Abstract
Background
Infectious complications are common after gastrointestinal surgery. Selective decontamination of the digestive tract (SDD) might reduce their incidence. SDD is used widely in colorectal resections, but its role in upper gastrointestinal resection is less clear. The aim of this study was to investigate the impact of SDD on postoperative outcome in upper gastrointestinal surgery.
Methods
Studies investigating SDD in upper gastrointestinal surgery were included after search of medical databases (PubMed, Ovid, Cochrane Library and Google Scholar). Results were analysed according to predefined criteria. The incidence of perioperative overall complications and death was pooled. Risk of bias was assessed using the revised Cochrane risk‐of‐bias tool.
Results
Some 1384 studies were identified, of which four RCTs were included in the final analysis. These studies included 415 patients, of whom 213 (51·3 per cent) received standard treatment/placebo and 202 (48·7 per cent) had SDD. The incidence of anastomotic leakage (odds ratio (OR) 0·39, 95 per cent c.i. 0·19 to 0·80; P = 0·010) and pneumonia (OR 0·42, 0·23 to 0·78; P = 0·006) was reduced in patients receiving SDD. Rates of surgical‐site infection (P = 0·750) and mortality (P = 0·130) were not affected by SDD.
Conclusion
SDD seems to be associated with reduction of anastomotic leakage and pneumonia following upper gastrointestinal resection, without affecting postoperative mortality.
This meta‐analysis investigated the influence of selective decontamination of the digestive tract (SDD) on outcomes after upper gastrointestinal surgery. SDD led to a reduction in anastomotic leakage (odds ratio (OR) 0·39, 95 per cent c.i. 0·19 to 0·80; P = 0·010) and pneumonia (OR 0·42, 0·23 to 0·78; P = 0·006). Further RCTs on SDD in upper gastrointestinal surgery are needed.

Suggestion of better outcomes but not much evidence
Antecedentes
Las complicaciones infecciosas son frecuentes tras la cirugía gastrointestinal. La descontaminación selectiva del tracto digestivo (Selective Decontamination of the Digestive tract, SDD) podría reducir su incidencia. Mientras que la SDD es ampliamente utilizada en las resecciones colorrectales, su papel en las resecciones del tracto gastrointestinal superior está menos definido. El objetivo de este estudio fue investigar el impacto de la SDD en el resultado postoperatorio en cirugía del tracto gastrointestinal superior.
Métodos
Se incluyeron estudios que investigasen la SDD en cirugía del tracto gastrointestinal superior después de una búsqueda de bases de datos médicas (Pubmed, Ovid, Cochrane‐Library y Google/Scholar). Los resultados se analizaron de acuerdo con criterios predefinidos. Se agrupó la incidencia de complicaciones perioperatorias globales y mortalidad. El riesgo de sesgo se analizó utilizando la herramienta de la colaboración Cochrane revisada para la evaluación del riesgo de sesgo.
Resultados
Se identificaron 1.380 estudios, de los cuales cuatro ensayos controlados aleatorizados fueron incluidos en el análisis final. Estos estudios incluían a 415 pacientes de los cuales 213 (51,3%) recibieron tratamiento estándar/placebo y 202 (48,7%) recibieron SDD. La incidencia de fugas anastomóticas (razón de oportunidades, odds ratio, OR: 0,39 (0,19–0,80); P = 0,010)) y neumonía (OR: 0,42 (0,23–0,78); P = 0,006)) se redujo en pacientes que recibieron SDD. Infecciones del sitio quirúrgico (P = 0,750) y la mortalidad (P = 0,130) no se relacionaron con la SDD.
Conclusión
SDD parece asociarse con reducción de fuga anastomótica y neumonía tras resecciones del tracto gastrointestinal superior, sin afectar a la mortalidad postoperatoria.
Introduction
Gastrointestinal surgery is frequently followed by infectious complications 1 , which are associated with longer hospital stay and increased costs 2 . Surgical‐site infection (SSI) contributes up to 20 per cent of all hospital‐acquired infections 3 . Several risk factors are known to be involved, including prolonged duration of surgery, low preoperative serum albumin level, high intraoperative blood loss, ASA grade, high BMI and perioperative hypothermia 4 , 5 , 6 .
The gut microbiome appears to play a critical role in the development of postoperative infectious complications 7 , 8 . For potentially contaminated surgical procedures, preoperative antibiotic prophylaxis with intraoperative repetition for longer procedures is considered to be the standard of care 9 . Preoperative decontamination of the digestive tract by intake of oral, commonly non‐absorbable, antibiotics can be added to this schedule. Selective decontamination of the digestive tract (SDD) is believed to target Staphylococcus aureus, yeast and pathogenic Gram‐negative bacteria, representing risk factors for postoperative infection, and is continued until there is normal passage of stool and food intake 10 , 11 .
In colorectal surgery 12 , SDD in combination with mechanical bowel preparation is used widely, and a recent RCT 13 demonstrated that SDD significantly reduced postoperative infectious complications. The situation is less clear regarding SDD in upper gastrointestinal surgery 10 , 14 . The aim of this study was to conduct a systematic review and meta‐analysis of the current evidence for the role of SDD, focusing on postoperative complications and mortality reported in RCTs in upper gastrointestinal surgery.
Methods
This study adhered to the PRISMA guidelines 15 for performance of the meta‐analysis.
Online medical databases (PubMed, Ovid, Cochrane Library and Google Scholar) were searched using a search term (Appendix S1, supporting information) and combinations of ‘selective digestive decontamination’, ‘gastrointestinal surgery’ and ‘upper GI’. The last online database search was performed on 3 June 2019. Relevant articles specified in the reference list of identified articles were included. The studies included investigated SDD before upper gastrointestinal surgery, and only RCTs involving human subjects were considered. Only studies with at least an English abstract and published within the last 25 years were included. Those focusing on lower gastrointestinal surgery and SDD in the setting of transplantation were excluded. Inclusion of the selected studies was validated independently by three researchers. Data extraction and the assessment of quality and risk of bias across studies was performed independently by two researchers. In case of any differences, the subject was discussed until consensus was reached.
Postoperative complications (SSI, pneumonia, anastomotic insufficiency) were analysed as reported by the study authors within the time frames reported using authors' definitions of specific complications. Postoperative mortality was assessed as stated by the authors, including death during the hospital stay as well as within 30 days from surgery.
The study was registered in the PROSPERO database (registration number CRD42020144720).
Statistical analysis
Review Manager version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used to conduct the meta‐analysis. Outcomes were encoded as dichotomous variables, and odds ratios (ORs) were calculated by assessing the incidence of respective outcomes. Study heterogeneity was assessed by estimating I 2. Studies with an I 2 statistic above 33 per cent were considered to have high heterogeneity. Assuming random differences by chance in treatment procedure, patient characteristics and local differences, the Mantel–Haenszel statistical method was used with a random‐effects model when I 2 was 33 per cent or above in the test for heterogeneity, and with a fixed‐effect model when I 2 was less than 33 per cent. For statistical significance, a bilateral 95 per cent c.i. was defined. For the analysis of risk of bias, the revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2) was employed. Funnel plots of included ORs were created using Review Manager version 5.3.
Results
The initial search resulted in 1384 studies investigating SDD in gastrointestinal surgery, of which four RCTs 10 , 14 , 16 , 17 met the inclusion criteria and were included in the final analysis (Fig. 1 ). The studies included patients undergoing gastrectomy and oesophagectomy, mainly for malignant disease. A total of 415 patients were analysed, of whom 213 received standard‐of‐care treatment/placebo and 202 had SDD before surgery. Three of the four studies used polymyxin B, tobramycin and amphotericin B as SDD, four times daily with a cephalosporin as the perioperative intravenous antibiotic (Table 1 ). A study by Tetteroo and colleagues 18 did not meet the inclusion criterion of publication within the last 25 years and was not included in the analysis.
Fig. 1.
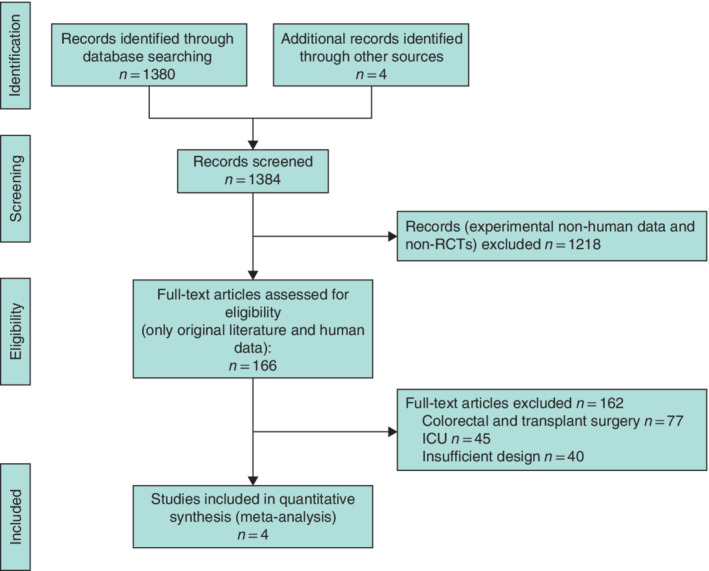
PRISMA diagram for the review
Table 1.
Details of selective decontamination for included RCTs
| No. of patients | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Year | Control | SDD | Resection | Perioperative parenteral antibiotics | SDD | Regimen |
| Schardey et al.16 | 1997 | 103 | 102 | Gastrectomy | Cefotaxime | Polymyxin B 100 mg, tobramycin 80 mg, amphotericin B 500 mg, vancomycin 125 mg | 4 times daily, from 1 day before surgery to POD7 |
| Riedl et al.20 | 2001 | 16 | 12 | Transthoracic resection of oesophagus and cardia | Cefazolin | Polymyxin B 100 mg, tobramycin 80 mg, amphotericin B 200 mg | 4 times daily, from 4–7 days before surgery to POD7 |
| Farran et al. 17 | 2008 | 51 | 40 | Gastrectomy, oesophagectomy | Amoxycillin/clavulanic acid | Erythromycin 500 mg, gentamicin 80 mg, nystatin sulphate 100 mg | 4 times daily, from 12 h before surgery to POD5 |
| Roos et al. 11 | 2011 | 43 | 48 | Oesophageal, gastric and hepatopancreatobiliary resections | Cefuroxime/metronidazole | Polymyxin B 100 mg, tobramycin 80 mg, amphotericin B 500 mg | 4 times daily, from 2 days before surgery until normal bowel function or minimum of POD3 |
SDD, selective decontamination of the digestive tract; POD, postoperative day.
Anastomotic leak
All 415 patients were eligible for the analysis of anastomotic insufficiency. This complication occurred in 28 of 213 patients (13·1 per cent) receiving standard‐of‐care treatment and in 12 of 202 (5·9 per cent) after SDD. SDD had a protective effect on anastomotic leakage (OR 0·39, 95 per cent c.i. 0·19 to 0·80; P = 0·010), and heterogeneity was low (I 2 = 0 per cent) (Fig. 2 ). In the study by Schardey and co‐workers 16 , leak rates were reduced significantly in the SDD group (2·9 per cent versus 10·6 per cent in the control group; P = 0·049) (Table 2 ). In the Schardey study, anastomotic insufficiency was defined as a total intestinal wall defect at the suture line, diagnosed by a positive dye test or radiological contrast study. Riedel et al. 17 found a reduction in anastomotic insufficiency by SDD of 16 versus 25 per cent, although no P value was given, nor was leakage defined. Farran and colleagues 14 reported no influence of SDD on anastomotic insufficiency (2·5 versus 5·9 per cent; P = 0·405) (Table 2 ), which was diagnosed by oral administration of methylene blue, clinical leak through a wound or drains, or radiological contrast examination. Roos et al. 10 reported a reduction in anastomotic insufficiency by SDD (12·5 per cent versus 23·3 per cent in the control group), but gave no P value; they defined leakage using a combination of clinical presentation, blood results, and confirmation by CT or X‐ray.
Fig. 2.
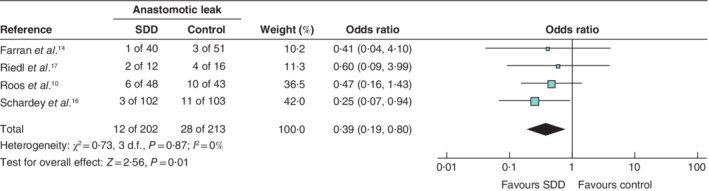
Forest plot comparing anastomotic insufficiency following selective decontamination of the digestive tract versus standard treatment in upper gastrointestinal resections A Mantel–Haenszel fixed‐effect model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals. SDD, selective decontamination of the digestive tract.
Table 2.
Outcomes of included studies
| Postoperative complication rate (%)* | Schardey et al.16 | Riedl et al.17 | Farran et al.14 | Roos et al.10 |
|---|---|---|---|---|
| Overall | 30·4 versus 44·7 | – | – | – |
| P | 0·049 | |||
| Pneumonia | 8·8 versus 22·3 | 42 versus 56 | 12·5 versus 19·6 | – |
| P | 0·012 | 0·269 | ||
| Anastomotic insufficiency | 2·9 versus 10·6 | 16 versus 25 | 2·5 versus 5·9 | 12·5 versus 23·3 |
| P | 0·049 | 0·405 | ||
| Surgical‐site infection | 4·9 versus 3·8 | 0 versus 0 | – | 33·3 versus 39·5 |
| P | 1·000 | |||
| Mortality | 4·9 versus 10·6 | 0 versus 6 | 5 versus 5·9 | – |
| P | 0·100 | 0·615 |
Selective decontamination of the digestive tract (SDD) versus control group respectively.
Pneumonia
The incidence of postoperative pneumonia in 324 eligible patients from three studies 14 , 16 , 17 indicated that 42 of 170 (24·7 per cent) in the control group were affected compared with 19 of 154 (12·3 per cent) after SDD, giving an OR of 0·42 (95 per cent c.i. 0·23 to 0·78; P = 0·006) in favour of SDD, with low heterogeneity (I 2 = 0 per cent) (Fig. 3 ). Schardey and colleagues 16 demonstrated a significant reduction in pneumonia after SDD (8·8 percent versus 22·3 per cent in the control group; P = 0·012) (Table 2 ). Pneumonia was diagnosed when four of the following five criteria occurred: body temperature above 38·5°C, leucocyte count greater than 104 or less than 5 × 103 cells per μl, positive auscultation examination, lung infiltration on X‐ray, or positive bacteriology. Riedl et al. 17 found a reduction in postoperative pneumonia from 56 to 42 per cent with SDD, although no P value was given (Table 2 ); they used the definition of pneumonia from the Robert Koch Institute. Farran and colleagues 14 reported no influence of SDD on postoperative pneumonia (12·5 versus 19·6 per cent; P = 0·269) (Table 2 ). Pneumonia was defined when two of the following characteristics were present: purulent respiratory secretion, fever, radiological infiltrates, rhonchi, positive auscultation of the chest, and positive bacteriology of respiratory secretions.
Fig. 3.
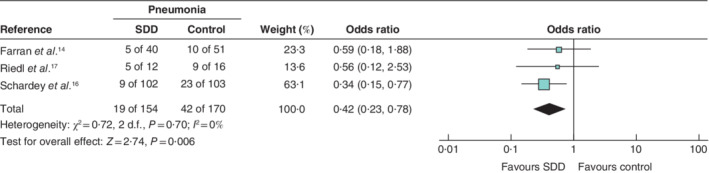
Forest plot comparing postoperative pneumonia following selective decontamination of the digestive tract versus standard treatment in upper gastrointestinal resections A Mantel–Haenszel fixed‐effect model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals. SDD, selective decontamination of the digestive tract.
Surgical‐site infection
SSI included wound infections as well as abscess formation, although definitions were inconsistent between the included studies. Of 324 patients, 21 of 162 (13·0 per cent) in the control group and 21 of 162 patients (13·0 per cent) in the SDD group were diagnosed as having an SSI (OR 0·89, 95 per cent c.i. 0·43 to 1·82; P = 0·750), with low heterogeneity (I 2 = 0 per cent) (Fig. 4 ). No individual study found a significant difference in SSI rates between control and SDD groups.
Fig. 4.
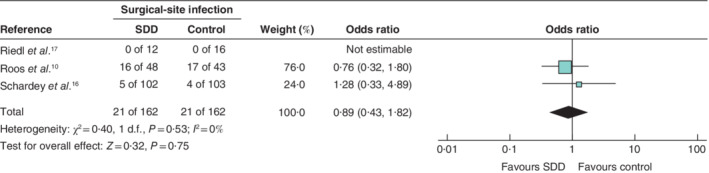
Forest plot comparing surgical‐site infection following selective decontamination of the digestive tract versus standard treatment in upper gastrointestinal resections A Mantel–Haenszel fixed‐effect model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals. SDD, selective decontamination of the digestive tract.
Mortality
Again, 324 patients were eligible for evaluation. Some 15 of 170 patients (8·8 per cent) died in the control group compared with seven of 154 (4·5 per cent) in the SDD group. Although there was a tendency in favour of SDD, significance was not reached (OR 0·50, 95 per cent c.i. 0·20 to 1·23; P = 0·130), with low heterogeneity (I 2 = 0 per cent) (Fig. 5 ). Of the three included studies, two 14 , 16 found no improvement in mortality and one 17 showed benefit for SDD (0 versus 6 per cent), but no P value was provided (Table 2 ).
Fig. 5.
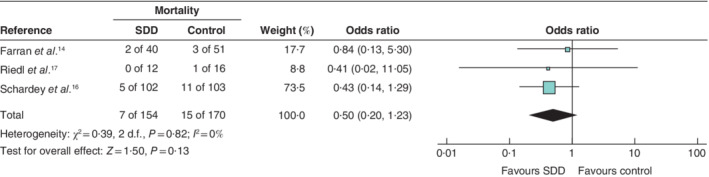
Forest plot comparing postoperative mortality following selective decontamination of the digestive tract versus standard treatment in upper gastrointestinal resections A Mantel–Haenszel fixed‐effect model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals. SDD, selective decontamination of the digestive tract.
Publication bias
Funnel plots showed even distribution within the pseudo 95 per cent c.i., suggesting no publication bias for incidence of anastomotic insufficiency, pneumonia, SSI or mortality (Fig. S1 , supporting information). Analysis using the Cochrane risk‐of‐bias tool (RoB 2) for randomized trials revealed ‘some concerns’ in the randomization process and deviations from intended interventions in the study by Riedl and colleagues 17 . The other included studies 10 , 14 , 16 had a low risk of bias (Fig. S2, supporting information).
Discussion
This meta‐analysis has demonstrated a reduction in anastomotic leakage and postoperative pneumonia after SDD for upper gastrointestinal surgery. Anastomotic leak was not defined uniformly, but adhered to a common basis. Three of the four studies identified a reduction in leak rates, and two of the three included studies showed a reduction in pneumonia, although in these two studies there was a threefold to fourfold difference in the prevalence of pneumonia, probably reflecting differences in definition.
In the study by Schardey et al. 16 , which used tobramycin, polymyxin B, amphotericin B and vancomycin for SDD, a concurrent effect of reduced bacterial oropharyngeal colonization was found 19 . This might relate to the reduced incidence of pneumonia, as microaspiration is associated with postoperative pneumonia 20 .
The number of eligible RCTs for this analysis was low, limiting its conclusions. An RCT by Tetteroo and colleagues 18 had different reporting of complications and was outdated and thus not included in the analysis. Overall morbidity was reported in only one study 16 . The type of resection varied, although most of the included patients underwent oesophagectomy or gastrectomy. The studies cover the time period for the introduction of minimal‐access approaches in upper gastrointestinal surgery. These operations are associated with a reduced risk of postoperative infectious morbidity. Of the included studies, only Roos and co‐workers 10 reported on the use of minimally invasive surgery, although the percentage of a specific procedure (e.g. gastric resection) was not apparent. Conversely, increasingly aggressive therapies for upper gastrointestinal cancers expose patients to increased risk of infection 21 .
In this analysis, three of the four included studies were underpowered with regard to their primary endpoint. Only the study of Roos et al. 10 was of sufficient size for analysis of infectious complications. Based on the metadata, analysis of anastomotic leakage would need 548 patients (13 per cent placebo versus 6 per cent SDD), pneumonia 278 patients (25 versus 12 per cent) and mortality 1276 patients (9 versus 5 per cent) to reach a power of 80 per cent with an α error of 5 per cent. As detrimental side‐effects and selection of resistant bacteria by SDD are uncommon, further well designed RCTs should investigate the impact of SDD in upper gastrointestinal surgery 22 . Hepatopancreatobiliary resections would be of particular interest, as preoperative biliary stenting is a known risk factor for postoperative infectious complications 8 .
Supporting information
Fig. S1 Funnel plots for included studies
Fig. S2 Cochrane risk‐of‐bias assessment
Acknowledgements
F.S. was funded by a German Society of Surgery scholarship (FORTÜNE programme) with D.R. as a mentor.
Disclosure: The authors declare no conflict of interest.
Funding information
German Society of Surgery (FORTÜNE programme)
References
- 1. Vazquez‐Aragon P, Lizan‐Garcia M, Cascales‐Sanchez P, Villar‐Canovas MT, Garcia‐Olmo D. Nosocomial infection and related risk factors in a general surgery service: a prospective study. J Infect 2003; 46: 17–22. [DOI] [PubMed] [Google Scholar]
- 2. Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical‐site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999; 20: 725–730. [DOI] [PubMed] [Google Scholar]
- 3. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017; 96: 1–15. [DOI] [PubMed] [Google Scholar]
- 4. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999; 20: 250–278. [DOI] [PubMed] [Google Scholar]
- 5. Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EG et al Is obesity a high‐risk factor for laparoscopic colorectal surgery? Surg Endosc 2002; 16: 855–858. [DOI] [PubMed] [Google Scholar]
- 6. Hedrick TL, Turrentine FE, Smith RL, McElearney ST, Evans HL, Pruett TL et al Single‐institutional experience with the surgical infection prevention project in intra‐abdominal surgery. Surg Infect (Larchmt) 2007; 8: 425–436. [DOI] [PubMed] [Google Scholar]
- 7. Alverdy JC, Hyoju SK, Weigerinck M, Gilbert JA. The gut microbiome and the mechanism of surgical infection. Br J Surg 2017; 104: e14–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Sargut M et al Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br J Surg 2017; 104: e182–e188. [DOI] [PubMed] [Google Scholar]
- 9. Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014; (5)CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roos D, Dijksman LM, Oudemans‐van Straaten HM, de Wit LT, Gouma DJ, Gerhards MF. Randomized clinical trial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br J Surg 2011; 98: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 11. Zandstra DF, Van Saene HKF. Selective decontamination of the digestive tract as infection prevention in the critically ill. A level 1 evidence‐based strategy. Minerva Anestesiol 2011; 77: 212–219. [PubMed] [Google Scholar]
- 12. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N et al Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg 2019; 43: 659–695. [DOI] [PubMed] [Google Scholar]
- 13. Abis GSA, Stockmann HBAC, Bonjer HJ, van Veenendaal N, van Doorn‐Schepens MLM, Budding AE et al; SELECT trial study group . Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg 2019; 106: 355–363. [DOI] [PubMed] [Google Scholar]
- 14. Farran L, Llop J, Sans M, Kreisler E, Miró M, Galan M et al Efficacy of enteral decontamination in the prevention of anastomotic dehiscence and pulmonary infection in esophagogastric surgery. Dis Esophagus 2008; 21: 159–164. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss A. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double‐blind, placebo‐controlled multicenter trial. Ann Surg 1997; 225: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riedl S, Peter B, Geiss HK, Aulmann M, Bach A, Lehnert T. Microbiological and clinical effects of selective bowel decontamination in transthoracic resection of carcinoma of the esophagus and cardia. Chirurg 2001; 72: 1160–1170. [DOI] [PubMed] [Google Scholar]
- 18. Tetteroo GWM, Wagenvoort JH, Castelein A, Tilanus HW, Ince C, Bruining HA. Selective decontamination to reduce Gram‐negative colonisation and infections after oesophageal resection. Lancet 1990; 335: 704–707. [DOI] [PubMed] [Google Scholar]
- 19. Schardey HM, Kamps T, Rau HG, Gatermann S, Baretton G, Schildberg FW. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother 1994; 38: 2564–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D'Haese J, de Keukeleire T, Remory I, van Rompaey K, Umbrain V, Poelaert J. Assessment of intraoperative microaspiration: does a modified cuff shape improve sealing? Acta Anaesthesiol Scand 2013; 57: 873–880. [DOI] [PubMed] [Google Scholar]
- 21. Cools KS, Sanoff HK, Kim HJ, Yeh JJ, Stitzenberg KB. Impact of neoadjuvant therapy on postoperative outcomes after pancreaticoduodenectomy. J Surg Oncol 2018; 118: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daneman N, Sarwar S, Fowler RA, Cuthbertson BH; SuDDICU Canadian Study Group . Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta‐analysis. Lancet Infect Dis 2013; 13: 328–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Funnel plots for included studies
Fig. S2 Cochrane risk‐of‐bias assessment


