Abstract
Background
Genetic alterations in fibroblast growth factor receptor (FGFR) and vascular endothelial growth factor receptor (VEGFR) signalling are observed in various tumours. We report a first-in-human phase I/IIa trial evaluating tolerability, pharmacokinetics and preliminary antitumour activity of ODM-203, a novel FGFR and VEGFR inhibitor.
Methods
Open-label, non-randomised, multicentre, phase I/IIa dose escalation and expansion study in patients with advanced or metastatic solid tumours.
Results
Overall, 84 patients received treatment; optimal tablet dose was found to be 400 mg/day with food. All patients experienced at least one adverse event; the majority (89.2%) were grade 1 or 2% and 70.4% were considered treatment related. The most commonly reported events were bilirubin increase-related events (75%) and diarrhoea (50%).
Overall response rate was 9.2% and median progression-free survival was 16.1 and 12.4 weeks for patients with aberrant or non-aberrant FGFR tumours. Median time on treatment was 10.1 weeks for all patients and 14.5 weeks for patients who received 400 mg tablets.
Conclusion
This study suggests ODM-203 400 mg/day results in sufficient plasma concentrations and acceptable tolerability in most patients. Preliminary signs of therapeutic activity of ODM-203 in patients with solid tumours was observed.
Trial registration number
Keywords: ODM-203, dose escalation study, phase I, solid tumours, FGFR and VEGFR inhibitor
Key questions.
What is already known about this subject?
Abnormal fibroblast growth factor receptor (FGFR) and vascular endothelial growth factor receptor (VEGFR) signalling is commonly observed in various tumour types, including breast, lung and gastric cancers.
Preclinical data suggest that the four FGFR tyrosine kinases (FGFR1–4) act distinctly from VEGFR, but in a synergistic manner, to promote tumour vascularisation through a network of downstream signalling pathways. This provides a compensatory angiogenic signal and potentially promotes the development of resistance to VEGFR inhibition
What does this study add?
We report a first-in-human phase I/IIa trial that evaluated the tolerability, pharmacokinetics and preliminary antitumour activity of a novel FGFR and VEGFR inhibitor ODM-203 in patients with advanced or metastatic solid tumours.
Evidence of ODM-203 activity on both FGFR and VEGFR pathways was found. Biomarker responses suggest that there is an exposure–response relationship between ODM-203.
How might this impact on clinical practice?
The P1 outcomes suggest a more selected tumour type selection for further studies to see the full potential benefits of combined FGFR and VEGFR inhibition.
Introduction
The receptor tyrosine kinases fibroblast growth factor receptor (FGFR) and vascular endothelial growth factor receptor (VEGFR) promote angiogenesis, which is essential for tumour growth, tissue invasion and metastasis.1 Abnormal FGFR and VEGFR signalling is commonly observed in various tumour types, including breast, lung and gastric cancers,2–4 and is associated with unfavourable survival outcomes.5–10 Preclinical data suggest that the four FGFR tyrosine kinases (FGFR1–4) act distinctly from VEGFR, but in a synergistic manner, to promote tumour vascularisation through a network of downstream signalling pathways. This provides a compensatory angiogenic signal and potentially promotes the development of resistance to VEGFR inhibition.11
Based on these observations, FGFR-selective tyrosine kinase inhibitors (TKIs) have been developed. The FGFR-2 and FGFR-3 TKI erdafitinib was the first such agent to be approved by the US Food and Drug Administration for the treatment of cancer; approval was based on phase II data showing an objective response rate of 40% in patients with previously treated metastatic urothelial carcinoma harbouring FGFR3 mutations or fusion genes involving FGFR-2 or FGFR-3.12 Several other selective FGFR TKIs are also currently under evaluation in clinical trials.
Given the interaction between the FGFR and VEGFR pathways, agents that inhibit signalling through both pathways are of particular interest.4 13 14 ODM-203 is a novel, selective and equipotent inhibitor of FGFR and VEGFR family kinases.13 ODM-203 also inhibits other kinases, including the RET proto-oncogene, at 50% inhibitory concentrations less than 100 nM.13 RET is essential for normal development and maintenance of numerous cell and tissue types,13 but dysregulated RET signalling is implicated in several human cancers, including lung and thyroid cancer.15 In vitro studies have shown that ODM-203 suppresses cell proliferation and FGFR, FGFR substrate 2 and extracellular signal-regulated kinase phosphorylation in cell lines with increased FGFR activity and known dependency on FGFR signalling.13 ODM-203 showed antitumour activity in xenograft models known to be dependent on FGFR-1, FGFR-2 or FGFR-3 and in an angiogenesis-dependent kidney capsule syngeneic model.13
We report a first-in-human phase I/IIa trial that evaluated the tolerability, pharmacokinetics (PK) and preliminary antitumour activity of ODM-203 in patients with advanced or metastatic solid tumours.
Methods
Study design
This was a two-part, open-label, non-randomised, multicentre, phase I/IIa study (KIDES-203; ClinicalTrials.gov: NCT02264418).
Part 1 was a dose escalation study using a standard 3+3 design to define the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of ODM-203 (online supplemental figure S1). Subjects with stable disease (SD) continued on study treatment until disease progression as assessed by the investigator or until experiencing an intolerable adverse event (AE) or until study end. In part 1 ODM-203 doses of 50–800 mg/day as capsule formulation were investigated. A single oral dose on day 1, after a standard low phosphate light breakfast was administered. Patients who did not experience any DLTs within 24 hours after administration of the first dose continued to receive ODM-203 once daily. A safety monitoring board (SMB) took decisions regarding dose adjustments or discontinuations, patient enrolment at dose levels, and PK sampling and safety assessments. The MTD was defined as the lowest studied dose level at which ≥2 of 6 patients experienced a DLT, or if the PK of ODM-203 suggested a plateau in exposure. Intra-patient dose escalation was not permitted, except in the first patient in cohort 1, in case very low exposures were observed, or at the time of disease progression. Patients who recovered from drug-induced toxicity other than a DLT could resume treatment at the same or lower dose level. At disease progression, the dose could be increased to the highest tolerated dose as determined by the SMB.
esmoopen-2020-001081supp001.pdf (541.4KB, pdf)
The objective of part 2 was to confirm the optimal starting dose, dose schedule and formulation (group A) and explore the antitumour activity of ODM-203 (group B). Group A used sequential cohorts of 3–6 or 12 patients to explore new dosing schemes, which were determined by the SMB based on clinical experience, but were not expected to exceed exposures of capsule formulation dosed at 800 mg/day. Part 2 also assessed a tablet formulation of ODM-203. For this assessment, patients received a single dose of ODM-203 in the capsule formulation, followed a week later by continuous daily doses of ODM-203 in tablet formulation until disease progression. Tablet doses, starting at 200 mg, were escalated following review of safety and PK data by the SMB at day 15/16.
Once optimal tablet dose of ODM-203 was established, part 2 group B aimed to investigate the antitumour effects of ODM-203 in a range of patients with various RET and FGFR aberrations and solid tumours. Doses in group B were based on those studied in group A and were determined by the sponsor and SMB. Patients in group B received the tablet formulation of ODM-203.
Patients
Male and female patients, aged ≥18 years with histologically or cytologically confirmed advanced or metastatic solid tumours, for which treatment according to the guidelines was no longer available, were eligible for inclusion. Patients in part 2 had to have ‘angiogenic tumours’ or tumour genetic aberrations, which included patients with tumours that had: (1) a previous radiological response to VEGFR inhibition, including those that had become VEGFR resistant; (2) a previous response to FGFR inhibition and had become resistant; (3) genetic alterations of any FGFR subtype, including fusions, mutations considered to be activating and gene amplification or (4) genetic alterations of RET, including fusions and mutations considered to be activating. Key exclusion criteria were prior severe or life-threatening AEs related to anti-VEGFR or anti-FGFR treatment, ongoing treatment with warfarin, and uncontrolled active central nervous system metastases. Full inclusion and exclusion criteria are provided in online supplemental table S2.
All patients provided written informed consent before any study procedure. The study was conducted according to the International Conference on Harmonization-Good Clinical Practice guidelines and local legislation.
Objectives and assessments
The primary objective was to evaluate the safety and tolerability of ODM-203, including determination of the MTD and DLT. The secondary objectives of the study were to characterise and evaluate: the PK profile of ODM-203 and its main metabolite, ORM-21444, after single and multiple doses at different dose levels; the relationship between ODM-203 dose, plasma exposure, pharmacodynamics (PD) and safety; the long-term safety and tolerability of ODM-203; the dose schedule and administration of ODM-203 recommended for further clinical trials; and the preliminary antitumour activity of ODM-203 according to Response Evaluation Criteria in Solid Tumours (RECIST) V.1.1 criteria, Eastern Cooperative Oncology Group (ECOG) performance status, time on treatment and overall survival. Tumour burden was assessed by CT or MRI at the screening visit, week 8 and every 8 weeks thereafter or if disease progression was suspected. ECOG performance status was assessed at the screening visit, visit 1, day 29, every 4 weeks up to week 24 and every 8 weeks thereafter. AEs and serious AEs (SAEs) were assessed at all visits. AEs of special interest, including bilirubin increase-related events, haemorrhagic and thrombotic events, hyperphosphataemia, effects on bone and cartilage, vasculitis, QT-interval prolongation, hypertension, ocular toxicities and decreased weight loss, were monitoried closely during the study.
PK assessment
Blood samples for PK evaluation were collected on Day 1 (single dose PK) and day 8 or 15 (multiple dose PK). Samples were collected before (0 hours) and at 0.1, 1, 2, 3, 4, 6, 8, 12 and 24 hours after study treatment administration. A 24-hour blood sample was collected before study treatment administration. In both parts 1 and 2, a single predose blood sample was collected at each study visit from visit 3 (day 8) onward for determination of pre-dose concentrations of ODM-203 and its active metabolite, ORM-21444, with multiple dosing.
The PK variables Cmax (maximum observed concentration), Tmax (time to reach Cmax) and AUC0–last (area under the concentration-time curve from time zero to the last sample with the quantifiable concentration calculated with linear trapezoidal rule) were analysed after log-transformation. Exposure was evaluated using a plasma-concentration versus dose curve; escalation was stopped if a plateau was observed prior to reaching the MTD. The relationship between ODM-203 exposure and different biomarkers was evaluated by percentage change from baseline (day 1 predose).
Assessment of PD variables
PD variables assessed were blood pressure, plasma phosphate, parathyroid hormone, fibroblast growth factor-23 (FGF23), soluble VEGFR2 (sVEGFR2) and sVEGFR1, VEGF, FGF2 and placental growth factor (PGF).
Blood pressure was evaluated at each study visit and was measured daily by patients at home from day 2 to week 12, and twice weekly thereafter. Measurements were taken in the morning and evening, twice each time with at least 1 min between measurements, and recorded in a diary. These data were used for medical management only and could be reviewed by the SMB.
Blood samples for assessment of phosphate were collected at the same time points as other safety laboratory assessments and, for assessment of parathyroid hormone, at baseline (visit 1), day 8 and day 29 with other safety laboratory assessments.
FGF23, sVEGFR2 and sVEGFR1, VEGF, FGF2 and PGF were assessed at baseline, day 8 and day 29. These markers were analysed in batches during or at the end of the study.
Statistical evaluation
The evaluations were summarised by dose levels, by the highest pre-dose exposure levels of ODM-203 or specified molecular aberration type. The proposed number of patients was based on clinical grounds and similar studies,14 and patients were initially analysed according to tumour characteristics.
The preliminary antitumour activity, including overall response rate, best response rate, time to response, duration of response, disease control rate (complete response+partial response+SD), progression-free survival and overall surivival of ODM-203 in patients with tumours harbouring specific molecular aberrations in the FGFR or RET pathway, or angiogenic tumours indicating VEGFR activity, was investigated. Safety population (N=84) includes all patients who received medication; efficacy population (n=76) includes patients who were evaluable by RECIST; 16-week disease progression population (N=71) was calculated from patients who had completed respective study visit. Further, patients without sufficient pharmacological exposure were excluded from best tumour response analyses (N=71).
Statistical testing was mainly performed using descriptive statistics. The Kaplan-Meier method was applied to estimate the time of progression-free survival. Data for patients who were progression free and alive, or with unknown status, were censored at the time of the last tumour assessment. All statistical analyses were performed using SAS for Windows (V.9.4).
Results
Between September 2014 and April 2019, a total of 104 patients were screened and 84 patients with advanced solid tumours were enrolled to receive treatment at eight centres in Europe. Baseline characteristics are shown in table 1. All patients had measurable disease at screening and the most common tumour types were cholangiocarcinoma (n=15), colorectal cancer (n=9), breast cancer (n=9) and sarcoma (n=7). All except one patient had received prior anticancer therapy.
Table 1.
Baseline patient and disease characteristics
| ODM-203 capsule | ODM-203 tablet | Total | |||||||
| 100 mg | 200 mg | 400 mg | 600 mg | 800 mg | 200 mg | 300 mg | 400 mg | (N=84) | |
| (n=1) | (n=3) | (n=7) | (n=30) | (n=7) | (n=3) | (n=3) | (n=30) | ||
| Mean age, years | 65.0 | 65.0 | 51.0 | 54.1 | 52.3 | 56.3 | 65.0 | 59.7 | 56.7 |
| Female, n (%) | 0 (0.0) | 2 (66.7) | 5 (71.4) | 18 (60.0) | 3 (42.9) | 3 (100) | 2 (66.7) | 21 (70.0) | 54 (64.3) |
| Race, n (%) | |||||||||
| Caucasian | 1 (100) | 3 (100) | 7 (100) | 28 (93.3) | 7 (100) | 1 (33.3) | 3 (100) | 29 (96.7) | 79 (94.0) |
| Black | 0 | 0 | 0 | 1 (3.3) | 0 | 0 | 0 | 1 (3.3) | 2 (2.4) |
| Other | 0 | 0 | 0 | 1 (3.3) | 0 | 2 (66.7) | 0 | 0 | 3 (3.6) |
| Cancer type, n (%) | |||||||||
| Cholangiocarcinoma | – | – | – | – | – | – | – | – | 15 (17.9) |
| Breast cancer | – | – | – | – | – | – | – | – | 9 (10.7) |
| Colorectal cancer | – | – | – | – | – | – | – | – | 9 (10.7) |
| Soft tissue sarcoma | – | – | – | – | – | – | – | – | 7 (8.3) |
| Endometrial cancer | – | – | – | – | – | – | – | – | 5 (6.0) |
| Ovarian cancer | – | – | – | – | – | – | – | – | 5 (6.0) |
| Medullary thyroid cancer | – | – | – | – | – | – | – | – | 5 (6.0) |
| Renal cell carcinoma | – | – | – | – | – | – | – | – | 4 (4.8) |
| Other | – | – | – | – | – | – | – | – | 25 (29.8) |
| ECOG, n (%) | |||||||||
| 0 | 1 (100) | 1 (33.3) | 4 (57.1) | 14 (46.7) | 4 (57.1) | 0 (0.0) | 2 (66.7) | 10 (33.3) | 36 (42.9) |
| 1 | 0 (0.0) | 2 (66.7) | 3 (42.9) | 16 (53.3) | 3 (42.9) | 3 (100) | 1 (33.3) | 20 (66.7) | 48 (57.1) |
ECOG, Eastern Cooperative Oncology Group.
MTD and selection of dose for further study
Thirty-one patients were enrolled in the dose-escalation part of the study and received ODM-203 in capsule formulation (100 mg, n=1; 200 mg, n=3; 400 mg, n=7; 600 mg, n=13; 800 mg, n=7). At a dose of 800 mg, 1 patient developed a DLT of punctate keratitis; no other DLTs were observed. Therefore, the MTD according to the protocol definition was not established. The SMB instead determined that ODM-203 as capsule 800 mg/day exceeded the limit of acceptable tolerability because all the patients experienced several AEs. Therefore, a capsule formulation dose of 600 mg/day was selected for further study.
Assessment of dose of tablet formulation
Of the 53 patients enrolled to the expansion part of the study, 17 received ODM-203 in the capsule formulation (600 mg/day, n=2; 600 mg/day 2 weeks on treatment, 1 week off (intermittent dosing regimen), n=15) and 36 received ODM-203 in the tablet formulation (200 mg, n=3; 300 mg, n=3; 400 mg, n=30). Although there were variations in PK, therapeutic exposure levels were achieved in all patients. Results indicated that 600 mg capsule exposure levels were similar to 400 mg tablet at steady state. Thus ODM-203 tablet formulation as tablet of 400 mg/day after a light meal was chosen as dosing regimen for further studies.
Safety and tolerability
All patients (N=84) experienced at least one AE during treatment (table 2); 70.4% of AEs were considered related to the treatment by the investigator. However, the majority of AEs (89.2%) were grade 1 or 2 in severity. Events resulting in treatment discontinuation occurred in 30 patients (35.7%), all of whom received an ODM-203 dose of at least 400 mg/day. Additionally, AEs led to treatment interruption in 61 patients (72.6%) and dose reductions in nine patients (10.7%). The most common events that resulted in treatment interruption were increase in blood bilirubin (16 patients) and hyperbilirubinaemia (8 patients) and those leading to dose reductions were palmar-plantar erythrodysesthesia syndrome (3 patients), followed by arthralgia (2 patients), increased blood bilirubin (2 patients), and stomatitis, asthenia, mucosal inflammation, hyperbilirubinaemia, platelet count decreased, decreased appetite and alopecia (2 patients each). Treatment was discontinued due to progression of disease in 69 patients (82.1%).
Table 2.
Safety summary (safety population)
| Patients, n (%) | Capsule | Tablet | Total | Grade | ||||||
| 100 mg | 200 mg | 400 mg | 600 mg | 800 mg | 200 mg | 300 mg | 400 mg | 3–5 | ||
| (n=1) | (n=3) | (n=7) | (n=30) | (n=7) | (n=3) | (n=3) | (n=30) | (N=84) | (n=84) | |
| Any AE | 1 (100) | 3 (100) | 7 (100) | 30 (100) | 7 (100) | 3 (100) | 3 (100) | 30 (100) | 84 (100) | |
| Related AE | 1 (100) | 2 (66.7) | 7 (100) | 30 (100) | 7 (100) | 3 (100) | 3 (100) | 29 (96.7) | 82 (97.6) | |
| Related SAEs | 0 | 0 | 0 | 8 (26.7) | 1 (14.3) | 0 | 1 (33.3) | 4 (13.3) | 14 (16.7) | |
| AE leading to death | 0 | 0 | 0 | 2 (6.7) | 0 | 0 | 0 | 5 (16.7) | 7 (8.3) | |
| AE leading to discontinuation | 0 | 0 | 1 (14.3) | 17 (56.7) | 3 (42.9) | 0 | 0 | 9 (30.0) | 30 (35.7) | |
| AE leading to treatment interruption | 1 (100) | 0 | 4 (57.1) | 22 (73.3) | 5 (71.4) | 0 | 1 (33.3) | 28 (93.3) | 61 (72.6) | |
| AE leading to dose reduction | 0 | 0 | 1 (14.3) | 1 (3.3) | 0 | 0 | 1 (33.3) | 6 (20.0) | 9 (10.7) | |
| Events occurring in ≥10% of patients | ||||||||||
| Blood bilirubin increased | 0 | 0 | 3 (42.9) | 13 (43.3) | 3 (42.9) | 2 (66.7) | 0 | 15 (50.0) | 36 (42.9) | 20 (23.8) |
| Diarrhoea | 0 | 0 | 1 (14.3) | 17 (56.7) | 4 (57.1) | 0 | 1 (33.3) | 19 (63.3) | 42 (50.0) | 5 (6.0) |
| Hyperbilirubinaemia | 0 | 0 | 2 (28.6) | 10 (33.3) | 4 (57.1) | 0 | 1 (33.3) | 11 (36.7) | 28 (33.3) | 16 (19.0) |
| Stomatitis | 0 | 0 | 1 (14.3) | 11 (36.7) | 4 (57.1) | 1 (33.3) | 0 | 17 (56.7) | 34 (40.5) | 3 (3.6) |
| Palmar-plantar eryrthodysaestesia | 0 | 0 | 2 (28.6) | 11 (36.7) | 1 (14.3) | 0 | 1 (33.3) | 15 (50.0) | 30 (35.7) | 4 (4.8) |
| Arthralgia | 0 | 0 | 1 (14.3) | 11 (36.7) | 4 (57.1) | 1 (33.3) | 2 (66.7) | 9 (30.0) | 28 (33.3) | 2 (2.4) |
| Dry mouth | 0 | 0 | 1 (14.3) | 14 (46.7) | 1 (33.3) | 1 (33.3) | 12 (40.0) | 29 (34.5) | 0 | |
| Fatigue | 0 | 0 | 1 (14.3) | 10 (33.3) | 2 (28.6) | 1 (33.3) | 0 | 9 (30.0) | 23 (27.4) | 5 (6.0) |
| Epistaxis | 0 | 1 (33.3) | 1 (14.3) | 8 (26.7) | 3 (42.9) | 0 | 1 (33.3) | 14 (46.7) | 28 (33.3) | 0 |
| Jaundice | 0 | 0 | 3 (42.9) | 10 (33.3) | 2 (28.6) | 0 | 1 (33.3) | 11 (36.7) | 27 (32.1) | 2 (2.4) |
| Asthenia | 0 | 1 (33.3) | 1 (14.3) | 8 (26.7) | 2 (28.6) | 0 | 3 (100) | 10 (33.3) | 25 (29.8) | 2 (2.4) |
| Decreased appetite | 0 | 0 | 1 (14.3) | 9 (30.0) | 1 (14.3) | 1 (33.3) | 2 (66.7) | 11 (36.7) | 25 (29.8) | 0 |
| Alopecia | 0 | 0 | 1 (14.3) | 8 (26.7) | 5 (71.4) | 1 (33.3) | 1 (33.3) | 10 (33.3) | 26 (31.0) | 1 (1.2) |
| Dysgeusia | 0 | 0 | 2 (28.6) | 5 (16.7) | 1 (14.3) | 1 (33.3) | 1 (33.3) | 10 (33.3) | 20 (23.8) | 0 |
| Hyperphosphataemia | 0 | 0 | 2 (28.6) | 6 (20.0) | 3 (42.9) | 0 | 1 (33.3) | 8 (26.7) | 20 (23.8) | 0 |
| Weight decreased | 0 | 0 | 1 (14.3) | 10 (33.3) | 1 (14.3) | 0 | 2 (66.7) | 5 (16.7) | 19 (22.6) | 1 (1.2) |
| Nasal dryness | 0 | 0 | 1 (14.3) | 5 (16.7) | 0 | 0 | 1 (33.3) | 6 (20.0) | 13 (15.5) | 0 |
| Conjugated bilirubin increased | 0 | 0 | 0 | 3 (10.0) | 0 | 0 | 1 (33.3) | 7 (23.3) | 11 (13.1) | 0 |
| Myalgia | 0 | 0 | 1 (14.3) | 4 (13.3) | 2 (28.6) | 1 (33.3) | 0 | 3 (10.0) | 11 (13.1) | 2 (2.4) |
| Hypertension | 0 | 0 | 0 | 5 (16.7) | 0 | 1 (33.3) | 0 | 8 (26.7) | 14 (16.7) | 3 (3.6) |
| Nausea | 0 | 0 | 0 | 4 (13.3) | 0 | 0 | 0 | 7 (23.3) | 11 (13.1) | 0 |
| Mucosal inflammation | 0 | 0 | 1 (14.3) | 1 (3.3) | 0 | 0 | 1 (33.3) | 7 (23.3) | 10 (11.9) | 0 |
| Vomiting | 0 | 0 | 1 (14.3) | 2 (6.7) | 0 | 0 | 1 (33.3) | 5 (16.7) | 9 (10.7) | 0 |
| Dry skin | 0 | 0 | 0 | 1 (3.3) | 1 (14.3) | 0 | 1 (33.3) | 7 (23.3 | 10 (11.9) | 0 |
| Onycholysis | 0 | 0 | 0 | 6 (20.0) | 2 (28.6) | 0 | 0 | 3 (10.0) | 11 (13.1) | 1 (1.2) |
AE, adverse event; SAE, serious adverse event.
The most commonly observed AEs related to treatment were bilirubin increase-related events (75% of patients), diarrhoea (50.0%, stomatitis (40.5%), palmar-plantar eryrthrodysesthesia (35.7%) and dry mouth (34.5%).
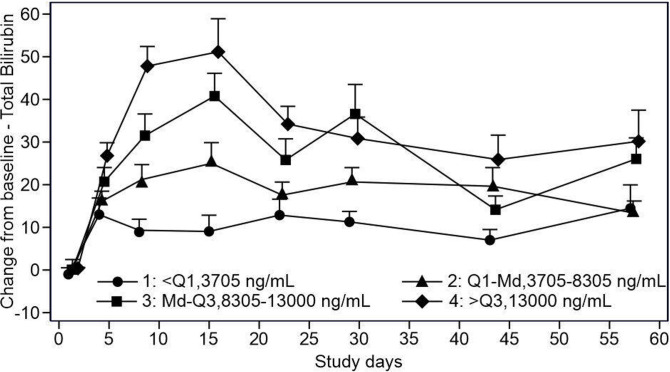
Bilirubin increase-related events (increased blood bilirubin, hyperbilirubinaemia, jaundice, conjugated bilirubin increase and ocular icterus) were observed in 63 (75%) patients. Of 30 patients treated with ODM-203 400 mg/day, 15 (50.0%) reported increased blood bilirubin and 11 (36.7%) reported hyperbilirubinaemia. Six of these 30 patients had increased aspartate transaminase levels and three had increased alanine transaminase levels. Increased total or unconjugated bilirubin was found to correlate with UGT1A1 enzyme inhibition by ODM-203 and was most common at doses above 400 mg/day.16 The magnitude and rate of bilirubin increase appeared related to ODM-203 dose (figure 1). Typically, increases in bilirubin were not associated with changes in transaminases, alkaline phosphatase or other indicators of hepatic injury and could be managed with dose modification or interruption.
Figure 1.
ODM-203 exposure versus total bilirubin.
Hyperphosphataemia is a frequently reported AE in clinical studies of FGFR inhibitors, and was reported in 16 patients (19.0%) in this study. The majority of events were grade 1 in severity.
The most commonly reported musculoskeletal AE during the study was arthralgia, reported by 34 patients (40.5%), two of whom had grade 3 events; myalgia and back pain affected 14 (16.7%; two grade 3) and 10 patients (11.9%), respectively. Most of the arthralgia and myalgia events were assessed as related to the treatment.
Hypertension, an AE commonly associated with VEGFR inhibitors, was observed in 16 patients (19%) and was considered related to study treatment in 14 patients. Grade 3 hypertension occurred in three patients (3.6%). All hypertension events except one occurred after the administration of either ODM-203 600 mg/day as capsules or ODM-203 400 mg/day as tablets.
Treatment-related SAEs occurred in 14 patients (16.7%). Seven deaths occurred and the AEs leading to death were dyspnoea (one patient), general physical health deterioration (two patients), acute respiratory distress syndrome (one patient), intestinal ischaemia (one patient), urosepsis (one patient) and Pneumocystis jirovecii pneumonia (one patient). Only intestinal ischaemia was considered to be related to treatment.
PK assessment
The PK profiles of ODM-203 and its metabolite (ORM-21444) were characterised after single and multiple (day 8 or day 15) dosing of ODM-203. In the dose escalation part, in which the ODM-203 capsule formulation was used, exposure increased with ODM-203 dose, although not directly dose proportionally. Compared with the capsule formulation, the tablet formulation showed higher exposure and lower variability.
As the tablet formulation is expected to be used in the future, results for this formulation are described. The key PK parameters are summarised in table 3.
Table 3.
Summary of key PK parameters of ODM-203 in expansion phase (tablet formulation)
| Day 1 | Day 15 | |||||
| 200 mg | 300 mg | 400 mg | 200 mg | 300 mg | 400 mg | |
| (n=3) | (n=3) | (n=25) | (n=3) | (n=3) | (n=24) | |
| Cmax, ng/mL | 1539 (9) | 2608 (46) | 1933 (49) | 3118 (28) | 4906 (148) | 9070 (81) |
| AUC0–last, h*ng/mL | 25 886 (7) | 36 708 (46) | 30 257 (59) | 58 612 (27) | 84 233 (213) | 170 304 (90) |
| Median (min, max) Tmax, h | 8.0 (7.7 to 11.1) | 6.0 (4.1 to 6.1) | 6.3 (3.1 to 24.7) | 8.0 (6.0 to 11.2) | 6.1 (3.1 to 8.7) | 6.0 (0.0 to 23.3) |
| Accumulation ratio* | – | – | – | 2.3 (23) | 2.3 (123) | 5.5 (76) |
| Mean (SD) metabolite to parent ratio† | 0.043 (0.022) | 0.061 (0.030) | 0.048 (0.034) | 0.085 (0.043) | 0.094 (0.004) | 0.117 (0.031) |
| Mean (SD) Caverage, ng/mL | – | – | – | 2560 (719) | 5652 (5460) | 8928 (5148) |
Values expressed as geometric mean (coefficient of variation (%)) unless otherwise stated.
*Calculated by dividing ODM-203 AUC0–last on day 15 by corresponding value on day 1.
†Calculated by dividing ORM-21444 AUC0–last by corresponding ODM-203 value.
AUC0–last, area under the concentration time-curve from time zero to last sample; Caverage, average concentration in plasma after multiple dosing; Cmax, maximum observed concentration of concentration-time curve; PK, pharmacokinetics.
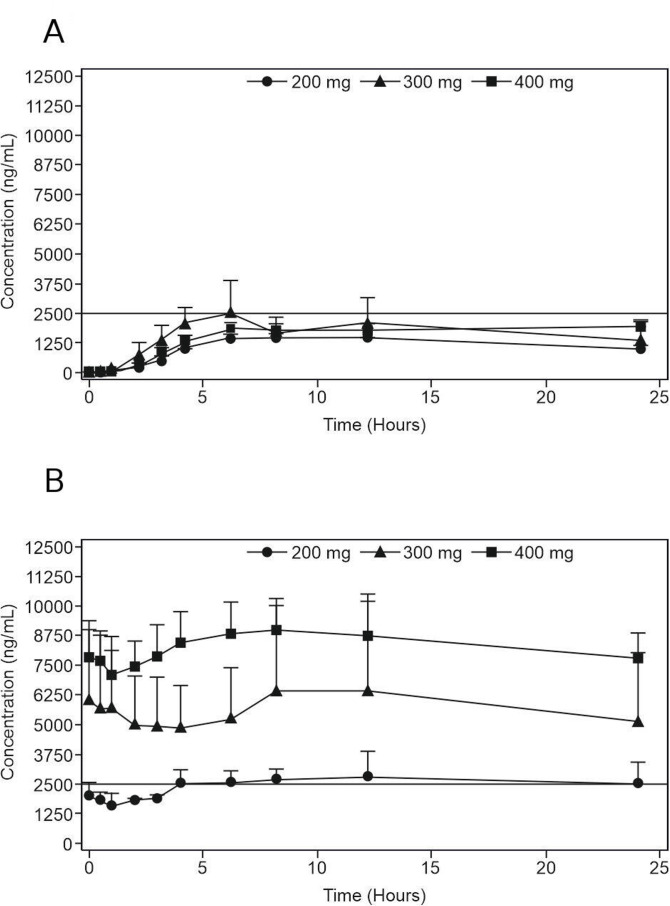
ODM-203 absorption was slow and variable; average Tmax values after a single dose in different cohorts were typically 6–10 hours, while the individual Tmax range was 3–24 hours. After repeated dosing, plasma-concentration curves were flat and Tmax values varied between 0 and 24 hours (figure 2A, B). The steady-state AUC was associated with considerable interindividual variability (coefficient of variation 90% in 400 mg tablet group at day 15). The elimination half-life of ODM-203 could not be reliably determined because concentrations were measured only up to 24 hours after dosing. The slow rate of elimination resulted in average accumulation ratios of 2.3–5.5 (based on AUC0–last) suggesting a half-life of 30–70 hours in different cohorts. Consistent with the slow elimination rate, the Tmax value for metabolite ORM-21444 on the first day of administration was typically ≥10 hours, with clear accumulation on repeated dosing of ODM-203. However, the half-life of ORM-21444 could not be reliably determined from 24 hours sampling. The AUC ratio was typically less than 0.15 at steady state, suggesting that ODM-203 is the main circulating drug-related material in plasma.
Figure 2.
The average (±SEM) plasma concentrations of ODM-203 after single (A) and repeated (B) dosing of ODM-203 tablet formulation (once daily dosing). Solid line at 2500 ng/mL represents the anticipated lower limit for target concentration range. SEM, Standard error of the mean.
Biomarkers of FGFR and VEGFR pathways
Evidence of ODM-203 activity on both FGFR and VEGFR pathways was found. Percentage mean changes in the soluble markers FGF23, VEGFR2, VEGF and PGF appeared to be dose dependent. Biomarker responses suggest that there is an exposure-response relationship between ODM-203 (online supplemental figure S2).
Tumour genetics
Based on tumour tissue profiling, 32 patients had genetic alterations in the FGFR pathway, including activating mutations (n=8), genomic rearrangements (n=4), amplification and a rearrangement (n=2), an amplification and an activating mutation (n=1) and an amplification (n=14; online supplemental table S3). Patients were classified as non-FGFR if no genomic aberrations in FGFR pathway genes were identified in the profiling assays used (n=6) or if profiling results were not available (n=8). Additionally, profiling revealed RET genomic aberrations in 10 patients. Of these, 6 patients had activating RET mutations and two had RET genomic rearrangements.
Efficacy
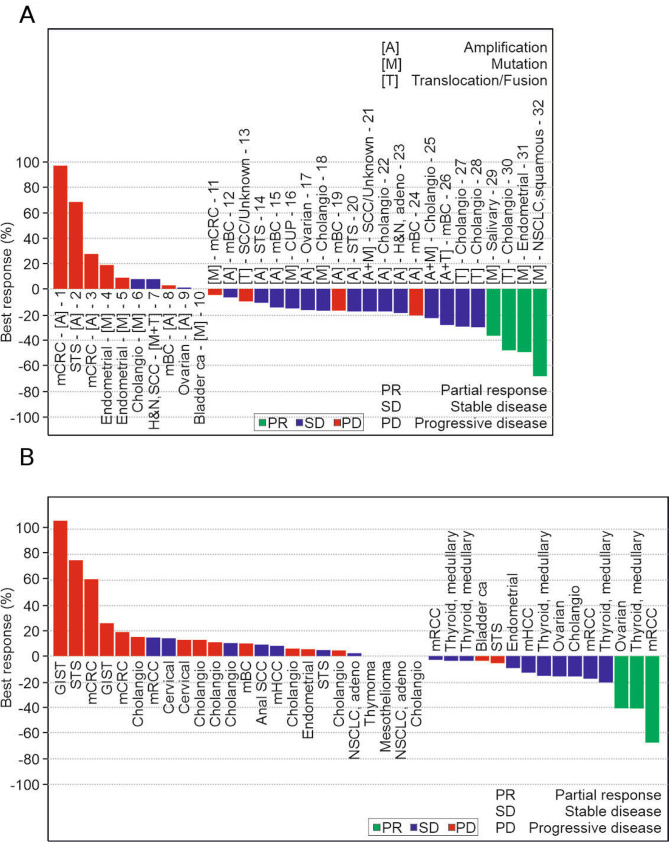
The overall response rate based on RECIST criteria was 9.2% (7/76 patients; online supplemental table S4); all responses were PRs. Patients with tumours with FGFR aberrations had an overall response rate of 12.5% (4/32), whereas those with non-FGFR tumours had an overall response rate of 6.8% (3/44). ODM-203 best tumour response (RECIST) (figure 3A, B) and the associated FGFR aberrations are shown in online supplemental table S3. The disease control rate was 57.9%, and was numerically higher in patients with tumours with FGFR aberrations (65.6%) than in those with non-FGFR tumours (52.3%). The proportion of patients without disease progression at 16 weeks of treatment was 33.8% (24/71) overall, 27.6% (8/29) for those with tumours with FGFR aberrations and 38.1% (16/42) for those with non-FGFR tumours.
Figure 3.
ODM-203 best tumour response (RECIST) for FGFR patients (A) and non-FGFR patients (B). ITT 76 patients, 4 patients with low exposure (100–200 mg) and one patient with non-evaluable non-target lesions (600 mg) are not included. Unscheduled visits are included in the data. Transcript of abbreviations: CUP, cancer of unknown primary; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stromal tumour; H&N, head and neck; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer; mHCC, metastatic hepatocellular carcinoma; NSCLC, non-small cell lung cancer; RECIST, Response Evaluation Criteria in Solid Tumours; SCC, squamous cell carcinoma; STS, soft-tissue sarcoma.
The median progression-free survival was 16.1 weeks for patients with FGFR aberration(s) and 12.4 weeks for non-FGFR patients. The median time (range) on ODM-203 treatment was 10.1 (1.1–62.9) weeks and the median time on treatment for patients who received ODM-203 400 mg tablets was 14.5 (2.6–62.9) weeks.
Discussion
This first-in-human study demonstrated that the MTD of ODM-203 was not reached at a dose of 800 mg once daily in the capsule formulation, and a dose of 400 mg once daily as tablet was selected for further studies. Data suggest that ODM-203 at 400 mg once daily in tablet formulation, administered with a light breakfast, produces effective ODM-203 plasma concentrations and has acceptable tolerability. The study also provides preliminary evidence of the therapeutic activity of ODM-203 in patients with advanced or metastatic solid tumours.
ODM-203 is a selective, equipotent inhibitor of FGFR and VEGFR, overall the observed AE profile was largely comparable to that seen with FGFR or VEGFR inhibitors and thus anticipated from the therapeutic mechanisms of action.4 14 17 18 The AEs reported were grade 1/2 in severity and manageable, and most commonly included increase in bilirubin, diarrhoea, stomatitis, arthralgia, decreased appetite, palmar-plantar erythrodysesthesia, asthenia, epistaxis and fatigue. Furthermore, bilirubin increase-related events, such as hyperbilirubinaemia, jaundice and ocular icterus were reported in 75% of patients. ODM-203 is a potent inhibitor of UGT1A1 in human liver microsomes (IC50 0.1 µM), this being the most likely mechanism behind the bilirubin increase.16 Increased total or unconjugated bilirubin was most common at doses above 400 mg/day. The magnitude and rate of bilirubin increase appeared related to ODM-203 dose. Typically, increases in bilirubin were not associated with changes in transaminases, alkaline phosphatase or other indicators of hepatic injury and could be managed with dose modification or interruption.
Hypertension is a documented adverse effect of VEGF/VEGFR inhibitors, which is attributed in part to vasoconstriction caused by VEGF inhibition.19 In this study, hypertension was reported in 19.0% of patients. Events were mainly mild at grade 1/2, with three events of grade 3 or higher. This rate is lower than that reported in a phase I/II trial with the selective FGFR1/2 and VEGFR1/2/3 inhibitor lucitanib,14 in which hypertension occurred in 91% of patients, with 57% having grade 3 events. Hyperphosphataemia, an AE often associated with selective FGFR inhibitors,4 20 was reported in 19% of patients and a blood phosphorus increase was reported in 5% of patients. These results are comparable to those for the FGFR-specific agents AZD4547 and ARQ087.18 21 Ocular toxicities have been reported with FGFR inhibitors.22 In this study, only a single case of grade 3 punctate keratitis was reported with ODM-203 and was classified as a DLT after administration of an 800 mg dose. Most of the ocular events reported with ODM-203 (19.0%) were mild in severity, with conjunctivitis being the most frequently reported event.
Conclusion
ODM-203 400 mg once daily in tablet formation results in sufficient plasma concentrations of ODM-203 along with acceptable tolerability in most patients. The AEs reported were for the most part anticipated from the therapeutic mechanisms of action of ODM-203, manageable and responsive to drug interruption and/or dose reduction. Based on the study results, guidance for clinicians regarding these dose modifications on the occurrence of specific AEs is recommended. Preliminary signs of therapeutic activity of ODM-203 in patients with solid tumours were also observed. A limitation of the study, however, is the small sample size. Only few patients with FGFR altered tumours were treated to accurately define the benefits and safety of ODM-203 in tablet formulation. As FGFR acts differently in different tumour types, patient selection should be a key consideration for phase II studies.
Acknowledgments
The authors would like to thank all patients and their families, and all sites and their staff for participating in this trial. Medical writing assistance was provided by Rachel Bell, Bioscript Group, Macclesfield, UK, and funded by Orion Pharma.
Footnotes
Twitter: @ulassen
Contributors: Authors (PB, CM, KJP, PH, CG, TI, MVJM and JAR) contributed to and were involved in the conception and design of the study, provision of study materials or patients (PB, CM, KJP, AA, AI, RSK, GC, UL and H-TA), collection and assembly of data, data analysis and interpretation, and paper writing (PB, CM, KJP, PH, CG, TI, MVJM and JAR). All authors (PB, CM, KJP, AA, AI, RSK, GC, UL, H-TA, PH, CG, TI, MVJM and JAR) read and approved the final paper.
Funding: The study was sponsored by Orion Corporation, Orion Pharma, Espoo, Finland.
Competing interests: PB reports personal fees from Orion Pharma, during the conduct of the study, personal fees from Bristol-Myers Squibb, MSD, Pfizer, Novartis, EUSA, Oncorena, TILT Biotherapeutics, Faron Pharmaceuticals, Ipsen and Herantis Pharma, outside the submitted work, and stock ownership: TILT Biotherapeutics and Terveystalo. At the time of the study PB was employed by Helsinki University Hospital. CM reports consultant/advisory fees from Amgen, Astellas, Astra Zeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion, outside the submitted work, he is also principal/sub-Investigator of clinical trials for Abbvie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, Innate Pharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, Xencor. KJP reports personal fees from MSD, BMS, Roche Pfizer, Varian and Ipsen, outside the submitted work, and stock ownership: Faron Pharmaceuticals. At the time of the study KJP was employed part time (6 months) by Orion Pharma. AA reports personal fees from Orion Pharma and Amcure, outside the submitted work. AI reports research grants and personal fees from Ipsen, Novartis, Bayer, Bristol-Myers Squibb, Epizyme, Immune Design, Daiichi Sankyo and MSD outside the submitted work. GC reports personal fees from Roche, Seattle Genetics, Daichii Sankyo, AstraZeneca and Lilly, and other from Roche and Pfizer outside the submitted work. UL reports personal fee from Bayer and Pfizer outside the submitted work, and research grants from Roche, BMS, Pfizer and GSK. H-TA is an employee of HCAHealthcare UK/Sarah Cannon and reports receiving speaker bureau honoraria from Pierre Fabre, Guardant, BeiGene, and Roche outside the submitted work. PH, CG, TI and MVJM were employed by Orion. JAR reports personal fees and other from Novartis, Kelun Pharmaceuticals/Klus Pharma, Spectrum Pharmaceuticals Inc., Pfizer and Bayer, personal fees from Eli Lilly, Orion Pharmaceuticals, Peptomyc, Roche Pharmceuticals, Ellipses Pharma, Certera, and Ionctura SA, and other from European Journal of Cancer, VHIO/ Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, SOLTI, Elsevier, GlaxoSmithKline, ESMO, Department of Defense, Merck Sharp & Dohme, Lousiania State University, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, WIN Consortium, Janssen, Tocagen, Symphogen, BioAlta, GenMab, CytomX, Kelun-Biotech, Takea-Millenium, and Ipsen outside the submitted work.
Patient consent for publication: Not required.
Ethics approval: The protocol was approved by the institutional review boards and independent ethics committees of the participating centres. The study was performed in accordance with the Declaration of Helsinki and was conducted in compliance with the International Conference on Harmonisation on Good Clinical Practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets generated and/or analysed during the current study are not publicly available due proprietary restrictions but are available from the corresponding author on a reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Bielenberg DR, Zetter BR. The contribution of angiogenesis to the process of metastasis. Cancer J 2015;21:267–73. 10.1097/PPO.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer 2017;17:318–32. 10.1038/nrc.2017.8 [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Jeong D, Han Y-S, et al. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 2015;89:1–8. 10.4174/astr.2015.89.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touat M, Ileana E, Postel-Vinay S, et al. Targeting FGFR signaling in cancer. Clin Cancer Res 2015;21:2684–94. 10.1158/1078-0432.CCR-14-2329 [DOI] [PubMed] [Google Scholar]
- 5.Carrillo de Santa Pau E, Arias FC, Caso Peláez E, et al. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer 2009;115:1701–12. 10.1002/cncr.24193 [DOI] [PubMed] [Google Scholar]
- 6.Cihoric N, Savic S, Schneider S, et al. Prognostic role of FGFR1 amplification in early-stage non-small cell lung cancer. Br J Cancer 2014;110:2914–22. 10.1038/bjc.2014.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbauomy Elsheikh S, Green AR, Lambros MBK, et al. FGFR1 amplification in breast carcinomas: a chromogenic in situ hybridisation analysis. Breast Cancer Res 2007;9:R23. 10.1186/bcr1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S, Sullivan CAW, Zerkowski MP, et al. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol 2008;39:1835–43. 10.1016/j.humpath.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murase H, Inokuchi M, Takagi Y, et al. Prognostic significance of the co-overexpression of fibroblast growth factor receptors 1, 2 and 4 in gastric cancer. Mol Clin Oncol 2014;2:509–17. 10.3892/mco.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozdemir F, Akdogan R, Aydin F, et al. The effects of VEGF and VEGFR-2 on survival in patients with gastric cancer. J Exp Clin Cancer Res 2006;25:83–8. [PubMed] [Google Scholar]
- 11.Saylor PJ, Escudier B, Michaelson MD. Importance of fibroblast growth factor receptor in neovascularization and tumor escape from antiangiogenic therapy. Clin Genitourin Cancer 2012;10:77–83. 10.1016/j.clgc.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 12.Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–48. 10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 13.Holmström TH, Moilanen A-M, Ikonen T, et al. ODM-203, a selective inhibitor of FGFR and VEGFR, shows strong antitumor activity, and induces antitumor immunity. Mol Cancer Ther 2019;18:28–38. 10.1158/1535-7163.MCT-18-0204 [DOI] [PubMed] [Google Scholar]
- 14.Soria J-C, DeBraud F, Bahleda R, et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol 2014;25:2244–51. 10.1093/annonc/mdu390 [DOI] [PubMed] [Google Scholar]
- 15.Mulligan LM. Ret revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014;14:173–86. 10.1038/nrc3680 [DOI] [PubMed] [Google Scholar]
- 16.Rodon Ahnert J, Garratt C, Laapas K, et al. Dose escalation study of ODM-203, a selective dual FGFR/VEGFR inhibitor, in patients with advanced solid tumours. JCO 2016;34:2576–76. 10.1200/JCO.2016.34.15_suppl.2576 [DOI] [Google Scholar]
- 17.Michael M, Bang Y-J, Park YS, et al. A phase 1 study of LY2874455, an oral selective pan-FGFR inhibitor, in patients with advanced cancer. Target Oncol 2017;12:463–74. 10.1007/s11523-017-0502-9 [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos KP, El-Rayes BF, Tolcher AW, et al. A phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer 2017;117:1592–9. 10.1038/bjc.2017.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey AK, Singhi EK, Arroyo JP, et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension 2018;71:e1–8. 10.1161/HYPERTENSIONAHA.117.10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol 2019;16:105–22. 10.1038/s41571-018-0115-y [DOI] [PubMed] [Google Scholar]
- 21.Paik PK, Shen R, Berger MF, et al. A phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers. Clin Cancer Res 2017;23:5366–73. 10.1158/1078-0432.CCR-17-0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis ME. Ocular toxicity of tyrosine kinase inhibitors. Oncol Nurs Forum 2016;43:235–43. 10.1188/16.ONF.235-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-001081supp001.pdf (541.4KB, pdf)