Abstract
About 75% of colorectal cancers are diagnosed as early stage, in which radical surgery is achievable. In the last decade, in Italy, the overall incidence of colorectal cancer has remained stable, while mortality gradually decreased, which is attributable to early diagnosis and improved medical, surgical and locoregional treatments. The Italian Medical Oncology Association formulated guidelines to manage early-stage colon cancer, including screening, diagnosis, treatment and follow-up, which we herein present.
Keywords: oncology, early colon cancer, italian guidelines
Introduction
Colorectal cancer (CRC) is the second most common cancer in Italy and second cause of death for cancer in both genders. Over 49.000 new CRC diagnoses were expected in 2019 (27.000 in men and 22.000 in women). Almost 20.000 CRC deaths were observed in 2016, of which 54% in men. In Italy, the 5-year survival rate is homogeneous between men and women and lies by 66% for colon cancer (CC) and 62% for rectum cancer.1
Mortality has declined progressively in many Western countries,2 3 probably because of cancer screening programmes able to early remove adenomas and to detect early cancerous lesions, and availability of more effective therapies. A more profound knowledge of biological disease characterisation and the application of personalised and patient-centred strategies associated with the evolution of multidisciplinary teams, led to some important advantages in diagnosis and treatment of CRC. In this changing scenario, the Italian Medical Oncology Association (AIOM) has developed evidence-based guidelines to provide oncologists, physicians and other healthcare professionals, comprehensive and updated CRC treatment strategy. Herein, we present the Italian guidelines on the management of early-stage CC, including the intraperitoneal portion of the rectum.
The working group
The AIOM CC guidelines working group is composed of several professional figures including 12 medical oncologists, 1 of which also specialised in cell/molecular biology, 1 surgeon specialised in CRC surgery, 1 radiation oncologist and 1 gastroenterologist. The methodology, systematic reviews and guideline development Unit of the Mario Negri Institute for Pharmacological Research IRCCS (Scientific Institute for Research, Hospitalization and Healthcare) was responsible for methodological support. Every year, the working group performs a systematic review of the literature in order to update, modify (when necessary) and improve CC guidelines. Updated guidelines are reviewed by both medical oncologists, considered opinion leaders in CRC and AIOM members, and different professional figures belonging to several scientific societies, such as Italian Association of Gastroenterology, Italian Association of Oncologic Radiotherapy, Italian Society of Pathology and Cytology, Italian Society of Oncologic Surgery, Italian Society of Human Genetic, Italian Society of General Medicine and Italian Society of Medical and Interventional Radiology (online supplemental table 1). Also one general practitioner (specialised in oncology), one nurse and one cancer survivor, who is a medical oncologist as well, review every year the updated guidelines.
esmoopen-2020-001001supp001.pdf (65.4KB, pdf)
The final report is published online on the AIOM website and presented annually at the Italian Congress of Medical Oncology. The 2019 CC guidelines have been also accepted and published on the website of the Italian National Health Institute.4
Methodology
As previously reported,5 the AIOM CC guidelines include recommendations based on evidence assessed according to both Scottish Intercollegiate Guidelines Network6 (SIGN) and approach Grading of Recommendations, Assessment, Development and Evaluations (GRADE).7 Specifically, until 2016 all the recommendations followed SIGN. Since 2016, all AIOM guidelines abandoned the SIGN quality assessment, replacing it with the GRADE approach, which based the certainty of evidence on five main dimensions (risk of bias, inconsistency, indirectness, imprecision and publication bias).
Applied to the single outcome, risk of bias refers to limitations in study design, inconsistency refers to heterogeneity among studies’ result, indirectness refers to the direct applicability of results between population, intervention, comparison, outcomes and evidence found, imprecision refers to the width of CI around the point estimate and it is related to optimal information size, as well, publication bias refers to the probability that evidence was published depending on the nature and direction of results.
Certainty of evidence is then synthesised into four levels (very low, low, moderated, high) and in table 1, we reported their meaning.
Table 1.
Grading of certainty of evidence
| Certainty of evidence | Meaning | Consequence |
| High | High confidence in results | It is very likely that the true effect of the treatment is similar to the estimated one. |
| Moderate | Moderate confidence in results | It is likely that the true effect of the treatment is similar to the estimated one but there is still the possibility that the effect is different. |
| Low | Results are not trustworthy | Confidence in the effect estimate is limited: the true effect could be substantially different from the estimated one. |
| Very low | Results are totally not trustworthy | Confidence in the effect estimate is very limited: it is likely that the true effect is substantially different from the estimated one. |
The strength of a recommendation reflects not only the certainty (assessed with either SIGN or GRADE), but also the clinical relevance of evidence.
To better suit to AIOM need the meaning of the strength of recommendation has been adapted and reported as ‘strong for’, ‘strong against’, ‘conditional for’ or ‘conditional against’, as explained in table 2.
Table 2.
Strength of recommendation according to the grade adaptation for AIOM
| Strength of recommendation | Meaning |
| Strong for | The intervention should be considered as the first treatment option (benefits are higher than risks). |
| Conditional for | The intervention can be considered as a possible treatment option (not sure if benefits are higher than risks). |
| Conditional against | The intervention should not be considered as the first treatment option; it could be considered in selected cases after discussion with the patient (not sureif risks are higher than benefits). |
| Strong against | The intervention must be not considered as a possible treatment option (risks are higher than benefits). |
AIOM, Italian Medical Oncology Association.
Access to CRC diagnosis and treatment
A few studies suggested a correlation between diagnostic delay and worsening prognosis.8 In order to accelerate diagnosis and treatment, training courses for general practitioners should be encouraged to improve early CRC detection skills and to define access to health facilities operating on the territory. Furthermore, a dedicated team in treatment hubs or alliances with dedicated teams allows quicker CRC diagnosis.
Regarding diagnosis, colon carcinomas or polyps should be first excluded in patients >50 years presenting recent rectal bleeding before assuming benign disease. All patients >50 years presenting new, significant or persistent symptoms related to CRC disease (eg, abdominal pain, mucorrhoea, rectal bleeding, weight loss, sideropenic anaemia, etc) must receive an accurate medical assessment, including anamnesis and physical examination with rectal inspection. Subsequent diagnostic tests should preferably be performed within 4 weeks. Patients <50 years presenting symptoms related to colorectal (CR) disease, in the absence of clinical worsening and/or family risk, may be carefully monitored for a few weeks: if symptoms persist the patient must promptly undergo diagnostic tests.8 9
Screening perspectives
Several randomised studies demonstrated improved CRC mortality rates if screening with faecal occult blood test (FOBT) or rettosigmoidoscopy (RSS) was offered.10–18 Four randomised phase III studies, with about 400 000 patients between 45 and 80 years, who underwent annual or biennial FOBT versus no intervention showed reduced CRC mortality ranging from 15% to 33% in favour of FOBT.9 10 12 Other four phase three studies evaluated the effect of RSS (performed once only between 55 and 64 years in three studies and twice between 55 and 74 years in one study) on mortality rates in more than 400 000 patients; RSS showed reduced CRC mortality from 31% to 22% compared with the no intervention group.13–15 An updated analysis by Atkin et al, considering more than 170 000 patients, confirmed a 30% reduced CRC mortality rate persisting over a period of 17 years, which reaffirms the effectiveness of the RSS even if once only performed between 55 and 64 years of age.19
Faecal immunochemical test is used for organised CRC screening in all Italian regions, with the exception of Piemonte.
Total colonoscopy has a greater diagnostic sensitivity than RSS, but should be performed by experienced endoscopists. Total colonoscopy is recommended in patients with positive FOBT or with advanced lesions in the rectum sigma (cancer or high-risk adenomas) due to increased probability of advanced lesions in the proximal colon.20 No evidences from randomised studies regarding colonoscopy efficacy in terms of CRC mortality reduction, nor about the frequency and the optimal range of age, are available.21 However, colonoscopy should be periodically proposed for surveillance in case of CR diseases (adenomas or chronic inflammatory disease) which are potentially associated with an increased CRC risk.22–24
Faecal markers25 should be considered an experimental screening method while the main indication for virtual colonoscopy is an incomplete colonoscopy even if it cannot yet be considered a standard screening method.26
CRC hereditary predisposition syndromes
The prevalence of inherited CRC syndromes, associated with known pathogenetic variants, is about 5%–6%.27
The most frequent CRC hereditary predisposition syndrome is the Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, which accounts for about 3% of all CRCs. It is an autosomal dominant inherited syndrome, due to a pathogenetic variant in the ‘mismatch repair’ (MMR) genes, which predominantly predisposes to CRC, endometrial cancer and other cancers at a younger age than the general population.28–31
The familial adenomatous polyposis (FAP) accounts for about 1% of all CRC. FAP can be categorised as Classical variant, when the number of polyps in the colon is more than 100, and Attenuated variant, when the polyp number is between 10 and 99. A further classification is based on pathogenetic variants, the most frequent due to a defect of the APC gene or the MUTYH gene.25 26
Patients with CRC should be referred to genetic counselling based on the following clinical suspicion criteria, adapted from Stjepanovic32:
-
Patient-related criteria:
< 50 years of age.
Multiple cancers (synchronous or metachronous), related to the inherited CRC syndromes, in the same patient.
Multiple polyps associated to CRC.
-
Family-related criteria:
Multiple cases of cancer in the same parental branch (maternal or paternal).
At least one first-degree or second-degree relative with one cancer diagnosed ≤50 years of age.
Known inherited CRC syndrome in the family.
-
Tumour-related criteria:
-
For Lynch syndrome only: MMR-deficient (dMMR) tumour, with loss of expression of proteins encoded by MMR genes at immunohistochemistry (IHC), or with high microsatellite instability (MSI-H) at PCR.
(dMMR tumours are often right-sided, G3, mucinous adenocarcinoma or signet ring cell adenocarcinoma, with lymphocytic infiltrate and Crohn-like reaction).
-
Diagnostic molecular tests differ according to different syndromes
-
Lynch syndrome:
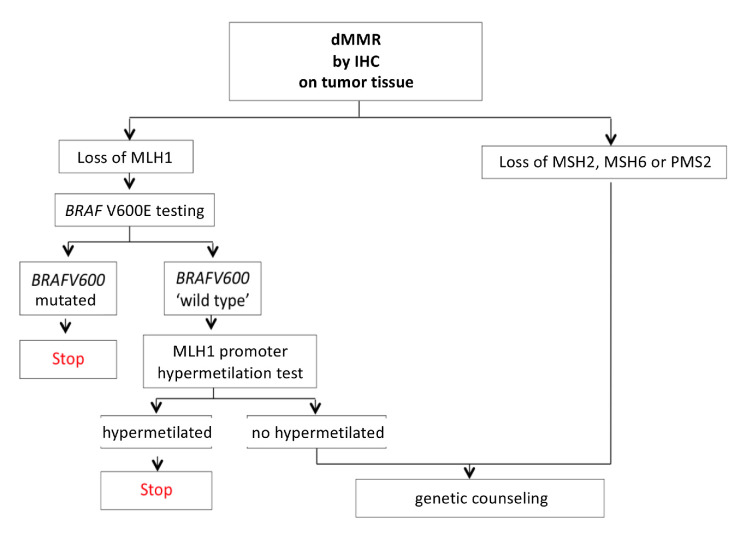
Prescreening tests on tumour tissue can identify dMMR, either by IHC, showing loss of protein expression encoded by MMR genes, or by PCR, showing an MSI-H. In case of dMMR with loss of MLH1 expression, it is recommended to perform BRAF V600 analysis and/or MLH1 promoter hypermethylation test on tumour tissue. The presence of BRAF mutation or MLH1 promoter hypermethylation can reasonably exclude the diagnosis of Lynch syndrome.33 34
Those with MLH1 loss, BRAF wt and with no MLH1 promoter hypermethylation, and those with MSH2, MSH6 or PMS2 loss should undergo genetic counselling and genetic testing (on a blood sample), in order to detect germline pathogenetic variants in one of the following 4 MMR genes: MLH1, MSH2, MSH6 and PMS2.35
-
Polyposis:
Genetic testing on a blood sample can detect germline pathogenetic variants, the most frequent in the APC and MUTYH genes.36
The use of multigene panels is recommended in selected cases only.
However, due to the low sensitivity of clinical suspicion criteria in diagnosing Lynch syndrome, universal screening through dMMR testing should be considered in all patients with CRC.
The AIOM CC working group estimated that the positive effects resulting from universal screening for Lynch syndrome and the subsequent identification of patients and their relatives to be referred to genetic counselling, screening and specific oncological follow-up, are significantly higher than the possible negative effects.37 However, no data assessing safety of universal screening have been published yet.
Endorsement of the universal screening programme on the national territory (figure 1), implementing the collaboration between oncologists, molecular pathologists and geneticists for Lynch syndrome diagnosis, is necessary. The subsequent surveillance, in accordance with different professionals such as oncologists (for surviving patients) and geneticists (for relatives at risk), could lead to a favourable cost/benefit balance.
Figure 1.
Algorithm for Lynch syndrome universal screening. dMMR, mismatch repair deficient; IHC, immunohistochemistry.
CRC survivors, with inherited syndromes, should receive specific and more intensive follow-up programmes as compared with non hereditary cases. The following recommendations are based on expert opinion only28
-
Lynch syndrome:
Colonoscopy every 1–2 years.
Annual gynaecological examination with transvaginal ultrasound and endometrial biopsy.
Prophylactic gynaecological surgery can be an option in women >35 years of age and/or in patients who completed childbearing.
-
Classical FAP:
Proctoscopy or pouchoscopy every 6–12 months, depending on the polyps load.
Gastroduodenoscopy every 6 months to 5 years according to Spigelman criteria.38
Abdominal ultrasound every 6–12 months after abdominal surgery in order to assess the onset of abdominal wall desmoids and/or mesenteric and retroperitoneal desmoids.
Thyroid ultrasound every 2 years for possible thyroid neoplasms.
Diagnosis and staging
Pancolonoscopy is considered the most important examination to diagnose CRC with a sensitivity of 96%–97% and specificity of 98%. Pancolonoscopy has a perforation risk of 0.1%, bleeding risk of >0.3% and mortality risk of 0.01%–0.03%.39–42 As an alternative to pancolonoscopy, RSS in combination with colon CT scan can be used, even if approximately 30% of patients should additionally undergo colonoscopy. Sensitivity and specificity of RSS are similar to colonoscopy but limited to the first 60 cm. Virtual colonoscopy cannot be proposed as a standard screening method yet, while it is useful to examine the colon in patients without complete colonoscopy screening.43 44
Histological assessment of colon neoformations should always be performed before surgery, but could be omitted in rare and well-selected cases of colic neoformations, not easily reachable by endoscopy and unequivocal iconography.45 Preoperative assessment of metastases must always be performed. Liver metastases should preferably be evaluated with a CT scan. Lung metastases should be excluded preferably with a chest CT scan. The use of different (and expensive) methods such as MR, bone scintigraphy and PET (positron emission tomography) scan should be reserved for special cases. The evaluation of preoperative CEA is recommended due to its prognostic role and its possible follow-up use. The determination of Ca 19.9, although widely used, is not supported by scientific evidence.46
Surgery: General information
Surgery is the main treatment option for early-stage CC, which should be performed as quickly as possible. Surgical mortality, perioperative complications and prognosis depend on the experience of the surgical team.47 The surgical report should include the description of the intraoperative procedure including technical details and the level of radicality.
A 2011 Cochrane analysis of 18 randomised trials including over 5800 patients demonstrated the equivalence between mechanical and non-mechanical bowel preparation, in terms of anastomotic dehiscences, perioperative mortality, surgical reinterventions and wound infections.48
The use of low molecular weight heparins prophylaxis for 30 days demonstrated lower bleeding risk and thrombosis and pulmonary embolism reduction.49
Prophylaxis with short-term antibiotic reduced infections from 30%–50% to 11% or less.50–52
Patients at risk for ostomy must receive adequate counselling before surgery. The site of the ostomy should be marked prior to surgery on the skin of the standing patient. The choice between colostomy (right) and ileostomy depends on the type and site of surgical resection, duration of the ostomy and clinical variables (eg, age, hydroelectrolytic balance, possible adjuvant treatment, etc).
Key points for the oncological radicality of CC surgery
Proximal and distal resection margin: 2 cm is the minimum acceptable limit of free margins.53–56
A total mesocolic excision must be always carried out to guarantee a complete locoregional removal.
In the right colon, standard lymphadenectomy must include ileo-colic lymph nodes and those of the right branch of the middle colic artery. In the left colon and sigma, standard lymphadenectomy must include lymph nodes at the origin of inferior mesenteric artery.57 At least 12 lymph nodes must be found in the surgical specimen to avoid surgical undertreatment.
Adjacent infiltrated organs have to be resected in block to ensure a radical resection.
Oncological outcomes of videolaparoscopic CC resection are equivalent to the laparotomy technique, but the technique has several advantages such as reduced postoperative pain, early resumption of eating and normal daily activities.58 Robotic technique needs still to be evaluated but costs are high.
Biopsies of any residual tumour and/or metastases should always be performed.
Criteria to define the risk of cancerised CR adenoma and its management
Cancerised adenoma is defined as an adenoma with neoplastic infiltration of the submucosa (pT1). Only an accurate histopathological evaluation can predict the risk of local recurrence and/or lymph node metastases, which is only possible if the polyp is completely removed, preferably in a single resection. The histological report of a cancerised adenoma should contain the following parameters, which define the risk of lymph node metastases (low risk: 2%–18%; high risk: 20%):
Grading of carcinoma (G1-G2 vs G3-G4) and presence of mucinous component.
Presence or absence of lymphovascular invasion.
Level of invasion of the submucosa (superficial, medium or deep);.
Free margin below the resection (present or absent).
Status of the endoscopic resection margin (cancer cells at less than 1 mm and/or included in the diathermocoagulation band).
Evaluation of tumour ‘budding’ (absent or present; low vs high grade) should be performed.
In case that all risk factors are absent, the probability of lymph node metastases is less than 1%, while it varies from 21% to 36% in case of presence of one or more risk factors. The risk of local recurrence is absent if the resection margin is free of neoplastic infiltration, while it rises to 33% if the margin is infiltrated.59
The presence of at least one of the risk parameters is associated to a high risk of lymph node metastases and it is an indication for surgical treatment. Surgical treatment consists of segmental resection, preferably laparoscopic.60 61
Adjuvant treatment
About 35% of radically resected early-CC patients develop disease recurrence, of which 80% occur within the first 3 years from surgery.62 Eight years after diagnosis, recurrences occur in less than 0.5% of cases. Local recurrences are rare in CC. The most frequent sites of recurrence are the liver, abdominal lymph nodes, peritoneum and lung. The grade of intestinal wall (T) infiltration has more influence on prognosis than lymph node involvement (N) and the ratio of positive/analysed lymph nodes is important to define prognosis.
Adjuvant treatment has the objective to reduce the risk of recurrence after CC radical surgery.
Stage I CC (pT1-2, N0) occurs in 15% of cases and 5-year overall survival (OS) after radical surgery is about 95%–100%. Thanks to its excellent prognosis, adjuvant chemotherapy is not indicated.
Stage II CC (pT3-4, N0) occurs in 20%–30% of cases and 5-year OS ranges from 85%, for pT3N0 without risk factors, to 55%, for pT4bN0. In this setting the indication for adjuvant chemotherapy is still controversial: overall, patients treated with 5-fluorouracil (5-FU) as monochemotherapy reach an absolute benefit in OS of 3%–4%.63–65 The choice of an adjuvant treatment is guided by a benefit/risk ratio evaluation for each patient and should be considered in case of poor prognostic factors (occlusion, perforation, T4, grading G3-4, inadequate number of analysed lymphnodes (<12), presence of vascular, lymphatic and/or perineural invasion), which should be discussed with the patient.66
The presence of MSI-H seems to identify patients with a better prognosis and no benefit from fluoropyrimidine adjuvant treatment. A retrospective analysis conducted on more than 1900 patients, enrolled in the QUASAR study, showed that the recurrence rate was doubled in radically resected CC patients with) versus MSI-H (26% vs 11%), with a risk ratio of 0.53 (95% CI 0.40 to 0.70; p=0.001).67 A further analysis conducted on 450 patients, randomised to receive a 5-FU-based adjuvant chemotherapy vs observation, demonstrated that adjuvant therapy did not significantly improve disease-free survival (DFS) in patients with MSI-H (HR 1.10; 95% CI 0.42 to 2.91; p=0.85).68
Oxaliplatin use in stage II CC may be considered in patients with multiple risk factors.69
Stage III CC (every T, pN1-2) occurs in 30%–40% of cases and 5-year OS ranges from 80%, for pT1-2N1, and 45%, for pT4N2. In this setting, adjuvant chemotherapy reduced the relative risk of death by 33%, with an absolute survival benefit of 10%–15%70; thus, adjuvant chemotherapy is always indicated, unless specific contraindications exist.
The combination of fluoropyrimidines with oxaliplatin is recommended as first adjuvant treatment option in patients radically resected for stage III CC with good performance status (PS), especially if <70 years. Several studies demonstrated that, in stage III CC, the combination of 5-FU and oxaliplatin, both with infusion regimens (FOLFOX4, validated in the registration study) and bolus (FLOX), compared with 5-FU alone, significantly improved DFS at 3 and 5 years and OS at 6 and 10 years.69 71–73 Data from the XELOXA study confirmed a benefit in DFS and OS also for the combination of capecitabine and oxaliplatin; a benefit that appeared comparable to that obtained with intravenous combinations.74 75
Based on results of 3 randomised trials, evaluating adjuvant treatment combinations containing irinotecan, which showed no advantage in DFS, OS and increased toxicity, irinotecan combinations must not be used in clinical practice.76–78
Monoclonal antibodies, such as bevacizumab and cetuximab, are not indicated in the adjuvant setting. Randomised clinical trials showed no benefit from the introduction of biological drugs in adjuvant treatment.79–82
In low-risk stage III (pT1-2, N1) or stage III patients with poor PS, significant comorbidity and/or elderly patients (>70 years), adjuvant treatment with fluoropyrimidine alone, either orally (capecitabine) or intravenously, can be considered.83 An analysis of the ACCENT group conducted on more than 11 900 patients with radically resected stage II/III CC treated with adjuvant chemotherapy, with or without oxaliplatin, suggested that the benefit of oxaliplatin was marginal in patients >70 years (HR for DFS 0.94; 95% CI, 0.78 to 1.13; HR for OS 1.04; 95% CI 0.85 to 1.27).77 However, recent data suggest that the indication for combination therapy in patients>70 years should be evaluated case by case.84
Until 2018, the standard duration of adjuvant treatment was 6 months. In the last 2 years, a shorter treatment period may be considered in a significant proportion of patients with stage III CC, based on risk factors and kind of administered fluoropyrimidine (capecitabine vs 5-FU). The IDEA trial,85 published in 2018, is a non-inferiority pooled analysis of six randomised clinical trials investigating the duration of adjuvant chemotherapy with oxaliplatin and fluoropyrimidines, comparing 3 months (experimental therapy) vs 6 months (standard therapy).
The 3-year DFS rate was 74.6% in the 3 months arm vs 75.5% in the 6 months arm (HR 1.07; 95% CI 1 to 1.15): since the upper limit of the CI exceeded the preplanned non-inferiority limit, the study was formally judged negative.
The rate of adverse events was significantly lower in the experimental arm: in particular, grade 3 or 4 neurotoxicity was 3% vs 16% in patients treated with a 3 vs 6 months FOLFOX regimen (p<0.0001), and 3% vs 9% in patients treated with a 3 vs 6 months XELOX regimen (p<0.0001). Considering low-risk stage III CC (pT1-3, N1), the absolute difference of 3-year DFS was 0.2% with the upper limit of CI not exceeding 1.12 (83.1% vs 83.3%; HR 1.01. 95% CI 0.90 to 1.12). In high-risk stage III CC (pT4 and/or pN2), the absolute difference of 3-year DFS was 1.7% (62.7% vs 64.4%; HR 1.12, 95% CI 1.03 to 1.23).
In patients treated with oxaliplatin and capecitabine, particularly in the low-risk subgroup, 3 months of treatment seemed to be superior in terms of DFS and less toxic in comparison with the standard arm.
Based on such considerations, the recommended duration should be 6 months for high-risk stage III CC (pT4 and/or pN2) patients, since the efficacy of 3 months therapy has been demonstrated to be inferior compared with the 6 months therapy. Instead, in low-risk stage III CC (pT1-3, N1) patients, especially if treated with CAPOX, 3 months of adjuvant therapy can be considered in specific circumstances, such as in case of significant onset of toxicity (particularly neurotoxicity) during therapy.
Final results of the IDEA trial, regarding OS and long-term DFS, have been recently presented at the ASCO Congress 2020 and the 5 year OS rate was 82.4% and 82.8% with 3 and 6 months of adjuvant therapy, respectively (HR 1.02; 95% CI 0.95 to 1.11). The 5-year DFS rate was 69.1% with 3 months of therapy and 70.8% with 6 months (HR 1.08; 95% CI 1.01 to 1.15).
In low-risk stage III CC (pT1–3, N1) patients, no loss (+2.3% absolute difference in 5-year OS rate) or a minimal loss (−0.3%) of efficacy was observed with 3 months of CAPOX and FOLFOX, respectively, in comparison to 6 months. Instead, in high-risk stage III CC (pT4 and/or pN2) patients, the absolute difference in 5-year OS rate between 3 and 6 months of therapy was −2.8% with FOLFOX and −1.0% with CAPOX.
Adjuvant chemotherapy should preferably be started within 6–8 weeks from surgery. A meta-analysis showed that delaying the start of adjuvant treatment beyond 8 weeks was associated with an OS reduction (risk ratio: 1.20; 95% CI 1.15 to 1.26).86 Nevertheless, a small benefit, starting adjuvant treatment between 8 weeks and 3 months from surgery was reported. A retrospective analysis conducted on 635 patients with stage III CC showed a 5-year relapse-free survival (RFS) of 70.9% (95% CI 65.7 to 76.5) in patients treated within 8 weeks from surgery vs 72.1% (95% CI 67.2 to 77) in those treated after more than 8 weeks, without a significant negative impact in terms of RFS (HR, 1.08; p=0.609).87 Therefore, in selected high-risk patients with postsurgical complications, in which the 8-week limit has been exceeded, adjuvant treatment should be considered, within 12 weeks although treatment initiation is recommended within 8 weeks from surgery.
The evaluation of RAS and BRAF status is not indicated in the adjuvant setting, because it does not improve the assessment of recurrence risk. Only few retrospective studies suggested a poorer prognosis for stage III MSS (microsatellite stable) CC harbouring KRAS or BRAF mutation,88 but these results are not sufficient to recommend such analysis in clinical practice.
The immunoscore test89 and the circulating tumour DNA analysis90 seem to be promising prognostic markers in the adjuvant setting, however, their use in clinical practice is not yet recommended.
Based on the recent recommendation of the (European Medicines Agency (EMA); EMA/125891/2020) and AIFA (Italian Medicines Agency; AIFA 2020.05.25) Pharmacovigilance Risk Assessment Committee, all patients who are candidates for fluoropyrimidine treatment should be tested for dihydropyrimidine dehydrogenase (DPYD) to prevent potentially serious adverse events. The working group recommends the analysis of the following mutations: c.1236G>A (c.1129–5923C>G), c.1679T>G, c.1905+1G>A and c.2846A>T.91 Furthermore, it may be useful to consider additional variants such as c.2194G>A in case of toxicity during treatment92–96 (table 3).
Table 3.
DPYD recommendations (adapted from https://www.aiom.it/raccomandazioni-2019-per-analisi-farmacogenetiche/)
| DPYD genotype | Recommended dose of fluoropyrimidine, % | |
| Wild-type | c.1236GG | 100 |
| c.1679TT | ||
| c.1905+1GG | ||
| c.2846AA | ||
| c.2194GG | ||
| Heterozygous | c.1236GA | 75 |
| c.1679TG | 50 | |
| c.1905+1GA | ||
| c.2846AT | ||
| c.2194GA | 85 | |
| Homozygous mutation | c.1236AA | 50 |
| c.1679GG | Fluoropyrimidine forbidden | |
| c.1905+1AA | ||
| c.2846TT | ||
| c.2194AA | 70 | |
DPYD, dihydropyrimidine dehydrogenase.
Follow-up and survivorship
The follow-up of patients with radically resected CC aims to early detect disease recurrence, second cancers and early as well as late sequelae related to previous treatment. Due to studies’ heterogeneity, it is not possible to define the kind of exams to be performed and the frequency or duration of the follow-up. About 80% of disease recurrence occurs within 3 years from surgery and 95% within 5 years.62 97 98 Therefore, timing of follow-up (4–6 months for the first 3 years; 6 months for the following 2 years) and the overall duration of the follow-up programme (5 years) have been defined based on these findings.
Despite these limits, an ‘intensive’ follow-up programme for patients with CC showed to improve OS in comparison to perform diagnostic exams at the onset of symptoms; therefore, an ‘intensive’ follow-up programme is strongly recommended. The first solid data, published in 2002, to support an ‘intensive’ follow-up derived from a meta-analysis of five randomised clinical trials, evaluating 1.342 patients, showed that an ‘intensive’ follow-up reduced cancer-related mortality by 9%–13% and anticipated the diagnosis of recurrence by 8.5 months.99
A meta-analysis, including 11 studies for a total of 4.055 patients and comparing ‘intensive’ follow-up, with no follow-up or minimal follow-up, improved OS (HR 0.75; 95% CI 0.66 to 0.86) and survival after recurrence (RR 2.13; 95% CI 1.24 to 3.69), increased the probability to identify asymptomatic recurrence (relative risk 2.59; 95% CI 1.66 to 4.06), increased the rate of curative surgery of metastases (relative risk 1.98; 95% CI 1.51 to 2.60) and anticipated the recurrence diagnosis by 5.23 months.100
However, what an ‘intensive’ follow-up means, remains unclear. Several studies evaluated different kind of follow-up, without evidence of significant difference in terms of survival.
Although no universally shared indications of the ideal follow-up procedure exist, the following guidelines should be followed:
Clinical examination every 4–6 months for the first 3 years; every 6 months for the following 2 years.
CEA every 4–6 months for the first 3 years, every 6 months for the following 2 years.
Colonoscopy: if complete and negative should be performed after 1 year from surgery; successively, after 3 years in the absence of adenomas and then every 5 years.
Chest-abdomen CT scan: every 6–12 months for the first 3–5 years depending on the recurrence risk.
Abdomen ultrasound and chest X-ray may be an alternative option to CT scan, but the lower sensitivity must be considered.
PET scan is not recommended.
Several evidences highlighted the importance of a correct lifestyle (physical activity and diet) in cancer survivors. In particular, several studies underlined the importance of a regular aerobic physical activity. A systematic review and meta-analysis of 7 studies showed that physical activity before and after diagnosis of CRC reduces the risk of mortality.101
The risk to develop late side effects after a CC diagnosis depends on several variables (tumour location, treatment type and duration, patient’s age, previous comorbidities). These effects can affect intestinal (chronic diarrhoea, bowel incontinence, perianal irritation and incomplete evacuations), genitourinary (incontinence, sexual dysfunction), neurological (residual neuropathy and cognitive deficits), reproductive (infertility) and psychological functions (chronic fatigue, anxiety-depressive syndrome and fear). All these issues might severely affect patients' quality of life.102 Thus, a correct monitoring of CC patients to perform an adequate and prompt management of such late adverse events represents a fundamental aspect.
Note
A summary of recommendations is provided in tables 4 and 5.
Table 4.
Summary of recommendations for screening and dignosis
| Recommendations for GPs and for early diagnosis of CRC | ||
|
Certainty of evidence |
Recommendations | Strength of recommendation |
| Low | GPs must be aware of access modalities to diagnostic (in particular endoscopy services) and therapeutic facilities. | Strong for |
| Low | Recent rectal bleeding in patients >50 years should never be attributed to benign disease without first excluding colon carcinomas or polyps. | Strong for |
| Low | All patients >50 years presenting new, significant or persistent symptoms related to CR disease (abdominal pain, mucorrhoea, rectal bleeding, weight loss, sideropenic anaemia, etc) must be accurately examined, including anamnesis and physical examination with rectal inspection). Subsequent diagnostic tests should preferably be performed within 4 weeks. | Strong for |
| Low | Patients <50 years presenting symptoms related to CR disease, in the absence of clinical evolution and/or family risk, may be carefully monitored for a few weeks: if symptoms persist the patient must promptly undergo diagnostic tests. | Strong for |
| Screening recommendations | ||
|
Certainty of evidence |
Recommendations | Strength of Recommendation |
| High | CRC screening is effective to reduce mortality risk for CRC. FOBT should be performed every 2 years between 50 and 69 years or RSS only once in life between 55 and 64 years, as proposed by regional Italian programmes. | Strong for |
| Low | High-risk patients with CRC should follow a different surveillance programme based on specific gastroenterology guidelines. | Strong for |
| Recommendations for inherited CRC syndromes | ||
|
Certainty of evidence |
Recommendations | Strength of recommendation |
| Low | Universal screening test for Lynch syndrome to identify dMMR should be considered in all patients with CRC. | Strong for |
| Low | Oncologists should send all patients with suspected inherited CRC syndromes to genetic counselling. | Strong for |
| Low | CRC patients should receive genetic counselling based on tumour-related, patient-related and/or family-related criteria. | Strong for |
| Low | Oncologists should assess family history using the minimum criteria proposed by the ASCO 'Expert Statement'103 | Conditional for |
| Low | Oncologists should propose specific and more intensive follow-up programmes to CRC patients with inherited CRC syndromes. | Conditional for |
| Recommendations for diagnosis and staging | ||
|
Certainty of evidence |
Recommendations | Strength of recommendation |
| Low | Patients with suspected CRC should perform a pancolonoscopy. | Strong for |
| Low | In pancolonoscopy cannot be performed, RSS in combination with colon CT is alternatively recommended. | Strong for |
| Low | Pancolonoscopy must be performed within 6–12 months after surgery if not already performed before surgery. | Strong for |
| Low | Liver metastases should preferably be investigated with a CT scan. | Strong for |
| Low | The presence of lung metastases should be investigated preferably with a chest CT scan. | Strong for |
| Low | No indication for routine use of MRI, bone scintigraphy and PET scan exist. | Conditional against |
| Low | CEA evaluation should be performed at the time of diagnosis. | Strong for |
*Working group opinion.
ASCO, American Society of Clinical Oncology; CEA, Carcino Embryonic Antigen; CR, colorectal; CRC, colorectal cancer; dMMR, mismatch repair deficient; FOBT, faecal occult blood test; GP, general practitioner; PET, positron emission tomography; RSS, rettosigmoidoscopy.
Table 5.
Summary of recommendations for treatment and follow-up
| Recommendations for surgery | ||
|
Certainty of evidence |
Recommendations | Strength of recommendation |
| * | The time between diagnosis and surgery should not exceed 4 weeks. | Conditional for |
| * | CC should be treated by surgeons with adequate training and experience. | Strong for |
| Low | Mechanical intestinal preparation can be useful in colon surgery even if not obligatory. | Conditional for |
| Moderate | In the absence of specific contraindications, the prophylactic use of low molecular weight heparin is recommended. | Conditional for |
| High | Preoperative antibiotic prophylaxis, based on second generation cephalosporin use, also active on anerobial germs, or amino glycosidic-methronidazole combination, administered in a single dose is recommended. The administration of the antibiotic may be prolonged for 24–48 hours depending on the extent of intraoperative contamination. | Conditional for |
| * | The site of the ostomy should be marked on the skin of the standing patient before surgery. The choice between ileostomy and colostomy (temporary) and its duration depend on clinical and intraoperative variables. In the late postoperative phase, the patient must be educated to manage the ostomy. | Strong for |
| High | The tumour must be removed intact with a section of at least 2 cm from the proximal and distal macroscopic margins of the tumour. The vascular peduncle must be linked to its origin. | Strong for |
| High | Regional lymph node dissection until the origin of the primary vascular peduncle must be performed. | Strong for |
| * | The radicality of the resection must be confirmed both by the absence of macroscopically disease and by subsequent histological examination (cancer-free margins). | Strong for |
| Moderate | Laparoscopic surgery in colon cancer is a preferred alternative to open surgery when performed by surgeons with adequate training. | Conditional for |
| High | Only cancerised adenomas with a well-differentiated cancer, absence of lympho-vascular invasion and negative margin, can be radically treated with endoscopic excision. | Conditional for |
| Recommendations for adjuvant treatment | ||
|
Certainty of evidence |
Recommendations | Strength of recommendation |
| * | Adjuvant chemotherapy is not recommended in stage I CC. | Strong against |
| High | Adjuvant chemotherapy (fluoropyrimidine±oxaliplatin) can be considered in high-risk stage II CC (occlusion, perforation, pT4, G3-4, inadequate number of examined lymph nodes, vascular and/or lymphatic and/or perineural invasion) patients. | Conditional for |
| Moderate | Follow-up alone can be considered in MSI-H stage II without risk factors CC patients, considering their good prognosis. | Strong for |
| Moderate | Adjuvant chemotherapy with a fluoropyrimidine can be considered in MSS stage II CC without risk factors. | Conditional for |
| High | Adjuvant chemotherapy should always be considered in stage III CC. The first option should be XELOX or FOLFOX. | Strong for |
| High | In low-risk stage III CC and/or patients with poor PS and/or elderly patients (>70 years) an adjuvant chemotherapy with fluoropyrimidine alone (oral or intravenously) can be considered. | Strong for |
| High | Adjuvant chemotherapy should preferably be started with 6–8 weeks from surgery. | Strong for |
| High | Monoclonal antibodies are not indicated in the adjuvant setting. | Strong against |
| * | RAS and BRAF evaluation should not be performed in the adjuvant setting. | Conditional against |
| Moderate | In stage III CC, a 3-month oxaliplatin-based adjuvant chemotherapy should not be considered as first option. | Conditional againts |
| Moderate | In high-risk stage III CC (pT4 and/or N2) a 3-month oxaliplatin-based adjuvant chemotherapy must not be considered. | Strong against |
| Moderate | In low-risk stage III CC (pT1-3, N1), a 3-month oxaliplatin-based adjuvant chemotherapy can be considered. | Conditional for |
| Recommendations for follow-up | ||
|
Certainty of evidence |
Recommendations | Strength of recommendation |
| High | An ‘intensive’ follow-up programme for CC patients is recommended. | Strong for |
| High | Considering that 95% of recurrences occurs within 5 years from surgery, the duration of follow-up should be 5 years. | Strong for |
| * | Follow-up should be considered to identify of late side effects (related to intestinal, genitourinary, neurological, reproductive and psychological functions) to ensure prompt management. | Conditional for |
| High | Although no universally shared indications for the ideal follow-up procedure exist, the following guidelines should be followed:
Abdomen ultrasound and chest X-ray may be an alternative option to CT scan, but the lower sensitivity must be considered. |
Strong for |
| Low | PET scan is not recommended in follow-up programmes. | Strong against |
| Low | A correct lifestyle (physical activity and diet) in cancer survivors should be recommended. | Conditional for |
*Working group opinion.
CC, colon cancer; CEA, Carcino Embryonic Antigen; MSI-H, high microsatellite instability; MSS, microsatellite stable; PET, positron emission tomography; PS, performance status.
Acknowledgments
We thank Dr Franziska Michaela Lohmeyer (Fondazione Policlinico Universitario A. Gemelli IRCCS) for English language editing.
Footnotes
Twitter: @Erikamartinelli
Contributors: All authors have made a substantial contribution to the information submitted for publication; all authors have read and approved the manuscript; the authors have no direct or indirect commercial financial incentive associated with publishing the article; and no extrainstitutional funding was obtained to complete report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article. All data to which these Italian Guidelines refer to, are available in published works.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.I Numeri del Cancro in Italia, 2019. Available: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf
- 2.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009;18:1688–94. 10.1158/1055-9965.EPI-09-0090 [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–907. 10.1158/1055-9965.EPI-10-0437 [DOI] [PubMed] [Google Scholar]
- 4. Available: https://snlg.iss.it/?cat=6
- 5.Salvatore L, Aprile G, Arnoldi E, et al. Management of metastatic colorectal cancer patients: guidelines of the Italian medical oncology association (AIOM). ESMO Open 2017;2:e000147. 10.1136/esmoopen-2016-000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Available: https://www.sign.ac.uk/
- 7.Trusted evidence Informed decisions.Better health. Available: https://training.cochrane.org/grade-approach
- 8.Lansdorp-Vogelaar I, von Karsa L, International Agency for Research on Cancer . European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Introduction. Endoscopy 2012;44 Suppl 3:SE15–30. 10.1055/s-0032-1308898 [DOI] [PubMed] [Google Scholar]
- 9.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 2003;124:544–60. 10.1053/gast.2003.50044 [DOI] [PubMed] [Google Scholar]
- 10.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota colon cancer control study. N Engl J Med 1993;328:1365–71. 10.1056/NEJM199305133281901 [DOI] [PubMed] [Google Scholar]
- 11.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467–71. 10.1016/S0140-6736(96)03430-7 [DOI] [PubMed] [Google Scholar]
- 12.Holme Øyvind, Bretthauer M, Fretheim A, et al. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev 2013;1:CD 009259. 10.1002/14651858.CD009259.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg 2008;95:1029–36. 10.1002/bjs.6136 [DOI] [PubMed] [Google Scholar]
- 14.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. 10.1016/S0140-6736(10)60551-X [DOI] [PubMed] [Google Scholar]
- 15.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst 2011;103:1310–22. 10.1093/jnci/djr284 [DOI] [PubMed] [Google Scholar]
- 16.Hoff G, Grotmol T, Skovlund E, et al. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ 2009;338:b1846. 10.1136/bmj.b1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472–7. 10.1016/S0140-6736(96)03386-7 [DOI] [PubMed] [Google Scholar]
- 18.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-Cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. 10.1056/NEJMoa1114635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK flexible sigmoidoscopy screening randomised controlled trial. Lancet 2017;389:1299–311. 10.1016/S0140-6736(17)30396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castells A, Bessa X, Quintero E, et al. Risk of advanced proximal neoplasms according to distal colorectal findings: comparison of sigmoidoscopy-based strategies. J Natl Cancer Inst 2013;105:878–86. 10.1093/jnci/djt117 [DOI] [PubMed] [Google Scholar]
- 21.Bretthauer M, Kaminski MF, Hassan C, et al. America, we are confused: the updated U.S. preventive services Task force recommendation on colorectal cancer screening. Ann Intern Med 2017;166:139–40. 10.7326/M16-1805 [DOI] [PubMed] [Google Scholar]
- 22.Laine L, Kaltenbach T, Barkun A, et al. Scenic international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639–51. 10.1053/j.gastro.2015.01.031 [DOI] [PubMed] [Google Scholar]
- 23.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task force on colorectal cancer. Gastroenterology 2012;143:844–57. 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 24.Strum WB. Colorectal adenomas. N Engl J Med 2016;374:1065–75. 10.1056/NEJMra1513581 [DOI] [PubMed] [Google Scholar]
- 25.Dickinson BT, Kisiel J, Ahlquist DA, et al. Molecular markers for colorectal cancer screening. Gut 2015;64:1485–94. 10.1136/gutjnl-2014-308075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar M, Cash BD. Screening and surveillance of colorectal cancer using CT colonography. Curr Treat Options Gastroenterol 2017;15:168–83. 10.1007/s11938-017-0121-7 [DOI] [PubMed] [Google Scholar]
- 27.Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of clinical oncology clinical practice guideline endorsement of the familial risk-colorectal cancer: European Society for medical oncology clinical practice guidelines. J Clin Oncol 2015;33:209–17. 10.1200/JCO.2014.58.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with Lynch syndrome. J Clin Oncol 2012;30:4409–15. 10.1200/JCO.2012.43.2278 [DOI] [PubMed] [Google Scholar]
- 29.Raymond VM, Mukherjee B, Wang F, et al. Elevated risk of prostate cancer among men with Lynch syndrome. J Clin Oncol 2013;31:1713–8. 10.1200/JCO.2012.44.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCCN guidelines Genetic/Familial high-risk assessment: colorectal, V 2/2019.
- 31.Syngal S, Brand RE, Church JM, et al. Acg clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–62. 10.1038/ajg.2014.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1558–71. 10.1093/annonc/mdz233 [DOI] [PubMed] [Google Scholar]
- 33.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for clinical pathology, College of American pathologists, association for molecular pathology, and the American Society of clinical oncology. J Clin Oncol 2017;35:1453–86. 10.1200/JCO.2016.71.9807 [DOI] [PubMed] [Google Scholar]
- 34.NICE dg27 Molecular testing strategies for Lynch syndrome in people with colorectal cancer. Nice org.uk/guidance/dg27 [Google Scholar]
- 35.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 1999;81:214–8. [DOI] [PubMed] [Google Scholar]
- 36.Grover S, Kastrinos F, Steyerberg EW, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA 2012;308:485–92. 10.1001/jama.2012.8780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrow P, Khan M, Lalloo F, et al. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg 2013;100:1719–31. 10.1002/bjs.9316 [DOI] [PubMed] [Google Scholar]
- 38.Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;2:783–5. 10.1016/S0140-6736(89)90840-4 [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Kim H-S, Park HJ. Adverse events related to colonoscopy: global trends and future challenges. World J Gastroenterol 2019;25:190–204. 10.3748/wjg.v25.i2.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benazzato L, Zorzi M, Antonelli G, et al. Colonoscopy-related adverse events and mortality in an Italian organized colorectal cancer screening program. Endoscopy 2020. 10.1055/a-1228-9225. [Epub ahead of print: 28 Jul 2020]. [DOI] [PubMed] [Google Scholar]
- 41.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services Task force. JAMA 2016;315:2576–9. 10.1001/jama.2016.3332 [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick-Lewis D, Ali MU, Warren R, et al. Screening for colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer 2016;15:298–313. 10.1016/j.clcc.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Byers T, Levin B, Rothenberger D, et al. American cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer: update 1997. American cancer Society detection and treatment Advisory group on colorectal cancer. CA Cancer J Clin 1997;47:154–60. 10.3322/canjclin.47.3.154 [DOI] [PubMed] [Google Scholar]
- 44.Desch CE, Benson AB, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of clinical oncology. J Clin Oncol 1999;17:1312. 10.1200/JCO.1999.17.4.1312 [DOI] [PubMed] [Google Scholar]
- 45.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479–516. 10.1093/annonc/mds236 [DOI] [PubMed] [Google Scholar]
- 46.Bast RC, Ravdin P, Hayes DF, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of clinical oncology. J Clin Oncol 2001;19:1865–78. 10.1200/JCO.2001.19.6.1865 [DOI] [PubMed] [Google Scholar]
- 47.Di Cataldo A, Scilletta B, Latino R, et al. The surgeon as a prognostic factor in the surgical treatment of rectal cancer. Surg Oncol 2007;16 Suppl 1:53–6. 10.1016/j.suronc.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 48.Guenaga KF, Matos D, Wille-Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev:CD001544. [DOI] [PubMed] [Google Scholar]
- 49.Kakkar VV, Cohen AT, Edmonson RA, et al. Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. The thromboprophylaxis Collaborative group. Lancet 1993;341:259–65. 10.1016/0140-6736(93)92614-y [DOI] [PubMed] [Google Scholar]
- 50.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophy- laxis for colorectal surgery. Cochrane Database Syst Rev 2014;5:CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dornfeld M, Lovely JK, Huebner M, et al. Surgical site infection in colorectal surgery: a study in antibiotic duration. Dis Colon Rectum 2017;60:971–8. 10.1097/DCR.0000000000000807 [DOI] [PubMed] [Google Scholar]
- 52.Ahn BK, Lee KH. Single-Dose antibiotic prophylaxis is effective enough in colorectal surgery. ANZ J Surg 2013;83:641–5. 10.1111/j.1445-2197.2012.06244.x [DOI] [PubMed] [Google Scholar]
- 53.Andreola S, Leo E, Belli F, et al. Adenocarcinoma of the lower third of the rectum surgically treated with a. Ann Surg Oncol 2001;8:611–5. [DOI] [PubMed] [Google Scholar]
- 54.de Haas-Kock DF, Baeten CG, Jager JJ, et al. Prognostic significance of radial margins of clearance in rectal cancer. Br J Surg 1996;83:781–5. 10.1002/bjs.1800830617 [DOI] [PubMed] [Google Scholar]
- 55.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894–9. 10.1001/archsurg.133.8.894 [DOI] [PubMed] [Google Scholar]
- 56.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–82. 10.1016/S0140-6736(86)91510-2 [DOI] [PubMed] [Google Scholar]
- 57.Killeen S, Mannion M, Devaney A, et al. Complete mesocolic resection and extended lymphadenectomy for colon cancer: a systematic review. Colorectal Dis 2014;16:577–94. 10.1111/codi.12616 [DOI] [PubMed] [Google Scholar]
- 58.Liang Y, Li G, Chen P, et al. Laparoscopic versus open colorectal resection for cancer: a meta-analysis of results of randomized controlled trials on recurrence. Eur J Surg Oncol 2008;34:1217–24. 10.1016/j.ejso.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 59.Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004;127:385–94. 10.1053/j.gastro.2004.04.022 [DOI] [PubMed] [Google Scholar]
- 60.Rosty C, Hewett DG, Brown IS, et al. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol 2013;48:287–302. 10.1007/s00535-012-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rickert RR, Auerbach O, Garfinkel L, et al. Adenomatous lesions of the large bowel: an autopsy survey. Cancer 1979;43:1847–57. [DOI] [PubMed] [Google Scholar]
- 62.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872–7. 10.1200/JCO.2008.19.5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benson AB, Schrag D, Somerfield MR, et al. American Society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408–19. 10.1200/JCO.2004.05.063 [DOI] [PubMed] [Google Scholar]
- 64.Köhne C-H. Should adjuvant chemotherapy become standard treatment for patients with stage II colon cancer? against the proposal. Lancet Oncol 2006;7:516–7. [PubMed] [Google Scholar]
- 65.Sobrero A. Should adjuvant chemotherapy become standard treatment for patients with stage II colon cancer? for the proposal. Lancet Oncol 2006;7:515–7. 10.1016/S1470-2045(06)70727-6 [DOI] [PubMed] [Google Scholar]
- 66.van Laarhoven HWM, Henselmans I, de Haes JHC. To treat or not to treat: who should decide? Oncologist 2014;19:433–6. 10.1634/theoncologist.2013-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–70. 10.1200/JCO.2010.30.1366 [DOI] [PubMed] [Google Scholar]
- 68.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–26. 10.1200/JCO.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the mosaic trial. J Clin Oncol 2009;27:3109–16. 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 70.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer International multicentre pooled analysis of colon cancer trials (impact) Investigators. Lancet 1995;345:939–44. [PubMed] [Google Scholar]
- 71.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with Weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198–204. 10.1200/JCO.2006.08.2974 [DOI] [PubMed] [Google Scholar]
- 72.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51. 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 73.André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the mosaic study. J Clin Oncol 2015;33:4176–87. 10.1200/JCO.2015.63.4238 [DOI] [PubMed] [Google Scholar]
- 74.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71. 10.1200/JCO.2010.33.6297 [DOI] [PubMed] [Google Scholar]
- 75.Schmoll H-J, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with Fluorouracil/Folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40. 10.1200/JCO.2015.60.9107 [DOI] [PubMed] [Google Scholar]
- 76.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456–61. 10.1200/JCO.2007.11.2144 [DOI] [PubMed] [Google Scholar]
- 77.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25. 10.1200/JCO.2008.21.6663 [DOI] [PubMed] [Google Scholar]
- 78.Ychou M, Raoul J-L, Douillard J-Y, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674–80. 10.1093/annonc/mdn680 [DOI] [PubMed] [Google Scholar]
- 79.de Gramont A, Van Cutsem E, Schmoll H-J, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225–33. 10.1016/S1470-2045(12)70509-0 [DOI] [PubMed] [Google Scholar]
- 80.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011;29:11–16. 10.1200/JCO.2010.30.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383–93. 10.1001/jama.2012.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taieb J, Tabernero J, Mini E, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862–73. 10.1016/S1470-2045(14)70227-X [DOI] [PubMed] [Google Scholar]
- 83.McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the accent database. J Clin Oncol 2013;31:2600–6. 10.1200/JCO.2013.49.6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brungs D, Aghmesheh M, de Souza P, et al. Safety and efficacy of oxaliplatin doublet adjuvant chemotherapy in elderly patients with stage III colon cancer. Clin Colorectal Cancer 2018;17:e549–55. 10.1016/j.clcc.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 85.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177–88. 10.1056/NEJMoa1713709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Des Guetz G, Nicolas P, Perret G-Y, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049–55. 10.1016/j.ejca.2010.01.020 [DOI] [PubMed] [Google Scholar]
- 87.Peixoto Renata D'Alpino, Kumar A, Speers C, et al. Effect of delay in adjuvant oxaliplatin-based chemotherapy for stage III colon cancer. Clin Colorectal Cancer 2015;14:25–30. 10.1016/j.clcc.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 88.Taieb J, Le Malicot K, Shi Q, et al. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. J Natl Cancer Inst 2017;109:5. 10.1093/jnci/djw272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391:2128–39. 10.1016/S0140-6736(18)30789-X [DOI] [PubMed] [Google Scholar]
- 90.Tie J, Cohen JD, Wang Y, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol 2019;5:1710–7. 10.1001/jamaoncol.2019.3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henricks LM, Lunenburg CATC, de Man FM, et al. Dpyd genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 2018;19:1459–67. 10.1016/S1470-2045(18)30686-7 [DOI] [PubMed] [Google Scholar]
- 92.Boige V, Vincent M, Alexandre P, et al. Dpyd genotyping to predict adverse events following treatment with Fluorouracil-Based adjuvant chemotherapy in patients with stage III colon cancer: a secondary analysis of the PETACC-8 randomized clinical trial.. JAMA Oncol 2016;2:655–62. 10.1001/jamaoncol.2015.5392 [DOI] [PubMed] [Google Scholar]
- 93.Del Re M, Cinieri S, Michelucci A, et al. DPYD*6 plays an important role in fluoropyrimidine toxicity in addition to DPYD*2A and c.2846A>T: a comprehensive analysis in 1254 patients.. Pharmacogenomics J 2019;19:556–63. 10.1038/s41397-019-0077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cecchin E, De Mattia E, Ecca F, et al. Host genetic profiling to increase drug safety in colorectal cancer from discovery to implementation.. Drug Resist Updat 2018;39:18–40. 10.1016/j.drup.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 95.Iachetta F, Bonelli C, Romagnani A, et al. The clinical relevance of multiple DPYD polymorphisms on patients candidate for fluoropyrimidine based-chemotherapy. an Italian case-control study. Br J Cancer 2019;120:834–9. 10.1038/s41416-019-0423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruzzo A, Graziano F, Galli F, et al. Dihydropyrimidine dehydrogenase pharmacogenetics for predicting fluoropyrimidine-related toxicity in the randomised, phase III adjuvant TOSCA trial in high-risk colon cancer patients.. Br J Cancer 2017;117:1269–77. 10.1038/bjc.2017.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guyot F, Faivre J, Manfredi S, et al. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol 2005;16:756–61. 10.1093/annonc/mdi151 [DOI] [PubMed] [Google Scholar]
- 98.Seo SI, Lim S-B, Yoon YS, et al. Comparison of recurrence patterns between ≤5 years and >5 years after curative operations in colorectal cancer patients. J Surg Oncol 2013;108:9–13. 10.1002/jso.23349 [DOI] [PubMed] [Google Scholar]
- 99.Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002;324:813. 10.1136/bmj.324.7341.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pita-Fernández S, Alhayek-Aí M, González-Martín C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol 2015;26:644–56. 10.1093/annonc/mdu543 [DOI] [PubMed] [Google Scholar]
- 101.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 2014;25:1293–311. 10.1093/annonc/mdu012 [DOI] [PubMed] [Google Scholar]
- 102.Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-Up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of clinical oncology clinical practice guideline endorsement. JCO 2013;31:4465–70. 10.1200/JCO.2013.50.7442 [DOI] [PubMed] [Google Scholar]
- 103.Lu KH, Wood ME, Daniels M, et al. American Society of clinical oncology expert statement: collection and use of a cancer family history for oncology providers. J Clin Oncol 2014;32:833–40. 10.1200/JCO.2013.50.9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-001001supp001.pdf (65.4KB, pdf)