation of microtubule-associated protein tau in Alzheimer’s disease brain. Acta Neuropathol. 1999;97(6):635–641. doi: 10.1007/s004010051040. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds M.R., Berry R.W., Binder L.I. Nitration in neurodegeneration: deciphering the “Hows” “nYs. Biochemistry. 2007;46(25):7325–7336. doi: 10.1021/bi700430y. [DOI] [PubMed] [Google Scholar]
- 14.de Calignon A., Fox L.M., Pitstick R., Carlson G.A., Bacskai B.J., Spires-Jones T.L., Hyman B.T. Caspase activation precedes and leads to tangles. Nature. 2010;464(7292):1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai H.C., Wang B.Y., Serrano-Pozo A., Frosch M.P., Spires-Jones T.L., Hyman B.T. Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer’s disease. Acta Neuropathol. Commun. 2014;2:146. doi: 10.1186/s40478-014-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai H.C., Serrano-Pozo A., Hashimoto T., Frosch M.P., Spires-Jones T.L., Hyman B.T. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am. J. Pathol. 2012;181(4):1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabezas-Opazo F.A., Vergara-Pulgar K., Pérez M.J., Jara C., Osorio-Fuentealba C., Quintanilla R.A. Mitochondrial dysfunction contributes to the pathogenesis of alzheimer’s disease. Oxid. Med. Cell. Longev. 2015;2015509654 doi: 10.1155/2015/509654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintanilla R.A., Matthews-Roberson T.A., Dolan P.J., Johnson G.V. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J. Biol. Chem. 2009;284(28):18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez M.J., Vergara-Pulgar K., Jara C., Cabezas-Opazo F., Quintanilla R.A. Caspase-cleaved tau impairs mitochondrial dynamics in alzheimer’s disease. Mol. Neurobiol. 2018;55(2):1004–1018. doi: 10.1007/s12035-017-0385-x. [DOI] [PubMed] [Google Scholar]
- 20.Quintanilla R.A., Dolan P.J., Jin Y.N., Johnson G.V. Truncated tau and Aβ cooperatively impair mitochondria in primary neurons. Neurobiol. Aging. 2012;33(3):619.e25–619.e35. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calkins M.J., Manczak M., Mao P., Shirendeb U., Reddy P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2011;20(23):4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy P.H., McWeeney S., Park B.S., Manczak M., Gutala R.V., Partovi D., Jung Y., Yau V., Searles R., Mori M., Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum. Mol. Genet. 2004;13(12):1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 23.Manczak M., Reddy P.H. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum. Mol. Genet. 2012;21(23):5131–5146. doi: 10.1093/hmg/dds360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 25.Busciglio J., Lorenzo A., Yeh J., Yankner B.A. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14(4):879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 26.Clark L.N., Poorkaj P., Wszolek Z., Geschwind D.H., Nasreddine Z.S., Miller B., Li D., Payami H., Awert F., Markopoulou K., Andreadis A., D’Souza I., Lee V.M., Reed L., Trojanowski J.Q., Zhukareva V., Bird T., Schellenberg G., Wilhelmsen K.C. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc. Natl. Acad. Sci. USA. 1998;95(22):13103–13107. doi: 10.1073/pnas.95.22.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R.C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J.M., Nowotny P., Che L.K., Norton J., Morris J.C., Reed L.A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P.R., Hayward N., Kwok J.B., Schofield P.R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B.A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport M., Dawson H.N., Binder L.I., Vitek M.P., Ferreira A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pallo S.P., Johnson G.V. Tau facilitates Aβ-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons. Neurosci. Lett. 2015;597:32–37. doi: 10.1016/j.neulet.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pallo S.P., DiMaio J., Cook A., Nilsson B., Johnson G.V.W. Mechanisms of tau and Aβ-induced excitotoxicity. Brain Res. 2016;1634:119–131. doi: 10.1016/j.brainres.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopeikina K.J., Hyman B.T., Spires-Jones T.L. Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 2012;3(3):223–233. doi: 10.2478/s13380-012-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McGowan E., Forster C., Yue M., Orne J., Janus C., Mariash A., Kuskowski M., Hyman B., Hutton M., Ashe K.H. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 34.Vossel K.A., Zhang K., Brodbeck J., Daub A.C., Sharma P., Finkbeiner S., Cui B., Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolarova M., García-Sierra F., Bartos A., Ricny J., Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers Dis. 2012;2012731526 doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso A.C., Li B., Grundke-Iqbal I., Iqbal K. Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2008;5(4):375–384. doi: 10.2174/156720508785132307. [DOI] [PubMed] [Google Scholar]
- 37.Di Xia Li, C.; Götz, J. (2015). Pseudophosphorylation of Tau at distinct epitopes or the presence of the P301L mutation targets the microtubule-associated protein Tau to dendritic spines. BBA Mol of Dis. 2015;1852(5):913–924. doi: 10.1016/j.bbadis.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Jin M., Shepardson N., Yang T., Chen G., Walsh D., Selkoe D.J. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. USA. 2011;108(14):5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jope R.S., Johnson G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Spittaels K., Van den Haute C., Van Dorpe J., Geerts H., Mercken M., Bruynseels K., Lasrado R., Vandezande K., Laenen I., Boon T., Van Lint J., Vandenheede J., Moechars D., Loos R., Van Leuven F. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 2000;275(52):41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- 41.Lucas J.J., Hernández F., Gómez-Ramos P., Morán M.A., Hen R., Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20(1-2):27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 43.Selkoe D.J. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 44.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. 1976. [DOI] [PubMed]
- 45.Albuquerque E.X., Pereira E.F., Alkondon M., Rogers S.W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 2009;89(1):73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sze C.I., Troncoso J.C., Kawas C., Mouton P., Price D.L., Martin L.J. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997;56(8):933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Ferrer I., Gullotta F. Down’s syndrome and Alzheimer’s disease: dendritic spine counts in the hippocampus. Acta Neuropathol. 1990;79(6):680–685. doi: 10.1007/BF00294247. [DOI] [PubMed] [Google Scholar]
- 48.Dorostkar M.M., Zou C., Blazquez-Llorca L., Herms J. Analyzing dendritic spine pathology in Alzheimer’s disease: problems and opportunities. Acta Neuropathol. 2015;130(1):1–19. doi: 10.1007/s00401-015-1449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasai H., Fukuda M., Watanabe S., Hayashi-Takagi A., Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33(3):121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Hoover B.R., Reed M.N., Su J., Penrod R.D., Kotilinek L.A., Grant M.K., Pitstick R., Carlson G.A., Lanier L.M., Yuan L.L., Ashe K.H., Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L., McInnes J., Wierda K., Holt M., Herrmann A.G., Jackson R.J., Wang Y-C., Swerts J., Beyens J., Miskiewicz K., Vilain S., Dewachter I., Moechars D., De Strooper B., Spires-Jones T.L., De Wit J., Verstreken P. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 2017;8:15295. doi: 10.1038/ncomms15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McInnes J., Wierda K., Snellinx A., Bounti L., Wang Y.C., Stancu I.C., Apóstolo N., Gevaert K., Dewachter I., Spires-Jones T.L., De Strooper B., De Wit J., Zhou L., Verstreken P. Synaptogyrin-3 mediates presynaptic dysfunction induced by tau. Neuron. 2018;97(4):823–835.e8. doi: 10.1016/j.neuron.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Balaji V., Kaniyappan S., Krüger L., Irsen S., Tepper K., Chandupatla R., Maetzler W., Schneider A., Mandelkow E., Mandelkow E-M. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017;12(1):5–5. doi: 10.1186/s13024-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeVos S.L., Corjuc B.T., Oakley D.H., Nobuhara C.K., Bannon R.N., Chase A., Commins C., Gonzalez J.A., Dooley P.M., Frosch M.P., Hyman B.T. Synaptic tau seeding precedes tau pathology in human alzheimer’s disease brain. Front. Neurosci. 2018;12:267. doi: 10.3389/fnins.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatch R.J., Wei Y., Xia D., Götz J. Hyperphosphorylated tau causes reduced hippocampal CA1 excitability by relocating the axon initial segment. Acta Neuropathol. 2017;133(5):717–730. doi: 10.1007/s00401-017-1674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mondragón-Rodríguez S., Trillaud-Doppia E., Dudilot A., Bourgeois C., Lauzon M., Leclerc N., Boehm J. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J. Biol. Chem. 2012;287(38):32040–32053. doi: 10.1074/jbc.M112.401240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., Eckert A., Staufenbiel M., Hardeman E., Götz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 58.Roberson E.D., Halabisky B., Yoo J.W., Yao J., Chin J., Yan F., Wu T., Hamto P., Devidze N., Yu G-Q., Palop J.J., Noebels J.L., Mucke L. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci. 2011;31(2):700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirazi S.K., Wood J.G. The protein tyrosine kinase, fyn, in Alzheimer’s disease pathology. Neuroreport. 1993;4(4):435–437. doi: 10.1097/00001756-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 60.Basurto-Islas G., Luna-Muñoz J., Guillozet-Bongaarts A.L., Binder L.I., Mena R., García-Sierra F. Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2008;67(5):470–483. doi: 10.1097/NEN.0b013e31817275c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohn T.T., Head E., Nesse W.H., Cotman C.W., Cribbs D.H. Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol. Dis. 2001;8(6):1006–1016. doi: 10.1006/nbdi.2001.0449. [DOI] [PubMed] [Google Scholar]
- 62.Rohn T.T., Rissman R.A., Davis M.C., Kim Y.E., Cotman C.W., Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol. Dis. 2002;11(2):341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 63.Gamblin T.C., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A.L., Lu M., Fu Y., Garcia-Sierra F., LaPointe N., Miller R., Berry R.W., Binder L.I., Cryns V.L. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2003;100(17):10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rissman R.A., Poon W.W., Blurton-Jones M., Oddo S., Torp R., Vitek M.P., LaFerla F.M., Rohn T.T., Cotman C.W. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest. 2004;114(1):121–130. doi: 10.1172/JCI200420640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fasulo L., Ugolini G., Cattaneo A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. J. Alzheimers Dis. 2005;7(1):3–13. doi: 10.3233/JAD-2005-7102. [DOI] [PubMed] [Google Scholar]
- 66.Matthews-Roberson T.A., Quintanilla R.A., Ding H., Johnson G.V. Immortalized cortical neurons expressing caspase-cleaved tau are sensitized to endoplasmic reticulum stress induced cell death. Brain Res. 2008;1234:206–212. doi: 10.1016/j.brainres.2008.07.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y., Choi H., Lee W., Park H., Kam T.I., Hong S.H., Nah J., Jung S., Shin B., Lee H., Choi T.Y., Choo H., Kim K.K., Choi S.Y., Kayed R., Jung Y.K. Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol. Dis. 2016;87:19–28. doi: 10.1016/j.nbd.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y., Tseng I.C., Heyser C.J., Rockenstein E., Mante M., Adame A., Zheng Q., Huang T., Wang X., Arslan P.E., Chakrabarty P., Wu C., Bu G., Mobley W.C., Zhang Y.W., St George-Hyslop P., Masliah E., Fraser P., Xu H. Appoptosin-Mediated Caspase Cleavage of Tau Contributes to Progressive Supranuclear Palsy Pathogenesis. Neuron. 2015;87(5):963–975. doi: 10.1016/j.neuron.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H., Zhang Y.W., Chen Y., Huang X., Zhou F., Wang W., Xian B., Zhang X., Masliah E., Chen Q., Han J.D., Bu G., Reed J.C., Liao F.F., Chen Y.G., Xu H. Appoptosin is a novel pro-apoptotic protein and mediates cell death in neurodegeneration. J. Neurosci. 2012;32(44):15565–15576. doi: 10.1523/JNEUROSCI.3668-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beal M.F. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 71.Praticò D., Clark C.M., Liun F., Rokach J., Lee V.Y., Trojanowski J.Q. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch. Neurol. 2002;59(6):972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 72.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beal M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 74.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Wang X., Su B., Fujioka H., Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am. J. Pathol. 2008;173(2):470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Su B., Lee H.G., Li X., Perry G., Smith M.A., Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sebastián D., Palacín M., Zorzano A. Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol. Med. 2017;23(3):201–215. doi: 10.1016/j.molmed.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Bertholet A.M., Delerue T., Millet A.M., Moulis M.F., David C., Daloyau M., Arnauné-Pelloquin L., Davezac N., Mils V., Miquel M.C., Rojo M., Belenguer P. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 2016;90:3–19. doi: 10.1016/j.nbd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Kerr J.S., Adriaanse B.A., Greig N.H., Mattson M.P., Cader M.Z., Bohr V.A., Fang E.F. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017;40(3):151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anand R., Wai T., Baker M.J., Kladt N., Schauss A.C., Rugarli E., Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 2014;204(6):919–929. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertholet A.M., Millet A.M., Guillermin O., Daloyau M., Davezac N., Miquel M.C., Belenguer P. OPA1 loss of function affects in vitro neuronal maturation. Brain. 2013;136(Pt 5):1518–1533. doi: 10.1093/brain/awt060. [DOI] [PubMed] [Google Scholar]
- 82.Gratuze M., Cisbani G., Cicchetti F., Planel E. Is Huntington’s disease a tauopathy? Brain. 2016;139(Pt 4):1014–1025. doi: 10.1093/brain/aww021. [DOI] [PubMed] [Google Scholar]
- 83.Mckee A.C., Daneshvar D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quintanilla R.A., Johnson G.V.W. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res. Bull. 2009;80(4-5):242–247. doi: 10.1016/j.brainresbull.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McIntosh G.C., Jameson H.D., Markesbery W.R. Huntington disease associated with Alzheimer disease. Ann. Neurol. 1978;3(6):545–548. doi: 10.1002/ana.410030616. [DOI] [PubMed] [Google Scholar]
- 86.Myers R.H., Sax D.S., Schoenfeld M., Bird E.D., Wolf P.A., Vonsattel J.P., White R.F., Martin J.B. Late onset of Huntington’s disease. J. Neurol. Neurosurg. Psychiatry. 1985;48(6):530–534. doi: 10.1136/jnnp.48.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moss R.J., Mastri A.R., Schut L.J. The coexistence and differentiation of late onset Huntington’s disease and Alzheimer’s disease. A case report and review of the literature. J. Am. Geriatr. Soc. 1988;36(3):237–241. doi: 10.1111/j.1532-5415.1988.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 88.Fernández-Nogales M., Cabrera J.R., Santos-Galindo M., Hoozemans J.J., Ferrer I., Rozemuller A.J., Hernández F., Avila J., Lucas J.J. Huntington’s disease is a four-repeat tauopathy with tau nuclear rods. Nat. Med. 2014;20(8):881–885. doi: 10.1038/nm.3617. [DOI] [PubMed] [Google Scholar]
- 89.Vuono R., Winder-Rhodes S., de Silva R., Cisbani G., Drouin-Ouellet J., Spillantini M.G., Cicchetti F., Barker R.A. The role of tau in the pathological process and clinical expression of Huntington’s disease. Brain. 2015;138(Pt 7):1907–1918. doi: 10.1093/brain/awv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goebel H.H., Heipertz R., Scholz W., Iqbal K., Tellez-Nagel I. Juvenile Huntington chorea: clinical, ultrastructural, and biochemical studies. Neurology. 1978;28(1):23–31. doi: 10.1212/WNL.28.1.23. [DOI] [PubMed] [Google Scholar]
- 91.Stahl W.L., Swanson P.D. Biochemical abnormalities in Huntington’s chorea brains. Neurology. 1974;24(9):813–819. doi: 10.1212/WNL.24.9.813. [DOI] [PubMed] [Google Scholar]
- 92.Reddy P.H., Shirendeb U.P. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim. Biophys. Acta. 2012;1822(2):101–110. doi: 10.1016/j.bbadis.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin Y.N., Yu Y.V., Gundemir S., Jo C., Cui M., Tieu K., Johnson G.V.W. Impaired mitochondrial dynamics and Nrf2 signaling contribute to compromised responses to oxidative stress in striatal cells expressing full-length mutant huntingtin. PLoS One. 2013;8(3):e57932. doi: 10.1371/journal.pone.0057932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J., Moody J.P., Edgerly C.K., Bordiuk O.L., Cormier K., Smith K., Beal M.F., Ferrante R.J. Mitochondrial loss, dysfunction and altered dynamics in Huntington’s disease. Hum. Mol. Genet. 2010;19(20):3919–3935. doi: 10.1093/hmg/ddq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shirendeb U., Reddy A.P., Manczak M., Calkins M.J., Mao P., Tagle D.A., Reddy P.H. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum. Mol. Genet. 2011;20(7):1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shirendeb U.P., Calkins M.J., Manczak M., Anekonda V., Dufour B., McBride J.L., Mao P., Reddy P.H. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum. Mol. Genet. 2012;21(2):406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song W., Chen J., Petrilli A., Liot G., Klinglmayr E., Zhou Y., Poquiz P., Tjong J., Pouladi M.A., Hayden M.R., Masliah E., Ellisman M., Rouiller I., Schwarzenbacher R., Bossy B., Perkins G., Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 2011;17(3):377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soneson C., Fontes M., Zhou Y., Denisov V., Paulsen J.S., Kirik D., Petersén A. Early changes in the hypothalamic region in prodromal Huntington disease revealed by MRI analysis. Neurobiol. Dis. 2010;40:531–543. doi: 10.1016/j.nbd.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costa V., Giacomello M., Hudec R., Lopreiato R., Ermak G., Lim D., Malorni W., Davies K.J., Carafoli E., Scorrano L. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Mol. Med. 2010;2(12):490–503. doi: 10.1002/emmm.201000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramos-Cejudo J., Wisniewski T., Marmar C., Zetterberg H., Blennow K., de Leon M.J., Fossati S. Traumatic brain injury and alzheimer’s disease: The cerebrovascular link. EBioMedicine. 2018;28(C):21–30. doi: 10.1016/j.ebiom.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I., Perl D.P. Stein; T. D.; Vonsattel, J. P.; Stewart, W.; Tripodis, Y.; Crary, J.F.; Bieniek, K.F.; Dams- O’Connor, K.; Alvarez, V.E.; Gordon, W. A.; Group, T. C. The first NINDS/NIBIB con- sensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Washington P.M., Morffy N., Parsadanian M., Zapple D.N., Burns M.P. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J. Neurotrauma. 2014;31(1):125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abisambra J.F., Scheff S. Brain injury in the context of tauopathies. J. Alzheimers Dis. 2014;40(3):495–518. doi: 10.3233/JAD-131019. [DOI] [PubMed] [Google Scholar]
- 104.Benaroya H. Brain energetics, mitochondria, and traumatic brain injury. Rev. Neurosci. 2020;31(4):363–390. doi: 10.1515/revneuro-2019-0086. [DOI] [PubMed] [Google Scholar]
- 105.Di Pietro V., Lazzarino G., Amorini A.M., Signoretti S., Hill L.J., Porto E., Tavazzi B., Lazzarino G., Belli A. Fusion or fission: The destiny of mitochondria in traumatic brain injury of different severities. Sci. Rep. 2017;7(1):9189. doi: 10.1038/s41598-017-09587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fischer T.D., Hylin M.J., Zhao J., Moore A.N., Waxham M.N., Dash P.K. Altered Mitochondrial Dynamics and TBI Pathophysiology. Front. Syst. Neurosci. 2016;10:29. doi: 10.3389/fnsys.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Q., Xia S.X., Li Q.Q., Gao Y., Shen X., Ma L., Zhang M.Y., Wang T., Li Y.S., Wang Z.F., Luo C.L., Tao L.Y. Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res. 2016;1630:134–143. doi: 10.1016/j.brainres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 108.Wu Q., Gao C., Wang H., Zhang X., Li Q., Gu Z., Shi X., Cui Y., Wang T., Chen X., Wang X., Luo C., Tao L. Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int. J. Biochem. Cell Biol. 2018;94(94):44–55. doi: 10.1016/j.biocel.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Nisoli E., Clementi E., Paolucci C., Cozzi V., Tonello C., Sciorati C., Bracale R., Valerio A., Francolini M., Moncada S., Carruba M.O. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 110.Cleeter M.W., Cooper J.M., Darley-Usmar V.M., Moncada S., Schapira A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 111.Clementi E., Brown G.C., Feelisch M., Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA. 1998;95(13):7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gillardon F., Rist W., Kussmaul L., Vogel J., Berg M., Danzer K., Kraut N., Hengerer B. Proteomic and functional alterations in brain mitochondria from Tg2576 mice occur before amyloid plaque deposition. Proteomics. 2007;7(4):605–616. doi: 10.1002/pmic.200600728. [DOI] [PubMed] [Google Scholar]
- 113.Chen G., Chen K.S., Knox J., Inglis J., Bernard A., Martin S.J., Justice A., McConlogue L., Games D., Freedman S.B., Morris R.G. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408(6815):975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 114.Reddy P.H., Manczak M., Yin X. Mitochondria-division inhibitor 1 protects against amyloid-β induced mitochondrial fragmentation and synaptic damage in alzheimer’s disease. J. Alzheimers Dis. 2017;58(1):147–162. doi: 10.3233/JAD-170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hollenbeck P.J., Saxton W.M. The axonal transport of mitochondria. J. Cell Sci. 2005;118(Pt 23):5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo X., Macleod G.T., Wellington A., Hu F., Panchumarthi S., Schoenfield M., Marin L., Charlton M.P., Atwood H.L., Zinsmaier K.E. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47(3):379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 117.Glater E.E., Megeath L.J., Stowers R.S., Schwarz T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173(4):545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fransson S., Ruusala A., Aspenström P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 2006;344(2):500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 119.Stowers R.S., Megeath L.J., Górska-Andrzejak J., Meinertzhagen I.A., Schwarz T.L. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36(6):1063–1077. doi: 10.1016/S0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 120.Stokin G.B., Lillo C., Falzone T.L., Brusch R.G., Rockenstein E., Mount S.L., Raman R., Davies P., Masliah E., Williams D.S., Goldstein L.S. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307(5713):1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 121.Iijima-Ando K., Hearn S.A., Shenton C., Gatt A., Zhao L., Iijima K. Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer’s disease. PLoS One. 2009;4(12):e8310. doi: 10.1371/journal.pone.0008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quintanilla R.A., von Bernhardi R., Godoy J.A., Inestrosa N.C., Johnson G.V. Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol. Dis. 2014;71(C):260–269. doi: 10.1016/j.nbd.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 123.Stoothoff W., Jones P.B., Spires-Jones T.L., Joyner D., Chhabra E., Bercury K., Fan Z., Xie H., Bacskai B., Edd J., Irimia D., Hyman B.T. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J. Neurochem. 2009;111(2):417–427. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vossel K.A., Xu J.C., Fomenko V., Miyamoto T., Suberbielle E., Knox J.A., Ho K., Kim D.H., Yu G.Q., Mucke L. Tau reduction prevents Aβ-induced axonal transport deficits by blocking activation of GSK3β. J. Cell Biol. 2015;209(3):419–433. doi: 10.1083/jcb.201407065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shahpasand K., Uemura I., Saito T., Asano T., Hata K., Shibata K., Toyoshima Y., Hasegawa M., Hisanaga S. Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease. J. Neurosci. 2012;32(7):2430–2441. doi: 10.1523/JNEUROSCI.5927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niewidok B., Igaev M., Sündermann F., Janning D., Bakota L., Brandt R. Presence of a carboxy-terminal pseudorepeat and disease-like pseudohyperphosphorylation critically influence tau’s interaction with microtubules in axon-like processes. Mol. Biol. Cell. 2016;27(22):3537–3549. doi: 10.1091/mbc.e16-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jonas E.A., Porter G.A., Jr, Beutner G., Mnatsakanyan N., Alavian K.N. Cell death disguised: The mitochondrial permeability transition pore as the c-subunit of the F(1)F(O) ATP synthase. Pharmacol. Res. 2015;99:382–392. doi: 10.1016/j.phrs.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pérez M.J., Quintanilla R.A. Development or disease: duality of the mitochondrial permeability transition pore. Dev. Biol. 2017;426(1):1–7. doi: 10.1016/j.ydbio.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 129.Bonora M., Bononi A., De Marchi E., Giorgi C., Lebiedzinska M., Marchi S., Patergnani S., Rimessi A., Suski J.M., Wojtala A., Wieckowski M.R., Kroemer G., Galluzzi L., Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12(4):674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du H., Guo L., Fang F., Chen D., Sosunov A.A., McKhann G.M., Yan Y., Wang C., Zhang H., Molkentin J.D., Gunn-Moore F.J., Vonsattel J.P., Arancio O., Chen J.X., Yan S.D. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14(10):1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Du H., Guo L., Zhang W., Rydzewska M., Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging. 2011;32(3):398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Du H., Yan S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: cyclophilin D and amyloid beta. Biochim. Biophys. Acta. 2010;1802(1):198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo L., Du H., Yan S., Wu X., McKhann G.M., Chen J.X., Yan S.S. Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer’s neurons. PLoS One. 2013;8(1):e54914. doi: 10.1371/journal.pone.0054914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gauba E., Chen H., Guo L., Du H. Cyclophilin D deficiency attenuates mitochondrial F1Fo ATP synthase dysfunction via OSCP in Alzheimer’s disease. Neurobiol. Dis. 2019;121:138–147. doi: 10.1016/j.nbd.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pérez M.J., Ponce D.P., Osorio-Fuentealba C., Behrens M.I., Quintanilla R.A. Mitochondrial bioenergetics is altered in fibroblasts from patients with sporadic alzheimer’s disease. Front. Neurosci. 2017;11:553–553. doi: 10.3389/fnins.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pérez M.J., Ponce D.P., Aránguiz A., Behrens M.I., Quintanilla R.A. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer’s disease. Redox Biol. 2018;19:290–300. doi: 10.1016/j.redox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johri A., Beal M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012;342(3):619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pérez M.J., Jara C., Quintanilla R.A. Contribution of tau pathology to mitochondrial impairment in neurodegeneration. Front. Neurosci. 2018;12:441. doi: 10.3389/fnins.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yao J., Irwin R.W., Zhao L., Nilsen J., Hamilton R.T., Brinton R.D. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L., Atwood C.S., Johnson A.B., Kress Y., Vinters H.V., Tabaton M., Shimohama S., Cash A.D., Siedlak S.L., Harris P.L., Jones P.K., Petersen R.B., Perry G., Smith M.A. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li X.C., Hu Y., Wang Z.H., Luo Y., Zhang Y., Liu X.P., Feng Q., Wang Q., Ye K., Liu G.P., Wang J.Z. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 2016;6:24756. doi: 10.1038/srep24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schulz K.L., Eckert A., Rhein V., Mai S., Haase W., Reichert A.S., Jendrach M., Müller W.E., Leuner K. A new link to mitochondrial impairment in tauopathies. Mol. Neurobiol. 2012;46(1):205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 143.Manczak M., Reddy P.H. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum. Mol. Genet. 2012;21(11):2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.David D.C., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., Ravid R., Dröse S., Brandt U., Müller W.E., Eckert A., Götz J. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 2005;280(25):23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 145.Lopes S., Teplytska L., Vaz-Silva J., Dioli C., Trindade R., Morais M. Tau deletion prevents stress-induced dendritic atrophy in prefrontal cortex: Role of synaptic mitochondria. Cereb. Cortex. 2016;27(4):2580–2591. doi: 10.1093/cercor/bhw057. [DOI] [PubMed] [Google Scholar]
- 146.Jara C., Aránguiz A., Cerpa W., Tapia-Rojas C., Quintanilla R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018;18:279–294. doi: 10.1016/j.redox.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cente M., Filipcik P., Pevalova M., Novak M. Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur. J. Neurosci. 2006;24(4):1085–1090. doi: 10.1111/j.1460-9568.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 148.Melov S., Adlard P.A., Morten K., Johnson F., Golden T.R., Hinerfeld D., Schilling B., Mavros C., Masters C.L., Volitakis I., Li Q.X., Laughton K., Hubbard A., Cherny R.A., Gibson B., Bush A.I. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One. 2007;2(6):e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rhein V., Song X., Wiesner A., Ittner L.M., Baysang G., Meier F., Ozmen L., Bluethmann H., Dröse S., Brandt U., Savaskan E., Czech C., Götz J., Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA. 2009;106(47):20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liska D.J. The detoxification enzyme systems. Altern. Med. Rev. 1998;3(3):187–198. [PubMed] [Google Scholar]
- 151.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 152.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dodson M., de la Vega M.R., Cholanians A.B., Schmidlin C.J., Chapman E., Zhang D.D. Modulating NRF2 in disease: Timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jo C., Gundemir S., Pritchard S., Jin Y.N., Rahman I., Johnson G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lavich I.C., de Freitas B.S., Kist L.W., Falavigna L., Dargél V.A., Köbe L.M., Aguzzoli C., Piffero B., Florian P.Z., Bogo M.R., de Lima M.N., Schröder N. Sulforaphane rescues memory dysfunction and synaptic and mitochondrial alterations induced by brain iron accumulation. Neuroscience. 2015;301:542–552. doi: 10.1016/j.neuroscience.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 156.Butterfield D.A., Drake J., Pocernich C., Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 2001;7(12):548–554. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 157.Maccioni R.B., Muñoz J.P., Barbeito L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch. Med. Res. 2001;32(5):367–381. doi: 10.1016/S0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 158.Sayre L.M., Smith M.A., Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001;8(7):721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- 159.Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E.K., Jones P.K., Ghanbari H., Wataya T., Shimohama S., Chiba S., Atwood C.S., Petersen R.B., Smith M.A. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 160.McManus M.J., Murphy M.P., Franklin J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011;31(44):15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Amadoro G., Corsetti V., Atlante A., Florenzano F., Capsoni S., Bussani R., Mercanti D., Calissano P. Interaction between NH(2)-tau fragment and Aβ in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiol. Aging. 2012;33(4):833.e1–833.e25. doi: 10.1016/j.neurobiolaging.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 162.Cieri D., Vicario M., Vallese F., D’Orsi B., Berto P., Grinzato A., Catoni C., De Stefani D., Rizzuto R., Brini M., Calì T. Tau localises within mitochondrial sub-compartments and its caspase cleavage affects ER-mitochondria interactions and cellular Ca2+ handling. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(10):3247–3256. doi: 10.1016/j.bbadis.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 163.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., Robbins J., Molkentin J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 164.Hom J.R., Quintanilla R.A., Hoffman D.L., de Mesy Bentley K.L., Molkentin J.D., Sheu S.S., Porter G.A., Jr The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev. Cell. 2011;21(3):469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Briston T., Selwood D.L., Szabadkai G., Duchen M.R. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol. Sci. 2019;40(1):50–70. doi: 10.1016/j.tips.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 166.Camilleri A., Ghio S., Caruana M., Weckbecker D., Schmidt F., Kamp F. 2020. [DOI] [PubMed]