Abstract
Background
Prehabilitation has emerged as a strategy to prepare patients for elective abdominal cancer surgery with documented improvements in postoperative outcomes. The aim of this study was to assess the evidence for prehabilitation interventions of relevance to the older adult.
Methods
Systematic searches were conducted using MEDLINE, Web of Science, Scopus, CINAHL and PsychINFO. Studies of preoperative intervention (prehabilitation) in patients undergoing abdominal cancer surgery reporting postoperative outcomes were included. Age limits were not set as preliminary searches revealed this would be too restrictive. Articles were screened and selected based on PRISMA guidelines, and assessment of bias was performed. Qualitative, quantitative and meta‐analyses of data were conducted as appropriate.
Results
Thirty‐three studies (3962 patients) were included. Interventions included exercise, nutrition, psychological input, comprehensive geriatric assessment and optimization, smoking cessation and multimodal (two or more interventions). Nine studies purposely selected high‐risk, frail or older patients. Thirty studies were at moderate or high risk of bias. Ten studies individually reported benefits in complication rates, with meta‐analyses for overall complications demonstrating significant benefit: multimodal (risk difference −0·1 (95 per cent c.i. −0·18 to −0·02); P = 0·01, I 2 = 18 per cent) and nutrition (risk difference −0·18 (−0·26 to −0·10); P < 0·001, I 2 = 0 per cent). Seven studies reported reductions in length of hospital stay, with no differences on meta‐analysis.
Conclusion
The conclusions of this review are limited by the quality of the included studies, and the heterogeneity of interventions and outcome measures reported. Exercise, nutritional and multimodal prehabilitation may reduce morbidity after abdominal surgery, but data specific to older patients are sparse.
This systematic review and meta‐analysis suggests that multimodal and nutrition‐alone prehabilitation interventions may reduce postoperative complications and length of hospital stay. More high‐quality research is needed specifically on prehabilitation for older patients.
Use multimodal prehabilitation to reduce morbidity
Antecedentes
La pre‐habilitación ha surgido como una estrategia para preparar a los pacientes para la cirugía electiva del cáncer abdominal con mejoras documentadas en los resultados postoperatorios. El objetivo de este estudio fue evaluar la evidencia sobre las intervenciones de pre‐habilitación relevantes en adultos de edad avanzada.
Métodos
Se realizaron búsquedas sistemáticas utilizando MEDLINE, Web of Science, Scopus, CINAHL y PsychINFO. Registro PROSPERO: CRD42019120381. Se incluyeron estudios de intervención preoperatoria (pre‐habilitación) en pacientes sometidos a cirugía oncológica abdominal que describiesen resultados postoperatorios. No se fijaron límites en la edad dado que las búsquedas preliminares revelaron que ello sería demasiado restrictivo. Los artículos fueron examinados y seleccionados en base a las guías PRISMA y se realizó una evaluación del sesgo. Se llevó a cabo un análisis cualitativo, cuantitativo y metaanálisis de los datos según fuese apropiado.
Resultados
Se incluyeron 33 estudios (3.962 patients). Las intervenciones incluyeron ejercicio, nutrición, intervención psicológica, evaluación geriátrica global y optimización, abandono del tabaquismo y multimodal (dos o más intervenciones). Nueve estudios seleccionaron expresamente una población de pacientes de elevado riesgo, frágiles o de edad avanzada. Treinta estudios presentaban un riesgo moderado/alto de sesgo. Diez estudios describieron de forma individual beneficios en las tasas de complicaciones con metaanálisis para las complicaciones globales demostrando un beneficio significativo: multimodal (diferencia de riesgo ‐0,1 (i.c. del 95% −0,18 a −0,02); P = 0,01, I2 = 18%) e intervención nutricional (diferencia de riesgo −0,18 (i.c. del 95% −0,26 a −0,10); P < 0,001, I2 = 0%). Siete estudios describieron reducciones en la duración de la estancia hospitalaria, sin diferencias en el metaanálisis.
Conclusión
Las conclusiones de esta revisión están limitadas por la calidad de los estudios incluidos, heterogeneidad de las intervenciones y descripción de las medidas de resultados. Las intervenciones de pre‐habilitación de ejercicio, nutricionales y multimodales puede reducir la morbilidad tras cirugía abdominal, pero los datos concretos en pacientes de edad avanzada son escasos.
Introduction
The majority of cancers in the UK are diagnosed in the older adult population (aged 65 years and above), with this population predicted to increase exponentially 1 . The pathogenesis and treatment of cancer can lead to a decline in cardiorespiratory fitness, weight loss and psychological morbidity 2 . Surgery remains the mainstay of curative treatment for many gastrointestinal, gynaecological and urological cancers, but outcomes are poorer in the older adult, making strategies to optimize this complex group increasingly important.
Adverse factors associated with ageing include co‐morbidity, polypharmacy, cognitive impairment, dependency and frailty, all of which are associated with increased all‐cause mortality in the general population 3 . When these at‐risk individuals are exposed to the stress of major cancer surgery, postoperative mortality and morbidity also increase 4 , 5 . Common lifestyle choices, including smoking, poor nutrition and sedentary behaviours, add to this risk. ‘Prehabilitation’, the process of enhancing an individual's functional capacity before elective surgery with the aim of improving tolerance to the anticipated physiological stress of major surgery, may have a role in improving postoperative outcomes 6 . Prehabilitation programmes vary in their components, but can include exercise programmes, nutritional or psychological interventions 7 . Where they encompass different types of intervention, they are referred to as ‘multimodal’ 8 . In the context of the older adult, programmes may also include preoperative comprehensive geriatric assessment (CGA) and optimization. A summary of intervention types is presented in Fig. 1 .
Fig. 1.
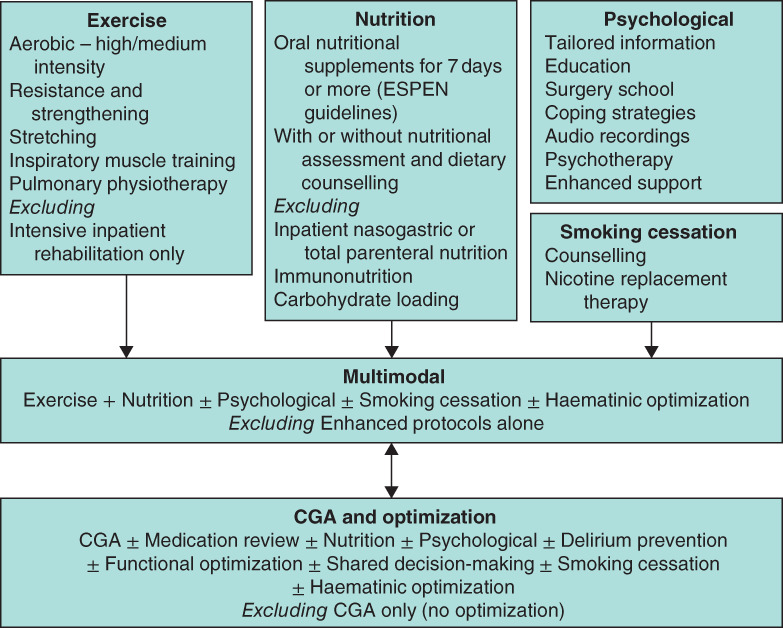
Summary of prehabilitation intervention components and exclusions ESPEN, European Society for Clinical Nutrition and Metabolism; CGA, comprehensive geriatric assessment.
Early prehabilitation studies focused on the safety and feasibility of unimodality interventions 9 . More recently, studies have been more likely to be multimodal and to involve higher‐risk populations 10 . Previous systematic reviews 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 focused predominantly on single‐modality prehabilitation in mixed surgical populations. This review addresses the need for an updated review of the entire spectrum of prehabilitation interventions in elective abdominal cancer surgery with particular relevance to the older patient.
Methods
This systematic review and meta‐analysis was conducted with reference to the Cochrane Handbook and is reported using the PRISMA guidelines 21 . The protocol was registered with PROSPERO (CRD42019120381). The primary objective was to determine whether any modality of prehabilitation (alone or in combination) before elective abdominal surgery leads to a reduction in either length of hospital stay (LOS) or complications (overall, pulmonary, wound infection rate, delirium, severe complications) compared with a control arm that does not include prehabilitation. The review was undertaken with particular relevance to older adults. Secondary objectives were to determine any effect on functional outcome measures (physical activity or walking capacity, weight loss, discharge independence) and psychological outcome measures (quality of life (QoL)).
Search strategy
Systematic searches were performed of the MEDLINE, Web of Science, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsychINFO and the Cochrane databases for papers published from database inception to January 2019. Preliminary searches revealed that limiting the searches to studies performed in older adults would be too restrictive and result in the exclusion of potentially relevant studies; therefore no age limits were set. Searches were limited to studies published in the English language as resources were not available to support translation. The search was constructed using the PICO (patient, intervention, comparison, outcome) framework: Patient (adults undergoing abdominal or gastrointestinal surgery); Intervention (prehabilitation or preoperative optimization); Comparator (standard care or rehabilitation only); and Outcome (primary: LOS or complication rates). Clinical.Trials.gov was also searched for trials that had been completed but not published. A sample search strategy is shown in Appendix S1 (supporting information).
Inclusion and exclusion criteria
Randomized, case–control, cohort or retrospective studies reporting on adults (aged 18 years or above) undergoing surgery with curative intent for any gastrointestinal (oesophagus, stomach, pancreas, liver, colorectal) or intra‐abdominal (urological or gynaecological) cancer were included. Studies including mixed surgical populations were included if they reported the cancer and non‐cancer results separately or if more than 50 per cent of the population were patients with cancer. Studies could test any prehabilitation intervention or preoperative optimization strategy, alone or in combination (multimodal), and had to report outcomes in a control group. Control groups could include standard care, placebo, postoperative rehabilitation programme only, information leaflet or verbal advice on preparing for surgery and positive behaviour change (for example smoking cessation or alcohol reduction) in line with current perioperative care guidelines. Studies of postoperative interventions only were excluded, as were studies that did not report on either of the primary outcomes. Studies published only in abstract form without full text were excluded. Reference lists of primary studies and relevant systematic reviews were also hand‐searched for additional studies.
Screening of all titles and abstracts was undertaken independently by two reviewers. Articles were considered for full‐text review if they met the study inclusion criteria or could not be excluded on the basis of the abstract alone. Full‐text articles were retrieved and assessed by the same two reviewers. Disagreements were addressed by discussion and consensus and, if required the opinion of a third reviewer was sought.
Definitions of eligible interventions
Eligible interventions included exercise interventions (either alone or in combination with pulmonary exercises), nutritional assessment and supplementation, psychological interventions, CGA and optimization, smoking cessation and multimodal (two or more modalities). These are summarized in Fig. 1 .
Assessment of study quality
Risk‐of‐bias assessment was performed using the Cochrane risk‐of‐bias tool 22 for randomized trials and the Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) 23 for non‐randomized trials. Randomized studies were graded for risk of bias (+, low risk; −, high risk; ?, unclear risk) in each of the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other source of bias. Non‐randomized studies were assessed on bias due to confounding, selection, classification of interventions, deviations from intended interventions, missing data, outcome measurement and reporting. Quality assessment was undertaken independently by two reviewers, and disagreements were resolved by consensus.
Data extraction
Data were extracted according to a predesigned pro forma, which included study characteristics, baseline data, intervention characteristics, adherence and outcomes. Studies were divided according to modality: exercise (alone or including pulmonary training), multimodal, nutrition, psychological, smoking, and CGA with optimization.
The primary outcomes, LOS and complication rates, were recorded as mean(s.d.) values and proportions respectively. Where the mean was not reported, an approximation was calculated from the median and range 22 . Complication rates were recorded as total, severe (Clavien–Dindo grade III or above) or pulmonary complications, wound infections and delirium within 30 days of surgery. Secondary outcomes were extracted where reported: change in functional outcome measures (preoperative change in 6‐minute walk test (6MWT) or cardiopulmonary exercise test (CPET) variables of physiological fitness, percentage preoperative weight loss or discharge independence), or psychological outcomes (postoperative Hospital Anxiety and Depression Scale (HADS), Short Form 36 Health Survey (SF‐36®; Rand Corporation, Santa Monica, California, USA) or European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 29 and 30 (EORTC QLQ‐C29/C30) score).
Statistical analysis
Qualitative analyses were performed for all studies that met the inclusion criteria. Studies were analysed according to the type of prehabilitation intervention. Meta‐analysis was performed using RevMan software (Review Manager version 5.3, 2014; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) where the number (greater than 3) and quality of studies permitted, if the 95 per cent c.i. overlapped and effect sizes were similar 24 . Meta‐analysis was performed using random‐effects models, assessing risk difference for both dichotomous and continuous outcomes. Heterogeneity was assessed using the I 2 statistic. Significance was set at α = 0·050.
Results
Searches were performed on 6 January 2019. Some 130 papers were identified for full text review; 97 were excluded, leaving 33 studies for inclusion (Fig. 2 ). There were 25 RCTs (including pilot and feasibility studies) 9 , 10 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , seven prospective cohort studies (with either contemporary or historical controls) 48 , 49 , 50 , 51 , 52 , 53 , 54 , and one retrospective study 55 . Three studies 32 , 33 , 35 reported two separate intervention groups, resulting in a total of 36 interventions for comparison (Table S1, supporting information).
Fig. 2.
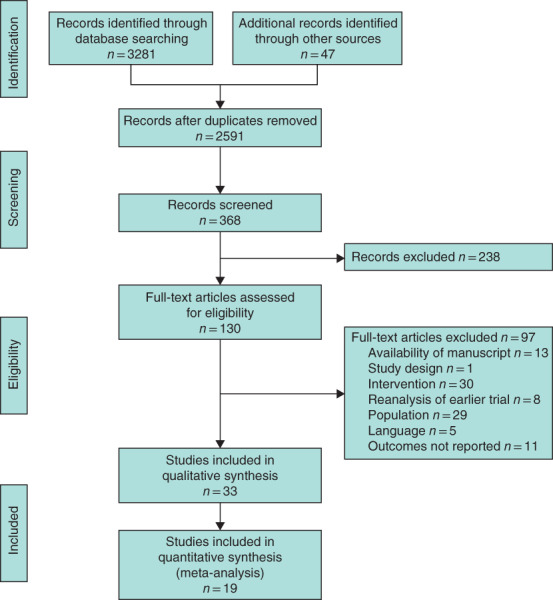
PRISMA diagram for the review
Baseline characteristics
The studies, published between 2000 and 2019, included 2028 patients undergoing prehabilitation and 1934 controls. Interventions comprised: exercise only (9 studies) 9 , 10 , 25 , 36 , 42 , 43 , 44 , 45 , 46 , multimodal (10 studies)26–29,47–52, nutrition only (7 studies) 30 , 31 , 32 , 33 , 40 , 41 , 53 , psychological only (2 studies) 34 , 35 , CGA with optimization only (4) 37 , 38 , 54 , 55 and smoking cessation only (1 study) 39 . Sample sizes ranged from 32 to 443 patients, with most having fewer than 100 patients in each arm; only four studies 36 , 37 , 54 , 55 had more than this, and were mostly non‐randomized. The wide range of sample sizes reflects the diverse primary outcomes on which power calculations were based, and also the fact that a small number were pilot or feasibility studies. Studies were predominantly single‐centre, with only eight studies 33 , 36 , 37 , 38 , 40 , 44 , 45 , 53 conducted across multiple centres. Studies were conducted in North America, Europe, Australasia, South‐East Asia and Brazil. A range of surgical populations were studied, including colorectal (16 studies), upper gastrointestinal, hepatobiliary and pancreatic (9 studies), urological (3 studies), and mixed populations of gastrointestinal and abdominal malignancies (5 studies) (Table S1, supporting information).
Twenty‐four studies involved patients with cancer exclusively, with a range of 52–78 per cent of patients with cancer in the remaining studies. Six studies included patients receiving neoadjuvant therapy. Although the average age range was 55–81 years, it was less than 70 years in the majority of studies. Three 48 , 50 , 52 of the ten multimodal studies and four 37 , 38 , 54 , 55 of the CGA studies had populations with an average age over 75 years (Table S1, supporting information). Nine studies 10 , 37 , 38 , 42 , 48 , 50 , 52 , 54 , 55 selected patients who were either assessed as frail (using a recognized frailty screen or criteria) or over a certain age cut‐off; however the method of detecting frailty, frailty criteria used, and age varied between studies. Two studies 40 , 41 selected patients who were malnourished, and one 28 selected patients with chronic liver injury (Table S1, supporting information).
Methodological quality assessment
The assessment of methodological quality is summarized in Tables 1 and 2 . Only three randomized studies blinded both participants and researchers, one 30 by using a placebo oral nutritional supplement, the second 36 by having all patients attend a preoperative physiotherapy appointment in which those in the control arm received only an information booklet whereas patients in the intervention arm learned breathing exercises, and the third 10 by using a double‐informed consent model where control and intervention arms were not aware of each other. The absence of blinding of either participants or study personnel was the most common reason for high risk of bias assessment. The majority of RCTs adequately described randomization, but allocation concealment was not as reported robustly. Half of the RCTs adequately described blinding of outcome assessment10,25,26,29,30,34–36,38–40,42,43. Only two studies 27 , 34 did not adequately report their outcome data (Table 1 ).
Table 1.
Cochrane risk‐of‐bias tool results for randomized studies
| Reference | Randomization (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other sources of bias (other bias) |
|---|---|---|---|---|---|---|---|
| Exercise alone | |||||||
| Banerjee et al. 25 | + | + | − | + | + | ? | ? |
| Barberan‐Garcia et al. 10 | + | + | + | + | + | + | ? |
| Boden et al. 36 | + | + | + | + | + | ? | ? |
| Carli et al. 9 | + | ? | − | ? | + | ? | ? |
| Dronkers et al. 42 | + | ? | − | + | + | ? | ? |
| Dunne et al. 43 | + | + | − | + | + | ? | ? |
| Santa Mina et al. 44 | ? | ? | − | ? | + | + | ? |
| Soares et al. 45 | ? | ? | − | − | + | + | ? |
| Yamana et al. 46 | ? | ? | − | − | + | ? | ? |
| Multimodal | |||||||
| Bousquet‐Dion et al. 47 | + | + | − | − | + | ? | ? |
| Gillis et al. 26 | + | + | − | + | + | ? | ? |
| Jensen et al. 27 | + | + | − | − | ? | + | ? |
| Kaibori et al. 28 | ? | ? | − | ? | + | ? | ? |
| Minnella et al. 29 | + | + | − | + | + | + | ? |
| Nutrition | |||||||
| Burden et al. 40 | + | + | − | + | + | + | ? |
| Gillis et al. 30 | + | + | + | + | + | ? | ? |
| Kabata et al. 31 | + | + | − | ? | + | ? | ? |
| Kong et al. 41 | + | ? | − | − | + | ? | ? |
| MacFie et al. 32 | ? | ? | − | ? | + | ? | ? |
| Smedley et al. 33 | ? | ? | − | ? | + | ? | ? |
| Psychological | |||||||
| Chaudhri et al. 34 | ? | ? | − | + | ? | ? | ? |
| Haase et al. 35 | ? | ? | − | + | + | ? | ? |
| CGA and optimization | |||||||
| Hempenius et al. 37 | + | + | − | ? | + | ? | ? |
| Ommundsen et al. 38 | + | + | − | + | + | ? | ? |
| Smoking | |||||||
| Sørensen and Jørgensen 39 | + | + | − | + | + | ? | ? |
+, Low risk of bias; −, high risk of bias; ?, unclear risk of bias. CGA, comprehensive geriatric assessment.
Table 2.
ROBINS‐I tool results for non‐randomized studies
| Reference | Type of study | Bias due to confounding | Bias in selection of participants | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of reported result |
|---|---|---|---|---|---|---|---|---|
| Multimodal | ||||||||
| Chia et al. 48 | Prospective, before and after intervention | Moderate | High | Low | Low | Low | Moderate | Low |
| Li et al. 49 | Prospective, before and after intervention | Moderate | Low | Low | Low | Low | Moderate | Low |
| Mazzola et al. 50 | Prospective cohort, retrospective control | Moderate | Low | Low | Low | Low | Moderate | Low |
| Nakajima et al. 51 | Prospective cohort, retrospective control | Moderate | Moderate | Low | Low | Low | Moderate | Low |
| Souwer et al. 52 | Prospective, before and after intervention | Moderate | Low | Low | Low | Low | Moderate | Low |
| Nutrition | ||||||||
| Maňásek et al. 53 | Prospective cohort, retrospective control | Moderate | Moderate | Low | Low | Low | Moderate | Low |
| CGA and optimization | ||||||||
| Indrakusuma et al. 55 | Retrospective cohort | Moderate | Moderate | Moderate | Low | Low | Moderate | Low |
| McDonald et al. 54 | Case–control (matched) | Moderate | Low | Low | Low | Low | Moderate | Low |
CGA, comprehensive geriatric assessment.
Seven 49 , 50 , 51 , 52 , 53 , 54 , 55 of the eight non‐randomized studies were graded as moderate risk of bias owing to bias in outcome measurements and due to confounding factors as they mainly used historical controls. One study 48 was judged to be at high risk of bias as the authors chose to include a wider age range in the intervention group than in controls (Table 2 ).
Interventions
Exercise‐based interventions
Unimodal exercise interventions were most commonly based in hospital and conducted under supervision 36 , 42 , 43 , 45 , 46 ; four studies 36 , 42 , 45 , 46 included specific pulmonary exercises or training. Exercise prehabilitation programmes varied in intensity from a single preoperative session 36 to one to three times per week, and ranged from 1 to 6 weeks in duration.
Multimodal interventions
Multimodal interventions were more likely to be home‐based 26 , 29 , 49 , 50 , 51 ; all included exercise and nutrition, with four 26 , 47 , 49 , 52 also including psychological interventions. The nutritional component of multimodal interventions commonly involved dietician assessment and supplementation if required. Two studies 28 , 48 did not mention supplementation. Two multimodal programmes specifically mentioned other behavioural modifications: alcohol reduction 49 and smoking cessation 50 .
Nutrition‐based interventions
All nutrition‐only prehabilitation studies 30 , 31 , 32 , 33 , 40 , 41 , 53 included oral nutritional supplementation, but the prescriptions varied from ‘ad libitum’ between meals to 400 ml three times a day, with duration varying from 1 to 4 weeks. Two studies 32 , 33 included separate intervention groups that received supplements both before and after surgery.
Psychology‐based interventions
The two psychological prehabilitation studies had different interventions; the study by Chaudhri and colleagues 34 looked at the impact of a community‐based stoma education intervention, whereas that by Haase and colleagues 35 involved giving patients audio recordings with either guided imagery or relaxation techniques to listen to before surgery.
Comprehensive geriatric assessment with optimization
All four CGA prehabilitation studies 37 , 38 , 54 , 55 involved preoperative CGA performed by a geriatrician‐led multidisciplinary team, nutritional optimization and medication reviews; two studies 37 , 54 included postoperative daily reviews by a geriatric specialist nurse. Two studies specified that they corrected anaemia with either blood transfusion 55 or supplementation 38 .
Smoking cessation
One study 39 of a smoking cessation intervention met the inclusion criteria; the intervention involved a single smoking cessation counselling session combined with nicotine replacement therapy.
Adherence
Adherence was reported in eight9,10,25,36,42–44,46 of the nine studies of exercise, five 26 , 27 , 29 , 47 , 49 of the ten multimodal studies, and four 30 , 32 , 40 , 41 of the seven nutrition prehabilitation studies, with percentages varying from 69 to 100 per cent, 59 to 98 per cent, and 75 to 99 per cent respectively. Adherence was not stated in studies of psychological, CGA with optimization, or smoking cessation interventions; as these were typically single preoperative interventions, adherence would not have been an issue.
Primary outcome
Twenty different primary outcomes were reported, and 12 of the 33 studies reported more than one primary outcome measure (Tables 3 –8). Four studies 25 , 27 , 42 , 44 reported feasibility as the primary outcome. Postoperative complications (overall complication rate, severe complications (Clavien–Dindo grade II or above, or III or above), pulmonary complications, delirium or site‐specific infection rate) were the most common postoperative outcome measures, and were reported in all except one study 34 . LOS was reported in all except two studies 31 , 46 .
Table 3.
Summary of outcomes and results for exercise prehabilitation
| Reference | Adherence (%) | Primary study outcome | Postoperative outcomes * | Functional outcomes * | Psychological outcomes * |
|---|---|---|---|---|---|
| Banerjee et al. 25 | 92 | Feasibility |
All complications: 4 of 30 versus 10 of 30, P = 0·075 CDC grade ≥ III: 1 of 30 versus 4 of 30 Pneumonia: 3 of 30 versus 2 of 30 LOS: median 7 (4–78) versus 7 (5–107) days |
Peak OP: +1·36 (95% c.i. 0·63, 2·10) ml/beat, P = 0·001 Peak VE: +7·49 (95% c.i. 2·86, 12·12) l/min. P = 0·02 Peak power output: +19 (95% c.i. 10, 27) W. P < 0·001 |
|
| Barberan‐Garcia et al. 10 | 87 | Any complications |
All complications: 20 of 62 versus 38 of 63, P = 0·001; RR 0·5 (95% c.i. 0·3, 0·8) Pulmonary: 4 of 63 versus 10 of 62, P = 0·155 Wound: 1 of 63 versus 1 of 62 LOS: mean(s.d.): 8(8) versus 13(20) days, P = 0·078 |
6MWT: no difference |
SF‐36®: PCS n.s. HADS anxiety and depression: no change in either group |
| Boden et al. 36 | 98 | Pulmonary complications within 14 days |
Any complication within 6 weeks: 74 of 192 versus 79 of 197 Pulmonary: 27 of 218 versus 58 of 214 (adjusted HR 0·48, 95% c.i. 0·30, 0·75, P = 0·001) Wound: 36 of 192 versus 40 of 197 LOS: median 8 (6–11) versus 9 (7–13) days |
||
| Carli et al. 9 | 79 | Change in 6MWT before and after surgery |
All complications: 22 of 56 versus 18 of 54 CDC grade ≥ III: 6 of 56 versus 3 of 54 LOS: mean(s.e.) 11·9(34·6) versus 6·6(3·6) days |
6MWT: baseline to preop. −10·6(7·3) versus + 8·7(6·8) Mean peak V o 2: +134 versus + 112 ml/min |
HADS anxiety: baseline to postop. follow‐up −1·8(0·7) versus −2·0(0·5), P n.s. HADS depression: −0·8(0·6) versus −0·4(0·5), P n.s. |
| Dronkers et al. 42 | 97 | Feasibility |
All complications: 9 of 22 versus 8 of 20 Pulmonary: 5 of 22 versus 5 of 20 LOS: mean(s.d.) 16·2(11·5) versus 21·6 (23·7) days |
EORTC QLQ‐C30: P n.s. | |
| Dunne et al. 43 | 92 | Oxygen uptake at AT |
All complications: 8 of 19 versus 7 of 15 CDC grade ≥ III: 3 of 19 versus 1 of 15 Pneumonia: 2 of 20 versus 3 of 17 Wound: 3 of 20 versus 0 of 17 LOS: median (range) 5 (4–6) versus 5 (4·5–7) days |
V o 2 at AT: +1·5 (95% c.i. 0·2, 2·9) ml per kg per min, P = 0·023 Peak work rate: +13 (95% c.i. 4, 22) W, P = 0·005 |
SF‐36 ® overall QoL score: +11 (95% c.i. 1, 21), P = 0·028 SF‐36 ® overall mental health score: +11 (1, 22), P = 0·037 |
| Santa Mina et al. 44 | 69 | Feasibility |
All complications: 18 of 44 versus 14 of 42 CDC grade ≥ III: 1 of 44 versus 1 of 42 LOS: mean(s.d.) 1·7(0·9) versus 1·76(1·0) |
6MWT preop.: +14·6(+14·5) (95% c.i. −13·87, 43·05), P = 0·313 | HADS anxiety postop.: difference estimate +0·47(0·68), P = 0·49 |
| Soares et al. 45 | Pulmonary function change and 6MWT |
Pulmonary: 5 of 16 versus 11 of 16, P = 0·03 LOS: median (range) 8·5 (4·8–12·3) versus 8·5 (6·5–17·3) days |
6MWT preop: 514·4 (460–557·5) versus 441·5 (412·3–505·9), P = 0·105 | ||
| Yamana et al. 46 | 100 | Pulmonary complications | Pulmonary (CDC grade ≥ III): 3 of 30 versus 5 of 30, P = 0·014 |
Comparative data show intervention and control results respectively. CDC, Clavien–Dindo classification; LOS, length of hospital stay; OP, oxygen pulse; VE, minute ventilation; RR, relative risk; 6MWT, 6‐minute walk test; SF‐36®, Short Form 36; PCS, physical component score; HADS, Hospital Anxiety and Depression Scale; HR, hazard ratio; V o 2, oxygen consumption; n.s., not significant; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; AT, anaerobic threshold; QoL, quality of life.
Postoperative, functional and psychological outcomes
Exercise studies
One study 10 reported a significant reduction in overall complications in the intervention arm (20 of 62 versus 38 of 63 in the control arm, P = 0·001; relative risk 0·5 m, 95 per cent c.i. 0·3 to 0·8). One study 9 found a non‐significant higher overall complication rate in the intervention arm (22 of 56 versus 18 of 54 for the control; P value not reported), which was attributed to poor compliance in the intervention group and an increase in physical activity in the control group. Meta‐analysis showed no significant difference in overall complications, but heterogeneity was high (Fig. 3a ).
Fig. 3.
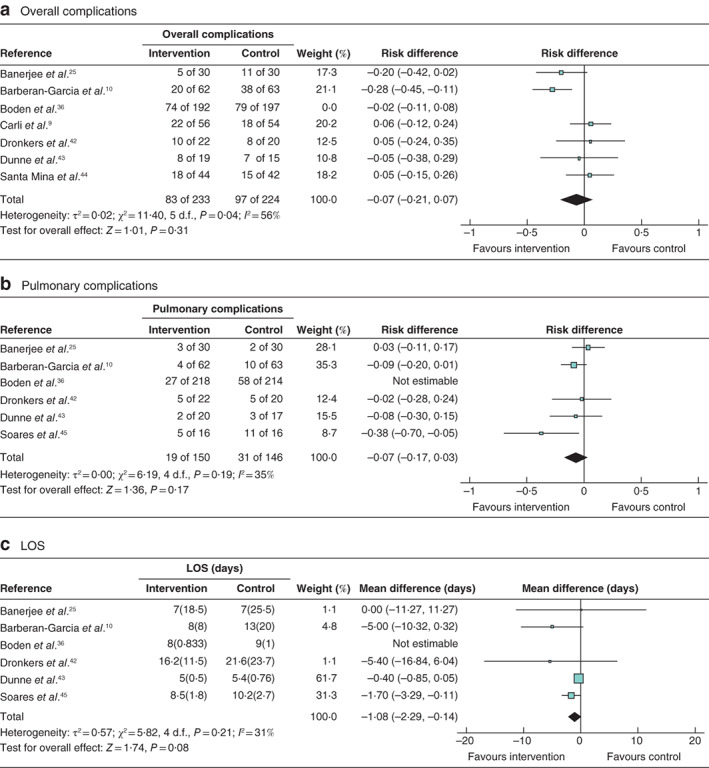
Forest plots showing the effect of exercise prehabilitation on overall and pulmonary complications, and length of hospital stay a Overall complications; b pulmonary complications; c mean(s.d.) length of hospital stay (LOS). a,b Mantel–Haenszel random‐effects models were used for meta‐analysis; risk differences are shown with 95 per cent confidence intervals. c An inverse‐variance model was used for meta‐analysis; mean differences are shown with 95 per cent confidence intervals.
Two studies reported lower rates of pulmonary complications in the intervention group: 27 of 218 versus 58 of 214 (adjusted hazard ratio 0·48, 95 per cent c.i. 0·30 to 0·75; P = 0·001) in the study by Boden and colleagues 36 , and five of 16 versus 11 of 16 (P = 0·03) in that of Soares and co‐workers 45 . Yamana et al. 46 also found a lower Clavien–Dindo grade of pulmonary complication with intervention (P = 0·014). Meta‐analysis of five studies (the study by Boden and colleagues 36 was excluded owing to a significantly different intervention) for pulmonary complications revealed a non‐significant trend in favour of the intervention (Fig. 3b ).
A non‐significant trend towards lower LOS was also observed on meta‐analysis (Fig. 3c and Table 3 ).
Two studies 25 , 43 that assessed preoperative change in CPET variables before and after intervention both demonstrated significant improvements in peak oxygen uptake and peak work rate (Table 3 ). Four studies 9 , 10 , 44 , 45 that assessed functional walking ability using the 6MWT demonstrated no preoperative differences between intervention and control groups. Of the five studies that reported psychological outcomes, only that by Dunne and colleagues 43 showed an improvement in overall QoL score measured using the SF‐36® (+11, 95 per cent c.i. 1 to 21; P = 0·028) and overall mental health score (+11, 1 to 22; P = 0·037) (Table 3 ).
Multimodal studies
One study 50 found a reduction in overall complications in the intervention group (17 of 41 versus 26 of 35 in the control group; P = 0·005) (Table 4 ). Meta‐analysis showed a significant reduction in overall complications after multimodal prehabilitation (Fig. 4a ). Mazzola and colleagues 50 (Clavien–Dindo grade II or above: 7 of 41 versus 15 of 35 respectively, P = 0·02) and Souwer and colleagues 52 (Clavien–Dindo grade III or above: 14 of 86 versus 24 of 75 respectively; odds ratio (OR) 0·4, 95 per cent c.i. 0·2 to 0·9, P = 0·03) both showed a reduction in severe complications with multimodal prehabilitation. No other studies demonstrated a reduction in severe complications, delirium, pulmonary or wound infection.
Table 4.
Summary of outcomes and results for multimodal prehabilitation
| Reference | Adherence (%) | Primary study outcome | Postoperative outcomes * | Functional outcomes * | Psychological outcomes * |
|---|---|---|---|---|---|
| Bousquet‐Dion et al. 47 | 98 | Exercise capacity 6MWT |
All complications: 14 of 37 versus 8 of 26 Wound: 5 of 37 versus 3 of 26 CDC grade ≥ II: 5 of 37 versus 4 of 26 CDC grade ≥ III: 2 of 41 versus 0 of 39 LOS: median (i.q.r.) 3 (3–4) versus 3 (2–4) days, P = 0·122 |
6MWT: mean(s.d.) difference +21(47) versus +10(30) m, P n.s. |
HADS anxiety score > 7: 35% versus 23% HADS depression score > 7: 11% versus 19% |
| Chia et al. 48 | LOS, complications |
Complications (CDC grade ≥ III): 3 of 57 versus 5 of 60, P = 0·511 LOS: 8·4 versus 11 days, P = 0·029 |
|||
| Gillis et al. 26 | 78 | 6MWT at 8 weeks |
All complications: 12 of 38 versus 17 of 39, P = 0·277 Wound: 3 of 38 versus 3 of 39 CDC grade ≥ III: 4 of 38 versus 6 of 39 Pulmonary: 1 of 38 versus 0 of 39 LOS: 4 (i.q.r. 3–5) versus 4 (3–7) days, P = 0·812 |
6MWT preop.: mean(s.d.) +25·2(50·2) versus −16·4(46) m; mean difference 41·7 (95% c.i. 19·8, 63·6) m; adjusted P < 0·001 | SF‐36®/HADS: P n.s. |
| Jensen et al. 27 | 59 | Feasibility |
All complications: 30 of 50 versus 34 of 57 LOS: median 8 (3–30) versus 8 (4–55), P = 0·68 |
||
| Kaibori et al. 28 | Whole body mass and fat mass |
All complications: 2 of 23 versus 3 of 23, P = 0·671 LOS: mean(s.d.) 13·7(4·0) versus 17·5(11·3), P = 0·12 |
|||
| Li et al. 49 | 70 (partial) | 6MWT at 8 weeks |
All complications: 15 of 42 versus 20 of 45 CDC grade ≥ III: 2 of 42 versus 1 of 45 LOS: median (i.q.r.) 4 (3–6) versus 4 (3–6) days |
6MWT preop.: 464(92) versus 402(57) m baseline (prehabilitation group only), P < 0·01 | SF‐36®: P n.s. |
| Mazzola et al. 50 | Mortality, complications |
All complications: 17 of 41 versus 26 of 35, P = 0·005 CDC grade ≥ III: 7 of 41 versus 15 of 35, P = 0·02 Pulmonary: 2 of 41 versus 1 of 35 LOS: median (range) 17 (7–76) versus 27 (8–146) days, P = 0·08 |
|||
| Minnella et al. 29 | 63 | 6MWT before and after surgery |
All complications: 14 of 24 versus 18 of 25 CDC grade ≥ II: 12 of 24 versus 16 of 25 CDC grade ≥ III: 6 of 24 versus 10 of 25 LOS: median (i.q.r.) 8 (5·75–11·75) versus 7 (5·5–12·5) days, P = 0·44 |
6MWT preop.: mean(s.d.) change +36·9(51·4) versus −22·8(52·5) m, P < 0·001 | |
| Nakajima et al. 51 | Preop. nutritional status and postop. course |
Complications (CDC grade ≥ III): 32 of 76 versus 38 of 76 Pneumonia: 1 of 76 versus 1 of 76 Wound: 2 of 76 versus 3 of 76 LOS: median (i.q.r.) 23 (16–34) versus 30 (21–40) days, P = 0·045 |
Prehabilitation (no control) 6MWT: median (i.q.r.) baseline 530 (470–571) to preop. 554 (499–620) m, P < 0·001 | ||
| Souwer et al. 52 | 1‐year mortality |
All complications: 24 of 86 versus 26 of 63 CDC grade ≥ III: 14 of 86 versus 24 of 75 (OR 0·4 (95% c.i. 0·2, 0·9), P = 0·03) Pulmonary: P = 0·3 LOS ≥ 14 days: 5 of 86 versus 17 of 63 days (OR 0·2 (0·1, 0·5), P = 0·001 |
Comparative data show intervention and control results respectively. 6MWT, 6‐minute walk test; CDC, Clavien–Dindo classification; LOS, length of hospital stay; n.s., not significant; HADS; Hospital Anxiety and Depression Scale; SF‐36®, Short Form 36; OR, odds ratio.
Fig. 4.
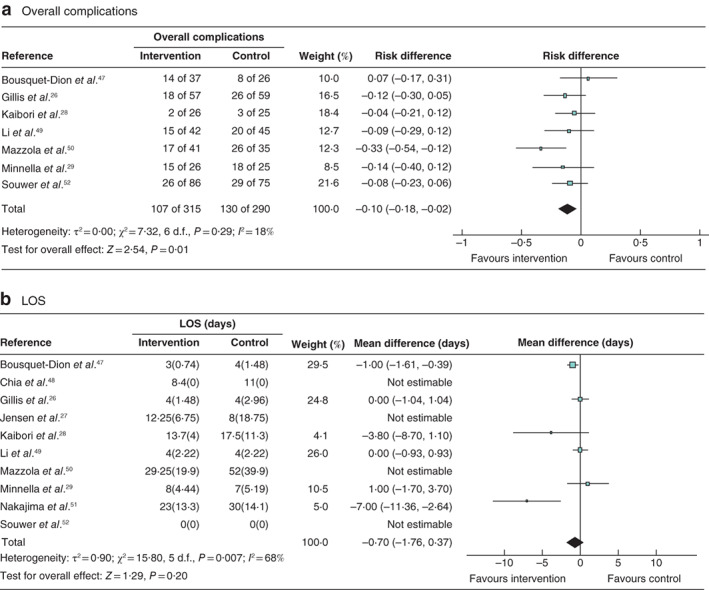
Forest plots showing the effect of multimodal prehabilitation on overall complications and length of hospital stay a Overall complications; b mean(s.d.) length of hospital stay (LOS). a A Mantel–Haenszel random‐effects model was used for meta‐analysis; risk differences are shown with 95 per cent confidence intervals. b An inverse‐variance model was used for meta‐analysis; mean differences are shown with 95 per cent confidence intervals.
Three studies reported a significant reduction in LOS in the intervention group: 8·4 versus 11 days in the control group (P = 0·029) in the study by Chia and colleagues 48 ; median LOS 23 (i.q.r. 16–34) versus 30 (21–40) days in the control group (P = 0·045) in the study by Nakajima and co‐workers 51 ; and LOS of 14 days or more in five of 86 versus 17 of 63 patients respectively (OR 0·2, 95 per cent c.i. 0·1 to 0·5; P = 0·001) in the study by Souwer and colleagues 52 (Table 4 ). Meta‐analysis for LOS including six studies was not significant; however, there were high levels of heterogeneity (Fig. 4b ).
Four multimodal studies 26 , 29 , 49 , 51 demonstrated significant preoperative improvements in functional walking ability using the 6MWT after the intervention (mean difference range 24–62 m; all P < 0·010) (Table 4 ). However, in two of these studies 49 , 51 walking ability was tested only in the intervention group. No differences in psychological outcomes were observed in multimodal studies 47 , 49 , 59 (Table 4 ).
Nutrition studies
Two studies reported a reduction in overall complications in the intervention group: eight of 54 versus 17 of 48 in the control group (P = 0·04) in the study by Kabata and colleagues 31 , and 15 of 32 versus 34 of 44 respectively (P < 0·050) for group 2 in the study by Smedley et al. 33 (Table 5 ). Meta‐analysis demonstrated significantly fewer overall complications following the intervention (the historical study of MacFie et al. 32 was excluded from meta‐analysis) (Fig. 5 ).
Table 5.
Summary of outcomes and results for nutrition prehabilitation
| Reference | Adherence (%) | Primary study outcome | Postoperative outcomes* | Functional outcomes* | Psychological outcomes* |
|---|---|---|---|---|---|
| Burden et al. 40 | 75 (estimated) | SSI or chest infection |
All complications: 23 of 54 versus 35 of 62, P = 0·114 Pneumonia: 5 of 54 versus 4 of 62 CDC grade ≥ III: 9 of 54 versus 10 of 62 SSI: 11 of 55 versus 17 of 45 (OR 0·41 (95% c.i. 0·16, 1·00), P = 0·044) LOS: median (i.q.r.) 7 (4–10·5) versus 7 (4–10) days, P = 0·63 |
% weight loss preop.: median (i.q.r.) 4·1 (1·7–7·0) versus 6·7 (2·6–10·8), P = 0·016 | |
| Gillis et al. 30 | 93·7–96·6 | 6MWT before and after surgery |
All complications: 8 of 22 versus 9 of 21 CDC grade ≥ III: 2 of 22 versus 2 of 21 Pneumonia: 0 of 22 versus 1 of 21 LOS: median 5 (3–13) versus 4 (3–10) days |
6MWT: mean(s.d.) +20·8(42·6) versus +1·2(65·5) m, P = 0·27 | SF‐36 ® postop.: PCS 41·3 (34·2–46·5) versus 36·5 (34·5–42·8); MCS 47·7 (38·1–53·8) versus 41·3 (35·6–55·8) |
| Kabata et al. 31 | – | Complications within 30 days |
All complications: 8 of 54 versus 17 of 48, P = 0·04 CDC grade ≥ III: 5 of 54 versus 11 of 48, P < 0·001 Wound: 1 of 54 versus 7 of 48 Pneumonia: 1 of 54 versus 0 of 48 |
% weight loss preop.: median 7·4 versus 6·3, P n.s. | |
| Kong et al. 41 | 99 (partial) | Postop. complications, CDC grade ≥ II |
Complications (CDC grade ≥ III): 9 of 65 versus 12 of 62 Wound: 7 of 65 versus 3 of 62 Pulmonary: 6 of 65 versus 4 of 62 LOS: mean(s.d.) 9·3(3·6) versus 9·7(5·9) days |
% bodyweight change preop.: −0·37 versus −0·97, P = 0·173 | EORTC‐QLQ: no difference |
| MacFie et al. 32 | Weight change and clinical outcomes | Weight loss preop.: P n.s. | |||
| Group 1 | 89·3 |
All complications: 7 of 24 versus 3 of 25 LOS: mean 12 versus 13 days |
HADS postop.: anxiety or depression, P n.s. | ||
| Group 2 | 80·7 |
All complications: 6 of 24 versus 3 of 25 LOS: mean 11 versus 13 days |
HADS postop.: anxiety or depression, P n.s. | ||
| Maňásek et al. 53 | Complications |
Wound: 3 of 52 versus 13 of 105 (RR 2·2) LOS: mean(s.d.) 9·4(5·0) versus 12·0(6·4) days, P = 0·002 |
% weight loss postop.: 2·6 versus 6·4, P n.s. | ||
| Smedley et al. 33 | Postop. change in bodyweight | ||||
| Group 1 | – |
All complications: 20 of 41 versus 34 of 44 Buzby definition 56 : minor 17 of 41 versus 30 of 44; major 3 of 41 versus 4 of 44 LOS: mean(s.d.) 12·8(4·5) versus 14·1(6·6) days |
– | SF‐36®: no difference | |
| Group 2 | – |
All complications: 15 of 32 versus 34 of 44, P < 0·05 Buzby definition 56 : minor 10 of 32 versus 30 of 44; major 5 of 32 versus 4 of 44 LOS: mean(s.d.) 11·7(5·1) versus 14·1(6·6) days |
Only group to gain weight before surgery; lost less weight over course of study, P = 0·05 | SF‐36®: no difference |
Comparative data show intervention and control results respectively. SSI, surgical‐site infection; CDC, Clavien–Dindo classification; OR, odds ratio; LOS, length of hospital stay; 6MWT, 6‐minute walk test; SF‐36®, Short Form 36; PCS, physical component score; MCS, mental component score; n.s., not significant; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HADS; Hospital Anxiety and Depression Scale; RR, relative risk.
Fig. 5.
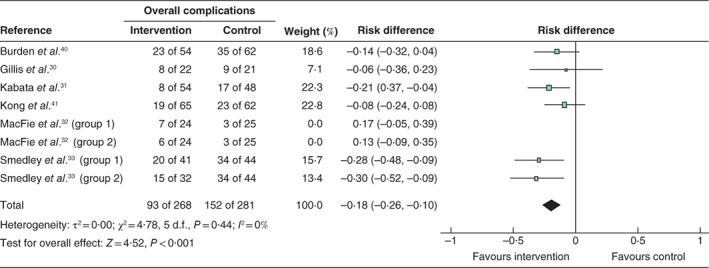
Forest plot showing the effect of nutrition prehabilitation on overall complications A Mantel–Haenszel random‐effects model was used for meta‐analysis; risk differences are shown with 95 per cent confidence intervals.
Kabata and colleagues 31 also reported a reduction in severe complications in the intervention group (Clavien–Dindo grade III or above: 5 of 54 versus 11 of 48 in the control group; P < 0·001) and Burden and co‐workers 40 found a reduction in surgical‐site infection (11 of 55 versus 17 of 45; OR 0·41, 95 per cent c.i. 0·16 to 1·00, P = 0·044) (Table 5 ). Only one study 53 reported a reduction in LOS with the intervention (mean(s.d.) 9·4(5·0) versus 12·0(6·4) days in the control group; P = 0·002) (Table 5 ), with no difference in LOS on meta‐analysis (data not shown).
Burden and colleagues 40 (median percentage weight loss 4·1 (i.q.r. 1·7–7·0) in the intervention group versus 6·7 (2·6–10·8) in the control group; P = 0·016) and Smedley et al. 33 (less weight loss in group 2, P = 0·05) were able to demonstrate a reduction in preoperative weight loss with their interventions that was not seen in other studies 31 , 32 , 41 . No differences in functional walking ability 30 or psychological outcomes 30 , 32 , 33 , 41 were found (Table 5 ).
Psychological studies
Chaudhri and co‐workers 34 reported a reduction in LOS in the intervention group (8 versus 10 days in the control group; P = 0·029), which was attributed to fewer delayed discharges owing to stoma proficiency (Table 6 ). Haase et al. 35 found no difference in overall complications between either of their interventions and the control. Neither psychological intervention had any effect on the measured psychological outcomes 34 , 35 (Table 6 ).
Table 6.
Summary of outcomes and results for psychological prehabilitation
| Reference | Primary study outcome | Postoperative outcomes* | Functional outcomes* | Psychological outcomes* |
|---|---|---|---|---|
| Chaudhri et al. 34 | Time to stoma proficiency, LOS | LOS: 8 versus 10 days, P = 0·029 | HADS postop.: anxiety 33% versus 32%; depression 17% versus 24% | |
| Haase et al. 35 | Systemic analgesic consumption via PCA | EORTC‐QLQ and GIQLI: P n.s. | ||
| Group 1 |
Wound infection: 3 of 20 versus 3 of 18 Delirium: 0 of 20 versus 0 of 18 LOS: overall median (range) 12·5 (11–14) days |
|||
| Group 2 |
Wound infection: 4 of 22 versus 3 of 18 Delirium: 1 of 22 versus 0 of 18 LOS: median (range) 12·5 (11–14) days |
Comparative data show intervention and control results respectively. LOS, length of hospital stay; HADS, Hospital Anxiety and Depression Scale; PCA, patient‐controlled analgesia; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; GIQLI, GastroIntestinal Quality of Life Index.
Comprehensive geriatric assessment with optimization
McDonald and colleagues 54 demonstrated a reduction in the mean number of complications per patient with the intervention (0·9 versus 1·4 in the control group, 95 per cent c.i. −0·13 to −0·89; P < 0·001), despite a significantly higher incidence of delirium in the intervention group (52 of 183 versus 8 of 143, 95 per cent c.i. 3·06 to 14·65; P < 0·001) (Table 7 ).
Table 7.
Summary of outcomes and results for comprehensive geriatric assessment with optimization prehabilitation
| Reference | Primary study outcome | Postoperative outcomes* | Functional outcomes* | Psychological outcomes* |
|---|---|---|---|---|
| Hempenius et al.37 | Postop. delirium |
Complications (> 1): 42 of 127 versus 38 of 133 (OR 1·24 (95% c.i. 0·73, 2·10)) Pulmonary: 31 of 127 versus 27 of 133 Wound: 13 versus 12, P = 0·37 Delirium: 12 of 127 versus 19 of 133 (OR 0·63 (0·29, 1·35)) LOS: 8 versus 8 days |
Independence on discharge: 76 of 127 versus 87 of 133 (OR 1·84 (1·01, 3·37)) | SF‐36® bodily pain same or better: 57 of 127 versus 41 of 133 (OR 0·49 (0·29, 0·82)) |
| Indrakusuma et al.55 | 30‐day mortality, delirium, LOS |
Pneumonia: 37 of 221 versus 31 of 222 Wound: 18 of 221 versus 26 of 222 Delirium: 22 of 221 versus 27 of 222 LOS: 7 (range 5–12) versus 9 (7–14) days; P = 0·001 |
||
| McDonald et al.54 | LOS, readmissions and level of care at discharge |
Complications: mean 0·9 versus 1·4 (95% c.i. −0·13, −0·89), P < 0·001 Delirium: 52 of 183 versus 8 of 143 (95% c.i. 3·06, 14·65), P < 0·001 Pulmonary: 18 of 183 versus 25 of 143 Wound: 4 of 183 versus 8 of 143 LOS: median 4 versus 6 days (95% c.i. −1·06, −4·21), P < 0·001 |
Discharge home with self‐care: 114 of 183 versus 73 of 143 (95% c.i. 1·02, 2·47), P = 0·04 | |
| Ommundsen et al.38 | Complications, CDC grade ≥ II |
Any complication: 40 of 52 versus 55 of 62 CDC grade ≥ II: 36 of 52 versus 47 of 62 LOS: 8 versus 8 days |
Discharged directly home: 38 of 57 versus 38 of 65, P = 0·2 |
Comparative data show intervention and control results respectively. OR, odds ratio; LOS, length of hospital stay; SF‐36®, Short Form 36; CDC, Clavien–Dindo classification.
Two studies demonstrated a significant reduction in LOS with intervention: median 4 versus 6 days respectively (95 per cent c.i. −1·06 to −4·21; P < 0·001) in the study by McDonald et al. 54 , and a median of 7 (range 5–12) versus 9 (7–14) days respectively (P = 0·001) in that by Indrakusuma and colleagues 55 . McDonald and co‐workers 54 demonstrated an improvement in independence on discharge with the intervention (114 of 183 versus 73 of 143 respectively, 95 per cent c.i. 1·02 to 2·47; P = 0·04). Hempenius et al. 37 observed an improvement in psychological outcome with intervention (SF‐36® bodily pain scores were the same or better in 57 of 127 versus 41 of 133 in the control group; OR 0·49, 95 per cent c.i. 0·29 to 0·82) (Table 7 ).
Smoking studies
The smoking cessation trial 39 did not find a reduction in either complications or LOS with intervention (Table 8 ).
Table 8.
Summary of outcomes and results for smoking cessation prehabilitation
| Reference | Primary study outcome | Postoperative outcomes* | Functional outcomes* | Psychological outcomes* |
|---|---|---|---|---|
| Sørensen and Jørgensen39 | Postop. wound and tissue complications within 30 days |
Any complication: 11 of 27 versus 13 of 30 Pneumonia: 3 of 27 versus 4 of 30 Wound: 3 of 27 versus 4 of 30 LOS: median (i.q.r.) 11 (10–13) versus 11 (8–14) days |
Comparative data show intervention and control results respectively. LOS, length of hospital stay.
Discussion
This systematic review has found evidence from a number of trials that exercise, multimodal, nutrition and CGA with optimization prehabilitation programmes may reduce the number of postoperative complications after elective surgery for gastrointestinal and urological cancers. It has shown evidence that multimodal, nutritional, psychological and CGA interventions (but not exercise interventions or smoking cessation alone) may reduce LOS. In particular, the small number of studies that selected high‐risk, frail or older patients were more likely to report improvements in either complications or LOS compared with studies that included all patients. Equally, studies conducted in patients undergoing oesophageal and upper gastrointestinal surgery, known to be associated with high levels of postoperative morbidity and mortality, were more likely to demonstrate reductions in pulmonary complications. However, conclusions are limited by the methodological quality of included studies, in particular the lack of blinding of participants in all except three studies. Significant heterogeneity of interventions also limits comparison. Adherence to exercise, multimodal and nutritional interventions was generally high; however, it is possible that participant selection bias and lack of blinding may have resulted in more motivated patients being recruited.
National and international guidelines 57 , 58 , 59 recommend that CGA should be performed in all patients over the age of 70 years with a diagnosis of cancer to try to predict treatment toxicity and postoperative complications, and to aid in shared decision‐making. However, there remain very few studies of CGA in surgical cancer populations, and the majority of these are limited to its role in risk prediction and prognostication 60 , 61 . This systematic review identified only two RCTs 37 , 38 evaluating CGA and tailored interventions. It is worth noting that the median age of patients in studies included in this review was only 68 years, with patients in the exercise‐alone interventions having a median age of only 63 years. Only seven of the 33 studies in this review had a median age greater than 75 years. This suggests that many prehabilitation studies to date either failed to recruit older patients due to the location or nature of the interventions or they excluded older patients owing to a perceived risk of the interventions, despite mounting evidence 62 , 63 that exercise‐based interventions are safe in older individuals.
This review also demonstrated that improvements in preoperative functional measures can be made with exercise prehabilitation (measured by CPET), multimodal interventions (measured using 6MWT) and nutritional prehabilitation (reduction in preoperative weight loss). However, the link between small statistically significant improvements in these variables and clinical outcomes is not clear.
A number of previous systematic reviews have examined individual components of prehabilitation in varying surgical populations: exercise 18 , 19 , 20 , 64 , 65 , exercise in frail individuals 16 , multimodal interventions 13 , 14 , 15 , multimodal interventions in frail individuals 12 , nutrition with and without exercise 66 , and psychological interventions 11 . All of these, including the present review, have been limited by the quality of the underlying evidence. This is the first review that included all modalities of prehabilitation of relevance to the older adult.
Prehabilitation programmes, regardless of the individual components they comprise, are complex multicomponent interventions, and thus should be evaluated as such. The Medical Research Council in the UK has published a clear framework for evaluating and conducting trials involving complex interventions 67 . Two of the potential reasons for negative findings in prehabilitation studies are either that the interventions are too standardized to enable reproducible delivery or that, in efforts to provide truly personalized programmes, no two individuals receive the same intervention. Equally, although there is accumulating evidence that multimodal prehabilitation is likely to be more beneficial than using a single modality, future trials that use methodologies designed for evaluating complex interventions will be able to determine which components are most beneficial for different patients and why.
This review is limited by the heterogeneity of outcomes reported. LOS and complications were selected as primary outcomes for this review; however, a number of studies were powered to detect changes in other primary outcomes and therefore may have been inadequately powered for the primary outcomes of this review. The majority of trials in prehabilitation are relatively small, and this may contribute towards reporting bias of trials with statistically significant outcomes. Heterogeneity of studies may have also contributed to some analyses attaining statistical significance inappropriately. The wide date range of included studies may have added to the heterogeneity, as perioperative care has evolved over the past 20 years with the introduction of enhanced recovery pathways and laparoscopic surgery. Another potential limitation is that diverse surgical procedures with a range of complication rates have been compared. This may have resulted in some analyses not reaching significance, and will have contributed towards heterogeneity on meta‐analysis. For the purpose of this review, a large number of studies were excluded at full‐text review due to lack of reporting of LOS or complications, which are considered core outcomes for surgical trials 68 , 69 . In particular, a number of trials of psychological interventions 70 , 71 , 72 , 73 , 74 , 75 were excluded for this reason. Of note, only one preoperative smoking cessation trial 39 and no studies in gynaecological cancer surgery met the inclusion criteria. The main strength of this review is the comprehensive nature, whereby all current prehabilitation modalities in abdominal cancer surgery were included. This means that the review is of relevance to a wide range of surgical specialties, identifies gaps in the current evidence base, and will be of interest to commissioners looking to fund prehabilitation services.
The reporting of outcomes presented a challenge in this review owing to the range of outcome measures used; this reflects complex interventions and the inability to compare them directly, and raises an important issue for researchers. The evidence base for prehabilitation might be stronger if a core outcome set could be used in all trials, irrespective of modality of prehabilitation or surgical population, to facilitate comparison of interventions. The StEP‐COMPAC group (Standardising Endpoints in Perioperative Medicine) have already made progress in this regard in perioperative medicine 76 , 77 , 78 , 79 . Initiatives such as the DiSCO (Defining Standards in Colorectal Optimisation) project led by researchers in the West of Scotland, which aims to create key sets of standards for prehabilitation in collaboration with patients, their caregivers and the public, will be vital in ensuring that results are relevant to service users as well as clinicians, and to the successful promotion of patient‐centred care. Future studies also need to evaluate strategies for implementation and the associated costs to enable adequate investment at a time of increasing healthcare costs.
Supporting information
Appendix S1 Search strategy
Table S1 General characteristics of the included studies
Acknowledgements
The authors acknowledge D. Hind, Clinical Trials Unit, School of Health and Related Research, University of Sheffield, for methodological advice.
This study received educational grant funding from the British Association of Surgical Oncology and the Bowel Disease Research Foundation.
Disclosure: The authors declare no conflict of interest.
Funding information
British Association of Surgical Oncology Educational Grant
Bowel Disease Research Foundation Educational Grant
References
- 1. Office for National Statistics . Cancer Registration Statistics, England: 2016 https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/final2016#breast‐prostate‐lung‐and‐colorectal‐cancers‐continue‐to‐be‐the‐most‐common [accessed 29 March 2019].
- 2. West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, Jack S et al Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth 2014; 112: 665–671. [DOI] [PubMed] [Google Scholar]
- 3. Audisio RA, Veronesi P, Ferrario L, Cipolla C, Andreoni B, Aapro M. Elective surgery for gastrointestinal tumours in the elderly. Ann Oncol 1997; 8: 317–326. [DOI] [PubMed] [Google Scholar]
- 4. Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA et al.; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016; 134: e653–e699. [DOI] [PubMed] [Google Scholar]
- 5. Audisio RA, Papamichael D. Treatment of colorectal cancer in older patients. Nat Rev Gastroenterol Hepatol 2012; 9: 716–725. [DOI] [PubMed] [Google Scholar]
- 6. Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 2005; 8: 23–32. [DOI] [PubMed] [Google Scholar]
- 7. Macmillan Cancer Support , Royal College of Anaesthetists and National Institute for Health Research Cancer and Nutrition Collaboration. Principles and Guidance for Prehabilitation within the Management and Support of People with Cancer 2019. https://www.macmillan.org.uk/_images/prehabilitation‐guidance‐for‐people‐with‐cancer_tcm9‐353994.pdf [accessed 16 August 2020].
- 8. Levett DZH, Edwards M, Grocott M, Mythen M. Preparing the patient for surgery to improve outcomes. Best Pract Res Clin Anaesthesiol 2016; 30: 145–157. [DOI] [PubMed] [Google Scholar]
- 9. Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ et al Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010; 97: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 10. Barberan‐Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R et al Personalised prehabilitation in high‐risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018; 267: 50–56. [DOI] [PubMed] [Google Scholar]
- 11. Tsimopoulou I, Pasquali S, Howard R, Desai A, Gourevitch D, Tolosa I et al Psychological prehabilitation before cancer surgery: a systematic review. Ann Surg Oncol 2015; 22: 4117–4123. [DOI] [PubMed] [Google Scholar]
- 12. McIsaac DI, Moloo H, Lavallee LT, Nantel J, Bryson GL, Gagne S et al Prehabilitation before cancer surgery to improve patient function in frail elderly. Anesth Analg 2017; 124: 1027–1028.28319536 [Google Scholar]
- 13. Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg 2017; 39: 156–162. [DOI] [PubMed] [Google Scholar]
- 14. Bolshinsky V, Li MHGG, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum 2018; 61: 124–138. [DOI] [PubMed] [Google Scholar]
- 15. Luther A, Gabriel J, Watson RP, Francis NK. The impact of total body prehabilitation on post‐operative outcomes after major abdominal surgery: a systematic review. World J Surg 2018; 42: 2781–2791. [DOI] [PubMed] [Google Scholar]
- 16. Bruns ERJJ, van den Heuvel B, Buskens CJ, van Duijvendijk P, Festen S, Wassenaar EB et al The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis 2016; 18: O267–O277. [DOI] [PubMed] [Google Scholar]
- 17. Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Deans DAC, Skipworth RJE. Prehabilitation before major abdominal surgery: a systematic review and meta‐analysis. World J Surg 2019; 43: 1661–1668. [DOI] [PubMed] [Google Scholar]
- 18. Orange ST, Northgraves MJ, Marshall P, Madden LA, Vince RV. Exercise prehabilitation in elective intra‐cavity surgery: a role within the ERAS pathway? A narrative review. Int J Surg 2018; 56: 328–333. [DOI] [PubMed] [Google Scholar]
- 19. Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J et al The ability of prehabilitation to influence postoperative outcome after intra‐abdominal operation: a systematic review and meta‐analysis. Surgery 2016; 160: 1189–1201. [DOI] [PubMed] [Google Scholar]
- 20. Mina DS, Clarke H, Ritvo R, Leung YW, Matthew AG, Katz J et al Effect of total‐body prehabilitation on postoperative outcomes: a systematic review and meta‐analysis. Physiotherapy 2014; 100: 196–207. [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT, Altman DG. Assessing risk of bias in included studies In: Cochrane Handbook for Systematic Reviews of Interventions, Higgins JPT, Green S. (eds). John Wiley: Chichester, 2008; 187–241. [Google Scholar]
- 23. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JAC, Harbord RM. Funnel plots in meta‐analysis. Stata J 2004; 4: 127–141. [Google Scholar]
- 25. Banerjee S, Manley K, Shaw B, Lewis L, Cucato G, Mills R et al Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer 2018; 26: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 26. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A et al Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014; 121: 937–947. [DOI] [PubMed] [Google Scholar]
- 27. Jensen BT, Laustsen S, Jensen JBJB, Borre M, Petersen AK. Exercise‐based prehabilitation is feasible and effective in radical cystectomy pathways – secondary results from a randomized controlled trial. Support Care Cancer 2016; 24: E652–E652. [DOI] [PubMed] [Google Scholar]
- 28. Kaibori M, Ishizaki M, Matsui K, Nakatake R, Yoshiuchi S, Kimura Y et al Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg 2013; 206: 202–209. [DOI] [PubMed] [Google Scholar]
- 29. Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg 2018; 153: 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gillis C, Loiselle SE, Fiore JFJ, Awasthi R, Wykes L, Liberman AS et al Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double‐blinded randomized placebo‐controlled trial. J Acad Nutr Diet 2016; 116: 802–812. [DOI] [PubMed] [Google Scholar]
- 31. Kabata P, Jastrzębski T, Kąkol M, Król K, Bobowicz M, Kosowska A et al Preoperative nutritional support in cancer patients with no clinical signs of malnutrition – prospective randomized controlled trial. Support Care Cancer 2015; 23: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacFie J, Woodcock NP, Palmer MD, Walker A, Townsend S, Mitchell CJ. Oral dietary supplements in pre‐ and postoperative surgical patients: a prospective and randomized clinical trial. Nutrition 2000; 16: 723–728. [DOI] [PubMed] [Google Scholar]
- 33. Smedley F, Bowling T, James M, Stokes E, Goodger C, O'Connor O et al Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br J Surg 2004; 91: 983–990. [DOI] [PubMed] [Google Scholar]
- 34. Chaudhri S, Brown L, Hassan I, Horgan AF. Preoperative intensive, community‐based vs. traditional stoma education: a randomized, controlled trial. Dis Colon Rectum 2005; 48: 504–509. [DOI] [PubMed] [Google Scholar]
- 35. Haase O, Schwenk W, Hermann C, Müller JM. Guided imagery and relaxation in conventional colorectal resections: a randomized, controlled, partially blinded trial. Dis Colon Rectum 2005; 48: 1955–1963. [DOI] [PubMed] [Google Scholar]
- 36. Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C et al Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. BMJ 2018; 360: j5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hempenius L, Slaets J, van Asselt D, de Bock G, Wiggers T, van Leeuwen B. Outcomes of a geriatric liaison intervention to prevent the development of postoperative delirium in frail elderly cancer patients: report on a multicentre, randomized, controlled trial. PLoS One 2013; 8: e64834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ommundsen N, Wyller TB, Nesbakken A, Bakka AO, Jordhøy MS, Skovlund E et al Preoperative geriatric assessment and tailored interventions in frail older patients with colorectal cancer: a randomized controlled trial. Colorectal Dis 2018; 20: 16–25. [DOI] [PubMed] [Google Scholar]
- 39. Sørensen LT, Jørgensen T. Short‐term pre‐operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Colorectal Dis 2003; 5: 347–352. [DOI] [PubMed] [Google Scholar]
- 40. Burden ST, Gibson DJ, Lal S, Hill J, Pilling M, Soop M et al Pre‐operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight‐losing patients with colorectal cancer: single‐blind randomized controlled trial. J Cachexia Sarcopenia Muscle 2017; 8: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kong SH, Lee HJ, Na JR, Kim WG, Han DS, Park SH et al Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery 2018; 164: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 42. Dronkers JJ, Lamberts H, Reutelingsperger IMMD, Naber RH, Dronkers‐Landman CM, Veldman A et al Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil 2010; 24: 614–622. [DOI] [PubMed] [Google Scholar]
- 43. Dunne DFJ, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ et al Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016; 103: 504–512. [DOI] [PubMed] [Google Scholar]
- 44. Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet‐Dion G, Alibhai SMHH et al Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol 2018; 27: 289–298. [DOI] [PubMed] [Google Scholar]
- 45. Soares SM, Nucci LB, da Silva MM, Campacci TC. Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: a randomized controlled trial. Clin Rehabil 2013; 27: 616–627. [DOI] [PubMed] [Google Scholar]
- 46. Yamana I, Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H et al Randomized controlled study to evaluate the efficacy of a preoperative respiratory rehabilitation program to prevent postoperative pulmonary complications after esophagectomy. Dig Surg 2015; 32: 331–337. [DOI] [PubMed] [Google Scholar]
- 47. Bousquet‐Dion G, Awasthi R, Loiselle SÈE, Minnella EM, Agnihotram RV, Bergdahl A et al Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol 2018; 57: 849–859. [DOI] [PubMed] [Google Scholar]
- 48. Chia CLK, Mantoo SK, Tan KY. ‘Start to finish trans‐institutional transdisciplinary care’: a novel approach improves colorectal surgical results in frail elderly patients. Colorectal Dis 2016; 18: O43–O50. [DOI] [PubMed] [Google Scholar]
- 49. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS et al Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013; 27: 1072–1082. [DOI] [PubMed] [Google Scholar]
- 50. Mazzola M, Bertoglio C, Boniardi M, Magistro C, De Martini P, Carnevali P et al Frailty in major oncologic surgery of upper gastrointestinal tract: how to improve postoperative outcomes. Eur J Surg Oncol 2017; 43: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 51. Nakajima H, Yokoyama Y, Inoue T, Nagaya M, Mizuno Y, Kadono I et al Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato‐pancreato‐biliary surgeries for malignancy. Ann Surg Oncol 2019; 26: 264–272. [DOI] [PubMed] [Google Scholar]
- 52. Souwer ETD, Bastiaannet E, de Bruijn S, Breugom AJ, van den Bos F, Portielje JEA et al Comprehensive multidisciplinary care program for elderly colorectal cancer patients: ‘from prehabilitation to independence’. Eur J Surg Oncol 2018; 44: 1894–1900. [DOI] [PubMed] [Google Scholar]
- 53. Maňásek V, Bezděk K, Foltys A, Klos K, Smitka J, Smehlik D. The impact of high protein nutritional support on clinical outcomes and treatment costs of patients with colorectal cancer. Klin Onkol 2016; 29: 351–357. [PubMed] [Google Scholar]
- 54. McDonald SR, Heflin MT, Whitson HE, Dalton TO, Lidsky ME, Liu P et al Association of integrated care coordination with postsurgical outcomes in high‐risk older adults the Perioperative Optimization of Senior Health (POSH) initiative. JAMA Surg 2018; 153: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Indrakusuma R, Dunker MS, Peetoom JJ, Schreurs WH. Evaluation of preoperative geriatric assessment of elderly patients with colorectal carcinoma. A retrospective study. Eur J Surg Oncol 2015; 41: 21–27. [DOI] [PubMed] [Google Scholar]
- 56. Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Kaakenson CM, McNeal GE et al Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Cin Nutr 1988; 47(Suppl): 366–381. [DOI] [PubMed] [Google Scholar]
- 57. Shipway D, Harari D, Dhesi J. Peri‐operative management of older people undergoing surgery. Rev Clin Gerontol 2014; 24: 78–92. [Google Scholar]
- 58. Griffiths R, Beech F, Brown A, Dhesi J, Foo I, Goodall J et al Peri‐operative care of the elderly 2014 : Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2014; 69(Suppl 1): 81–98. [DOI] [PubMed] [Google Scholar]
- 59. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen‐Heijnen ML, Extermann M et al International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014; 32: 2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramesh HSJ, Pope D, Gennari R, Audisio RA. Optimising surgical management of elderly cancer patients. World J Surg Oncol 2005; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pope D, Ramesh H, Gennari R, Corsini G, Maffezzini M, Hoekstra HJ et al Pre‐operative assessment of cancer in the elderly (PACE): a comprehensive assessment of underlying characteristics of elderly cancer patients prior to elective surgery. Surg Oncol 2006; 15: 189–197. [DOI] [PubMed] [Google Scholar]
- 62. Jack S, West M, Grocott MPW. Perioperative exercise training in elderly subjects. Best Pract Res Clin Anaesthesiol 2011; 25: 461–472. [DOI] [PubMed] [Google Scholar]
- 63. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC et al Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007; 39: 1435–1445. [DOI] [PubMed] [Google Scholar]
- 64. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta‐analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg 2020; 24: 1375–1385. [DOI] [PubMed] [Google Scholar]
- 65. O'Doherty AF, West M, Jack S, Grocott MPW. Preoperative aerobic exercise training in elective intra‐cavity surgery: a systematic review. Br J Anaesth 2013; 110: 679–689. [DOI] [PubMed] [Google Scholar]
- 66. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos‐Reed N et al Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta‐analysis. Gastroenterology 2018; 155: 391–410. [DOI] [PubMed] [Google Scholar]
- 67. Craig P, Dieppe P, Macintyre S, Health P, Unit S, Michie S et al Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Avery KNL, Chalmers KA, Brookes ST, Blencowe NS, Coulman K, Whale K et al Development of a core outcome set for clinical effectiveness trials in esophageal cancer resection surgery. Ann Surg 2018; 267: 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McNair AGK, Whistance RN, Forsythe RO, Macefield R, Rees J, Pullyblank AM et al Core outcomes for colorectal cancer surgery: a consensus study. PLoS Med 2016; 13: e1002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pinar G, Kurt A, Gungor T. The efficacy of preopoerative instruction in reducing anxiety following gyneoncological surgery: a case control study. World J Surg Oncol 2011; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. O'Connor G, Coates V, O'Neill S. Randomised controlled trial of a tailored information pack for patients undergoing surgery and treatment for rectal cancer. Eur J Oncol Nurs 2014; 18: 183–191. [DOI] [PubMed] [Google Scholar]
- 72. Garcia ACM, Simão‐Miranda TP, Carvalho AM, Elias PC, da Graça Pereira M, de Carvalho EC. The effect of therapeutic listening on anxiety and fear among surgical patients: randomized controlled trial. Rev Lat Am Enfermagem 2018; 26: e3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Parker PA, Pettaway CA, Babaian RJ, Pisters LL, Miles B, Fortier A et al The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 2009; 27: 3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thornton AA, Perez MA, Meyerowitz BE. Patient and partner quality of life and psychosocial adjustment following radical prostatectomy. J Clin Psychol Med Settings 2004; 11: 15–30. [Google Scholar]
- 75. Ali N, Khalil HZ. Effect of psychoeducational intervention on anxiety among Egyptian bladder cancer patients. Cancer Nurs 1989; 12: 236–242. [PubMed] [Google Scholar]
- 76. Buggy DJ, Freeman J, Johnson MZ, Leslie K, Riedel B, Sessler DI et al; StEP‐COMPAC Group . Systematic review and consensus definitions for standardised endpoints in perioperative medicine: postoperative cancer outcomes. Br J Anaesth 2018; 121: 38–44. [DOI] [PubMed] [Google Scholar]
- 77. Haller G, Bampoe S, Cook T, Fleisher LA, Grocott MPW, Neuman M et al; StEP‐COMPAC Group . Systematic review and consensus definitions for the standardised endpoints in perioperative medicine initiative: clinical indicators. Br J Anaesth 2019; 123: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abbott TEF, Fowler AJ, Pelosi P, Gama de Abreu M, Møller AM, Canet J et al; StEP‐COMPAC Group . A systematic review and consensus definitions for standardised end‐points in perioperative medicine: pulmonary complications. Br J Anaesth 2018; 120: 1066–1079. [DOI] [PubMed] [Google Scholar]
- 79. Myles PS, Grocott MPW, Boney O, Moonesinghe SR, Biccard B, Chan M et al; COMPAC‐StEP Group . Standardizing end points in perioperative trials: towards a core and extended outcome set. Br J Anaesth 2016; 116: 586–589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy
Table S1 General characteristics of the included studies


