Abstract
Background
International guidelines in 2008 recommended orchidopexy for undescended testis at 6–12 months of age to reduce the risk of testicular cancer and infertility. Using administrative data from England, Finland, Ontario (Canada), Scotland and Sweden (with data from Victoria (Australia) and Iceland in supplementary analyses), the aim of this study was to investigate compliance with these guidelines and identify potential socioeconomic inequities in the timing of surgery before 1 and 3 years.
Methods
All boys born in 2003–2011 with a diagnosis code of undescended testis and procedure codes indicating orchidopexy before their fifth birthday were identified from administrative health records. Trends in the proportion of orchidopexies performed before 1 and 3 years of age were investigated, as were socioeconomic inequities in adherence to the guidelines.
Results
Across all jurisdictions, the proportion of orchidopexies occurring before the first birthday increased over the study period. By 2011, from 7·6 per cent (Sweden) to 27·9 per cent (Scotland) of boys had undergone orchidopexy by their first birthday and 71·5 per cent (Sweden) to 90·4 per cent (Scotland) by 3 years of age. There was limited evidence of socioeconomic inequities for orchidopexy before the introduction of guidelines (2008). Across all jurisdictions for boys born after 2008, there was consistent evidence of inequities in orchidopexy by the first birthday, favouring higher socioeconomic position. Absolute differences in these proportions between the highest and lowest socioeconomic groups ranged from 2·5 to 5·9 per cent across jurisdictions.
Conclusion
Consistent lack of adherence to the guidelines across jurisdictions questions whether the guidelines are appropriate.
International guidelines recommend orchidopexy before 12 months of age. This study, using international administrative health data, found that this was achieved in only a minority of cases and that there were socioeconomic inequities in this outcome.

Guidelines not widely applied
Antecedentes
En el 2008, las guías internacionales recomendaban efectuar una orquidopexia para los testículos no descendidos entre los seis y los 12 meses de edad para reducir los riesgos de cáncer testicular e infertilidad. Utilizando datos administrativos de Inglaterra, Finlandia, Ontario (Canadá), Escocia y Suecia (con datos de Victoria, Australia e Islandia para análisis complementarios), el objetivo de este estudio fue investigar el cumplimiento de estas guías y la identificación de posibles desigualdades socioeconómicas con relación al momento de la cirugía antes de 1 y 3 años de edad.
Métodos
A partir de los registros administrativos de salud, se identificaron todos los niños nacidos entre 2003 y 2011 con código diagnóstico de testículos no descendidos y con código de procedimiento correspondiente a orquidopexia antes de cumplir 5 años. Se investigaron las tendencias en la proporción de orquidopexias realizadas antes de 1 y 3 años de edad, respectivamente, al igual que las desigualdades socioeconómicas en el cumplimiento de las directrices de las guías.
Resultados
En todas las jurisdicciones, la proporción de orquidopexias realizadas antes del primer año de vida aumentó durante el periodo de estudio. En 2011, del 7,6% (Suecia) al 27,9% (Escocia) de los niños habían sido sometidos a orquidopexia en su primer año de vida y del 71,5% (Suecia) al 90,4% (Escocia) a los 3 años de edad. Hubo evidencia limitada de las inequidades socioeconómicas para la orquidopexia antes de la introducción de las guías (2008). En todas las jurisdicciones para los niños nacidos después de 2008, hubo evidencia consistente de inequidades para la práctica de una orquidopexia en el primer año de vida en favor de una posición socioeconómica más alta (socioeconomic position, SEP). Las diferencias absolutas en estas proporciones entre los grupos SEP más altos y más bajos oscilaron entre el 2,5% y el 5,9% en todas las jurisdicciones.
Conclusión
La falta de adherencia a las guías observada consistentemente en todas las jurisdicciones cuestiona si las guías son apropiadas.
Introduction
Undescended testis is common, affecting around 3–9 per cent of full‐term boys at birth 1 . Most undescended testes spontaneously descend, but the problem persists beyond 1 year of age in around 1 per cent of individuals 1 . When uncorrected, undescended testis is associated with an increased risk of testicular cancer in adulthood as well as reduced testicular volume, sperm count and hormonal levels, with potential risks for fertility that may impact on psychosexual well‐being 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 . Early surgical placement of the testis into the scrotum is recommended to reduce these risks.
Two European consensus statements 2 , 4 were published in 2008, followed by one from the British Association of Paediatric Urologists 12 in 2011, recommending surgical intervention between 6 and 12 months of age. Although guidelines from the American Urological Association 13 in 2014 recommended orchidopexy before the age of 18 months, a systematic review 14 concluded that surgery between 6 and 12 months of age may optimize fertility and protect against malignancy. Older, single jurisdiction, studies have found poor adherence to these guidelines 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 .
Many healthcare systems provide free universal care. Equity of access is a key goal of these systems. A number of investigations have found, however, that people of lower socioeconomic position (SEP) are less likely to access healthcare services after adjusting for clinical need 24 , 25 , 26 , 27 , 28 . There are also concerns that public health interventions that do not address root causes of inequities in access to healthcare may lead to increased health inequity overall 29 . Orchidopexy is a useful exemplar condition to assess inequities in healthcare access, because the occurrence of undescended testis is not known to be associated with SEP 30 and the condition is routinely screened for at birth.
The aims of this study were to investigate trends in the proportion of orchidopexies performed before 1 and 3 years of age in five jurisdictions (England, Finland, Ontario (Canada), Scotland and Sweden), and to investigate potential socioeconomic inequities in the proportion of boys from high‐ versus low‐SEP families having orchidopexy before 1 and 3 years of age, stratified according to the introduction of the guidelines in 2008.
Methods
Rolling yearly male‐only birth cohorts were identified using administrative health data sets in five jurisdictions (England, Finland, Ontario, Scotland and Sweden) from 2003 to 2011. Owing to data limitations, the 2003 birth cohort was unavailable for Finland. All singleton live births among boys surviving to at least 6 months of age who had orchidopexy before their fifth birthday were included; the population denominator was the number of all singleton live‐born males in each respective year.
The data set for each jurisdiction was a whole‐population administrative data set from health services, from which it was possible to construct birth cohorts. Detail on each data set is available in Table S1 (supporting information). Each data set contained information on demographic and clinical characteristics of the patients, as well as dates and procedure codes associated with their hospital admissions. Complementary data were also supplied for Iceland and Victoria (Australia), but were excluded from the main analyses because of data limitations. In Iceland, the number of boys undergoing orchidopexy in each year was very small; in Victoria, birth cohorts could not be constructed and cross‐sectional data only were provided. Direct comparisons with these jurisdictions were therefore not feasible. Results for these two regions are presented in the supporting information, as indicated in the results section.
Case definition
A case was defined as any child born in 2003–2011 with hospital records indicating a diagnosis of undescended testis and who underwent orchidopexy by the age of 5 years. Orchidopexies were identified using jurisdiction‐specific procedure codes (Table S1 , supporting information). As orchidopexy is also used to treat testicular torsion, patients were included only if they had an ICD‐10 cryptorchidism diagnosis code (Table S1 , supporting information) recorded either on the same admission record as their orchidopexy or previously. Only the first operation was counted (revisions or second stages were ignored). Patients were followed up to their fifth birthday to reduce the risk of counting acquired ascending testis, which can occur during later childhood 31 .
Boys born preterm (at less than 37 weeks' gestation) were excluded, as were those with a congenital anomaly at birth, identified by any ICD‐10 diagnosis code indicating the presence of congenital anomalies in the first 3 months of life ( Appendix S1, supporting information) 32 .
Outcomes
Trends in the proportion of cases by age 5 years, where the first orchidopexy procedure was performed before 1 and 3 years of age, were plotted. Inequity ratios were then calculated for boys born before (2003–2006) and after (2008–2011) the introduction of European guidance recommending orchidopexy before 1 year of age. Inequity ratios were based on the distribution of births into quintiles (or quartiles for Finland) according to SEP using either household‐ or area‐level measures recorded in the birth or subsequent admission records.
Ethical approval
Access to data was granted following appropriate approvals in each jurisdiction. In England, researchers had a data‐sharing agreement with National Health Service Digital to use a deidentified extract of Hospital Episode Statistics linked to Office for National Statistics death registration data, so that ethical approval to use English data sets was not required. In Iceland, the study was approved by the Data Protection Authority and National Bioethics Committee (7 March 2017, VSN–17–044), the Directorate of Health (20 January 2017, 1701096/5.6.1) and Statistics Iceland (24 April 2017, 2017/01). No study permission was required in Finland, as only aggregated data were provided for the study group. The use of encoded Ontario data, accessed at ICES in this project, was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a Research Ethics Board. The Public Benefit and Privacy Panel for Health and Social Care (reference number 1516‐0405) and the Privacy Advisory Committee (number XRB13020) provided permission in Scotland. The Swedish part of this study was approved by the Regional Ethics Committee in Stockholm in January 2016 (dnr 2016/5:1), and in Victoria this was covered by RCH HREC 37164.
Small cell counts were suppressed in accordance with the requirements of each jurisdiction.
Statistical analysis
Trends based on the annual cumulative incidence of orchidopexy by age 5 years in each jurisdiction were plotted over time, based on year of birth. Trends in the proportion of patients undergoing orchidopexy before 1 and 3 years of age in each region by year of birth were also plotted. These proportions are cumulative: the proportion having orchidopexy by 3 years of age includes those who had it by 1 year.
Inequity in age at orchidopexy was measured by taking the proportion of patients in the three highest (2 highest in the case of Finland) SEP groups over the proportion of patients in the two lowest SEP groups who received their first orchidopexy by 1 or 3 years of age. These ratios are termed inequity ratios. A ratio of 1 indicates equality between the patients with higher and lower SEP. Ratios above 1 indicate inequities in favour of patients with a higher SEP, and those below 1 indicate inequities in favour of patients with a lower SEP. Ninety‐five per cent confidence intervals were also calculated. To assess inequities before and after the publication of the guidelines, inequity ratios were calculated for boys born in 2003–2006 (2004–2006 in Finland), and again for those born in 2008–2011.
Following up boys to their fifth birthday may have introduced bias into the analyses as later operations may nonetheless be for primary undescended testis. Sensitivity analyses were therefore carried out by extending follow‐up to the tenth birthday. Data were available for these analyses only from England, Ontario, Scotland and Sweden.
Analyses were conducted in each region independently, based on a detailed specification of the cohort and codes required. The statistical software used in each region included R (R Foundation for Statistical Computing, Vienna, Austria) (England, Iceland, Victoria), Stata® (StataCorp, College Station, Texas, USA) (Scotland) and SAS® (SAS Institute, Cary, North Carolina, USA) (Finland, Ontario and Sweden). Aggregate results were entered into Microsoft Excel® (Microsoft, Redmond, Washington, USA) for further analysis.
Results
Characteristics of boys born in 2011 in each jurisdiction are given in Table 1 (all years are in Appendix S2, supporting information). In 2011, 331 104 boys were born in England, 69 177 in Ontario, 54 400 in Sweden, 30 566 in Finland, and 28 099 in Scotland. Further descriptive data on the distribution of maternal age, birthweight and the numbers excluded due to death at less than 6 months of age are given in Appendix S2 (supporting information).
Table 1.
Characteristics of all male births in the latest birth year by region
| England | Finland | Ontario | Scotland | Sweden | |
|---|---|---|---|---|---|
| Latest birth year | 2011 | 2011 | 2011 | 2011 | 2011 |
| No. of male births | 331 104 | 30 566 | 69 177 | 28 099 | 54 400 |
| Socioeconomic position at birth | |||||
| Most deprived | 90 122 (27·9) | 6027 (23·5) | 19 145 (27·7) | 7349 (26·2) | 15 938 (29·4) |
| 2nd | 73 403 (22·7) | 11 513 (45·0) | 14 145 (20·4) | 6003 (21·4) | 10 300 (19·0) |
| 3rd | 59 757 (18·5) | 5325 (20·8) | 13 272 (19·2) | 5407 (19·3) | 8980 (16·6) |
| 4th | 51 690 (16·0) | 2744 (10·7) | 12 517 (18·1) | 4949 (17·6) | 10 269 (18·9) |
| Least deprived | 48 548 (15·0) | –* | 10 098 (14·6) | 4379 (15·6) | 8722 (16·1) |
| Any congenital anomaly | 9543 (2·9) | 1514 (5·0) | 2118 (3·1) | 743 (2·6) | 1122 (2·1) |
| Premature birth (< 37 weeks' gestation) | 17 210 (5·7) | 1764 (5·8) | 5755 (8·3) | 1590 (5·7) | 2657 (4·9) |
| Congenital anomaly or prematurity | 24 988 (8·2) | 2759 (9·1) | 7213 (10·4) | 2180 (7·8) | 3634 (6·7) |
Values in parentheses are percentages (calculated by excluding missing values for each variable); data for all years and missing values are shown in Appendix S2 (supporting information), where data for Iceland and Victoria, Australia, can also be found.
Finland's socioeconomic position categorization is on a four‐point scale only.
Fig. 1 and Appendix S3 (supporting information) show the cumulative incidence of cases over time in each region. Rates in England, Scotland and Ontario remained stable from 2003 to 2011. In Finland, the rate rose from 79·9 per 10 000 for boys born in 2004 to 105·6 for those born in 2011. Sweden observed an increased rate for boys born between 2006 and 2008, which then decreased from 2009 to 2011.
Fig. 1.

Cumulative incidence of orchidopexy by age 5 years in each region, 2003–2011 Error bars show 95 per cent confidence intervals.
Across all jurisdictions, a minority of boys had orchidopexy before the recommended age of 1 year, but a large majority had undergone the procedure by 3 years (Fig. 2 and Appendix S3, supporting information). All regions, except Sweden, showed increases in the proportions of boys having orchidopexy by the age of 1 year, with the steepest rises in Scotland between 2007 and 2009 and Finland between 2006 and 2007. In Sweden, there was a rise in this proportion to 2007, which had then decreased by 2009 to 2004–2005 levels. The proportions receiving orchidopexy by age 3 years were more stable across all birth years, except for marked increases in Sweden from 2003 to 2007.
Fig. 2.

Percentage of orchidopexies performed by 1 and 3 years of age in each region, 2003–2011 Orchidopexies by a 1 year and b 3 years of age. Note the different scales on the y‐axes.
Fig. 3 and Appendix S4 (supporting information) give the inequity ratios in time to surgery in England, Finland, Ontario, Scotland and Sweden for the 1‐year threshold for birth years 2003–2006 (2004–2006 for Finland) (Fig. 3a ) and 2008–2011 (Fig. 3b ). The ratios for the 3‐year threshold are shown in Fig. 3c,d . There was limited evidence of socioeconomic inequity in age at orchidopexy for births between 2003 and 2006, with small relative and absolute differences between boys with higher and lower SEP. For births between 2008 and 2011, however, there was consistent evidence across all jurisdictions of inequities for the first birthday guideline, favouring boys with a higher SEP. Boys born in 2008–2011 with a higher SEP were 25–48 per cent more likely to have had their operation by 1 year of age, depending on region, compared with those with a lower SEP. The absolute differences were small, and ranged from 2·5 to 5·9 per cent across each region.
Fig. 3.
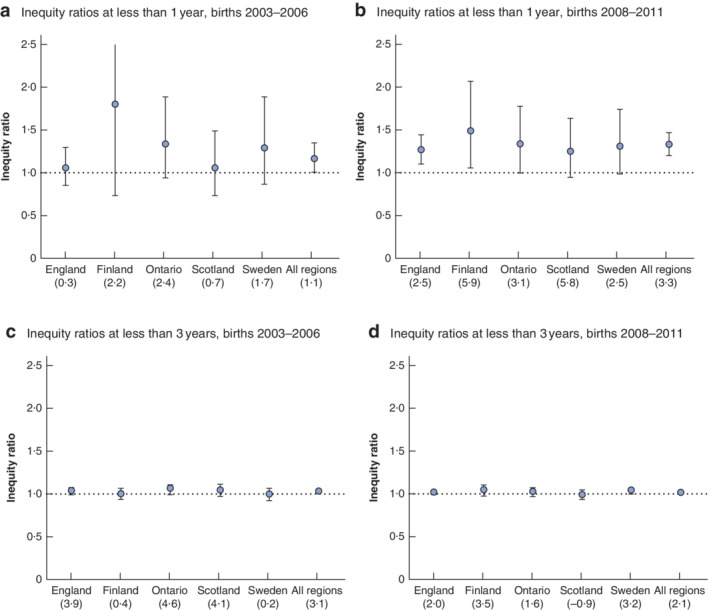
Inequity ratios for the timing of orchidopexy between the most deprived and least deprived patients, 2003–2006 and 2008–2011 a,b Inequity ratios at less than 1 year for births in a 2003–2006 (2004–2006 for Finland) and b 2008–2011. c,d Inequity ratios at less than 3 years for births in c 2003–2006 (2004–2006 for Finland) and d 2008–2011. Error bars show 95 per cent confidence intervals. The values in parentheses below each region show the absolute percentage difference (higher socioeconomic position (SEP) minus lower SEP).
There was limited evidence of very small inequities in the 3‐year threshold for births in 2003–2006 or 2008–2011.
Results of the sensitivity analyses are given in Appendices S5 and S6 (supporting information). There were no substantive differences between the main and sensitivity analyses in inequity ratios.
Discussion
Across all jurisdictions, a small but growing minority (between 7·6 and 27·9 per cent in 2011) of boys had orchidopexy before the recommended age of 1 year, although most underwent the operation by 3 years. There was evidence of socioeconomic inequity in the proportion of boys born after the introduction of guidelines in 2008 who received orchidopexy by 1 year of age, favouring those of higher SEP. There was no clear evidence of inequity for those born before 2008 or for orchidopexy by 3 years of age before or after the introduction of guidelines. The findings showed remarkable consistency across regions.
Each jurisdiction had its own measure of SEP (Table S1 , supporting information) 33 , 34 . However, relative comparisons between quintiles within countries should be stable, rendering within‐country estimates reliable. There were small numbers of cases within each quintile, hence the necessity to dichotomize SEP, which may have masked more subtle gradations. The administrative data resources do not have adequate data on referrals and so it was not possible to analyse separate parts of the pathway between referral and treatment. It may, for example, be that any observed inequities are due to later diagnosis, referral or decision to treat. Despite possible different referral pathways in each jurisdiction or in the availability of operating theatre space, these results were consistent across jurisdictions, suggesting that results may be similar in other countries.
Undescended testis is a relatively common condition, which was clearly and consistently coded within jurisdictions. It is screened for at birth and that process is not associated with SEP (after excluding preterm births and boys with congenital anomalies). A limitation in the diagnostic system used, however, is that it was not possible to separate congenital from acquired cryptorchidism. In this study, operations beyond 5 years of age were censored to minimize the inclusion of acquired cryptorchidism, but it seems probable that such patients may explain some of the high rates of late operation. This study spanned a sufficiently long period to cover the introduction of international guidelines in 2008 and therefore captured what should have been a wave of innovation adoption across all SEP groups.
Other studies 15 , 16 , 17 , 19 have noted that guidelines are not being met routinely. In the present study, it was observed not only that this was a persisting issue but that, by using comparable methods across jurisdictions, it was consistent in different countries. There are several potentially overlapping reasons for non‐adherence. Operating after 1 year of age may result from delayed diagnosis 15 or lack of knowledge about the recommended age of orchidopexy, thus causing later referral and older age at orchidopexy 14 . Healthcare systems must balance the need to perform early orchidopexy against the need to carry out other procedures, where there may also be delays and non‐adherence to timing guidelines 35 . Evidence about long‐term fertility and malignancy risk, particularly regarding whether orchidopexy should be performed at less than 1 year or later, is still uncertain given the limitations of existing studies, with small sample sizes or proxy outcomes. In a systematic review 14 evaluating the optimal age for orchidopexy, only two 36 , 37 of 24 studies examined fertility as opposed to a proxy. As for the association with malignancy, current literature has focused mostly on children undergoing orchidopexy at a later age (comparing children having the procedure at age above 10 or 13 versus less than 10 or 13 years) 7 , and it remains unclear whether findings showing that earlier surgery reduces risk for children aged under 10 years can be extrapolated to infants. The present findings of overwhelming non‐adherence might in part be due to these limitations in the evidence, particularly if practitioners are weighing the risks of long‐term harm with the risks and contraindications of early surgery 38 . This should therefore lead to the generation of stronger and more robust evidence of the balance of harms and benefits according to the timing of orchidopexy.
This study also showed socioeconomic inequities, which occurred most clearly after introduction of the guidelines. This suggests possible inequities in the adoption of the ‘innovation’ regarding surgery in the first year. Diffusion of innovation in healthcare takes time 39 , and it may be that those in better socioeconomic circumstances, as a result of either their own resources or interactions with the healthcare system, are more likely to be ‘early adopters’ through better access to the healthcare system or being more informed or questioning parents 29 . It has been suggested 28 , in the context of waiting times, that people with a higher SEP, by having higher levels of education and social capital, may be better equipped to articulate their needs and engage actively in waiting list systems. As these people are likely to have better working conditions, it might also be easier for them to attend appointments 28 . This is not to suggest that people with a lower SEP are less likely to seek healthcare, as there is evidence to the contrary 24 , but that they may be at a disadvantage when dealing with a complex system. If the findings of the present study are the result of diffusion of innovation, it may be that those with a lower SEP will ‘catch up’ and close the currently modest inequities in age at orchidopexy.
Across five jurisdictions, orchidopexy was not performed consistently before a child's first birthday, as recommended by international guidelines, for the majority of patients. Although lack of adherence to recommended practice may reflect balancing the risks of delaying surgery with those of performing an operation under general anaesthesia early in life, there may be systems‐level factors, such as limited availability of hospital space, that cause delay. Evidence of long‐term harms associated with performing orchidopexy before or after 1 year of age, however, remains very weak. Long‐term monitoring of outcomes and further research into the precise timing of events within the diagnosis–referral–treatment pathway still need to be carried out. National and international guidelines should be reassessed to account better for the risks of early versus delayed surgery.
Supporting information
Appendix S1 Congenital anomaly ICD‐10 codes
Appendix S2 Cumulative incidence and proportions of orchidopexy cases
Appendix S3 Characteristics of birth cohorts by region
Appendix S4 Inequity ratios in time to surgery
Appendix S5 Sensitivity analyses for cumulative incidence and proportions of cases
Appendix S6 Sensitivity analyses by region
Table S1 Description of the data sets
Acknowledgements
This work uses data provided by patients and collected by the English and Scottish National Health Services as part of their care and support. Hospital Episode Statistics source data can be accessed by researchers applying to National Health Service Digital (© 2019, reused with the permission of National Health Service Digital). All rights reserved.
Staff at the Directorate of Health (K. Jónsson and G. K. Guðfinnsdóttir) and Statistics Iceland (K. Stefánsson) in Reykjavík are thanked for their support in the collection of the Icelandic data.
This study was not preregistered in an independent, institutional registry.
R.G. and L.W. were (in part) supported by the National Institutes of Health Research (NIHR) Children and Families Policy Research Unit, but not commissioned by the NIHR Policy Research Programme. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. A.A., A.H., G.G. and S.H.J. were supported by a grant from EU Horizon 2020. Research at University College London Great Ormond Street Institute of Child Health is supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre.
The Ontario portion of this analysis was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent of the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the authors, and not necessarily those of CIHI. A.G. was supported by a Canadian Institute for Health Research Applied Chair in Reproductive and Child Health Services and Policy Research, which also funded the Ontario analyses.
The funders had no role in the design or conduct of the study, or in its write‐up or publication.
Disclosure: G.G. was Chief Medical Officer for Iceland, Directorate of Health, in 2010–2014. The authors declare no other conflict of interest.
Funding information
National Institute for Health Research Children and Families Policy Research Unit
EU Horizon 2020
National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre
Canadian Institute for Health Research
Ontario Ministry of Health and Long‐Term Care
References
- 1. Sijstermans K, Hack WW, Meijer RW, van der Voort‐Doedens LM. The frequency of undescended testis from birth to adulthood: a review. Int J Androl 2008; 31: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Gapany C, Frey P, Cachat F, Gudinchet F, Jichlinski P, Meyrat B et al Management of cryptorchidism in children: guidelines. Swiss Med Wkly 2008; 138: 492–498. [DOI] [PubMed] [Google Scholar]
- 3. Tekgül S, Dogan H, Hoebeke P, Kočvara R, Nijman J, Radmayr C et al Guidelines on Paediatric Urology. European Association of Urology: Arnhem, 2015. [Google Scholar]
- 4. Ritzen EM. Undescended testes: a consensus on management. Eur J Endocrinol 2008; 159(Suppl 1): S87–S90. [DOI] [PubMed] [Google Scholar]
- 5. Kollin C, Karpe B, Hesser U, Granholm T, Ritzén EM. Surgical treatment of unilaterally undescended testes: testicular growth after randomization to orchiopexy at age 9 months or 3 years. J Urol 2007; 178: 1589–1593. [DOI] [PubMed] [Google Scholar]
- 6. Kim S‐O, Hwang EC, Hwang IS, Oh KJ, Jung SI, Kang TW et al Testicular catch up growth: the impact of orchiopexy age. J Urol 2011; 78: 886–889. [DOI] [PubMed] [Google Scholar]
- 7. Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O. Age at surgery for undescended testis and risk of testicular cancer. NEJM 2007; 356: 1835–1841. [DOI] [PubMed] [Google Scholar]
- 8. Walsh TJ, Dall'Era MA, Croughan MS, Carroll PR, Turek PJ. Prepubertal orchiopexy for cryptorchidism may be associated with lower risk of testicular cancer. J Urol 2007; 178: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 9. Wood HM. Editorial comment. J Urol 2007; 178: 1446. [Google Scholar]
- 10. Strader CH, Weiss NS, Daling JR, Karagas MR, Mcknight B. Cryptorchism, orchiopexy, and the risk of testicular cancer. Am J Epidemiol 1988; 127: 1013–1018. [DOI] [PubMed] [Google Scholar]
- 11. Chung E, Brock GB. Cryptorchidism and its impact on male fertility: a state of art review of current literature. Can Urol Assoc J 2011; 5: 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. British Association of Paediatric Urologists . UDT Conesus Statement; 2011. http://www.bapu.org.uk/udt‐consensus‐statement/ (accessed 15 March 2018).
- 13. American Urological Association . Evaluation and Treatment of Cryptorchidism; 2014. https://www.auanet.org/guidelines/cryptorchidism (accessed 16 September 2019).
- 14. Chan E, Wayne C, Nasr A. Ideal timing of orchiopexy: a systematic review. Pediatr Surg Int 2014; 30: 87–97. [DOI] [PubMed] [Google Scholar]
- 15. Bradshaw CJ, Corbet‐Burcher G, Hitchcock R. Age at orchidopexy in the UK: has new evidence changed practice? J Pediatr Urol 2014; 10: 758–762. [DOI] [PubMed] [Google Scholar]
- 16. Bruijnen CJ, Vogels HD, Beasley SW. Age at orchidopexy as an indicator of the quality of regional child health services. J Pediatr Chil Health 2012; 48: 556–559. [DOI] [PubMed] [Google Scholar]
- 17. Hrivatakis G, Astfalk W, Schmidt A, Hartwig A, Kugler T, Heim T et al The timing of surgery for undescended testis: a retrospective multicenter analysis. Dtsch Arztebl Int 2014; 111: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dave S, Liu K, Garg AX, Shariff SZ. Secular trends in the incidence and timing of surgical intervention for congenital undescended testis and surgically treated hypospadias in Ontario, Canada between 1997 and 2007. J Pediatr Urol 2018; 14: 552–552. [DOI] [PubMed] [Google Scholar]
- 19. McCabe JE, Kenny SE. Orchidopexy for undescended testis in England: is it evidence based? J Pediatr Surg 2008; 43: 353–357. [DOI] [PubMed] [Google Scholar]
- 20. Nah SA, Yeo CS, How GY, Allen JC Jr, Lakshmi NK, Yap TL et al Undescended testis: 513 patients' characteristics, age at orchidopexy and patterns of referral. Arch Dis Child 2014; 99: 401–406. [DOI] [PubMed] [Google Scholar]
- 21. Ahn H, Lee HE, Park K, Choi H. Reasons for delayed orchiopexies in a Korean tertiary care hospital. Kor J Urol 2014; 55: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kokorowski PJ, Routh JC, Graham DA, Nelson CP. Variations in timing of surgery among boys who underwent orchidopexy for cryptorchidism. Pediatrics 2010; 126: e576–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Springer A, Huber C, Reck CA, Fengler D, Horcher E. Delayed referral despite appropriate knowledge in cryptorchidism as a cause of delayed orchidopexies in Austria. Klin Padiatr 2010; 222: 248–251. [DOI] [PubMed] [Google Scholar]
- 24. Morris S, Sutton M, Gravelle H. Inequity and inequality in the use of health care in England: an empirical investigation. Soc Sci Med 2005; 60: 1251–1266. [DOI] [PubMed] [Google Scholar]
- 25. Stirbu I, Kunst AE, Mielck A, Mackenbach JP. Inequalities in utilisation of general practitioner and specialist services in 9 European countries. BMC Health Serv Res 2011; 11: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Doorslaer E, Koolman X, Jones AM. Explaining income‐related inequalities in doctor utilisation in Europe. Health Econ 2004; 13: 629–647. [DOI] [PubMed] [Google Scholar]
- 27. van Doorslaer E, Wagstaff A, van der Burg H, Christiansen T, De Graeve D, Duchesne I et al Equity in the delivery of health care in Europe and the US. J Health Econ 2000; 19: 553–583. [DOI] [PubMed] [Google Scholar]
- 28. Laudicella M, Siciliani L, Cookson R. Waiting times and socioeconomic status: evidence from England. Soc Sci Med 2012; 74: 1331–1341. [DOI] [PubMed] [Google Scholar]
- 29. Darling EK, Ramsay T, Manuel D, Sprague AE, Walker MC, Guttmann A. Association of universal bilirubin screening with socioeconomic disparities in newborn follow‐up. Acad Pediatr 2017; 17: 135–143. [DOI] [PubMed] [Google Scholar]
- 30. Gurney JK, McGlynn KA, Stanley J, Merriman T, Signal V, Shaw C et al Risk factors for cryptorchidism. Nat Rev Urol 2017; 14: 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hutson JM, Vikraman J, Li R, Thorup J. Undescended testis: what paediatricians need to know. J Pediatr Chil Health 2017; 53: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 32. Hardelid P, Dattani N, Gilbert R. Estimating the prevalence of chronic conditions in children who die in England, Scotland and Wales: a data linkage cohort study. BMJ Open 2014; 4: e005331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey SG. Indicators of socioeconomic position (part 1). J Epidemiol Community Health 2006; 60: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey SG. Indicators of socioeconomic position (part 2). J Epidemiol Community Health 2006; 60: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wright JG, Menaker RJ; Canadian Paediatric Surgical Wait Times Study Group . Waiting for children's surgery in Canada: the Canadian Paediatric Surgical Wait Times project. Can Med Assoc J 2011; 183: E559–E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee PA, O'Leary LA, Songer NJ, Coughlin MT, Bellinger MF, LaPorte RE. Paternity after unilateral cryptorchidism: a controlled study. Pediatrics 1996; 98: 676–679. [PubMed] [Google Scholar]
- 37. Kumar D, Bremner DN, Brown PW. Fertility after orchiopexy for cryptorchidism: a new approach to assessment. Br J Urol 1989; 64: 516–520. [DOI] [PubMed] [Google Scholar]
- 38. Arts DL, Voncken AG, Medlock S, Abu‐Hanna A, van Weert HC. Reasons for intentional guideline non‐adherence: a systematic review. Int J Med Informat 2016; 89: 55–62. [DOI] [PubMed] [Google Scholar]
- 39. Berwick DM. Disseminating innovations in health care. JAMA 2003; 289: 1969–1975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Congenital anomaly ICD‐10 codes
Appendix S2 Cumulative incidence and proportions of orchidopexy cases
Appendix S3 Characteristics of birth cohorts by region
Appendix S4 Inequity ratios in time to surgery
Appendix S5 Sensitivity analyses for cumulative incidence and proportions of cases
Appendix S6 Sensitivity analyses by region
Table S1 Description of the data sets


