Abstract
Background:
The purpose of this study was to evaluate the diagnostic value of transrectal real- time strain elastography (RTE) in identifying prostatic carcinoma (PCa).
Methods:
60 patients suspected of having PCa based on abnormal digital rectal examination and raised prostate specific antigen levels underwent transrectal ultrasound (TRUS), color Doppler (CD) and RTE. Elastograms were scored on a five point scale based on distribution of strain in relation to hypoechoic area on TRUS. Twelve core systematic biopsy as well as targeted biopsy was performed from suspicious areas on TRUS and RTE. Diagnostic performance of sonoelastography was evaluated using histopathology as reference standard.
Results:
Histopathology revealed cancer in 28 out of 60 patients (47%) studied. Gleason score ranged from 6 to 9. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of TRUS in detecting prostate cancer were 78.57%, 81.25%, 78.57%, and 81.25%, respectively. On CD evaluation 87.5% (n = 28) of benign lesions showed symmetric, radial flow compared to 14.3% (n = 4) of malignant lesions (P < 0.0001). The sensitivity and specificity of RTE was 89.29% and 56.25% with PPV and NPV being 58.13% and 82.35%, respectively. Higher elastography score was found to be significantly associated with malignant histopathology (P = 0.004). Cancer detection rate with RTE was greater for tumors with higher Gleason score.
Conclusion:
RTE was found to have better sensitivity than TRUS as well as combination of TRUS and CD. Although less specific, RTE can be an effective adjuvant tool to TRUS for guidance of biopsy and improve detection rate of PCa.
Keywords: Elastography, prostate cancer, ultrasonography
INTRODUCTION
Prostate carcinoma (PCa) is the second most frequently diagnosed cancer in males all over the world and is a major healthcare concern associated with significant morbidity and mortality. The burden of PCa is estimated to grow to 1.7 million new cases and 499000 new deaths by 2030 as a result of growth and aging of the global population.[1]
Digital rectal examination (DRE), screening for prostate specific antigen (PSA), and imaging techniques such as transrectal ultrasound (TRUS) and magnetic resonance imaging (MRI) are the methods commonly employed for detection of PCa. DRE is limited by its low sensitivity, subjective nature, and depends on the experience of the clinician. Although PSA testing has been widely used for screening of prostatic cancer, it is not disease specific and can be elevated in patients with prostatitis as well as benign prostatic hyperplasia.[2,3] In a patient with suspicion of prostatic cancer, current guidelines involve histopathologic examination of systematic biopsy cores to confirm or rule out cancer.[4] However, the conventional biopsy protocol misses significant cancer in a large proportion of patients.[5] Further many insignificant cancers that do not need immediate treatment are also detected leading to overdiagnosis and treatment.[6] Use of TRUS for detecting prostate cancer has limitations such as poor specificity of focal abnormalities, frequent multifocality of cancer within the prostate, and substantial percentage of isoechoic prostatic carcinoma (PCa).[7] Although MRI results are promising, it is expensive and not always available. In view of these limitations, improved noninvasive imaging modalities that can provide better characterization of prostatic lesions and increase detection of prostatic cancer are needed.
Ultrasound elastography (USE) is a dynamic technique that uses ultrasound to estimate tissue stiffness by measuring the degree of distortion under application of an external force. As cancerous tissue has greater stiffness than a benign lesion USE has been used to differentiate malignant from benign lesions in breast, thyroid, liver, and cervix. Recent studies have demonstrated the utility of sonoelastography in detecting prostate cancer.[8,9,10]
Data on the usefulness of transrectal strain elastography (SE) in evaluation of prostatic cancer and its correlation with histopathology are relatively few, especially from India. The purpose of the present study was to evaluate the usefulness and diagnostic performance of transrectal SE in identifying prostatic cancer with histopathologic diagnosis obtained by needle biopsy of prostate as reference standard.
MATERIALS AND METHODS
This prospective study was performed in a tertiary care center in South India between December 2014 and September 2016. 60 consecutive patients who were referred to the department of Radiology with suspicion of PCa based on abnormal DRE and elevated PSA levels (>4 ng/ml) were enrolled. The institutional review board approved the study protocol and the protocol was in compliance with tenets of the Declaration of Helsinki (IRB approval Number: 14/399). Informed consent was obtained from all participants. Exclusion criteria consisted of patients with bleeding disorders, inflammatory bowel disease and surgical absence of rectum or ileoanal pouch. All patients underwent TRUS (grey scale and Doppler) and SE as well as needle biopsy of prostate in the same session.
TRUS of prostate was done using an endocavitary transducer (Siemens Accuson S3000, Siemens Healthineers, Erlangen, Germany) with 9–4 MHz range and 174 degree field of vision, capable of user selectable multi hertz imaging. Xylocaine gel was applied over a probe cover applied onto the probe. All patients were examined in the left lateral decubitus position. Prostate was imaged in both axial and sagittal planes with assessment of volume, echogenicity, surface, calcification, vascularity, and the presence of nodules. Each nodule was assessed for size, margins, location in the gland, morphology, and echogenicity.
Glands with homogeneous echotexture or minimal heterogeneity on TRUS were labeled as benign while those with ill-defined echotexture abnormality, focal bulge or hypoechoic mass were categorized as malignant. For Color Doppler (CD) imaging, capsular/periurethral flow and symmetric radial flow from capsular branches were considered benign while asymmetric or increased flow with disorganized pattern was labeled malignant.[11]
Using the same probe transrectal real-time strain elastography (RTE) of prostate was done by a single experienced radiologist. The probe was placed over the region such that the lesion is in the center of the image. Elastograms were obtained by slight compression and decompression of the prostate. The appropriate frequency and pressure of the compression/decompression was optimized with the help of real time display of quality index. Elastograms obtained with quality index equal to or >60 were only included. At least a 5 mm thickness of normal adjacent tissue was included, to assess the lesion stiffness in relation with the average elasticity of the surrounding tissue. A standardized color coding system was used, where red indicated soft, more compliant areas and blue indicated stiff areas. Green color indicated average strain in the region of interest. The elastograms along with conventional B-mode images were displayed side by side on split screen mode. Multiple frames were acquired and the best fit B mode-elastography image pairs were selected for examination. The elasticity patterns were scored using a five point scale proposed by Kamoi et al.[10] [Table 1] The cut off for malignancy was set between scores of 2 and 3 [Figures 1-4].
Table 1.
Sonoelastography scoring system proposed by Kamoi et al.
| Elastography score | Description |
|---|---|
| Score 1 | Normal - homogeneous strain |
| Score 2 | Probably normal - symmetric heterogeneous strain |
| Score 3 | Indeterminate - asymmetric focal stiff lesion which is not related to any hypoechoic area |
| Score 4 | Probably carcinoma (strain at the periphery of the hypoechoic lesion with sparing of the center |
| Score 5 | Definitely carcinoma (no strain in the entire hypoechoic lesion or in the surrounding area) |
Figure 1.
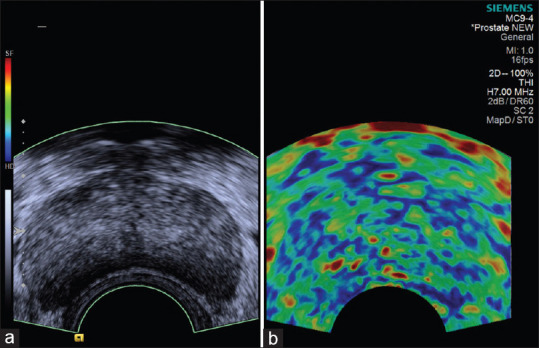
(a) Transrectal ultrasonography image showing mild enlargement of prostate. (b) Sonoelastography showing heterogeneous appearance with symmetrical mosaic pattern of green and blue (Score 2 – probably normal). Histopathology revealed hyperplasia of prostate with benign glandular pattern
Figure 4.
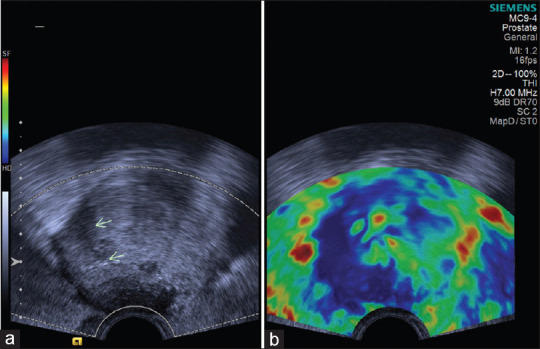
(a) Transrectal ultrasonography image showing hypoechoic lesion with irregular margin in the right peripheral zone (arrows) of prostate. (b) Sonoelastography showing stiffness in the entire hypoechoic lesion and in the surrounding area; the entire lesion appears blue (score 5 – definitely carcinoma). Histopathology confirmed adenocarcinoma prostate
Figure 2.
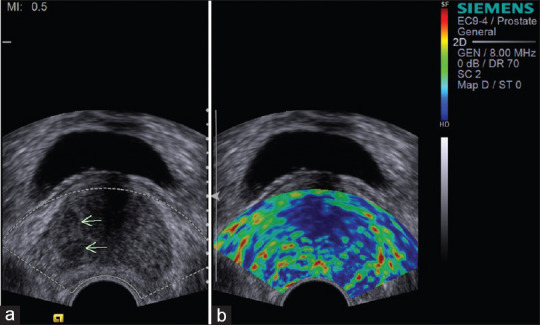
(a) Transrectal ultrasonography image showing hypoechoic lesion in the right peripheral zone (arrows) of prostate. (b) Sonoelastography showing focal asymmetric stiff lesion not related to hypoechoic area (score 3 – indeterminate). Histopathology was suggestive of adenocarcinoma prostate
Figure 3.
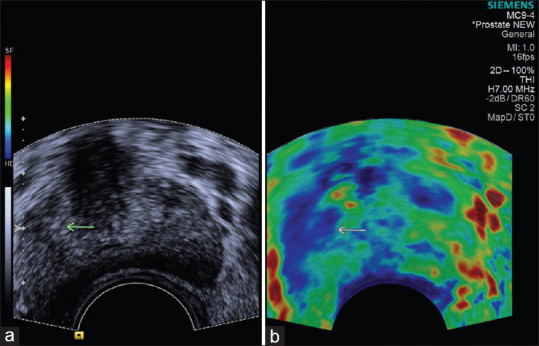
(a) Transrectal ultrasonography image showing hypoechoic lesion in the right peripheral zone (arrow) of prostate. (b) Sonoelastography showing stiffness in the center of the lesion and strain at the periphery; the peripheral part of lesion appears green and the central part appears blue (score 4 – probably carcinoma). Histopathology confirmed adenocarcinoma prostate
Sampling of the prostate was performed either in the sagittal or in the axial plane. Biopsies were taken using an 18 G biopsy gun under strict aseptic precautions. Extended core biopsy protocol (12 cores) was used. Additional targeted biopsy was carried out on suspicious areas identified by B mode US and elastography. The grade (degree of aggressiveness) of prostate cancer was evaluated on the basis of the Gleason score. No major complications were encountered after the procedure.
The findings of TRUS, Doppler and RTE were interpreted by consensus by two experienced radiologists who were blinded to patients' clinical profile and biopsy results. The histopathologic results were considered as the reference standard, and utility of RTE was evaluated.
Statistical analysis
Data was entered in the Statistical Package for Social Sciences version 24 (SPSS Inc., Chicago, IL, USA). Descriptive statistics was presented as mean ± standard deviation. Qualitative variables were presented in the form of frequency and percentages. The diagnostic sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated with histopathologic analysis as reference standard. For analyzing the combined diagnostic performance of tests, the test was considered positive if either of them was suspicious for malignancy and negative if neither showed suspicious features. Pearson Chi-square test was used to study the association between categorical variables. P < 0.05 was considered as statistically significant.
RESULTS
The age of the patients ranged from 53 to 88 years (Mean: 69.16 ± 9.40 years). Serum PSA levels ranged between 4.3 and 178.6 ng/ml (Mean 48.54 ± 56.69 ng/ml). No Significant difference in PSA levels were noted between patients with carcinoma and benign lesions of the prostate (P = 0.083). In 60 patients, 784 biopsy cores were harvested out of which 720 was according to the extended core biopsy protocol and 64 were targeted (36 and 28 targeted to areas found suspicious on B-mode US and RTE, respectively). Histopathology revealed cancerous focus in at least one biopsy core in 28/60 (47%) patients. Gleason score ranged from 6 to 9 (Mean: 7.42 ± 1.13). Among the 32 patients with benign histopathology results, 20 (62.5%) were found to have benign prostatic hyperplasia and 12 (37.5%) had prostatitis. The mean prostatic volume was 38.20 ± 18.25 cm3 (range: 8–89 cm3). The mean age of patients with benign and malignant lesions were 66.78 ± 9.56 years and 71.89 ± 8.59 years, respectively (P = 0.035).
Out of the 60 patients, 28 (47%) were suspected of having prostate cancer by TRUS. The sensitivity, specificity, PPV, and NPV of TRUS in detecting prostate cancer were 78.57%, 81.25%, 78.57%, and 81.25%, respectively, with histopathology as reference standard. On CD evaluation 87.5% (n = 28) of benign lesions showed symmetric, radial flow compared to 14.3% (n = 4) of malignant lesions (P < 0.0001).
Table 2 shows the correlation of elastography scores and histopathologic diagnoses of lesions. Out of the 32 patients with benign histopathology, 14 were judged as negative for malignancy at RTE. There were 3 false negative patients on elastography with score of 2. The sensitivity and specificity of RTE was 89.29% and 56.25% with positive and NPV of 58.13% and 82.35%, respectively. Higher elastography score was found to be significantly associated with malignant histopathology (P = 0.004).
Table 2.
Correlation between elastography score and histopathologic diagnosis of lesions
| Elastogram pattern | Benign (n=32) | Malignant (n=28) | Total (n=60) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 14 | 3 | 17 |
| 3 | 18 | 4 | 22 |
| 4 | 0 | 9 | 9 |
| 5 | 0 | 12 | 12 |
Table 3 shows the relation between Gleason score and elastography scores. Approximately 84% of patients with a Gleason score ≤6 were detected by RTE whereas for scores 8–9, the detection rate was 100%.
Table 3.
Relation between Gleason score and elastography scores
| Gleason score | ES 1 | ES 2 | ES 3 | ES 4 | ES 5 |
|---|---|---|---|---|---|
| ≤6 | 0 | 1 | 0 | 2 | 3 |
| 7 | 0 | 2 | 2 | 6 | 2 |
| 8 | 0 | 0 | 0 | 1 | 1 |
| 9 | 0 | 0 | 2 | 0 | 6 |
ES: Elastography score
The diagnostic performance of TRUS, combined modalities (TRUS and Doppler) and RTE are shown in Table 4.
Table 4.
Diagnostic performance of transrectal ultrasound, combined modalities (transrectal ultrasound and Doppler) and real-time strain elastography
| Performance measure | TRUS | TRUS and doppler | RTE |
|---|---|---|---|
| Sensitivity (%) | 78.57 | 85.71 | 89.29 |
| Specificity (%) | 81.25 | 75 | 56.25 |
| Positive predictive value (%) | 78.57 | 75 | 58.13 |
| Negative predictive value (%) | 81.25 | 85.71 | 82.35 |
TRUS: Transrectal ultrasound, RTE: Real-time strain elastography
DISCUSSION
In this prospective study from South India, we evaluated the diagnostic performance of transrectal real-time elastography (RTE) in detection of prostatic cancer in patients with abnormal DRE and elevated PSA levels. RTE showed superior sensitivity of nearly 90% versus sensitivity of 79% by TRUS and 86% by a combination of TRUS and CD ultrasound.
Ever since the description of principles of elastography by Ophir et al., elasticity imaging has been applied to a wide variety of tissues such as breast, thyroid, liver, lymph nodes and prostate.[12]
The two US elastography techniques in clinical use are SE and shear wave elastography. Several studies have evaluated the utility of SE in diagnosing prostatic cancer and have shown varied results. While few have compared the diagnostic accuracy with needle biopsy of prostate,[13,14,15] others have used histopathologic examination of radical prostatectomy specimen as the reference standard.[8,9,16] The heterogeneous results can be attributed to the varied inclusion criteria, demographic characteristics of patients, serum PSA levels, location of tumors, reference standard, number of biopsy cores, systematic or targeted biopsies and Gleason score of the tumors examined.
The age of patients with malignancy of prostate was significantly higher compared to those with benign lesions in our study (P = 0.035). Age is considered to be an established risk factor for prostatic cancer and study of age-specific incidence curves have shown that risk of prostate cancer begins to rise sharply after 55 years of age and peaks at 70–74, declining slightly thereafter.[17]
In our study, TRUS revealed hypoechoic/focal lesions in 28 patients (47%). Out of these, 22 were confirmed as adenocarcinoma prostate while 6 turned out to be benign on histopathology. The results from our study in terms of specificity and PPV of TRUS in detecting prostatic cancer are similar to that of Ferrari et al. who had evaluated 84 patients suspected of prostatic cancer by TRUS, RTE and transperineal prostate biopsy.[18] B-mode US demonstrated 56% sensitivity, 80% specificity, 70% PPV and 67% NPV in diagnosing prostatic cancer. A hypoechoic lesion in the peripheral zone of prostate is suggested to be more likely malignant, the proposed cause being replacement of normal loose glandular tissue by a packed mass of tumor cells which results in fewer reflecting interfaces. Hyperplasia, prostatitis, fibrosis and cysts of prostate are other lesions that appear hypoechoic on TRUS.[19] Nearly 25%–40% of prostate cancers are isoechoic and missed by TRUS.[20]
CD and Power Doppler (PD) have been reported to be useful in detecting isoechoic tumors which may be missed by conventional ultrasound.[21,22] In 2 patients with prostatic cancer in our study, Doppler evaluation revealed asymmetric, increased flow while TRUS was not suspicious for malignancy. Few authors have shown that increased CD signal correlates positively with grading and staging of prostate cancer as well as risk of recurrence after treatment.[23,24]
On CD evaluation, 87.5% (n = 28) of benign lesions showed symmetric, radial flow compared to 14.3% (n = 4) of malignant lesions (P < 0.0001) in our study. A combination of TRUS and Doppler revealed superior diagnostic performance with sensitivity and NPV of nearly 86% compared to TRUS. However, benign lesions like prostatitis can also show increased flow on CD and CD does not reveal microscopic vessels of prostatic cancer that do not possess enough flow to cause Doppler shift.[25,26] Despite these limitations, our findings indicate that CD imaging can be a useful adjunct to improve tumor detection by TRUS.
Prostatic cancer tends to be stiffer than benign lesions due to replacement of normal glandular tissue by neoplastic cells.[27] Stiffer tissues demonstrate less strain to deformation, and RTE helps in differentiation of benign and malignant tissues based on distribution of strain. Miyagawa et al. evaluated the usefulness of RTE in detecting prostatic cancer prior to systematic biopsy and have noted a diagnostic sensitivity of 72.6% for elastography and 89.5% for combination of TRUS and elastography.[14] Kamoi et al. used a five point subjective scale based on degree and distribution of strain in relation to hypoechoic area and found that RTE had 68% sensitivity and 81% specificity in detecting prostatic cancer. The combination of RTE with PD US further increased the sensitivity to 78%.[10]
Using the cutoff value of 3 (suggesting focal asymmetric lesion without strain unrelated to hypoechoic lesion) proposed by Kamoi et al., RTE was found to have sensitivity of 89.29% and specificity of 56.25% in our study. Of 43 patients with RTE findings suspicious for carcinoma, 25 were confirmed to be malignant on histopathology. Benign entities such as benign prostatic hyperplasia, adenomyomatosis, fibrosis and prostatitis can be associated with increased tissue stiffness and therefore may be difficult to distinguish from carcinoma prostate. This can be responsible for the relatively low specificity and PPV observed in our study. Because of the high frequency of false-positive results, RTE should be used in combination with TRUS to increase the detection rate.
In 5 of 28 patients with prostatic cancer (17.9%), B mode US was normal but RTE was suggestive of carcinoma. While systematic biopsy picked up malignancy in 3 patients, targeted biopsy based on RTE findings resulted in detection of cancer in 2 patients. The role of RTE targeted biopsy in detecting prostatic cancer has been reported by several authors. Pallwein et al. compared a 5-core RTE targeted biopsy with a 10-core systematic biopsy and concluded that cancer detection rate per patient was not significantly higher for targeted approach compared to systematic approach but an RTE targeted core was 2.9 fold more likely to be positive for cancer than a systematic core.[28] Salomon et al. analyzed the incremental detection rate of 4-core RTE targeted biopsy in addition to randomized 10-core in 1024 patients.[29] Additional use of RTE resulted in an incremental detection rate of 18.3%. 34 patients who harbored high-grade PCa (Gleason score ≥4) were diagnosed by RTE targeted biopsy only. A recent meta-analysis on the role of RTE targeted biopsy has shown that the detection rate of malignancy is enhanced when systematic biopsies are combined with RTE targeted biopsies.
Further RTE targeted biopsy can make a nearly equivalent diagnosis with fewer cores.[30]
In our study RTE detected 5 of 6 patients with prostatic cancer of Gleason score ≤6, 10 of 12 with score 7 and all with Gleason score 8 or 9. Our observations are similar to that of other authors who have noted that RTE detection rate of prostate cancer with higher Gleason score was greater than that of prostatic cancer with a lower Gleason score.[9,31] This might be explained by the greater cell density in high grade tumors resulting in stiffer tissue. In contrast, Tsutsumi et al. have noted a lower RTE detection rate for high grade tumors.[32]
Our study has few limitations. The sample size was small and no interobserver variability was studied since the RTE findings were interpreted by consensus. Another main limitation of RTE is that the degree of manual compression influences the elasticity image and may compromise diagnostic accuracy of RTE. However all examinations were performed by a single experienced radiologist and only images with optimal compression as displayed by the quality index were included in the study. Recent literature suggests that multiparametric MRI has greater sensitivity and specificity for detection of clinically significant prostate cancer.[33]
Although the incidence of prostatic cancer is low in Asian countries compared to the west, demographic and epidemiological transition in recent times have resulted in increase in the burden of various cancers including prostate in India. Prostate is reported to be the second leading site of cancer among males in several cities in India and cancer projection data estimate that the number of cases will become doubled by 2020.[34]
CONCLUSION
In this initial study from South India, RTE was found to have better sensitivity than TRUS as well as combination of TRUS and CD. With its greater sensitivity and NPV, RTE can be an effective adjuvant tool to guide biopsy and thereby increase the detection rate of prostatic cancer. Further prospective studies with larger sample size are needed before recommending RTE along with conventional TRUS for routine use in the evaluation of suspected prostatic malignancy in India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Banu NA, Azim FA, Kamal M, Rumi MA, Barua AR, Khan KH. Inflammation and glandular proliferation in hyperplastic prostates: Association with prostate specific antigen value. Bangladesh Med Res Counc Bull. 2001;27:79–83. [PubMed] [Google Scholar]
- 3.Ferrero Doria R, Pérez Flores D, Terrer Artes C, Guzmán Martínez-Valls PL, Morga Egea JP, Tomás Ros M, et al. Impact of prostatic benign hyperplasia and prostatic inflammation on the increase of prostate specific antigen levels. Actas Urol Esp. 1997;21:100–4. [PubMed] [Google Scholar]
- 4.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Lecornet E, Ahmed HU, Hu Y, Moore CM, Nevoux P, Barratt D, et al. The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: A computer simulation. J Urol. 2012;188:974–80. doi: 10.1016/j.juro.2012.04.104. [DOI] [PubMed] [Google Scholar]
- 6.Borza T, Konijeti R, Kibel AS. Early detection, PSA screening, and management of overdiagnosis. Hematol Oncol Clin North Am. 2013;27:1091–110, vii. doi: 10.1016/j.hoc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Lemaître L, Villers A, Mouton D, Puech P. Transrectal ultrasound and biopsy of the prostate. J Radiol. 2006;87:201–9. doi: 10.1016/s0221-0363(06)73994-4. [DOI] [PubMed] [Google Scholar]
- 8.Pallwein L, Mitterberger M, Struve P, Pinggera G, Horninger W, Bartsch G, et al. Real-time elastography for detecting prostate cancer: Preliminary experience. BJU Int. 2007;100:42–6. doi: 10.1111/j.1464-410X.2007.06851.x. [DOI] [PubMed] [Google Scholar]
- 9.Sumura M, Shigeno K, Hyuga T, Yoneda T, Shiina H, Igawa M. Initial evaluation of prostate cancer with real-time elastography based on step-section pathologic analysis after radical prostatectomy: A preliminary study. Int J Urol. 2007;14:811–6. doi: 10.1111/j.1442-2042.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 10.Kamoi K, Okihara K, Ochiai A, Ukimura O, Mizutani Y, Kawauchi A, et al. The utility of transrectal real-time elastography in the diagnosis of prostate cancer. Ultrasound Med Biol. 2008;34:1025–32. doi: 10.1016/j.ultrasmedbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Halpern EJ, Ramey JR, Strup SE, Frauscher F, McCue P, Gomella LG. Detection of prostate carcinoma with contrast-enhanced sonography using intermittent harmonic imaging. Cancer. 2005;104:2373–83. doi: 10.1002/cncr.21440. [DOI] [PubMed] [Google Scholar]
- 12.Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 13.König K, Scheipers U, Pesavento A, Lorenz A, Ermert H, Senge T. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174:115–7. doi: 10.1097/01.ju.0000162043.72294.4a. [DOI] [PubMed] [Google Scholar]
- 14.Miyagawa T, Tsutsumi M, Matsumura T, Kawazoe N, Ishikawa S, Shimokama T, et al. Real-time elastography for the diagnosis of prostate cancer: Evaluation of elastographic moving images. Jpn J Clin Oncol. 2009;39:394–8. doi: 10.1093/jjco/hyp026. [DOI] [PubMed] [Google Scholar]
- 15.Brock M, von Bodman C, Palisaar RJ, Löppenberg B, Sommerer F, Deix T, et al. The impact of real-time elastography guiding a systematic prostate biopsy to improve cancer detection rate: A prospective study of 353 patients. J Urol. 2012;187:2039–43. doi: 10.1016/j.juro.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi M, Miyagawa T, Matsumura T, Endo T, Kandori S, Shimokama T, et al. Real-time balloon inflation elastography for prostate cancer detection and initial evaluation of clinicopathologic analysis. AJR Am J Roentgenol. 2010;194:W471–6. doi: 10.2214/AJR.09.3301. [DOI] [PubMed] [Google Scholar]
- 17.Gann PH. Risk factors for prostate cancer. Rev Urol. 2002;4(Suppl 5):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari FS, Scorzelli A, Megliola A, Drudi FM, Trovarelli S, Ponchietti R. Real-time elastography in the diagnosis of prostate tumor. J Ultrasound. 2009;12:22–31. doi: 10.1016/j.jus.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar S, Das S. A review of imaging methods for prostate cancer detection. Biomed Eng Comput Biol. 2016;7:1–5. doi: 10.4137/BECB.S34255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara K, Wheeler TM, Scardino PT. The appearance of prostate cancer on transrectal ultrasonography: Correlation of imaging and pathological examinations. J Urol. 1989;142:76–82. doi: 10.1016/s0022-5347(17)38666-4. [DOI] [PubMed] [Google Scholar]
- 21.Newman JS, Bree RL, Rubin JM. Prostate cancer: Diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology. 1995;195:86–90. doi: 10.1148/radiology.195.1.7534429. [DOI] [PubMed] [Google Scholar]
- 22.Okihara K, Kojima M, Nakanouchi T, Okada K, Miki T. Transrectal power Doppler imaging in the detection of prostate cancer. BJU Int. 2000;85:1053–7. doi: 10.1046/j.1464-410x.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 23.Cornud F, Belin X, Piron D, Chrétien Y, Flam T, Casanova JM, et al. Color Doppler-guided prostate biopsies in 591 patients with an elevated serum PSA level: Impact on Gleason score for nonpalpable lesions. Urology. 1997;49:709–15. doi: 10.1016/S0090-4295(96)00632-2. [DOI] [PubMed] [Google Scholar]
- 24.Ismail M, Petersen RO, Alexander AA, Newschaffer C, Gomella LG. Color Doppler imaging in predicting the biologic behavior of prostate cancer: Correlation with disease-free survival. Urology. 1997;50:906–12. doi: 10.1016/S0090-4295(97)00403-2. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S. Imaging and intervention in prostate cancer: Current perspectives and future trends. Indian J Radiol Imaging. 2014;24:139–48. doi: 10.4103/0971-3026.134399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taverna G, Morandi G, Seveso M, Giusti G, Benetti A, Colombo P, et al. Colour Doppler and microbubble contrast agent ultrasonography do not improve cancer detection rate in transrectal systematic prostate biopsy sampling. BJU Int. 2011;108:1723–7. doi: 10.1111/j.1464-410X.2011.10199.x. [DOI] [PubMed] [Google Scholar]
- 27.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20:260–74. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 28.Pallwein L, Mitterberger M, Struve P, Horninger W, Aigner F, Bartsch G, et al. Comparison of sonoelastography guided biopsy with systematic biopsy: Impact on prostate cancer detection. Eur Radiol. 2007;17:2278–85. doi: 10.1007/s00330-007-0606-1. [DOI] [PubMed] [Google Scholar]
- 29.Salomon G, Drews N, Autier P, Beckmann A, Heinzer H, Hansen J, et al. Incremental detection rate of prostate cancer by real-time elastography targeted biopsies in combination with a conventional 10-core biopsy in 1024 consecutive patients. BJU Int. 2014;113:548–53. doi: 10.1111/bju.12517. [DOI] [PubMed] [Google Scholar]
- 30.Tu X, Qiu S, Chang T, Jin K, Bao Y, Yang L, et al. The role of real-time elastography-targeted biopsy in the detection and diagnosis of prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e0220. doi: 10.1097/MD.0000000000010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallwein L, Mitterberger M, Pinggera G, Aigner F, Pedross F, Gradl J, et al. Sonoelastography of the prostate: Comparison with systematic biopsy findings in 492 patients. Eur J Radiol. 2008;65:304–10. doi: 10.1016/j.ejrad.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsumi M, Miyagawa T, Matsumura T, Kawazoe N, Ishikawa S, Shimokama T, et al. The impact of real-time tissue elasticity imaging (elastography) on the detection of prostate cancer: Clinicopathological analysis. Int J Clin Oncol. 2007;12:250–5. doi: 10.1007/s10147-007-0669-7. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389:815–22. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 34.Jain S, Saxena S, Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–605. doi: 10.1016/j.mgene.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


