Abstract
Skin cancer affects 1 in 5 Americans, resulting in significant morbidity and mortality. Treatment costs and rates of skin cancer and melanoma continue to rise, making preventative measures increasingly important. However, there is conflicting evidence about efficacy of primary and secondary prevention strategies in decreasing incidence and improving early diagnosis. The US Preventative Services Task Force 2016 guidelines did not endorse routine skin cancer screening because of “insufficient evidence.” Yet, countries like Australia have shown the feasibility and cost-effectiveness of primary sun safety interventions and secondary prevention measures such as routine skin cancer surveillance. Additional emerging evidence shows that regular skin cancer screening in high-risk populations improves early detection and decreases melanoma mortality. New technology may enhance prevention, promote accurate diagnoses, and improve management of melanoma and nonmelanoma skin cancers. Here, we place rising rates of melanoma within historical context, review costs, efficacy, and evidence for primary and secondary skin cancer prevention and examine the evolving role of novel technologies in the field.
Keywords: Melanoma, Primary prevention, Secondary prevention, Sunscreen, Sun safety
INTRODUCTION: BURDEN OF SKIN CANCER AND MELANOMA IN HISTORICAL PERSPECTIVE
In the last 2 decades, the United States has seen a dramatic rise in the rates of both melanoma and nonmelanoma skin cancers, which are linked to ultraviolet radiation exposure.1 Nonmelanoma skin cancer, which include basal cell carcinoma and squamous cell carcinoma, are the most common malignancies in the United States. Melanoma is the fifth most common cancer, and it has been projected that nearly 100,000 new cases of melanoma will be diagnosed in this country by the end of 2019.2
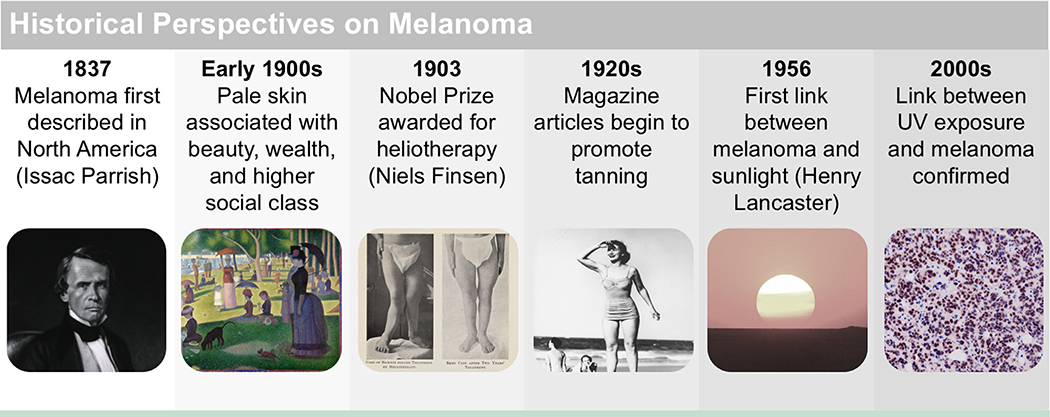
Melanoma as a distinct nosological entity was first described in Europe in 1804 and in North America in 1837.3 It was a rare cancer at the beginning of the 20th century, but the 1930s saw a rise in skin cancer incidence with a dramatic increase in associated deaths between 1950 and 1980. This shift coincided with the promotion of sun exposure, including increased use of heliotherapy to treat diseases and a change in public attitude toward tanning resulting from cultural shifts in beauty ideals (Figure 1).4
Figure 1.
Timeline of the history of melanoma. Melanoma was first described in Europe in 1804 and in North America in 1837. Historically, tanning as a fashion trend is a relatively new phenomenon that was popularized in the 1920s. Prior to the 1900s, pale skin was associated with beauty, wealth, and higher social class, whereas tanned skin was associated with manual labor. These attitudes were captured in artwork of the time, for example in Seurat’s A Sunday Afternoon on the Island of La Grande Jatte. However, in the early 20th century, attitudes shifted toward sun-seeking behavior rather than protection, and this shift was reflected in magazine articles and advertisements of the time. Increased sun exposure in the 1920s contributed to the rise in incidence of melanoma seen decades later during the mid-20th century. Following popularization of the tan, the bikini was introduced in 1946, and the first commercial tanning center was developed in 1978.
Attributions: Issac Parrish, courtesy of National Library of Medicine. A Sunday on La Grande Jatte, Georges Seurat, 1884, courtesy of Art Institute of Chicago. Before and after photography for therapy for rickets, courtesy of Wellcome Collection Gallery. Cherry Walker modeling swimsuits at Surfers Paradise, courtesy of John Oxley Library. Spinning Top Sunset, courtesy of Jessie Eastland.
Early in the 20th century, 80%−90% of children in Northern Europe and the United States suffered from vitamin D deficiency.5 The medical community began to recognize a role for sunlight in treating conditions such as tuberculosis and rickets.6 By 1919, heliotherapy became the standard treatment for rickets, inspiring deeper investigations into the role of ultraviolet radiation in vitamin D synthesis. In 1956, Henry Lancaster made the first connection between sunlight and melanoma.7 However, a causal relationship was not established until the 2000s because of the sustained latency between ultraviolet radiation exposure and tumor development.
Both ultraviolet A and ultraviolet B radiation are carcinogenic, inducing DNA double-stranded breaks and formation of cyclobutane pyrimidine dimer and pyrimidine (6–4)-pyrimidone photoproducts, respectively. Whole exome sequencing of primary and metastatic melanoma found that 76% of primary melanomas and 84% of metastatic melanomas had a ultraviolet signature mutation.8 There is no doubt that most melanomas are related to the ultraviolet exposure. In fact, melanomas can be classified by high-, medium-, or low-mutation burden, which correspond with degree of sun exposure.9 Chronically sun-exposed skin has a different genetic profile than skin in areas without chronic exposure and from extracutaneous mucosal and choroidal melanomas,10 underscoring the etiologic relationship between ultraviolet radiation and melanoma. Ultraviolet radiation signature mutations are also well characterized in nonmelanoma skin cancers. Mutations in P53, a tumor suppressor gene involved in DNA repair and apoptosis of mutant cells, contributes to carcinogenesis and to the development of actinic keratoses, squamous cell carcinomas, and basal cell carcinomas; 71% of these mutations are attributed to ultraviolet radiation.11,12
According to the Centers for Disease Control and Prevention (CDC), the costs to treat skin cancer increased 5 times faster than costs of other cancers between 2002 and 2011 ($3.6 billion between 2002 and 2006; $8.1 billion between 2007 and 2011).13 Of the $8.1 billion expenditures, $3.3 billion was for melanoma.13 These numbers do not include the cost of new agents that are now standard of care for stage III and IV melanoma. For example, the oncolytic virus talimogene laherparepvec (T-VEC), an intralesional injection given for treatment of advanced melanoma, can cost up to $40,000 per single injection. In combination with ipilimumab, T-VEC improves response rates to 38.8%, compared to 18% with ipilimumab alone. The cost of gaining 1 additional progression-free quality-adjusted life-year, 1 progression-free life-year, or having 1 additional patient attain objective response is about $1.6 million with T-VEC.14
The costs associated with skin cancer treatment far exceed those of photoprotective strategies. Importantly, there are many opportunities for these interventions both at the national and individual level. Nonetheless, the preventative measures may carry other hidden costs to individual patients and to society as a whole because of the potential for overdiagnosis and treatment. There is conflicting evidence surrounding the efficacy of public health campaigns, sunscreen use, and skin cancer screening. Some of these benefits, controversies, and pitfalls will be discussed in this article. Here, we contextualize the rising rates of melanoma and nonmelanoma skin cancer, evaluate the evidence surrounding primary and secondary prevention, and take a glimpse at emerging new technologies to aid in melanoma prevention and diagnosis.
ROLE OF PRIMARY PREVENTION IN REDUCING SKIN CANCER INCIDENCE
Ultraviolet radiation, in the form of sun exposure or indoor tanning, is a significant risk factor for the development of skin cancer. It is estimated that ultraviolet radiation causes nearly 70% of melanomas and 90% of nonmelanoma skin cancers, suggesting many such tumors can be prevented.15–17 Unsurprisingly, basal cell carcinomas and squamous cell carcinomas are most frequently found on sun-exposed body areas of the body, especially the head and neck.18 In 2014, the “Surgeon General’s Call to Action to Prevent Skin Cancer” addressed the increasing prevalence of skin cancer in the United States and identified opportunities to reduce skin cancer through primary prevention and education.
More than 400,000 annual cases of skin cancer, approximately 6000 of which are melanoma, are estimated to be related to indoor tanning in the United States. Despite the hazards of ultraviolet radiation, nearly 1 of every 3 white women ages 16–25 years engages in indoor tanning.19 Another especially vulnerable population is children; their skin is thinner and more sensitive to ultraviolet radiation with a higher density of finer, shorter vellus hair follicles compared to adult terminal hair follicles, allowing greater percutaneous absorption of ultraviolet radiation.20–22 Children spend an average of 1.5 to 5.1 hours outdoors and are exposed to higher levels of ultraviolet radiation.20 Hence, approximately 50% of total lifetime ultraviolet radiation exposure occurs before the age of 18.20,23 The damage incurred during childhood appears decades after exposure. Given the high mortality of melanoma and its aggressive treatment, preventative measures focused on reducing ultraviolet radiation exposure through environmental and behavioral modifications are a highly desirable alternative.
The first country to successfully implement public health campaigns to reduce the incidence of skin cancer was Australia. Between 1985 and 1989, the age-standardized incidence rate of melanoma was 96 cases per million compared with 44 cases per million young Australians between 2010 and 2014.24 SunSmart is an Australian skin cancer prevention program initiated in 1988 with the goal of reducing skin cancer incidence, morbidity, and mortality through a targeted prevention and early detection program. Known as the Slip! Slop! Slap! Seek! Slide! media campaign, it advocates: “slipping on clothes, slopping on sunscreen, slapping on a hat, seeking shade, and sliding on sunglasses” from a young age.25 The campaign promotes Sun-safe behaviors, including the use of protective clothing, applying broad-spectrum sunscreens of sun protection factor (SPF) 30+ containing ultrafine particulate zinc and titanium, avoiding the midday sun, using shade-providing structures, and avoiding indoor tanning. This campaign is estimated to have prevented nearly 50,000 cancers and 1,400 deaths from skin cancer between 1988 and 2011, with net cost savings of $92 million.26 Despite the thinning ozone layer over Australia and a very high-risk population, the incidence of cutaneous melanoma has stabilized in Australia and the incidence of invasive melanoma in Australians younger than age 55 is decreasing.27 These interventions have proven to be highly cost-effective, with savings of approximately $60 million with an expenditure of less than $16 million.28
Sun safety and educational programs have been implemented in the United States, including Ray and the Sunbeatables: A Sun Safety Curriculum, which was launched by the MD Anderson Cancer Center and disseminated by the CATCH Global Foundation in 2015.29 This educational program is geared toward preschool to first-grade students, parents, and teachers, focusing on the development of sun safety skills through interactive lessons. Outcomes of these programs may not be evident for many decades.
Today, there is no national or state recommendation in the United States for the use of sunscreen as a primary skin cancer prevention measure. This is partly because of the controversy stemming from prior studies showing either no change or even an increase in skin cancer incidence with sunscreen use, concerns about vitamin D deficiency, and issues regarding sunscreen product ingredient safety.30–32 These early studies had many limitations. As the effects of ultraviolet radiation exposure present decades later, a follow-up period of less than 10 years may be inadequate to capture any reduction in skin cancer incidence.33 Further confounding the picture, sunscreen may create a sense of false security, thereby encouraging greater ultraviolet radiation exposure.34 Sunscreen users typically apply only 25% of the recommended amount of sunscreen needed to achieve the specified SPF of the labeled product.35 Most important, the sunscreen formulations tested in these early studies were primitive compared to currently recommended sunscreens. Some studies tested SPF 15 sunscreen, which by current standards is considered inadequate (minimum SPF 30 recommended) or used chemical instead of physical sunscreens.
Another deterrent to the regular use of sunscreen is concern for sunscreen-induced vitamin D deficiency. Early studies of sunscreens with low SPF (10–15) demonstrated differing effects on vitamin D production in the skin.36,37 SPF 50+ sunscreens decrease cutaneous production of vitamin D 25(OH)D336 and likely decrease serum levels. Based on these risks, the Skin Cancer Foundation recommends dietary vitamin D supplementation.
Concerns have also been raised about percutaneous absorption of chemical sunscreens, possibly leading to endocrine disruption.38 The US Food and Drug Administration (FDA) considers a steady-state blood level of <0.5 ng/mL to be a safe threshold for systemic absorption of photoprotective sunscreen ingredients.39 A recent study showed that 4 active ingredients in chemical sunscreens exceeded plasma concentrations of 0.5 ng/mL, although the clinical significance of these concentrations is unknown.39 The FDA classifies physical sunscreens titanium dioxide or zinc oxide as “generally recognized as safe and effective (GRASE),” and advises their use as a substitute for potentially harmful ingredients.40,41 In 2019, the FDA proposed banning trolamine and p-aminobenzoic acid (PABA) from sunscreens because they are not GRASE and requested further safety and effectiveness data on 12 additional chemical sunscreen ingredients.
In the United States, socioeconomic barriers need to be addressed to successfully implement sunscreen use as primary prevention on a population level. The annual cost of sunscreen in the United States for an adult male is estimated to be between $200 and $400 for generic and name-brand sunscreens, respectively,42 which is a barrier to use in the population; higher income has been associated with increased sunscreen use.43 Trying to address this issue, nonprofit organizations have implemented free sunscreen dispenser initiatives.44 However, these initiatives may not provide sunscreens that meet FDA standards. Today, public sunscreen dispensers are being installed globally including in the United States (Florida, New York), the United Kingdom, and Africa.45,46 Although these local efforts are gaining traction across the globe, standardized, national interventions to collect data on skin cancer incidence are needed.
Recent data assessing the impact of sunscreen use in reducing skin cancer is encouraging. An Australian study found that adults 25- to 75-years old who regularly applied sunscreen for a 5-year period had a lower incidence of primary melanomas up to 10 years following the conclusion of the trial.47 Another study examining the association of self-reported lifetime sunscreen use in adults 18- to 40-years old found that regular sunscreen use in childhood reduced the risk of developing melanoma by 40% compared to those who rarely used sunscreen.48
SKIN CANCER SCREENING AS SECONDARY PREVENTION
There is no national consensus in the United States on recommendations regarding skin cancer screening. In 2016, the United States Preventative Services Task Force (USPSTF) found insufficient evidence to recommend routine skin cancer screening for early detection, with a recent Cochrane Review supporting this conclusion.49 Indeed, indiscriminate screening may result in overdiagnosis of melanoma and skin cancer, raising similar concerns to overdiagnosis of breast, prostate, and other cancers50 and potential for a surge in treatment costs with limited mortality benefit. The benefits and risks of over- and underdiagnosis of skin cancer and melanoma need to be balanced, but no data is currently available to inform this decision. Among specialists, it is generally accepted that regular screening of high-risk populations is worthwhile. Focusing on patients whose risk is the highest may prevent delayed diagnosis of melanoma, which may necessitate costly surgical procedures and require systemic therapy as opposed to simple local excision. Delayed treatment can also lead to cosmetic disfigurement, functional loss, decreased quality of life, and a fatal outcome.
The Melanoma Prevention Working Group published a response to the USPSTF’s conclusions (Table 1) and recommended an updated set of guidelines (Table 2)51 to address this population-based screening controversy. The benefits of screening on a national level have been observed through population-based surveillance in Europe. In 2003, a skin cancer screening campaign (SCREEN) was launched in Schleswig-Holstein, Germany. Five years following the launch of SCREEN, there was a 47% and 49% decrease in melanoma mortality rates in men and women, respectively.52,53 When SCREEN ended in 2008, the melanoma mortality rates returned to the levels observed prior to the intervention. This led Germany to implement a national screening program with biennial examinations for adults older than age 35. In the United States, University of Pittsburgh researchers investigated whether annual full-body examinations improved early melanoma detection. Primary care physicians (PCPs) were trained in melanoma detection, and all patients older than age 35 were offered annual screening. On average, melanomas detected in the screened group were almost 50% thinner than those detected in the unscreened group, suggesting earlier diagnosis and supporting a benefit of full-body screens.54 This study demonstrates that skin examinations performed by PCPs can be effective interventions.
Table 1.
The US Preventative Services Task Force Concerns and the Melanoma Prevention Working Group’s Response on Behalf of Dermatologists, 2016
| USPSTF Concerns | Melanoma Prevention Working Group Response |
|---|---|
| Increased detection of basal cell carcinomas is harmful to patients because it leads to unnecessary procedures with a limited effect on life expectancy. | • Basal cell carcinomas may look clinically similar to squamous cell carcinomas. |
| • It is important to biopsy the lesion to confirm the diagnosis because squamous cell carcinomas can be aggressive and require further management. | |
| • Identification of a basal cell carcinoma does not mandate immediate surgical removal. | |
| • Basal cell carcinomas can be treated by number of topical and oral drugs. | |
| • Watchful waiting supervised by a dermatologist may also be acceptable for some. | |
| A high biopsy number is needed to diagnose a skin cancer. | • The detection rates with biopsy are relatively high. |
| • An estimated 1 per 28 excisions are needed to detect melanoma and 1 per 9 excisions are needed for basal cell carcinoma detection.64 | |
| • Biopsy technique is associated with low risk and morbidity. | |
| There are adverse cosmetic outcomes after procedures. | • The study cited evaluated outcomes from cosmetic procedures rather than diagnostic procedures. |
| • General practice techniques are rarely disfiguring. | |
| • Cosmetic outcomes are far worse when skin cancer is left untreated and allowed to progress. |
USPSTF = US Preventative Services Task Force.
Table 2.
Recommended 2017 Guidelines in Response to the 2016 USPSTF Findings
| Adults ages 35–75 years with 1 or more of the following risk factors should be screened annually with a total body skin examination: | |
| Personal History | • Melanoma, actinic keratosis, or keratinocyte carcinoma |
| • CDKN2A (or other high-penetrance gene) mutation carrier | |
| • Immunocompromised | |
| Family History | • Melanoma in 1 or more family members |
| • Family history suggestive of a hereditary predisposition to melanoma | |
| Physical Features | • Light skin (Fitzpatrick I-III) |
| • Blonde or red hair | |
| • >40 total nevi | |
| • 2 or more atypical nevi | |
| • Many freckles | |
| • Severely sun-damaged skin | |
| Ultraviolet-Radiation Exposure | • History of blistering or peeling sunburn |
| • History of indoor tanning | |
Australia, New Zealand, the Netherlands, and the United Kingdom recommend screening high-risk patients for melanoma.51 A prospective observational study found a 4-year cumulative melanoma risk of 18.2% in the high-risk population, indicating that screening this population should be worthwhile.55 Identifying and monitoring high-risk patients improves outcomes through early detection and is cost-effective.56
Screening by performing total-body skin examinations of high-risk populations is a safe and cost-effective strategy.57 In the general population, screening frequency is proportional to number of lives saved (5.2 quality-adjusted life-years per 1000 people screened annually).57 Despite the utility of this intervention, only 15% of US workers have ever received a skin examination.58 Additionally, only 8% of patients who had seen their physician within the past year had undergone a skin examination, highlighting the underuse of this cost-effective method of surveillance.58 It appears that empowering PCPs to conduct skin cancer screening may allow for risk stratification of the general population, improved early detection, and decreased morbidity and mortality from melanoma. Focusing on PCPs offers an attractive solution to this and other issues, such as of shortage of dermatology services in some areas, allowing those in need to receive necessary preventative care.
NEW TOOLS FOR SKIN CANCER SCREENING
New technologies are emerging for screening to aid both dermatologists and PCPs. These include image-analysis software, dermoscopy, multispectral digital skin lesion analysis (MSDSLA), and artificial intelligence (AI). Future advances in these technologies may further increase the cost-effectiveness of screening high-risk populations.
Total-body mole mapping has been used for decades to document the skin surface with reproducible photos. Several companies have developed software to digitally compare baseline and follow-up images, flagging evolving lesions for further examination aiding in melanoma diagnosis. These programs, using digital dermoscopic images, have rather low sensitivity for detecting malignancy.59
Dermoscopy, or epiluminescence microscopy (ELM), uses noninvasive polarized light capable of penetrating the epidermis and superficial dermis (up to 1 mm) to evaluate skin microstructures. It improves diagnostic accuracy in skilled hands and has been used in clinical practice to analyze skin lesions.55,60 Training of PCPs in dermatoscope use leads to increased sensitivity in melanoma diagnosis.61
MSDSLA analyzes pigmented lesions up to 2.5 mm below the skin surface and has been shown to be a valuable screening tool, achieving up to a 98.2% sensitivity for melanoma detection in highly preselected pigmented lesions. Its specificity remains low for diagnosing melanoma, but it was higher than that of the average nonspecialist clinician (9.5% compared with 3.7%, respectively), approaching that of a specialist.62 These developments are encouraging and suggest a greater role for similar software in the future.
Recently, AI systems for skin cancer detection have shown promise in detecting melanomas. A convolutional neural network trained on 129,450 clinical images matched the performance of 21 experienced clinical dermatologists across multiple classifications.63 This technology is deployable on mobile devices and could be used by non-specialists or to improve diagnostic accuracy. Barriers to the implementation of AI for the diagnosis of skin cancer include the need for hundreds of thousands of optimal photos for algorithm training. The use of AI in clinical practice will help to improve diagnostic accuracy.
CONCLUSION
Primary prevention through sunscreen use, public health campaigns, and educational programs that have been implemented successfully in other countries can reduce rising melanoma rates throughout the world. Early detection through skin cancer screenings is a critical secondary intervention in high-risk groups. Integration of technological advances into practice will improve surveillance and diagnosis.
CLINICAL SIGNIFICANCE.
Primary prevention can halt the rise of melanoma and decrease morbidity and mortality.
Effectiveness of public health primary care campaigns has been demonstrated in several countries through lives and costs saved. However, the United States has not yet adopted sun-safety campaigns on the national level.
The US Melanoma Working Group recommends annual screenings for high-risk individuals ages 35–75 years.
Skin screenings by primary care physicians can improve early diagnosis.
Acknowledgments
Funding: None.
Footnotes
Conflicts of Interest: MHT, DQ, FHS, DRB, LJG report none. RDC serves as a consultant to Astra Zeneca, BMS, Castle Biosciences, Foundation Medicine, Immunocore, Incyte, Merck, Novartis, Roche, Compugen, I-Mab, PureTech Health, Sanofi Genzyme, and Sorrento Therapeutics; he also on the advisory boards for Aura Biosciences, Chimeron, and Rgeniz.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA CancerJ Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Netscher DT, Leong M, Orengo I, Yang D, Berg C, Krishnan B. Cutaneous malignancies: melanoma and nonmelanoma types. Plast Reconstr Surg 2011;127(3):37e–56e. [DOI] [PubMed] [Google Scholar]
- 3.Rebecca VW, Sondak VK, Smalley KSM. A brief history of melanoma: from mummies to mutations. Melanoma Res 2012;22 (2):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JM, Ghaferi JM, Cummins DL, et al. Changes in skin tanning attitudes. Fashion articles and advertisements in the early 20th century. Am J Public Health 2009;99(12):2140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006;116(8):2062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris RI. Heliotherapy in surgical tuberculosis. Am J Public Health (New York, NY: 1912) 1926;16(7):687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster HO. Some geographical aspects of the mortality from melanoma in Europeans. Med J Aust 1956;43(26):1082–7. [PubMed] [Google Scholar]
- 8.Genomic classification of cutaneous melanoma. Cell 2015;161 (7):1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012;44 (9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol 2014;9:239–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature 1994;372(6508):773–6. [DOI] [PubMed] [Google Scholar]
- 12.Bolshakov S, Walker CM, Strom SS, et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res 2003;9(1):228–34. [PubMed] [Google Scholar]
- 13.Guy GP Jr., Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002−2006 and 2007 −2011. Am J Prev Med 2015;48(2):183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almutairi AR, Alkhatib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol 2019;155(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res 1993;3(6):395–401. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Estimated number of incident cases from 2018 to 2040, all cancers, both sexes, all ages. Available at:http://gco.iarc.fr/tomorrow/graphic-isotype?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0 Accessed April 17, 2019.
- 17.World Health Organization. Global disease burden from solar ultraviolet radiation. Available at:https://www.who.int/uv/resources/archives/fs305/en/ Accessed April 17, 2019.
- 18.Karjalainen S, Salo H, Teppo L. Basal cell and squamous cell carcinoma of the skin in Finland. Site distribution and patient survival. Int J dermatol 1989;28(7):445–50. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. Reports of the Surgeon General.. The Surgeon General’s Call to Action to Prevent Skin Cancer. Washington (DC): Office of the Surgeon General (US); 2014. [Google Scholar]
- 20.Green AC, Wallingford SC, McBride P. Childhood exposure to ultraviolet radiation and harmful skin effects: epidemiological evidence. Prog Biophys Mole Biol 2011;107(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez Garcia AM, McLaren CE, Meyskens FL Jr.. Melanoma: is hair the root of the problem? Pigment Cell Melanoma Res 2011;24(1):110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Protheroe MD, Al-Jumaily AM, Chalmers AN, Paul SP, Fu X. Simulation of UV power absorbed by follicular stem cells during sun exposure and possible implications for melanoma development. J Opt Soc Am A Opt Image Sci Vis 2019;36(4):628–35. [DOI] [PubMed] [Google Scholar]
- 23.Stern RS, Weinstein MC, Baker SG. Risk reduction for nonmelanoma skin cancer with childhood sunscreen use. JAMA Dermatol 1986;122 (5):537–45. [PubMed] [Google Scholar]
- 24.Australian Institute of Health and Welfare. Cancer in Adolescents and Young Adults in Australia 2018. Available at:https://www.aihw.gov.au/reports/cancer/cancer-adolescents-young-adults/contents/table-of-contents Accessed November 12, 2019.
- 25.Slip!Slop!Slap! Original SunSmart Campaign. Available at:http://www.sunsmart.com.au/tools/videos/past-tv-campaigns/slip-slop-slap-original-sunsmart-campaign.html Accessed April 21, 2019.
- 26.Shih STF, Carter R, Heward S, Sinclair C. Skin cancer has a large impact on our public hospitals but prevention programs continue to demonstrate strong economic credentials. Aust N Z J Public Health 2017;41(4):371–6. [DOI] [PubMed] [Google Scholar]
- 27.Curchin DJ, Harris VR, McCormack CJ, Smith SD. Changing trends in the incidence of invasive melanoma in Victoria, 1985–2015. Med J Aust 2018;208(6):265–9. [DOI] [PubMed] [Google Scholar]
- 28.Doran CM, Ling R, Byrnes J, et al. benefit cost analysis of three skin cancer public education mass-media campaigns implemented in New South Wales, Australia. PloS One 2016;11(1):e0147665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Release MAN. MD Anderson joins CATCH Global Foundation to boost child health, prevent cancer in later years. Available at: https://www.mdanderson.org/newsroom/md-anderson-joins-catch-global-foundation-to-boost-child-health-.h00-158985078.html Accessed May 24, 2019.
- 30.Westerdahl J, Ingvar C, Masback A, Olsson H. Sunscreen use and malignant melanoma. Int J Cancer 2000;87(1):145–50. [DOI] [PubMed] [Google Scholar]
- 31.Wolf P, Quehenberger F, Mullegger R, Stranz B, Kerl H. Phenotypic markers, sunlight-related factors and sunscreen use in patients with cutaneous melanoma: an Austrian case-control study. Melanoma Res 1998;8(4):370–8. [DOI] [PubMed] [Google Scholar]
- 32.Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999;354(9180):723–9. [DOI] [PubMed] [Google Scholar]
- 33.van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol, Biomarkers Prev 2006;15(12): 2546–8. [DOI] [PubMed] [Google Scholar]
- 34.Autier P, Boniol M, Dore JF. Sunscreen use and increased duration of intentional sun exposure: still a burning issue. Int JCancer 2007;121 (1):1–5. [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Oh BH, Lee YW, Choe YB, Ahn KJ. The relation between the amount of sunscreen applied and the sun protection factor in Asian skin. J Am Acad Dermatol 2010;62(2):218–22. [DOI] [PubMed] [Google Scholar]
- 36.Libon F, Courtois J, Le Goff C, et al. Sunscreens block cutaneous vitamin D production with only a minimal effect on circulating 25-hydroxyvitamin D. Arch Osteoporos 2017;12(1):66. [DOI] [PubMed] [Google Scholar]
- 37.Marks R, Foley PA, Jolley D, Knight KR, Harrison J, Thompson SC. The effect of regular sunscreen use on vitamin D levels in an Australian population. Results of a randomized controlled trial. Arch-Dermatol 1995;131(4):415–21. [PubMed] [Google Scholar]
- 38.Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl 2012;35(3):424–36. [DOI] [PubMed] [Google Scholar]
- 39.Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA 2019; 321:2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KB, Kim YW, Lim SK, et al. Risk assessment of zinc oxide, a cosmetic ingredient used as a UV filter of sunscreens. J Toxicol Environ Health B Critical Rev 2017;20(3):155–82. [DOI] [PubMed] [Google Scholar]
- 41.DiNardo JC, Downs CA. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J Cosmet Dermatol 2018;17(1):15–9. [DOI] [PubMed] [Google Scholar]
- 42.Johal R, Leo MS, Ma B, Sivamani RK. The economic burden of sunscreen usage. Dermatol Online J 2014;20(6). [PubMed] [Google Scholar]
- 43.Weig EA, Tull R, Chung J, Brown-Joel ZO, Majee R, Ferguson NN. Assessing factors affecting sunscreen use and barriers to compliance: a cross-sectional survey-based study [epub ahead of print]. J Dermatolog Treat 2019:1–3. 10.1080/09546634.2019.1587147. [DOI] [PubMed] [Google Scholar]
- 44.Lief E Take me out to the ballgame … sunscreen dispenser. 2017. Available at:https://www.acsh.org/news/2017/07/19/take-me-out-ball-game-sunscreen-dispenser-11574 Accessed May 24, 2019.
- 45.Charity STKCSC. Skcin and the Colin Bloomfield Melanoma Appeal. Available at:http://www.skcin.org/ourWork/colinBloomfieldMelanomaAppeal.htm Accessed May 24, 2019.
- 46.IPG. FB Africa and Nivea Protect Kids With Sunslide. Available at: https://www.interpublic.com/our-agencies/recent-work/strongerpost?id=8362&casename=FCB+Africa+and+NIVEA+Protect+Kids+with+SunSlide Accessed May 24, 2019. [Google Scholar]
- 47.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011;29(3):257–63. [DOI] [PubMed] [Google Scholar]
- 48.Watts CG, Drummond M, Goumas C, et al. Sunscreen use and melanoma risk among young Australian adults. JAMA Dermatol 2018;154 (9):1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson M, Brodersen J, Gøtzsche PC, Jørgensen KJ. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev 2019;6:CD012352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamson AS, Pignone MP. Eliminating copayments for skin cancer screening—a public health policy with insufficient evidence [epub ahead of print]. JAMA Dermatol 2019. 10.1001/jamadermatol.2019.2797. [DOI] [PubMed] [Google Scholar]
- 51.Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag 2017;4(1):13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boniol M, Autier P, Gandini S. Melanoma mortality following skin cancer screening in Germany . BMJ Open 2015;5(9):e008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol 2012;66 (2):201–11. [DOI] [PubMed] [Google Scholar]
- 54.Ferris LK, Saul MI, Lin Y, et al. A large skin cancer screening quality initiative: description and first-year outcomesa large skin cancer screening quality initiativea large skin cancer screening quality initiative. JAMA Oncol 2017;3(8):1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol 2014;150(8):819–27. [DOI] [PubMed] [Google Scholar]
- 56.Watts CG, Cust AE, Menzies SW, Coates E, Mann GJ, Morton RL. Specialized surveillance for individuals at high risk for melanoma: a cost analysis of a high-risk clinic. JAMA Dermatol 2015;151(2): 178–86. [DOI] [PubMed] [Google Scholar]
- 57.Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol 2007; 143(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol 2008;59(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.del Rosario F, Farahi JM, Drendel J, et al. Performance of a computer-aided digital dermoscopic image analyzer for melanoma detection in 1,076 pigmented skin lesion biopsies. J Am Acad Dermatol 2018;78 (5):927–934.e926. [DOI] [PubMed] [Google Scholar]
- 60.Halpern AC, Marchetti MA, Marghoob AA. Melanoma surveillance in “high-risk” individuals. JAMA Dermatol 2014;150(8):815–6. [DOI] [PubMed] [Google Scholar]
- 61.Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol 2000;143(5):1016–20. [DOI] [PubMed] [Google Scholar]
- 62.Monheit G, Cognetta AB, Ferris L, et al. The performance of Mela-Find: a prospective multicenter study. JAMA Dermatol 2011;147(2): 188–94. [DOI] [PubMed] [Google Scholar]
- 63.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017;542 (7639):115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol 2012;148(8):903–10. [DOI] [PubMed] [Google Scholar]