Abstract
Ketogenic diets have the potential to lower glucose availability to cancer cells. However, the effect that the resulting increase in ketone bodies has on cancer cells is not fully understood. The present study explored the effect of β-hydroxybutyrate (BHB) on glucose-deprived MCF-7 and T47D breast cancer cells. Cell proliferation was decreased in response to lower glucose conditions, which could not be rescued consistently by 10 or 25 mM BHB supplementation. In addition, gene expression levels were altered when cells were glucose deprived. Reducing glucose availability of cancer cells to 225 mg/l for 4 days significantly decreased the expression of 113 genes and increased the expression of 100 genes in MCF-7 breast cancer cells, and significantly decreased the expression of 425 genes and increased the expression of 447 genes in T47D breast cancer cells. Pathway enrichment analysis demonstrated that glucose deprivation decreased activity of the Hippo-Yap cell signaling pathway in MCF-7 breast cancer cells, whereas it increased the expression of genes in the NRF2-pathaway and genes regulating ferroptosis in T47D breast cancer cells. Treatment of glucose-deprived cells with 10 or 25 mM BHB significantly changed the expression of 14 genes in MCF-7 breast cancer cells and 40 genes in T47D breast cancer cells. No significant pathway enrichment was detected when glucose-deprived cells were treated with BHB. Both cell lines expressed the enzymes (OXCT1/2, BDH1 and ACAT1/2) responsible for metabolizing BHB to acetyl-CoA, yet expression of these enzymes was not altered by either glucose deprivation or BHB treatment. In the publicly available The Cancer Genome Atlas (TCGA), increased expression of ketone body-catabolizing enzymes was observed in various types of cancer based on mRNA expression z-scores. Increased expression of BDH1 and ACAT1 significantly decreased overall survival of patients with breast cancer in TCGA studies, while decreased OXCT1 expression non-significantly decreased overall survival. In conclusion, neither MCF-7 nor T47D breast cancer cells were affected by BHB during glucose deprivation; however, screening of tumors for activation of ketone body-metabolizing enzymes may be able to identify patients that will benefit from ketogenic diet interventions.
Keywords: breast cancer cells, ketone bodies, β-hydroxybutyrate, ferroptosis, Hippo-Yap
Introduction
The energy metabolism of cancer cells and breast cancer cells in particular is different than that of most non-proliferating epithelial cells (1,2). This altered energy metabolism, variously termed the Warburg effect or aerobic glycolysis, is characterized by breast cancer cells importing vast amounts of glucose from the blood, metabolizing it through the glycolysis pathway and releasing most of the produced pyruvate as lactate. While this type of metabolism is found in certain anaerobic organisms and temporarily under hypoxic conditions in mammalian cells, it is a permanent feature for almost all cancer cells, even in the presence of sufficient oxygen levels, hence ‘aerobic’ glycolysis. The discovery of this peculiar effect has been made almost a century ago (3), however its potential for therapeutic exploitation has only recently started to be elucidated. In 1995, Nebeling and colleagues (4) placed two pediatric patients with advanced astrocytoma on a low carbohydrate diet, with the idea to reduce the availability of glucose to the cancer cells. Both patients went into remission and remained progression-free while on the diet. While a small case study, it indicated that glucose deprivation through dietary adaptation (ketogenic diets or low-carbohydrate diets) could be detrimental to tumor and be useful for cancer patient treatment. Ketogenic diets are characterized by replacing the almost all calories from carbohydrate with lipids and protein, with calories from carbohydrates as low as 5% of total calories (5). These diets are used clinically to treat refractory epilepsy, obesity, and type-2 diabetes (6–8). This leads to hepatic activation of ketone body production as alternative fuel primarily for the central nervous system, ketone bodies however can be used by all cell types as metabolic fuel (9). Since the 1995 case study report ketogenic diets have been trialed for their effect against tumors in both mouse and human studies with varying results (10–13). In vitro studies have mainly focused on the role of ketone bodies, specifically β-hydroxybutyrate (BHB) as a treatment of cancer cells under regular glucose conditions (14,15). They show similar discrepancies as clinical studies in that some show no effect on breast cancer cell behavior (15,16), while others do demonstrate reduced survival or metabolic changes in cancer cells (17,18). However, if breast cancer cells artificially express enzymes for ketone body metabolism, they are able to survive and even thrive in low glucose conditions (19). Similar effects were observed in glioblastoma patients that failed to respond to ketogenic diet interventions. Their tumors had developed the ability to metabolize ketone bodies (20). Even if ketone bodies are not metabolized for energy, recent research demonstrates that BHB may also act as a signaling molecule that could potentially have other effects on breast cancer cells besides being an inert metabolic substrate (21). Since ketogenic diets are increasingly used in clinical practice for adjuvant cancer treatment, there is a need to better understand how cancer cells react when confronted with ketogenic environments.
Here we present the results of exposing luminal-A type breast cancer cells MCF-7 and T47D (22) to ketogenic environments. As such we reduced glucose exposure to these cells to 5% of their usual amount and supplemented with up to 25 mM BHB. We examined the breast cancer cell's rate of proliferation and analyzed gene expression changes under these conditions.
Materials and methods
Cell culture
Human breast cancer cell lines MCF-7 (cat. no. HTB-22) and T47D (cat no. HTB-133) were purchased from the American Tissue Culture Collection (ATCC) and routinely maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Atlanta Biologicals; R&D Systems), 2 mM glutamine (Gibco; Thermo Fisher Scientific, Inc.) and 50 ng/ml gentamycin (Lonza Biologicals). Human breast epithelial cells MCF-10A (cat. no. CRL-10317) cells were purchased from ATCC and routinely maintained in DMEM/F-12 Medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 5% Horse Serum (Sigma-Aldrich; Merck KGaA), 10 µg/ml human insulin (Sigma-Aldrich; Merck KGaA), 0.5 µg/ml hydrocortisone (Sigma-Aldrich; Merck KGaA) 20 µg/ml human epidermal growth factor (Invitrogen; Thermo Fisher Scientific, Inc.), 100 ng/ml cholera toxin (Sigma-Aldrich; Merck KGaA), 50 ng/ml gentamycin (Lonza). Sodium β-hydroxybutyrate was purchased from Sigma-Aldrich; Merck KGaA. Cell lines were incubated at 37°C humidified 5% CO2-supplemented air. Cell lines were routinely maintained in 75 cm2 tissue culture flasks with filtered lids (Thermo Fisher Scientific, Inc.) and sub-cultured when reaching approximately 80% confluence. Cell cultures were inspected daily for visible contamination and consistent growth rates. After purchase from ATCC, cell lines were maintained exclusively within the research group and handled by qualified researchers. The cell culture facility is only accessible to qualified researchers and does not maintain HeLa cells. Cell stock were stored in a locked designated cryo-vessel and inventory records were maintained including clear labeling of cryo-tubes and flasks with full cell line name, date, passage number and initials of the researcher. Trypsin (0.25%) (Gibco; Thermo Fisher Scientific, Inc.) was maintained in 10 ml aliquots at −20°C in sterile 15 ml falcon tubes (Thermo Fisher Scientific, Inc.). Fetal bovine serum (R&D Systems) was maintained in 50 ml aliquots at −20°C in sterile 50 ml falcon tubes (Thermo Fisher Scientific, Inc.). Cells were routinely maintained in mycoplasma suppressing antibiotic gentamycin and prepared medium was used for a maximum of three weeks. Experiments on cell lines were performed within 25 passages of receipt from ATCC.
Cell proliferation
In 96-well dishes, 5×103 cells were plated in 100 µl of regular DMEM and incubated overnight. After removing all medium, cells were treated with decreasing glucose concentrations [4.5, 2.25, 1.5, 1., 0.75, 0.5, 0.225, 0.125, 0 (all in g/l)] alone, or in combination with 10 or 25 mM BHB for either 48 or 72 h. Four hours prior to analysis, cells were supplemented with 1 mg/ml MTT (Thermo Fisher Scientific, Inc.) solution. After finalizing the indicated treatment periods, all media was removed from the wells and 200 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to each well. After incubating the plates at room temperature for 15 min with gentle shaking, absorbance of each well was read at 540 nm using a BioRad Mark plate reader. For MCF-7 and MCF-10A at least three experiments with six replicates for each treatment were performed, while a single experiment with six replicates for each treatment was performed for T47D cells.
In 10 cm dishes, 105 MCF-10A breast epithelial cells or MCF-7 breast cancer cells were plated and treated for 18 days with regular DMEM and either 225 mg/l glucose or 225 mg/l glucose with 10 or 25 mM β-hydroxybutyrate (BHB). Medium and treatment conditions were renewed every four days. Cells were counted every day using Trypan Blue exclusion counts. Cells were observed with a Nikon Microscope and brightfield pictures were captured at ×40 magnification daily. Experiments were performed in duplicates for a total of three experiments.
RNA sequencing
In 10 cm dishes, 1×106 MCF-7 or T47D breast cancer cells were incubated in regular DMEM medium or treated with 225 mg/l (5% of glucose in regular DMEM medium) alone or in combination with 10 mM BHB or 25 mM BHB for four days for a total of two experiments. Then total RNA was extracted using Qiagen's RNeasy Mini Kit (Qiagen) and submitted for RNA sequencing using Psomagen's mRNA sequencing service, which included library development and delivered in excess of 6×106 150 bp pair-ended reads per sample (Psomagen Inc.). We performed two independent experiments for each cell line and treatment condition.
RNA sequence analysis
After obtaining sequences, abundances were quantified from raw fastq files and mapped against human Ensembl v96 transcriptomes pre-indexed files using Lior Pachter's kallisto (23). Abundance files were analyzed using Bioconductor's DESeq2 (version 1.28.1) package for R (24). Data quality control was established using Bioconductor's principal component analysis (PCA) to detect variance within samples. PCA results are displayed as two-dimensional graphs showing contribution of the highest component variance in each sample comparison. Results were reduced for expression changes of over 1.5-fold and analyzed for false discoveries as previously described (25). P-adjusted values of <0.05 were considered to be statistically significant. We used Bioconductor version 3.11 (26,27) and R version 4.0 (28) on RStudio version 1.3.959.
Gene ontology and over representation analysis
Gene ontology analysis was performed using the WikiPathway package (29) for R and further processed for pathway enrichment (30) in Cytoscape version 3.8.0 (31), using a hypergeometric test with a Benjamini-Hochberg false discovery rate correction (32). A P-value of less than 0.05 was used to identify enriched pathways. Selected pathways that had been identified were visualized using WikiPathway (33) or using clusterProfiler for pathway visualization (34).
Comparison of catabolic ketone metabolism enzymes in The Cancer Genome Atlas
We queried The Cancer Genome Atlas (TCGA) PanCancer Atlas studies at cBioPortal (cBioPortal.org) (35,36) for expression of BDH1, OXCT1, and ACAT1 genes encoding enzymes for catabolic metabolism of ketone bodies. This returned 32 studies with 10,953 patient samples. Additionally, we selected the Breast Invasive Carcinoma (TCGA, PanCancer Atlas) database and plotted mRNA expression levels (high: Above 1.5-fold; low: Below −1.5-fold expression based on mRNA expression z-scores relative to all samples, with a z-score threshold of ±2.0) of BDH1, OXCT1 and ACAT1 against overall survival.
Statistical analysis
Cell proliferation data from MTT assays are presented as bars representing interquartile range including the observed median (horizontal line). Whiskers represent highest and lowest observed values. Outliers detected by Tukey's method are shown in each graph if present.
Cell proliferation data were analyzed by two-way ANOVA, followed by Holm-Sidak post-hoc corrections to test for statistical differences of survival of each condition (control; 25 mM BHB) at any glucose concentration compared to regular glucose concentration of 4.5 g/l. Cell proliferation data were also analyzed using multiple t-tests with Holm-Sidak corrections for multiple analyses to test for statistical differences between non-BHB and BHB treated samples at every glucose concentration. Cell proliferation data in Fig. S1 are presented as the mean of two independent experiment with six replicates. Error bars represent standard deviation. Cell proliferation data in Fig. S1 were analyzed for statistical differences between each BHB concentration and control using Dunnett's corrections following two-way ANOVA for T47D cells and MCF-7 cells and Tukey correction following one-way ANOVA for MCF-10A cells. Cell count data (Fig. 1G) are presented by means of three experiments with two replicates per experiments. Error bars were omitted for clarity. Cell count data were analyzed by multiple t-tests using the Holm-Sidak method to determine statistical significance for count differences between all three experimental conditions on each day (control; no glucose no BHB; no glucose 25 mM BHB). A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism software v.8.4.3 (GraphPad Software, Inc.). Statistical analysis of the RNA sequencing workflow was integrated into the DESeq2 analysis as previously described (24). Kaplan-Meier curves were drawn to compare overall survival in patients with breast cancer where tumors express high or low mRNA levels of BDH1, OXCT1 or ACAT1. A log rank test was used for detection of statistical differences of patient survival data was performed using cBioPortal built-in analysis. A P-value of <0.05 was considered statistically significant.
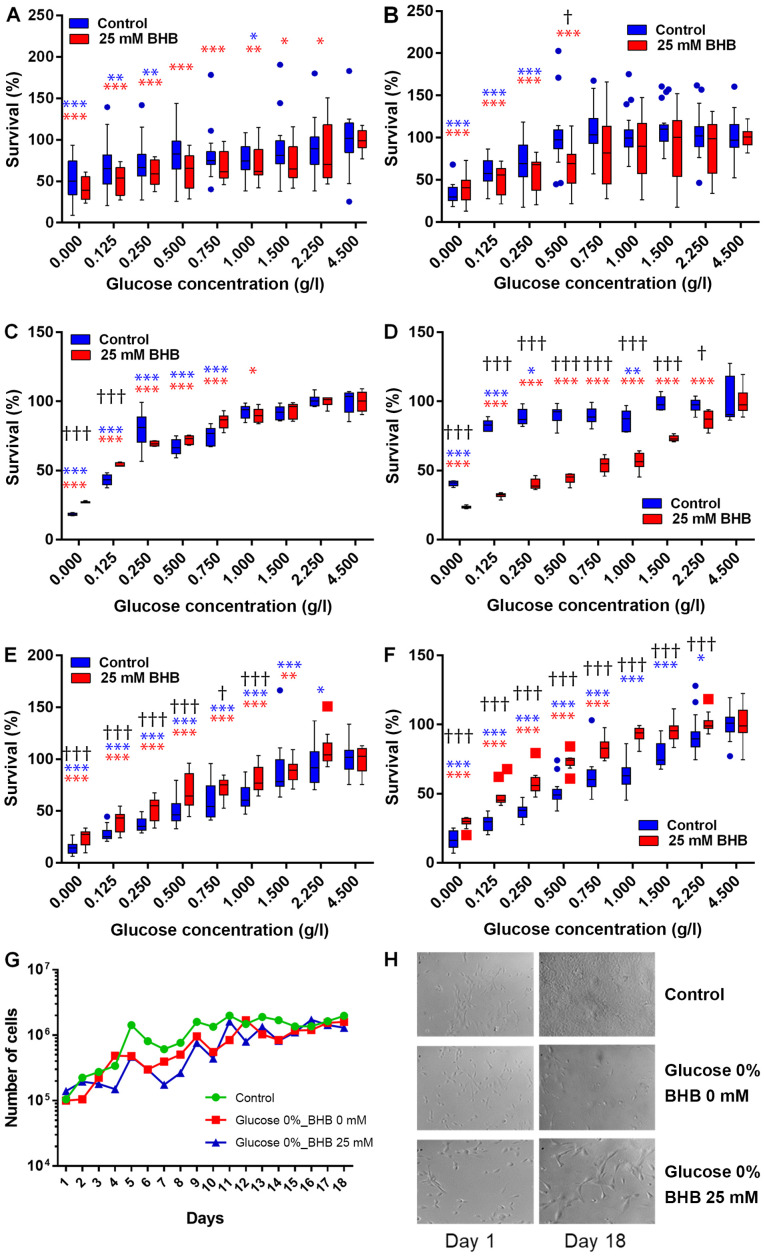
Figure 1.
Cell proliferation analysis in glucose starved human cells. (A and B) MCF-7, (C and D) T47D human breast cancer cells and (E and F) MCF-10A human breast epithelial cells were incubated in decreasing amounts of glucose alone (blue) or in the presence of 25 mM BHB (red) for (A, C and E) 48 h or (B, D and F) 72 h. Bars represent interquartile range including the observed median (horizontal line). Whiskers represent highest and lowest observed values. Outliers detected by the Tukey-method are indicated by blue circles and red squares for not-BHB- and BHB-supplemented cells, respectively. *Indicate significant difference of the respective treatment [not-BHB supplemented (blue); BHB supplemented (red)] between the glucose concentration it is associated with and the respective cell proliferation level observed at 4.5 g/l glucose as calculated by two-way ANOVA followed by Holm-Sidak post hoc corrections for multiple comparisons. †Indicate significant difference between not-BHB- and BHB-supplemented cells at the specific glucose concentration as calculated by multiple t-tests with Holm-Sidak method corrections. Significant levels: */†P<0.05; **P<0.01; ***/†††P<0.001. (G) MCF-7 breast cancer cells were incubated in regular high-glucose DMEM medium (green), in glucose depleted DMEM medium (red) or in glucose depleted medium supplemented with 25 mM BHB (blue) for up to 18 days. Line graphs represent mean cell count of two independent experiments. Error bars for standard deviation were omitted for clarity. There were no significant differences in proliferation at any time-point. (H) Brightfield pictures at ×40 magnification of MCF-10A human breast epithelial cells after 18 days of exposure to glucose deprivation with or without BHB supplementation. BHB, β-hydroxybutyrate.
Results
Ketone bodies are tolerated by breast cancer cells
We performed kill curves of BHB on all cell lines of up to 200 mM using MTT assays (Fig. S1). No significant impact on cell proliferation was observed for BHB concentrations of up 25 mM, which was chosen as the highest BHB treatment concentration, while 10 mM BHB corresponds to a high physiological level of BHB in medically therapeutic uses of ketogenic diets (37).
Ketone bodies do not rescue breast cancer cells during glucose deprivation
MCF-7 and T47D breast cancer cells and MCF-10A breast epithelial cells were incubated in decreasing glucose concentrations with 25 mM BHB for 48 h or 72 h (Fig. 1A-F). Cell proliferation decreased in both cell lines with decreasing glucose concentrations. For MCF-7 cells, cell proliferation significantly decreased after reducing glucose concentrations to 1 g/l and below glucose concentrations of 250 mg/l when compared to proliferation at 4.5 g/l glucose after 48 h. When supplementing with 25 mM BHB cell proliferation decreased significantly after treatment with 2.25 g/l glucose and below compared to proliferation at 4.5 g/l glucose and 25 mM BHB after 48 h. There was no significant difference in cell proliferation between not BHB- and BHB-supplemented MCF-7 cells at any of the different glucose treatment concentrations (Fig. 1A). Cell proliferation significantly decreased after treatment of MCF-7 cells with reduced glucose of 250 mg/l and below compared to proliferation at 4.5 g/l glucose after 72 h. When supplementing with 25 mM BHB cell proliferation decreased significantly after treatment with 500 mg/l glucose and below compared to proliferation at 4.5 g/l glucose and 25 mM BHB after 72 h. We observed a significant difference in proliferation between not-BHB- and BHB-supplemented MCF-7 cells after treatment with 1 g/l glucose (Fig. 1B).
For T47D cells, cell proliferation significantly decreased after reducing glucose concentrations below 750 mg/l when compared to proliferation at 4.5 g/l glucose after 48 h. When supplementing with 25 mM BHB cell proliferation decreased significantly after treatment with 1 g/l glucose and below compared to proliferation at 4.5 g/l glucose and 25 mM BHB after 48 h. There was a significantly higher levels of cell proliferation in BHB-supplemented T47D cells compared to not-BHB-supplemented cells at very low glucose concentration (0 g/l and 125 mg/l) (Fig. 1C), indicating that BHB may be metabolized by these cells when there is no glucose present. Cell proliferation significantly decreased after treatment of T47D cells with reduced glucose concentrations of 1 g/l and below glucose concentrations of 250 mg/l when compared to proliferation at 4.5 g/l glucose after 72 h. When supplementing with 25 mM BHB cell proliferation decreased significantly after treatment with 2.25 g/l glucose and below compared to proliferation at 4.5 g/l glucose and 25 mM BHB after 72 h. Conversely to 48 h treatments, there was significantly lower proliferation in BHB-supplemented cells compared to not-BHB supplemented cells at all glucose treatment conditions of 2.25 g/l and below (Fig. 1D).
We were interested if extending the incubation time beyond 72 h would demonstrate adaptation of the breast cancer cells to the ketogenic environment as a reflection of the transition period observed during ketogenic diets in patients. When using cell counts, we did not find any significant difference between treatment conditions and control (Fig. 1G).
For MCF-10A cells, cell proliferation significantly decreased after reducing glucose concentrations below 2.25 g/l when compared to proliferation at 4.5 g/l glucose after 48 h. When supplementing with 25 mM BHB cell proliferation decreased significantly after treatment with 1.5 g/l glucose and below compared to proliferation at 4.5 g/l glucose and 25 mM BHB after 48 h. There was a significantly higher levels of cell proliferation in BHB-supplemented MCF-10A cells compared to not-BHB-supplemented cells at glucose concentration of 1 g/l and below (Fig. 1E). Cell proliferation significantly decreased after treatment of MCF-10A cells with reduced glucose concentrations of 2.25 g/l and below when compared to proliferation at 4.5 g/l glucose after 72 h. When supplementing with 25 mM BHB cell proliferation was significantly decreased after treatment with 750 mg/l glucose and below compared to proliferation at 4.5 g/l glucose and 25 mM BHB after 72 h. Complementary to 48 h treatments, there was significantly higher proliferation in BHB-supplemented cells compared to not-BHB supplemented cells at all glucose treatment conditions of 2.25 g/l and below (Fig. 1F).
When treatment times of MCF-10A breast epithelial cells was extended to 18 days we observed that glucose deprivation decreased proliferation, but the BHB supplementation increased proliferation and cell size (Fig. 1H). This indicates that non-cancerous epithelial cells can metabolize ketone bodies such as BHB and would be able to survive under ketogenic conditions.
Differential gene expression in low glucose environments
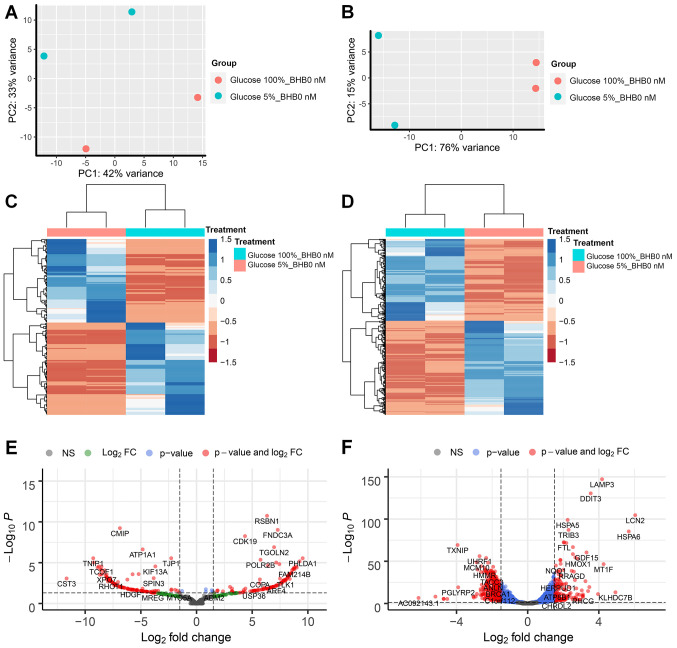
We performed RNA sequencing analysis on MCF-7 and T47D breast cancer cells and compared treatments in three pairs per cell line. The first comparison examined the effect of low glucose conditions on gene expression changes in breast cancer cells without BHB supplementation compared to cells grown in normal (4.5 g/l) glucose concentrations. We observed that for MCF-7 breast cancer cells 221 genes had at least 1.5-fold difference in expression and less than 0.05 p-adjusted value and 1182 genes for T47D breast cancer cells (Fig. 2E and F). Both comparisons showed distinction between the treatment methods based on PCA (Fig. 2A and B). Comparison between the two cell lines demonstrated that there are almost five times as many genes that show differential expression in T47D breast cancer cells compared to MCF-7 breast cancer cells, while the ratio of up- and down-regulated gene expression is similar in both cell lines (MCF-7 100 genes up, 113 gene down; T47D 447 gene up and 425 down). Unsupervised cluster analysis demonstrated gene expression changes in MCF-7 and T47D breast cancer cells (Fig. 2C and D). Volcano plots between differences in expression levels and significant show high levels of expression changes with relatively low levels of significance in MCF-7 cells (Fig. 2E), while T47D cells demonstrate higher levels of significance on the expression of several genes (Fig. 2F).
Figure 2.
RNA sequencing analysis of MCF-7 and T47D breast cancer cells after glucose deprivation. Principle component analysis of (A) MCF-7 cells and (B) T47D) cells. Unsupervised clustering analysis and heatmap of differentially expressed genes in (C) MCF-7 and (D) T47D cells after glucose deprivation. Volcano plot of differentially expressed genes in (E) MCF-7 cells and (F) T47D cells. Vertical dotted lines indicate borders for genes with 1.5-fold expression change; horizontal dotted line indicates P-value of 0.05. BHB, β-hydroxybutyrate.
Differential gene expression in low glucose environments with BHB treatment
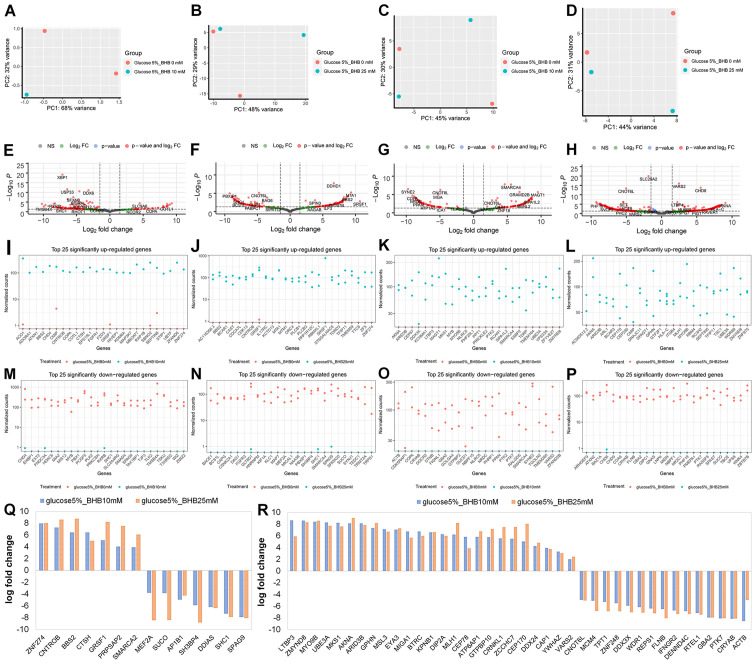
Next, we examined the effect of BHB supplementation on gene expression in MCF-7 and T47D breast cancer cells. The comparisons in this analysis were comparing either 225 mg/l glucose-treated cells with 225 mg/ml glucose and 10 mM BHB-treated cells, or 225 mg/l glucose-treated cells with 225 mg/ml glucose and 25 mM BHB-treated cells for both MCF-7 and T47D cells. One of the samples for MCF-7 breast cancer cells treated with 5% glucose and 10 mM BHB failed during library creation, thus this treatment has only a single replicate (Fig. 3A). For MCF-7 cells we observed 163 differentially expressed genes after treatment with 10 mM BHB (Fig. 3E) and 177 differentially expressed genes after treatment with 25 mM BHB (Fig. 3F). Since we did not observe any ontological clustering for MCF-7 glucose deprived breast cancer cells treated with 25 mM BHB (Fig. 4), the 25 most upregulated (Fig. 3I and J) and downregulated (Fig. 3M and N) genes are displayed. In total we observed 14 differentially expressed genes with BHB treatment in both 10 and 25 mM BHB treated groups (Fig. 3Q). Metabolically interesting is the increased expression of PRPSAP2, which codes for a protein associated with ribosyl-phosphate production (38).
Figure 3.
RNA sequencing analysis after 10 mM BHB or 25 mM BHB treatment of glucose deprived MCF-7 and T47D breast cancer cells. Principle component analysis of (A and B) MCF-7 cells and (C and D) T47D cells treated with (A and C) 10 mM BHB or (B and D) 25 mM BHB compared to cells treated with 225 mg/l glucose. Volcano plot of differentially expressed genes in (E and F) MCF-7 cells and (G and H) T47D cells treated with (E and G) 10 mM BHB or (F and H) 25 mM BHB compared to cells treated with 225 mg/l glucose. Vertical dotted lines indicate borders for genes with 1.5-fold expression change; horizontal dotted line indicates P-value of 0.05. Highest fold change of differentially expressed genes in (I, J, M and N) MCF-7 cells and (K, L, O and P) T47D cells treated with (I, K, M and O) 10 mM BHB and (J, L, N and P) 25 mM BHB compared to cells treated with 225 mg/l glucose; Graphs are separates by (I-L) significantly upregulated or (M-P) significantly downregulated gene expression. Genes that are significantly differentially expressed in (Q) MCF-7 cells and (R) T47D cells in both 10 mM BHB and 25 mM BHB treatments. BHB, β-hydroxybutyrate.
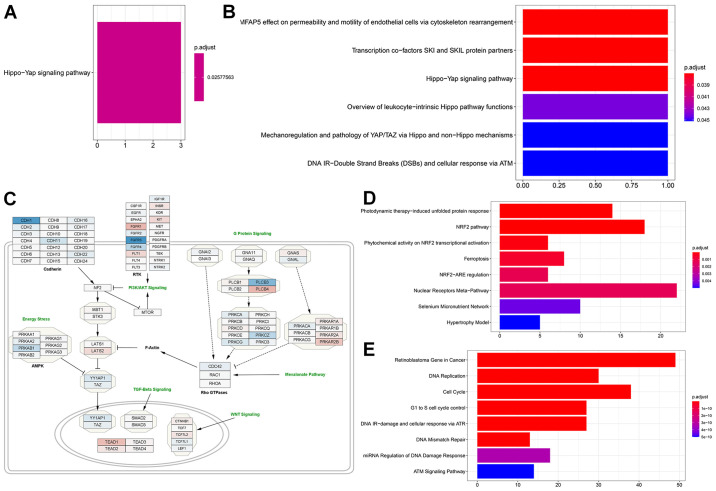
Figure 4.
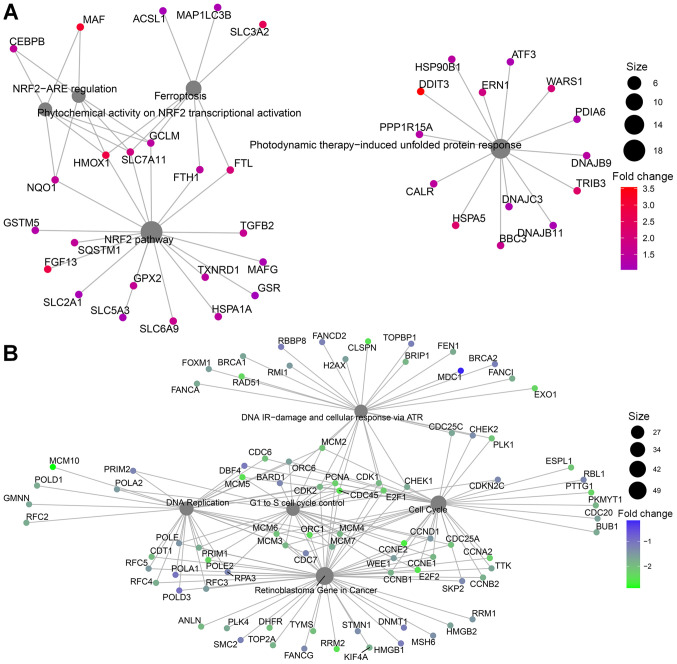
Gene ontology, over representation analysis and pathway enrichment analysis. (A) MCF-7 cells enrichment after glucose deprivation and (B) after treatment with 10 mM BHB compared to cells treated with 225 mg/l glucose alone. (C) WikiPathways regulating Hippo signaling (WP4540) overlaid with fold-change differentially expressed genes in MCF-7 cells after glucose deprivation compared to cells grown with 4.5 g/l glucose (blue, downregulation; red, upregulation). (D) Over representation analysis results for significantly higher expressed genes in T47D cells after glucose deprivation and (E) for significantly lower expressed genes. BHB, β-hydroxybutyrate.
For T47D cells we observed 156 differentially expressed genes after treatment of glucose deprived cells with 10 mM BHB (Fig. 3G) and 394 differentially expressed genes after treatment with 25 mM BHB (Fig. 3H). Despite the number of changes in genes expression, similar to MCF-7 cells, there were no clusters identified in glucose deprived T47D cells following treatment with wither 10 or 25 mM BHB. The 25 most upregulated (Fig. 3K and L) and downregulated (Fig. 3O and P) are displayed. In total we found 40 differentially expressed genes with BHB treatment in both 10 and 25 mM BHB treated groups (Fig. 3R).
Glucose deprivation increases expression of HIPPO pathway inhibitors
When we performed gene ontology, in MCF-7 cells genes that were significantly increased in expression associated with the Hippo-Yap signaling pathway (Fig. 4A) after glucose deprivation (comparison 1), while down-regulated genes were not associated with any pathway. Interestingly gene ontology analysis also indicated that genes that were upregulated when glucose starved cells were compared to those supplemented with 10 mM BHB (comparison 2) returned the Hippo signaling pathway (Fig. 4B), however only one gene (LATS1) was responsible for this result. Since we did not find the same results after treatment with 25 mM BHB (comparison 3), we did not follow up on this discovery. We downloaded the WikiPathway No. WP4540 to Cytoscape and superimposed the results from comparison 1 for MCF-7 cells. Glucose deprivation of MCF-7 breast cancer cells lead to a decrease in Hippo signaling due to the higher expression of LATS2 and the reduced expression of YAP1 and TAZ (Fig. 4C). Additionally, several members of pathways interacting with the Hippo pathway are identified as being differentially expressed in glucose deprived MCF-7 cells such as TEAD1, and the reduced expression of members of the AMPK pathway.
Glucose deprivation activates ferroptosis in T47D breast cancer cells
In T47D breast cancer cells, gene ontology indicated different pathway involvements for comparison 1 between normal glucose and low glucose treatments. Genes with increased expression after glucose deprivation clustered around the NRF2 pathway, regulation of ferroptosis and unfolded protein response (Figs. 4D and 5A). Within differentially regulated genes around the NRF2 pathway there is increased expression of SLC2A1, the gene coding for the glucose transporter GLUT1 as well as other members of the solute carrier family (SLC5A3, SLC6A9, SLC3A2, SLC7A11). Additionally, several genes regulating ferroptosis showed increased expression, indicating that T47D cells may be susceptible to this unusual cell suicide pathway following glucose deprivation. Genes that showed decreased expression after glucose deprivation are primarily involved in cell cycle control and DNA replication (Figs. 4E and 5B). This is in line with the finding of decreased proliferation in Fig. 1D and E.
Figure 5.
Pathway enrichment analysis of (A) significantly higher expressed genes and (B) significantly lower expressed genes in T47D cells after glucose deprivation.
Expression of enzymes that catalyze ketone body catabolism in MCF-7 and T47D cells and patient samples
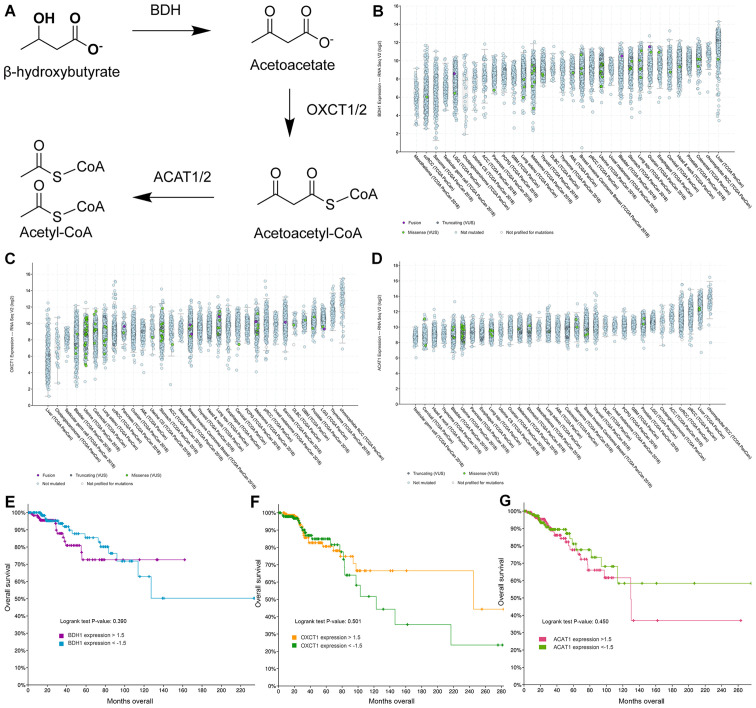
Ketone body catabolism transforms BHB to Acetyl-CoA for immediate oxidation in the TCA cycle (Fig. 6A). The enzymes responsible for this process are BDH, OXCT and ACAT, with OXCT being the rate limiting step in the process. When we extracted the expression results of these genes, we did not find any significant difference in their expression with any treatment (Table SI). Using fragments per kilobase per million mapped reads (FPKM) to provide a means of comparing expression levels, OXCT1 expression is higher in MCF-7 cells than in T47D cells and vice versa for BDH1 expression. In cBioPortal 32 studies with samples from 10953 patients were selected spanning 32 cancer sites and queried for gene expression of BDH1 (Fig. 6B), OXCT1 (Fig. 6C), and ACAT1 (Fig. 6D). All genes showed high expression levels in all queried cancer types. Testicular cancer samples were consistently the lowest expressing samples for all three genes. Interestingly, liver cancer samples showed high expression of BDH1 and ACAT1, but the lowest expression of OXCT1. Meanwhile renal cell carcinoma patient samples consistently showed high expression among all three genes. Among all patient samples though BDH1/2 and OXCT1/2 enzymes tended to be co-expressed (Table SII). Genetic or chromosomal mutations of either BDH1 (624/10953), or OXCT1 (377/10953), or ACAT1 (173/10953) in patient samples was a rare occurrence (Fig. 6B-D).
Figure 6.
Expression of enzymes responsible for catabolic metabolism of ketone bodies in cancer patient samples. (A) Schematic overview of catabolism of ketone bodies BHB and acetoacetate in neural and peripheral tissues. (B-D) Log2-fold gene expression in 32 different cancer sites of (B) BDH1, (C) OXCT1, and (D) ACAT1. Each dot represents the expression level of a single patient. Colors represent gene or chromosomal mutations as labelled in each figure. Cancer studies are ordered by median expression within each study. A total of 10,953 patient samples are represented in these graphs. (E-G) Gene expression levels of above 1.5-fold or below −1.5-fold change based on mRNA expression z-scores relative to all samples are represented from breast cancer patients only and plotted against overall survival for (E) BDH1, (F) OXCT1 and (G) ACAT1.
Next, we selected the Breast Invasive Carcinoma (TCGA, PanCancer Atlas) database in cBioPortal and queried the database for gene expression levels of above 1.5-fold or below −1.5-fold change based on mRNA expression z-scores relative to all samples. Low and high expression of BDH1 (Fig. 6E), OXCT1 (Fig. 6F), and ACAT1 (Fig. 6G) were plotted against overall survival. High expression of BDH1 or ACAT1 was significantly correlated with lower survival, while there was no significant difference in survival between low and high OXCT1 expressing breast cancer tumors. In fact, there is a non-significant trend to higher survival for patients with tumors overexpressing OXCT1.
Discussion
Metabolic alterations during cancer etiology are essential for carcinogenesis and have been identified as an emerging hallmark of all cancers (39). At the time of writing there are four clinical trials registered at clinicaltrials.gov that resulted from a query of ‘breast cancer’ and ‘ketogenic diet’ and seven trials when queried for ‘breast cancer’ and ‘low carbohydrate’. This demonstrates that dietary intervention trials for the treatment of breast cancer is of interest. The molecular actions of ketone bodies on breast cancer cells during low glucose have not been elucidated. This is important because breast cancer cells may start metabolizing ketone bodies, instead of their preferred fuel glucose, under these conditions. In the presented study we aimed to determine the effects of BHB, the main circulating hepatic ketone body during starvation or ketogenic diets, on two breast cancer cell lines in low glucose conditions.
Reduction in glucose availability decreased proliferation in breast cancer cells. Similarly, glucose restrictions affected breast cancer cells in vitro in long term glucose deprivation experiments (40). We did not observe any changes in cell proliferation when breast cancer cells were treated with BHB in low glucose conditions (Fig. 1G). For breast cancer cells it was observed that BHB treatment decreased cell proliferation further than glucose deprivation alone. The only exception was for T47D cells with 125 mg/l or 0 g/l glucose after 48 h treatment. However this effect was no longer observed when treatment times were extended to 72 h. Similar results were observed for BT20, BT474, HBL100, MCF-7, MDA-MB 231, MDA-MB 468, and T47D breast cancer cells under hypoxic conditions (15). Glucose deprivation may be useful in reducing the growth of breast tumor and a ketogenic approach can be utilized for this purpose since breast cancer cells do not seem to increase proliferation in high ketone body environments. It can also be concluded that this effect is specific to cancer cells, since treatment of MCF-10A breast epithelial cells showed higher proliferation with BHB supplementation than without during glucose deprivation.
In MCF-7 breast cancer cells, we found that genes with decreased expression clustered around the Hippo-Yap cell signaling pathway when glucose was removed from the medium. Interestingly pathway enrichment analysis demonstrated that LATS1/2 was further decreased in expression when we compared 10 mM BHB with no BHB supplementation in glucose deprived MCF-7 breast cancer cells. The Hippo-Yap pathway is a conserved pathway involved in organ growth originally discovered in Drosophila. This pathway is a kinase cascade that is activated by G-protein coupled receptors which results in Merlin/NF-2 activation. The signal is further transduced through SAV1-MST1/2 complexes (human ortholog to Drosophila's Hippo) and LATS1/2 before ending in inactivation of YAP/TAZ transcription factors. When active these translocate to the nucleus and dimerize with TEAD1/2 or other co-activators (41). It has been implicated in cancer growth and includes the known tumor suppressor gene NF-2 (42) and implicates a TEAD1 mutation in excessive lesions retina and optical nerve leading to a rare autosomal dominant disease called Sveinsson's chorioretinal atrophy (SCRA) (43). The Hippo pathway transcription factor targets YAP/TEAD have recently been found to accumulate in the nucleus of trastuzumab resistance HER2-positive breast cancer patient samples (44). Additionally, YAP activation was implicated in FAK mediated development of triple-negative breast cancer cells (45). Metabolically the inactivation of the Hippo pathway is involved in maintaining and mediating glucose metabolism in breast cancer cells (46–48). Based on our findings and the supporting literature the Hippo pathway may be involved in mediating the aerobic glycolysis phenotype at least in MCF-7 breast cancer cells.
In T47D breast cancer cells glucose deprivation increased gene expression of genes associates with the NRF2-Ferroptosis axis. Ferroptosis is a recently discovered pathway of programmed cell death driven by iron-dependent lipid peroxidation and distinct from other forms of apoptosis. Cell death is caused by iron mediated lipid reactive oxygen species accumulation exceeding the cell's antioxidant defenses (49,50). Activation of ferroptosis following glucose deprivation in breast cancer cells has not been shown previously and the mechanistic induction is open to speculation. However, several approaches have been used to induce ferroptosis in pancreatic cancer cells (51), hepatocellular (52), and breast cancer cells (53).
One way in which ferroptosis can be activated is through the NRF2 pathway, which our data indicates is activated in response to glucose deprivation in T47D breast cancer cells. Indeed, previous studies found that NRF2 protein levels increased in glucose deprived T47D breast cancer cells and knockdown of NRF2 impaired survival during glucose deprivation (54). Thus, it may be possible that glucose deprivation activated NRF2 signaling as a protective measure against increased autophagic flux, while simultaneously increasing ferroptosis.
In glucose deprived breast cancer cells treated with different levels of BHB we did not find any pathways associated with the treatment. We propose this as another incremental evidence that breast cancer cells do not or cannot utilize ketone bodies for their metabolic functions and support the use of ketogenic diets in cancer therapy.
T47D and MCF-7 breast cancer cells were chosen for this analysis since both are luminal A breast cancer subtype and it was expected that their reaction to glucose deprivation would be similar. However, T47D cells reacted by significantly changing 1182 genes compared to 221 genes for MCF-7. Additionally, MCF-7 cell's proliferation remained at ~50% without any glucose for up to 72 h (Fig. 1A). When the incubation time was extended up to 18 days, MCF-7 cells showed no difference in proliferation between any of the treatment groups (Fig. 1G). Compared to T47D cells, MCF-7 cells are therefore more resistant to glucose deprivation and it can be suggested that they are utilizing an alternative metabolic fuel. Our results rule out that MCF-7 cells utilize BHB as fuel and we suggest that the alternative could be glutamine. We retained glutamine in the medium, since most ketogenic diets do not include reduction in protein intake and may even promote increased protein intake in order to increase satiety and palatability of ketogenic diets (55). In other works, glutamine deprivation of MCF-7 cells resulted in decreased proliferation and increased apoptosis (56,57), supporting this assertion. It was also observed that genes coding for enzymes able to metabolize ketone bodies to acetyl-CoA are expressed in our breast cancer cells (Table SI) and that patient samples indicate decreased survival in patients with tumors expressing high levels of BDH1 and ACAT1. Thus while the breast cancer cells in this study are not utilizing BHB as energetic substrate, the inherent ability of cells to sustain energetic requirements through a number of different metabolic fuels mandates fuller investigation into the metabolic patterns that breast cancer cells display before engaging in therapeutic interventions. It may be catastrophic to alter diets of cancer patients that inadvertently provide their tumors with a metabolic fuel, including glutamine and BHB, they are adapted to consume.
We aimed to elucidate the gene expression changes that occur when breast cancer cells are adapting their metabolism to a ketogenic environment and are required to use BHB as an energetic metabolite. However, some of the observed gene expression changes may be due to a secondary function of BHB, i.e. its ability to act as a signaling molecule (21,58,59). While the signaling ability of BHB has primarily been demonstrated to activate the starvation response, it is possible that BHB may have effects on breast cancer cells independently of its role as energetic substrate, which may explain some of the changes we have observed. This may in part explain why a substantial number of genes were differentially expressed in glucose deprived cells treated with BHB, without demonstrating any known cell signaling pathway involvement.
In conclusion, glucose deprivation through ketogenic diets may provide a useful tool in battling breast cancer. Similar to a recent study (15), we did not observe any effect of BHB on cell proliferation in breast cancer cells, suggesting that breast cancer cells are unable to use BHB as a fuel to drive proliferation. However, it may be necessary to screen patients for the presences of ketone catabolic enzymes OXCT1/2, BDH1/2, and ACAT1/2, which show increased expression in a variety of patient samples. A ketogenic diet may be disastrous for this subgroup of patients. Additionally, further research is required to understand the different responses we observed in just two breast cancer cell lines to glucose deprivation. This heterogeneity in breast cancer cells will likely impact the ongoing clinical trials using ketogenic diets and possibly lead to dismissing ketogenic diets for cancer treatments, when there might be a substantial subgroup of patients that will benefit from this intervention.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Dirk Geerts (Department of Medical Biology, Academic Medical Center Amsterdam, Amsterdam, Netherlands) for consulting on the experimental conditions for the RNA sequencing samples and the Epigenetics Core (Department of Native Hawaiian Health, John A. Burns School of Medicine, University of Hawai'i at Manoa, Honolulu, HI, USA) for consultation on the RNA sequencing analysis. The authors would also like to acknowledge undergraduate interns Mr. Colin Andres-Paguirigan, Ms. Alyssa Sato, Ms. Leslie Ann Villanueva and Ms. Chanya Techasurungkul (School of Natural Sciences and Mathematics, Chaminade University of Honolulu, Honolulu, HI, USA).
Funding
This study was funded by a grant from the Alana Dung Research Foundation. MW received pilot funding from the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS), IDeA Networks of Biomedical Research Excellence (INBRE), Award no. P20GM103466.
Availability of data and materials
The sequencing datasets generated for this study can be found in the Gene Expression Omnibus (GEO) DataSets (https://www.ncbi.nlm.nih.gov/gds) under the accession no. GSE153830. Cell proliferation datasets are available from the corresponding author upon reasonable request.
Authors' contributions
Conception and design of the study: RM, CAT and MW. Acquisition of data: RM, CAT, CS, AD, KU and MW. Analysis and interpretation of data: RM, AD, KU, CAT and MW. Drafting the manuscript: RM, KU and MW. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Arora R, Schmitt D, Karanam B, Tan M, Yates C, Dean-Colomb W. Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget. 2015;6:662–678. doi: 10.18632/oncotarget.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: Two case reports. J Am Coll Nutr. 1995;14:202–208. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Heber D. Ketogenic diets. JAMA. 2020;323:386. doi: 10.1001/jama.2019.18408. [DOI] [PubMed] [Google Scholar]
- 6.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178–1187. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 7.Martin-McGill KJ, Jackson CF, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev. 2018;11:CD001903. doi: 10.1002/14651858.CD001903.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifton P, Carter S, Headland M, Keogh J. Low carbohydrate and ketogenic diets in type 2 diabetes. Curr Opin Lipidol. 2015;26:594–595. doi: 10.1097/MOL.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 9.Masino SA, Rho JM. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Jasper's Basic Mechanisms of the Epilepsies. 4th edition. National Center for Biotechnology Information; Bethesda, MD: 2012. Mechanisms of ketogenic diet action; pp. 1483–1516. [PubMed] [Google Scholar]
- 10.Erickson N, Boscheri A, Linke B, Huebner J. Systematic review: Isocaloric ketogenic dietary regimes for cancer patients. Med Oncol. 2017;34:72. doi: 10.1007/s12032-017-0930-5. [DOI] [PubMed] [Google Scholar]
- 11.Klement RJ. Beneficial effects of ketogenic diets for cancer patients: A realist review with focus on evidence and confirmation. Med Oncol. 2017;34:132. doi: 10.1007/s12032-017-0991-5. [DOI] [PubMed] [Google Scholar]
- 12.Klement RJ, Champ CE, Otto C, Kämmerer U. Anti-tumor effects of ketogenic diets in mice: A meta-analysis. PLoS One. 2016;11:e0155050. doi: 10.1371/journal.pone.0155050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kämmerer U, Coy JF, Weller M, Steinbach JP. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44:1843–1852. doi: 10.3892/ijo.2014.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadochi Y, Mori S, Fujiwara-Tani R, Luo Y, Nishiguchi Y, Kishi S, Fujii K, Ohmori H, Kuniyasu H. Remodeling of energy metabolism by a ketone body and medium-chain fatty acid suppressed the proliferation of CT26 mouse colon cancer cells. Oncol Lett. 2017;14:673–680. doi: 10.3892/ol.2017.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartmann C, Janaki Raman SR, Flöter J, Schulze A, Bahlke K, Willingstorfer J, Strunz M, Wöckel A, Klement RJ, Kapp M, et al. Beta-hydroxybutyrate (3-OHB) can influence the energetic phenotype of breast cancer cells, but does not impact their proliferation and the response to chemotherapy or radiation. Cancer Metab. 2018;6:8. doi: 10.1186/s40170-018-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang D, Li T, Wang L, Zhang L, Yan R, Li K, Xing S, Wu G, Hu L, Jia W, et al. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress. Cell Res. 2016;26:1112–1130. doi: 10.1038/cr.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao GW, Chen YS, He DM, Wang HY, Wu GH, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev. 2015;16:2061–2068. doi: 10.7314/APJCP.2015.16.5.2061. [DOI] [PubMed] [Google Scholar]
- 18.Poff AM, Ari C, Arnold P, Seyfried TN, D'Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135:1711–1720. doi: 10.1002/ijc.28809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11:3964–3971. doi: 10.4161/cc.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz KA, Noel M, Nikolai M, Chang HT. Investigating the ketogenic diet as treatment for primary aggressive brain cancer: Challenges and lessons learned. Front Nutr. 2018;5:11. doi: 10.3389/fnut.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman JC, Verdin E. β-Hydroxybutyrate: A signaling metabolite. Annu Rev Nutr. 2017;37:51–76. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8:3131–3141. doi: 10.7150/jca.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens M. False discovery rates: A new deal. Biostatistics. 2017;18:275–294. doi: 10.1093/biostatistics/kxw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12:115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team: R: A language and environment for statistical computing. https://www.R-project.org/ [Feb 10;2015 ];
- 29.Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, Mélius J, Cirillo E, Coort SL, Digles D, et al. WikiPathways: A multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018;46(D1):D661–D667. doi: 10.1093/nar/gkx1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustavsen JA, Pai S, Isserlin R, Demchak B, Pico AR. RCy3: Network biology using cytoscape from within R. F1000Res. 2019;8:1774. doi: 10.12688/f1000research.20887.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 33.Kutmon M, Lotia S, Evelo CT, Pico AR. WikiPathways app for cytoscape: Making biological pathways amenable to network analysis and visualization. F1000 Res. 2014;3:152. doi: 10.12688/f1000research.4254.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu G, Wang LG, Han Y, He QY. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanItallie TB, Nufert TH. Ketones: Metabolism's ugly duckling. Nutr Rev. 2003;61:327–341. doi: 10.1301/nr.2003.oct.327-341. [DOI] [PubMed] [Google Scholar]
- 38.Katashima R, Iwahana H, Fujimura M, Yamaoka T, Ishizuka T, Tatibana M, Itakura M. Molecular cloning of a human cDNA for the 41-kDa phosphoribosylpyrophosphate synthetase-associated protein. Biochim Biophys Acta. 1998;1396:245–250. doi: 10.1016/S0167-4781(97)00217-0. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Mathews EH, Visagie MH, Meyer AA, Joubert AM, Mathews GE. In vitro quantification: Long-term effect of glucose deprivation on various cancer cell lines. Nutrition. 2020;74:110748. doi: 10.1016/j.nut.2020.110748. [DOI] [PubMed] [Google Scholar]
- 41.Badouel C, McNeill H. SnapShot: The hippo signaling pathway. Cell. 2011;145:484–484.e1. doi: 10.1016/j.cell.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Araki N, Takeshima H, Saya H. Neurofibromatosis type 2 (NF2) Gan To Kagaku Ryoho. 1997;24:1427–1431. (In Japanese) [PubMed] [Google Scholar]
- 43.Fossdal R, Jonasson F, Kristjansdottir GT, Kong A, Stefansson H, Gosh S, Gulcher JR, Stefansson K. A novel TEAD1 mutation is the causative allele in Sveinsson's chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration) Hum Mol Genet. 2004;13:975–981. doi: 10.1093/hmg/ddh106. [DOI] [PubMed] [Google Scholar]
- 44.González-Alonso P, Zazo S, Martín-Aparicio E, Luque M, Chamizo C, Sanz-Álvarez M, Minguez P, Gómez-López G, Cristóbal I, Caramés C, et al. The hippo pathway transducers YAP1/TEAD induce acquired resistance to trastuzumab in HER2-positive breast cancer. Cancers (Basel) 2020;12:1108. doi: 10.3390/cancers12051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigiracciolo DC, Nohata N, Lappano R, Cirillo F, Talia M, Scordamaglia D, Gutkind JS, Maggiolini M. IGF-1/IGF-1R/FAK/YAP transduction signaling prompts growth effects in triple-negative breast cancer (TNBC) cells. Cells. 2020;9:1010. doi: 10.3390/cells9041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin C, Xu X. YAP1-TEAD1-Glut1 axis dictates the oncogenic phenotypes of breast cancer cells by modulating glycolysis. Biomed Pharmacother. 2017;95:789–794. doi: 10.1016/j.biopha.2017.08.091. [DOI] [PubMed] [Google Scholar]
- 48.Zheng X, Han H, Liu GP, Ma YX, Pan RL, Sang LJ, Li RH, Yang LJ, Marks JR, Wang W, Lin A. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36:3325–3335. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han C, Liu Y, Dai R, Ismail N, Su W, Li B. Ferroptosis and its potential role in human diseases. Front Pharmacol. 2020;11:239. doi: 10.3389/fphar.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi Y, Kasukabe T, Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52:1011–1022. doi: 10.3892/ijo.2018.4259. [DOI] [PubMed] [Google Scholar]
- 52.Ou W, Mulik RS, Anwar A, McDonald JG, He X, Corbin IR. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597–607. doi: 10.1016/j.freeradbiomed.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS, Lee HC, Tseng LM. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget. 2017;8:114588–114602. doi: 10.18632/oncotarget.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker A, Singh A, Tully E, Woo J, Le A, Nguyen T, Biswal S, Sharma D, Gabrielson E. Nrf2 signaling and autophagy are complementary in protecting breast cancer cells during glucose deprivation. Free Radic Biol Med. 2018;120:407–413. doi: 10.1016/j.freeradbiomed.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr. 2008;87:44–55. doi: 10.1093/ajcn/87.1.44. [DOI] [PubMed] [Google Scholar]
- 56.Gwangwa MV, Joubert AM, Visagie MH. Effects of glutamine deprivation on oxidative stress and cell survival in breast cell lines. Biol Res. 2019;52:15. doi: 10.1186/s40659-019-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: Implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12:1085–1097. doi: 10.4161/cbt.12.12.18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rojas-Morales P, Tapia E, Pedraza-Chaverri J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal. 2016;28:917–923. doi: 10.1016/j.cellsig.2016.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing datasets generated for this study can be found in the Gene Expression Omnibus (GEO) DataSets (https://www.ncbi.nlm.nih.gov/gds) under the accession no. GSE153830. Cell proliferation datasets are available from the corresponding author upon reasonable request.