Abstract
Background
Little is known on early irreversible effects of increased intra-abdominal pressure (IAP). Therefore, timing of abdominal decompression among patients with abdominal compartment syndrome remains challenging. The study objective was to determine the relation between IAP and respiratory parameters, hemodynamic parameters, and early intestinal ischemia.
Methods
Twenty-five anesthetized and ventilated male Sprague-Dawley rats were randomly assigned to five groups exposed to IAPs of 0, 5, 10, 15, or 20 mm Hg for 3 hours. Respiratory parameters, hemodynamic parameters, and serum albumin-cobalt binding (ACB) capacity as measure for systemic ischemia were determined. Intestines were processed for histopathology.
Results
IAP was negatively associated with mean arterial pressure at 90 (Spearman correlation coefficient; Rs=−0.446, p=0.025) and 180 min (Rs=−0.466, p=0.019), oxygen saturation at 90 min (Rs=−0.673, p<0.001) and 180 min (Rs=−0.882, p<0.001), and pH value at 90 (Rs=−0.819, p<0.001) and 180 min (Rs=−0.934, p<0.001). There were no associations between IAP and lactate level or ACB capacity. No histological signs for intestinal ischemia were found.
Discussion
Although increasing IAP was associated with respiratory and hemodynamic difficulties, no signs for intestinal ischemia were found.
Level of evidence
Prognostic and epidemiologic study, level II.
Keywords: multiple trauma, animal experimentation, abdominal injuries, compartment syndromes
Introduction
Intra-abdominal pressure (IAP) is the pressure concealed within the abdominal cavity. A substantial increase in IAP ≥12 mm Hg is termed intra-abdominal hypertension (IAH). If IAH exceeds 20 mm Hg, reduced intra-abdominal arterial perfusion pressure will result in organ dysfunction which is termed abdominal compartment syndrome (ACS). This condition is related to high morbidity and mortality.1 2 Risk factors and treatment options for ACS have been listed by the World Society of the Abdominal Compartment Syndrome in the “consensus definitions and clinical practice guidelines”.3 According to these guidelines, a decompression laparotomy should be performed if non-invasive measures failed.
An IAP of 20 to 25 mm Hg for a period of only 60 min may already reduce mucosal blood flow of the intestines of rats and deteriorate intestinal-barrier function.4 Moreover, IAH may cause irreversible intestinal ischemia before alterations in cardiac output or mean arterial pressure (MAP) become noticeable.5 6 Therefore, early surgical decompression before the development of ACS is becoming increasingly common.7 Since this treatment is related to high morbidity, the surgeon must be sure whether organ dysfunction is caused by IAH.8
The detrimental effects of ACS and persistent high IAP are well known.9 However, the early irreversible effects of subclinical or transient IAH with intra-abdominal pressures up to 20 mm Hg are unknown. Knowledge of such effects may help surgeons in decision-making on early surgical abdominal decompression. As it is hardly feasible to study this in humans, an animal model should be used. The aim of this study was to determine the relation between early increased IAP and respiratory parameters, hemodynamic parameters, and the development of intestinal ischemia in rats.
Methods
All animal experiments were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, and under the regulation and permission of the local Animal Care Committee. Adult (8–10 weeks old) male Sprague-Dawley rats (300–350 g, specific pathogen-free; Harlan Laboratories, Boxmeer, The Netherlands) were supplied with standard laboratory rat chow and water ad libitum, housed per two/three in individually ventilated cages, maintained on a 12:12 hours light–dark cycle, and acclimated for at least 1 week before the experiment. This manuscript was reported in line with the ARRIVE statement.10
Experimental model and IAH induction
Twenty-five anesthetized and ventilated rats were randomly assigned to five groups and exposed in random order to an IAP of 0, 5, 10, 15, or 20 mm Hg for 3 hours. From a pilot study, it was known that exposing rats to higher levels of IAP or for a prolonged period of time was not feasible in this model. This was due to the detrimental effects on hemodynamic and respiratory parameters. Rats were anesthetized with intra-peritoneal ketamine hydrochloride (50 µg/g), ventilated following tracheostomy, and kept warm by a warming pad and tin foil. A capnograph and pulse oxymeter (both Siemens SC9000 XL monitor; Siemens Medical Systems, EM-PCS, Danvers, USA) were installed to measure end-tidal CO2 concentrations in expired air (EtCO2) and oxygen saturation. The carotid artery and internal jugular vein were cannulated (PE-50) for blood draw access and monitoring the arterial blood pressures (systolic, diastolic, and mean), heart rate (HR), and central venous pressure (CVP). The tail vein was cannulated for anesthetic and fluid infusion (10 µL per gram body weight of KMA mix, consisting of 0.72 mL 100 mg/mL ketamine, 0.08 mL 1 mg/mL medetomidine, and 0.3 mL 0.5 mg/mL atropine, in 20 mL normal saline). Administration of antibiotics was considered as non-contributing since the experiment lasted only 3 hours. An intraperitoneal catheter (12 Ch Redon drain) was placed for fluid instillation and IAP monitoring by a midline laparotomy. The abdomen was closed with a running suture, including all layers of the abdominal wall.
The model used in this study has been described in detail previously.11 All animals were allowed to stabilize for 30 min before baseline measurements of MAP, CVP, HR, end tidal CO2 (EtCO2), and saturation. Direct continuous measurement of IAP was performed via the intraperitoneal catheter. After baseline analysis, the IAP was increased by instillation of warmed (40°C) Gelafundin (gelafundingelatine polysuccinate 4%; B. Braun Medical B.V., Oss, The Netherlands) and placing the fluid bag on specific level. A plaster cuff was applied to the abdomen of rats in order to reduce the required volume of Gelafundin (not in original model). The IAP and positive end expiratory pressure were kept at the same level during the experiment. Ventilatory and hemodynamic adaptations were made to compensate for deterioration during IAH. The respiratory rate and peak inspiratory pressure were increased to maintain EtCO2 between 4.5 and 6.0 kPa. MAP was kept between 70 and 110 mm Hg by Voluven administering (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride; Fresenius Kabi B.V. Zeist, The Netherlands). After completion of the experiment, all animals were sacrificed by exsanguination.
Blood sampling
Blood samples (with a capillary) were drawn at baseline, at 90 and 180 min for analyzing blood gases and serum lactate (ABL800 FLEX analyzer; Radiometer, Copenhagen, Denmark). All analyses were done once. At the same time points, single blood samples (0.6 mL) were drawn for duplicate determination of albumin-cobalt binding (ACB) capacity according to Bar-Or et al, as a measure of systemic ischemia.12 This biomarker is a highly sensitive and rapid marker for ischemia, but it is non-specific for tissue type and therefore a marker for systemic ischemia. The ACB test is a low-cost test which is easy to perform and well available. Also, the assay showed promising results for the detection of ischemia in an animal model of ACS.13 The ACB test indicates systemic ischemia when its absorbance reaches above 0.4 absorbance units (ABSU), measured using a microplate reader (Wallac 1420 Victor2; Perkin Elmer, Groningen, the Netherlands).
Histological examination
For each rat, five cross-sectional samples were taken at random locations of the intestine. If macroscopic damage was visible, samples were taken there as well. Samples were fixed with 10% formalin, embedded in paraffin and sliced in 4–5 µm sections, stained with routine H&E, and examined under a light microscope by a pathologist (KM) and clinical researcher (SGS); discrepancy was discussed. Histopathology was graded according to the Parks and Chiu/Park scoring systems for intestinal mucosal injury (Parks score for inflammation and necrosis and Park/Chiu score for mucosal lifting).14–16 All samples were scored at the most extensively affected areas. Mean scores of the five samples were calculated.
Statistical methods
The sample size calculation was based on an expected correlation coefficient between IAP and Parks/Chiu score of 0.95. With an alpha error of 0.05 (two-tailed) and a beta of 0.2, five animals per group were needed. Data for all animals were analyzed using SPSS statistical software, V.21.0. All data were non-parametric and are displayed as median with corresponding quartiles (P25–P75). Kruskal-Wallis test was used in order to test differences in body weight between groups. Spearman rank correlation tests was used in order to test the association between IAP and the individual variables.
Results
Basic characteristics
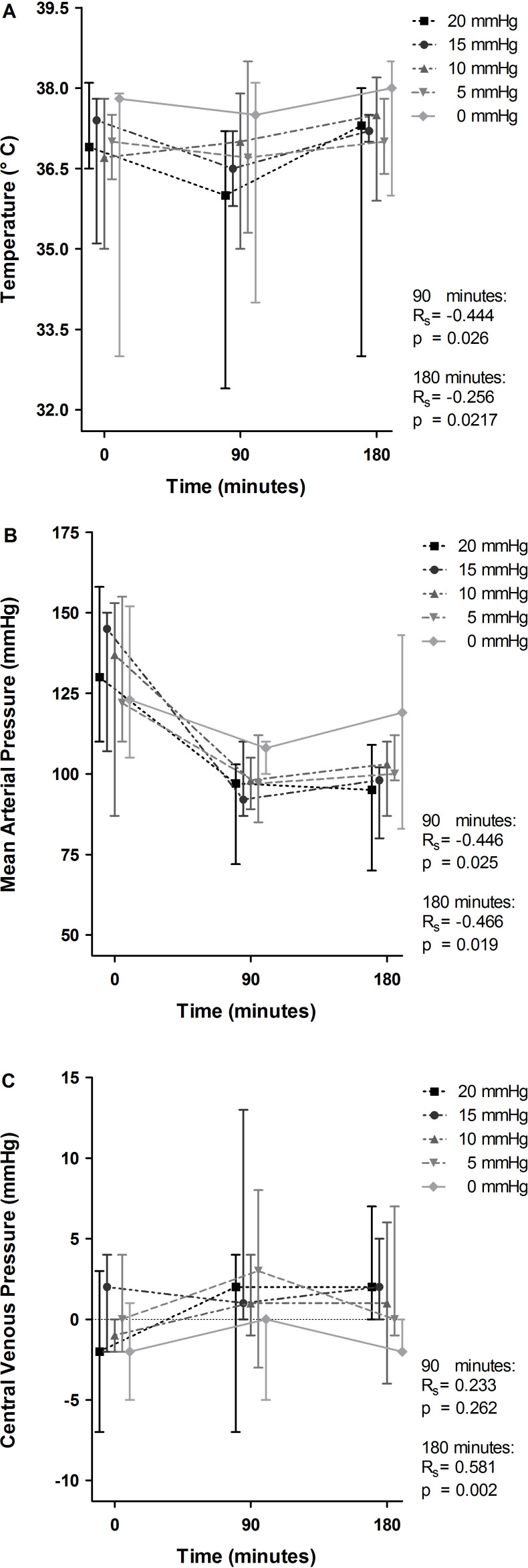
The median body weight of the rats was 377 g (P25–P75, 368–392 g), and there was no association between body weight and the IAP group the rats were assigned to (p=0.767). The median body temperature decreased statistically significantly to 36.0°C at 90 min (figure 1A). At that time point, temperature was negatively correlated with IAP (Spearman rank correlation coefficient; Rs=−0.444, p=0.026). At 180 min, body temperature had normalized again. A decrease in the median MAP was noted in all groups (figure 1B). MAP was negatively correlated with IAP, both at 90 min (from MAP 108 mm Hg in the control group to 97 mm Hg in the highest IAP group; Rs=−0.446, p=0.025) and at 180 min (from MAP 119 mm Hg to 95 mm Hg, respectively; Rs=−0.466, p=0.019). CVP remained fairly constant across time in all groups (figure 1C). At 180 min, the median CVP ranged from −2 mm Hg in the control group to 2 mm Hg in the highest IAP group (positive correlation with IAP, Rs=0.581, p=0.002). All animals completed the experiment and were used in the analyses.
Figure 1.
Effect of intra-arterial pressure (IAP) increase on temperature, mean arterial pressure and central venous pressure at 90 and 180 min. Temperature (A), mean arterial pressure (B) and central venous pressure (C) at 0, 90, and 180 min are demonstrated for the individual IAP groups and displayed as median with upper and lower limits. The Spearman correlation coefficient (Rs) and p value (p) represent correlation between IAP and the individual variables with corresponding statistical significance at 90 and 180 min.
Respiratory characteristics
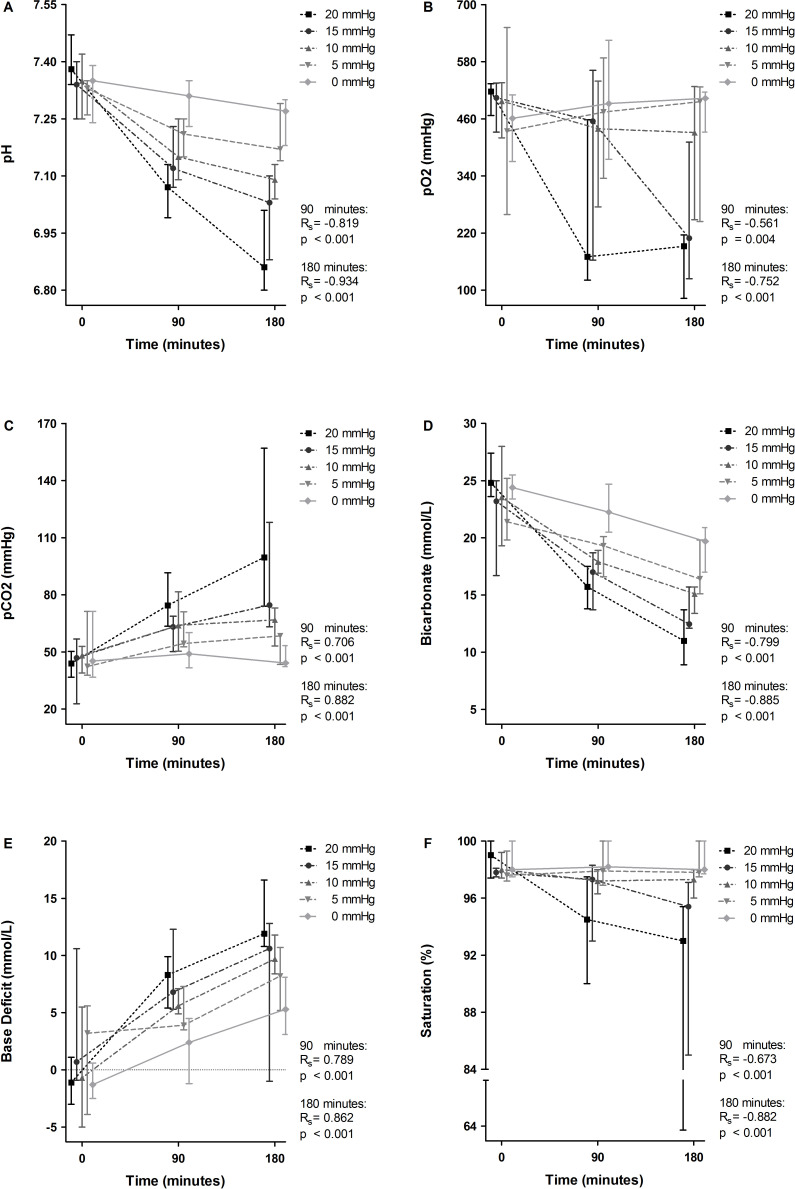
IAP was positively correlated with median EtCO2 at 180 min (from 4.5 kPa in the control group to 5.4 kPa in the highest IAP group; Rs=0.639, p=0.001). Respiratory deterioration was reflected in all individual parameters of arterial blood gas analysis by a dose-dependent correlation with IAP and time (figure 2). Most notably, a decrease was seen in median pH (ranging from 7.27 in the control group to 6.86 in the highest IAP group; figure 2A) and median pO2 (ranging from 503 mm Hg to 192 mm Hg; figure 2B) at 180 min. pH and pCO2 values were negatively correlated with IAP at that time point (Rs=−0.934, p<0.001 and Rs=−0.752, p<0.001 respectively). Bicarbonate levels and oxygen saturation also decreased, and demonstrated a negatively correlation with IAP (figure 2D and F). Positive correlations were seen between IAP and pCO2 (Rs=0.882, p<0.001; figure 2C) and base deficit (Rs=0.862, p<0.001; figure 2E) at 180 min. Median pCO2 increased over time with a range from 44.2 mm Hg in the control group to 99.6 mm Hg in the highest IAP group, and median base deficit increased with a range from 5.3 to 11.9 for the same groups at 180 min.
Figure 2.
Effect of intra-arterial pressure (IAP) increase on arterial blood gas values at 90 and 180 min. pH (A), pO2 (B), pCO2 (C), bicarbonate (D), base deficit (E), and saturation (F) at 0, 90, and 180 min are demonstrated for the individual IAP groups and displayed as median with upper and lower limits. The Spearman correlation coefficient (Rs) and p value (p) represent correlation between IAP and the individual variables with corresponding statistical significance at 90 and 180 min.
Outcome characteristics
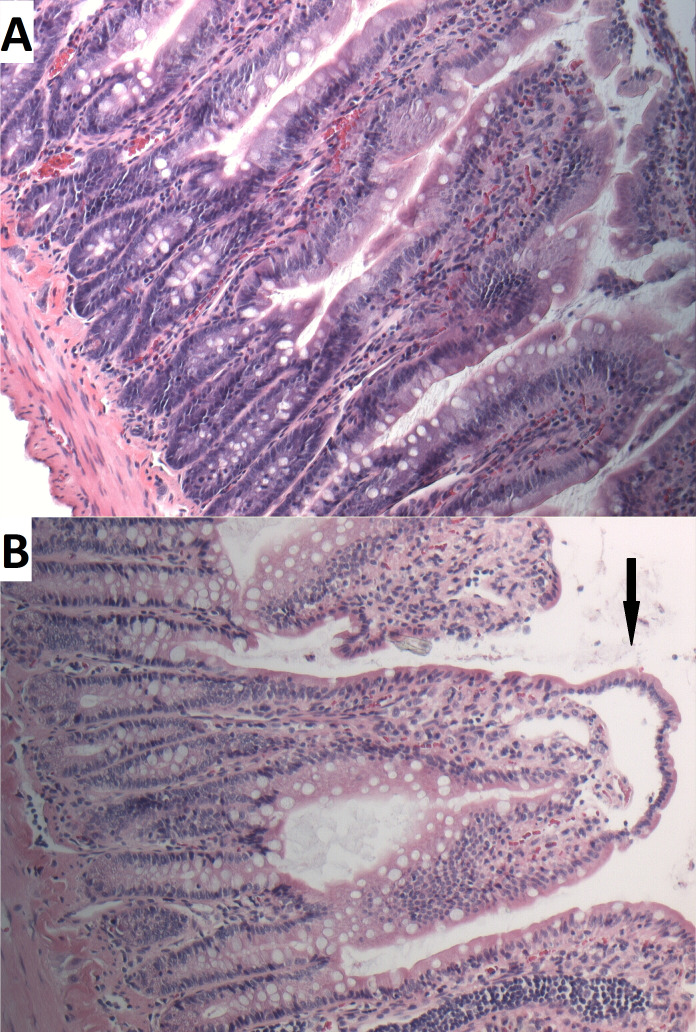
During the last phase of this experiment, serum lactate levels increased. No significant correlation was found between this and IAP (Rs=0.178, p=0.417; figure 3A). Although a decrease was seen in ACB capacity (demonstrated by an increase of ABSU), group medians did not reach the threshold of 0.4 ABSU for systemic ischemia. No correlation between ACB capacity and IAP was observed (figure 3B). This finding was confirmed by histopathological examination, and no evident signs for ischemic damage were found in the H&E-stained sections (figure 4). The Parks score was comparable in all groups: no inflammation or necrosis was found in any of the specimens. The Park/Chiu score for mucosal lifting ranged from 0 to 4; no significant correlation between Park/Chiu score and IAP was found (Rs=−0.141, p=0.501).
Figure 3.
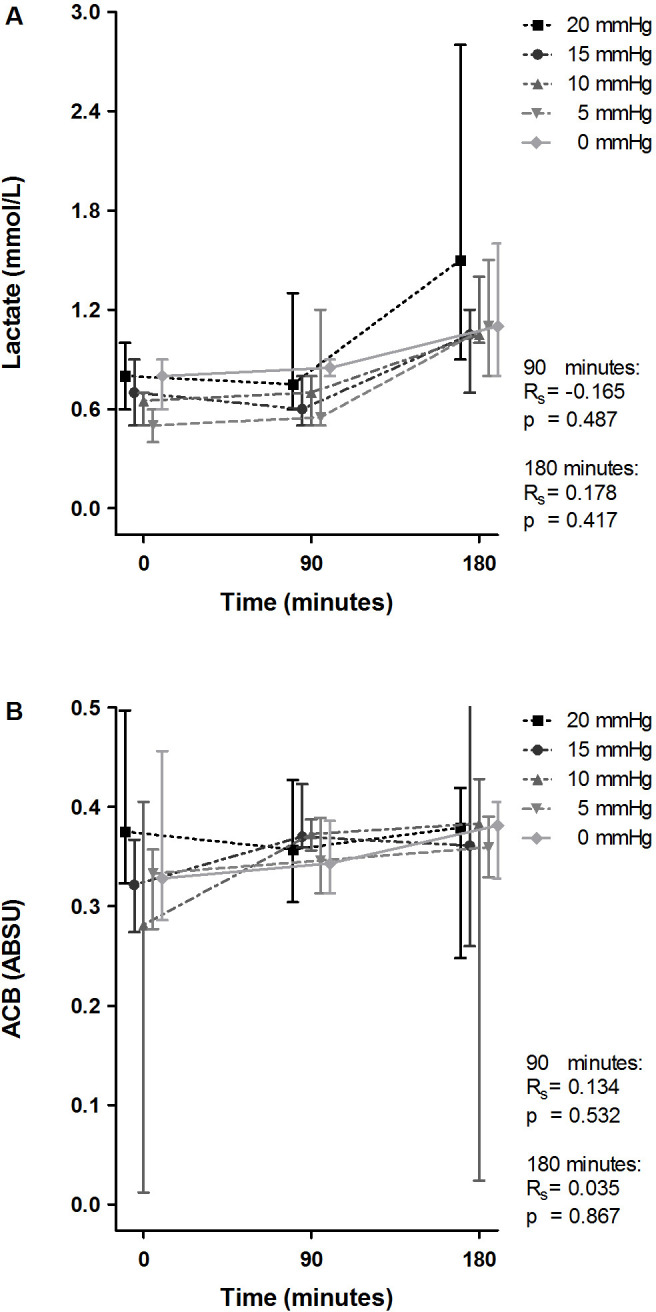
Effect of intra-arterial pressure (IAP) increase on serum lactate and albumin-cobalt binding at 90 and 180 min. ABSU, absorbance units; ACB, albumin-cobalt binding capacity. Lactate (A) and albumin-cobalt binding (B) at 0, 90, and 180 min are demonstrated for the individual groups and displayed as median with upper and lower limits. The Spearman correlation coefficient (Rs) and p value (p) represent correlation between IAP and the individual variables with corresponding statistical significance at 90 and 180 min.
Figure 4.
H&E sections of the least and most extensively damaged mucosa. In most sections of rats in all groups, no lesions were found in the small intestine (A). Grade 3 Parks/Chiu lesions of ischemia (ie, mucosal lifting down sides of the villi; see arrow) were seen sporadically (B).
Discussion
In this model of IAH, an increase in IAP did not result in significant ischemic complications within the first 3 hours. Even though a relation was seen between increasing IAP and hemodynamic deterioration, no histopathologic signs of irreversible damage to the intestines were found; nor were there any signs of systemic ischemia throughout the experiment as tested using the ACB assay. This is in contrast with the results of the only other similar study in rabbits. In this study, rabbits were exposed to an IAP of 25 mm Hg for 1 hour. During this period, histopathology and the ACB assay demonstrated a statistically significant increase in intestinal ischemia.13
The findings of this study may indicate that in the earliest phase of increasing IAP, physicians have some time to focus on adequate respiratory and hemodynamic support before preventive open abdomen decompression is applied. In the first phase of IAH, respiratory deterioration may already be very profound without causing irreversible damage to the intestines. During this period, the possible effects of less-invasive measures can be awaited with no further harm being done. This supports the theory that effects of percutaneous catheter decompression (PCD) can be awaited for a period of 4 hours; if PCD was not effective, urgent open abdomen decompression should be initiated.7
The respiratory and hemodynamic deterioration observed in this study demonstrated the suitability of the model used. Apart from the metabolic acidosis in the 0 mm Hg group, outcomes seem to be an adequate reflection of IAH in humans.17 18 The metabolic acidosis in the 0 mm Hg group was unexpected, a finding possibly due to the rats being relatively hypovolemic. In order to keep rats as normovolemic as possible, rats in the 0 mm Hg group only received resuscitation fluids for compensation of blood collection. As confirmed by the negative CVPs, this minimal support seems to have been insufficient.
All animals were sacrificed at 180 min for histopathological evaluation; reperfusion following decompression was not awaited. Theoretically, during reperfusion, free radicals may cause significant oxidative damage.19 The oxidative damage might even be more extensive than the damage induced by IAH itself. Demonstrating this, however, was not the aim of this study.
Possible limitations of this study were the relatively small sample size and the small size of the animals used in this experiment. It is known that the abdominal wall elasticity of small animals significantly differs from the elasticity in humans.20 Even though a plaster cuff was placed around the abdomen of the rats, this may have influenced the results of the present study. Moreover, the pathophysiology of ACS in critically ill humans is likely different from ACS in the otherwise healthy rats used in the current model. Translation of this IAH model to the human situation should therefore be done with care.
The period of 180 min was relatively short compared with patients who are observed and conservatively managed after bowel obstruction or non-operatively managed blunt abdominal trauma. This is inherent to the selected rat model. From a pilot study, it was known that exposing the rats to IAP levels of 25 or 30 mm Hg resulted in death within 1 hour. Even at 20 mm Hg IAP, the rats’ hemodynamic and respiratory parameters progressed to lethal levels, making it impossible to keep the animals alive longer than 180 min. At that time point, arterial blood gas values reached morbid levels in the highest IAP group. In one case, the rat died instantaneously when the IAP was relieved. Nevertheless, this experiment was suitable for demonstrating a principle. The outcomes of this study require confirmation in larger study groups or larger animals.
In conclusion, during this experimental study of increased IAP, no signs of early irreversible ischemic damage were found, while profound deterioration of respiratory and hemodynamic parameters were already present. These findings may indicate that in the early phase of increasing IAP, physicians have some time to focus on adequate respiratory and hemodynamic support before preventive open abdomen decompression is applied. Non-invasive measures which prevent a further increase of IAP seem preferable.
Acknowledgments
Jaqueline Voorbeijtel and Patricia Specht (laboratory animal technicians) are greatly acknowledged for their assistance during the preparation and execution of the experiments.
Footnotes
Contributors: Each author contributed significantly to the design, data acquisition, analysis and interpretation of data, and drafting or critical revision of the manuscript, and each author provided final approval of the submitted manuscript. SGS: design, data acquisition, analysis and interpretation, drafting and critical revision of the manuscript. BvdH: design, data acquisition and interpretation, critical revision of the manuscript. KM: data acquisition, interpretation of data, critical revision of the manuscript. SA: design, data acquisition. EMMvL: design, data analysis and interpretation, drafting and critical revision of the manuscript. OJFvW: design, data interpretation, critical revision of the manuscript. MHJV: data interpretation, critical revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All animal experiments were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, and under the regulation and permission of the local Animal Care Committee (DEC Nr. EMC 2089 [142-10-03]).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request from EMMvL (Erasmus MC, University Medical Center Rotterdam Trauma Research Unit Department of Surgery, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands; e.vanlieshout@erasmusmc.nl).
References
- 1.Malbrain MLNG, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, et al. . Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 2005;33:315–22. 10.1097/01.CCM.0000153408.09806.1B [DOI] [PubMed] [Google Scholar]
- 2.Chiaka Ejike J, Humbert S, Bahjri K, Mathur M. Outcomes of children with abdominal compartment syndrome. Acta Clin Belg 2007;62:141–8. 10.1179/acb.2007.62.s1.018 [DOI] [PubMed] [Google Scholar]
- 3.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain MLNG, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. . Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190–206. 10.1007/s00134-013-2906-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diebel LN, Dulchavsky SA, Brown WJ. Splanchnic ischemia and bacterial translocation in the abdominal compartment syndrome. J Trauma 1997;43:852–5. 10.1097/00005373-199711000-00019 [DOI] [PubMed] [Google Scholar]
- 5.Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma 1992;33:279–83. 10.1097/00005373-199208000-00019 [DOI] [PubMed] [Google Scholar]
- 6.Diebel LN, Dulchavsky SA, Wilson RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma 1992;33:45–9. 10.1097/00005373-199207000-00010 [DOI] [PubMed] [Google Scholar]
- 7.Cheatham ML, Safcsak K. Percutaneous catheter decompression in the treatment of elevated intraabdominal pressure. Chest 2011;140:1428–35. 10.1378/chest.10-2789 [DOI] [PubMed] [Google Scholar]
- 8.Mayberry JC, Burgess EA, Goldman RK, Pearson TE, Brand D, Mullins RJ. Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: the plain truth. J Trauma 2004;57:157–63. 10.1097/01.TA.0000102411.69521.80 [DOI] [PubMed] [Google Scholar]
- 9.Uysal E, Kirdak T, Korun N. Alterations in thyroid hormones due to increased intraabdominal pressure in rats. J Invest Surg 2015;28:317–22. 10.3109/08941939.2015.1020400 [DOI] [PubMed] [Google Scholar]
- 10.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med 2010;12:561–3. 10.1002/jgm.1473 [DOI] [PubMed] [Google Scholar]
- 11.Meier C, Schramm R, Holstein JH, Seifert B, Trentz O, Menger MD. Measurement of compartment pressure of the rectus sheath during intra-abdominal hypertension in rats. Intensive Care Med 2006;32:1644–8. 10.1007/s00134-006-0366-4 [DOI] [PubMed] [Google Scholar]
- 12.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med 2000;19:311–5. 10.1016/S0736-4679(00)00255-9 [DOI] [PubMed] [Google Scholar]
- 13.Unlüer EE, Kiliç TY, Akgöl E, Işgüven D, Vardar E, Bayol U, Yilmaz O, Erkan N, Gökmen N. The role of cobalt-albumin binding analysis in the diagnosis of experimental abdominal compartment syndrome in rabbits. Ulus Travma Acil Cerrahi Derg 2010;16:491–6. [PubMed] [Google Scholar]
- 14.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology 1982;82:9–15. 10.1016/0016-5085(82)90115-9 [DOI] [PubMed] [Google Scholar]
- 15.Chiu C-J. Intestinal mucosal lesion in low-flow states. Arch Surg 1970;101:478–83. 10.1001/archsurg.1970.01340280030009 [DOI] [PubMed] [Google Scholar]
- 16.Park PO, Haglund U, Bulkley GB, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 1990;107:574–80. [PubMed] [Google Scholar]
- 17.Lozen Y. Intraabdominal hypertension and abdominal compartment syndrome in trauma: pathophysiology and interventions. AACN Clin Issues 1999;10:104–12. 10.1097/00044067-199902000-00010 [DOI] [PubMed] [Google Scholar]
- 18.Cullen DJ, Coyle JP, Teplick R, Long MC. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med 1989;17:118–21. 10.1097/00003246-198902000-00002 [DOI] [PubMed] [Google Scholar]
- 19.Saracoglu KT, Saracoglu A, Umuroglu T, Ugurlu MU, Deniz M, Gogus FY. The preventive effect of dopamine infusion in rats with abdominal compartment syndrome. J Invest Surg 2013;26:334–9. 10.3109/08941939.2013.808289 [DOI] [PubMed] [Google Scholar]
- 20.Yoshino O, Quail A, Oldmeadow C, Balogh ZJ. The interpretation of intra-abdominal pressures from animal models: the rabbit to human example. Injury 2012;43:169–73. 10.1016/j.injury.2011.04.011 [DOI] [PubMed] [Google Scholar]