Abstract
Many studies have demonstrated the ability of chronically implanted multielectrode arrays (MEAs) to extract information from the motor cortex of both humans and nonhuman primates. Similarly, many studies have shown the ability of intracortical microstimulation to impart information to the brain via a single or a few electrodes acutely implanted in sensory cortex of nonhuman primates, but relatively few microstimulation studies characterizing chronically implanted MEAs have been performed. Additionally, device and tissue damage have been reported at the levels of microstimulation used in these studies. Whether the damage resulting from microstimulation impairs the ability of MEAs to chronically produce physiological effects, however, has not been directly tested. In this study, we examined the functional consequences of multiple months of periodic microstimulation via chronically implanted MEAs at levels capable of evoking physiological responses, that is, electromyogram (EMG) activity. The functionality of the MEA and neural tissue was determined by measuring impedances, the ability of microstimulation to evoke EMG responses, and the recording of action potentials. We found that impedances and the number of recorded action potentials followed the previously reported trend of decreasing over time in both animals that received microstimulation and those which did not receive microstimulation. Despite these trends, the ability to evoke EMG responses and record action potentials was retained throughout the study. The results of this study suggest that intracortical microstimulation via MEAs did not cause functional failure, suggesting that MEA-based microstimulation is ready to transition into subchronic (<30 days) human trials to determine whether complex spatiotemporal sensory percepts can be evoked by patterned microstimulation.
Keywords: micro-electrode array, motor cortex, feline, electromyogram, impedance, microstimulation
Introduction
Multielectrode arrays (MEAs) are a promising technology for use in intracortical neuroprosthetics. For such devices to be clinically successful in recording applications, MEAs must be able to acquire sufficient neural data to control an effector device, such as a prosthetic arm, for the life span of the patient. While the action potential recordings currently obtained using MEAs are not stable over these timeframes (Dickey et al., 2009; Linderman et al., 2006; Suner et al., 2005), studies have shown that having well-isolated action potential recordings is not necessary for successfully decoding movements (Fraser et al., 2009; Rivera-Alvidrez et al., 2010). Numerous studies have also demonstrated the utility of MEAs for intracortical motor prosthetic applications in nonhuman primates (Aggarwal et al., 2009; Musallam, 2004; Nicolelis, 2003; Santhanam et al., 2006; Schwartz et al., 2006; Taylor, 2002; Wessberg, 2000). Further, chronically implanted MEAs have been used to control a variety of effectors in human patients (Hochberg and Donoghue, 2006; Hochberg et al., 2006; Serruya et al., 2002).
MEAs can also potentially be used as a high-resolution interface for the injection of charge directly into brain tissue. Such electrical microstimulation activates neurons, which can generate or modulate a neurophysiological process, such as sensory perception or movement. Numerous studies have evaluated the effects of acute microstimulation on different neural systems. These studies include experiments in the auditory system of rodents (Otto et al., 2005a; Rousche et al., 2003), and the somatosensory system (Romo et al., 1998), visual system (DeYoe, 2005; Murphey and Maunsell, 2007, 2008; Tehovnik, 2006; Tehovnik and Slocum, 2009), and motor system (Cooke, 2003; Fitzsimmons et al., 2007; Graziano, 2005; Graziano et al., 2002; Salzman et al., 1990; Schmidt and McIntosh, 1990) of nonhuman primates. Only a few studies, however, have investigated the consequences of chronic intracortical microstimulation to effect through a chronically implanted MEA (Bradley, 2004; Rousche and Normann, 1999). Human studies have demonstrated the ability to evoke percepts through intracortical microstimulation, but these experiments have been limited to intraoperative time frames (Bak et al., 1990) or to a single chronically implanted patient (Schmidt et al., 1996). Significant insight into the perceptual effects of microstimulation was obtained in these few human experiments, suggesting that further studies utilizing microstimulation via highdensity MEAs in subchronic clinical trials will be valuable. Prior to transitioning such research into human patients, however, the functional effects of microstimulation must be evaluated in a nonhuman system to demonstrate both safety and efficacy over chronic timescales.
The consequences of chronic microstimulation are disputed. In vitro stimulation studies and investigations of the histological response of brain tissue to microstimulation have shown that damage to both device and tissue can arise from microstimulation (Cogan, 2004; McCreery et al., 2010; Merrill et al., 2005; Negi et al., 2010; Troyk et al., 2004). Intracortical stimulating macroelectrodes, such as deep brain stimulating electrodes, have also been shown to cause tissue reactivity with the application of stimulation (Moss, 2004). Despite the damage caused by stimulating macroelectrodes, however, such devices can deliver functional stimulation for years, especially when stimulation is titrated to effect (Deuschl et al., 2006). While histological markers, including antibodies for reactive astrocytes and neurons can indicate that tissue is damaged, histology cannot indicate whether or not stimulation was effective or whether tissue response would have had an effect on performance. Additionally, histology can only be collected at experimental end points, which means that tissue response cannot be tracked through the course of multiple months of implantation and microstimulation without the sacrifice of many animals at several time points. In order to analyze the performance of microstimulation via microelectrodes over the course of a multiple-month implantation, electrophysiological markers of performance, rather than histological markers of safety, must beused. Electrophysiological markers, such as recorded action potentials, can demonstrate not only the viability of tissue in the vicinity of electrodes, but also that device integrity is maintained.
In this study, we investigate the in vivo performance of sputtered iridium oxide film (SIROF) metalized MEAs used to deliver chronic, physiologically effective intracortical microstimulation. Four felines were implanted with MEAs and two were stimulated to physiological effect, as measured by electromyogram (EMG). Functionality was evaluated using impedance measurements, electrophysiological recordings, and the ability of microstimulation to evoke EMG responses, to determine if device and/or tissue damage occurred with periodic microstimulation. Using these measures, we found that microstimulation efficacy could be maintained after several months of implantation and many microstimulation sessions. Further, we found that microstimulation did not appear to adversely impact either impedances or the ability to perform electrophysiological recordings over the course of the study.
Methods
Surgical procedures
Implantations and all other procedures were performed in accordance with protocols approved by the University of Utah Institutional Animal Care and Use Committee. Four felines (Felis catus) were used in this study. Felines each had an array implanted in motor cortex (Ghosh, 1997) so that applied stimulation would result in motor response measurable by EMGs recorded in either forelimb or hindlimb muscles. All implants were performed by the same clinical neurosurgeon to ensure consistency of implant technique and array placement. Anesthesia was induced using ketamine/xylazine and then continued using Isoflurane. Under sterile conditions, a midline incision was performed, and a craniotomy over the targeted area was made by means of a neurosurgical drill. The dura was reflected, and the array was pneumatically inserted into motor cortex (Rousche and Normann, 1992). Following implantation, the titanium percutaneous connector was attached to the skull using bone screws. A dural replacement (DuraGen, Integra Life Sciences, Plainsboro, NJ) was used to cover the array, and silicone polymer (Kwik-cast, World Precision Instruments, Sarasota, FL) was used to fill the craniotomy, if necessary. The scalp was sutured closed and the animal given at least 24 h to recover before data acquisition was attempted.
Electrode arrays
For Felines 1 and 2, arrays were obtained from Cyberkinetics, Inc. (Salt Lake City, UT) with electrode tips that had been coated by EIC Laboratories (Norwood, MA) with SIROFs. For Felines 3 and 4, 96-electrode SIROF Utah Electrode Arrays were commercially obtained from Blackrock Microsystems, Inc. (Salt Lake City, UT). Arrays were manufactured as described elsewhere (Jones et al., 1992) under Design Controls specified by the United States Food and Drug Administration. Electrodes were 1 mm long and spaced 400 μm apart. The SIROF used to coat the conductive electrode tips increased the charge injection capacity of the electrodes and reduced the possibility of electrode dissolution (Cogan, 2008; Cogan et al., 2009). Active electrode tips were ~40 μm in length, yielding ~4000 μm2 of SIROF surface area per electrode (Negi et al., 2010; VanWagenen, 2004). The remainder of the array was coated with Parylene-C insulation for electrical isolation and biocompatibility. A summary of arrays used is presented in Table 1.
Table 1.
Arrays used
| Animal | Array material, manufacture date, location | Implant date | Preinsertion impedances (kΩ) |
|---|---|---|---|
| Feline 1 | SIROF 2006, | January 2007 | Mean 16, Median 11, Min 4, Max 110 |
| Cyberkinetics/EIC | |||
| Feline 2 | SIROF 2006, | October 2008 | Mean 23, Median 14, Min 5, Max 160 |
| Cyberkinetics/EIC | |||
| Feline 3 | SIROF 2009, | July 2009 | Mean 50.4, Median 50, Min 42, Max 74 |
| Blackrock Microsystems | |||
| Feline 4 | SIROF 2010, | July 2010 | Mean 46.9, Median 47, Min 40, Max 63 |
| Blackrock Microsystems |
Four SIROF arrays were chronically implanted in feline motor cortex. Felines 3 and 4 were microstimulated, while Felines 1 and 2 were used to obtain comparison recording-only data for long-term patterns of 1 kHz impedance and electrophysiological measures of chronic performance.
Data acquisition
A 128-channel Cerebus data acquisition system (Blackrock Microsystems) was used to acquire neural data. The 96 channels of electrode data from the UEA were fed to a front-end amplifier using a Cereport patient cable.
Impedance measurements
One kilohertz (kHz) impedance measurements were made using a routine in the Cerebus data acquisition system. Briefly, a small sinusoidal current at 1 kHz was passed through a reference electrode, and impedance was simultaneously computed on all electrodes. Chronic impedance readings were taken throughout the multiple-month course of implantation in all four felines. Acute impedance readings were also taken pre- and post-stimulation for each microstimulation session in Felines 3 and 4.
Electrophysiological recordings
Neural recordings were obtained from awake felines to examine device performance over time both with and without the application of microstimulation. Felines were placed in a pet carrier inside an electrically shielded chamber to minimize noise, and connected to the Cerebus. Recordings were made at least weekly in all felines, as well as prior to and following every stimulation session in Felines 3 and 4. Recordings were made in several-minute sessions using band-pass filter settings of 0.3 Hz–7.5 kHz and sampled at 30 kHz in Felines 1–3, and band-pass filter settings of 0.3 Hz–2.5 kHz sampled at 10 kHz in Feline 4 to reduce file size.
Electromyography
EMGs were used periodically in Felines 3 and 4 to test the ability to evoke physiological responses via intracortical microstimulation. Following a control neural data recording, the animal was anesthetized with Telazol administered intramuscularly at 0.01 mg/kg. Sterile, clinical fine-wire electrodes were placed in the biceps femoris muscle of Feline 3 and either the triceps or extensor carpi muscle of Feline 4. Reference electrodes were placed subcutaneously near the intramuscular electrode. EMG activity in response to stimulation was recorded at 2 kHz using the Cerebus data acquisition system described above. For the EMG sessions performed prior to 29/Sep/2009, a MA300-18-002 commercial EMG system (Motion Lab Systems, Baton Rouge, LA) was used. For subsequent EMG sessions, a one-channel AC differential amplifier (DAM 80, World Precision Instruments) was employed. Stimulus markers were output to Cerebus using in-house LabView code (National Instruments, Austin, TX).
Microstimulation
Six daisy-chained RX-7 stimulators (Tucker-Davis Technologies Inc., Alachua, FL) were used for microstimulation. These stimulators are capable of applying current-controlled waveforms with a voltage excursion of −24 to 24 V. Stimulation was controlled with in-house Matlab (The Mathworks, Natick, MA) and LabView code. Microstimulation was applied to Felines 3 and 4.
Stimulus waveforms
Stimuli were applied in charge-balanced square waveforms to prevent charge buildup (Merrill et al., 2005). For each session, pulses of a square, charge-balanced, biphasic waveform at 0.2 ms per phase were applied in trains of 25, 50, or 100 pulses at 100 Hz, settings chosen for their efficacy at evoking responses in perceptual microstimulation studies (Table 5). Trains of pulses were applied in rounds such that every electrode was stimulated at a given amplitude before the current used to stimulate was increased.
Table 5.
Summary of selected intracortical microstimulation studies
| Citation | Model system | Electrode type | Reporter of efficacy | Current range applied | Frequency (Hz) | Impedance range 1 kHz | Damage? |
|---|---|---|---|---|---|---|---|
| Murphey and Maunsell (2008) | Macaque | Platinum/iridium, single electrode | Behavioral task (visual) | Up to 50 μA | 200 | 0.2–1.5 MΩ | No |
| Murphey and Maunsell (2007) | Macaque | Platinum/iridium, single electrode | Behavioral task (visual) | 3–12 μA | 200 | 0.2–1.5 MΩ | No |
| McCreery et al. (2010) | Feline | Activated iridium, 16 microelectrode array | N/A | 10–20 μA | 50 | Not reported | Yes |
| McCreery et al. (2002) | Feline | Activated iridium, 16 microelectrode array | Evoked potentials in medulla | 0–32 μA | 50 | 0.2–1.5 MΩ | No |
| Graziano (2005) | Macaque | Tungsten, single microelectrode | Arm movements | 5–100 μA | 100–250 | 1–2 MΩ | No |
| Graziano et al. (2002) | Macaque | Tungsten, single microelectrode | Arm movements | Up to 150 μA | 200 | 0.5–2 MΩ | No |
| Tehovnik et al. (2003) | Macaque | Platinum/iridium, single microelectrodes | Behavioral task (visual) | Up to 30 μA | 200 | 1–2 MΩ | No |
| Tehovnik et al. (2002) | Macaque | Platinum/iridium, single microelectrodes | Behavioral task (visual) | Up to 40 μA | 200 | 1–1.5 MΩ | No |
| Romo et al. (2000) | Macaque | Platinum/tungsten, seven microelectrodes | Behavioral task (flutter discrimination) | 65–100 μA | 50–200 | 1–1.5 MΩ | No |
| Romo et al. (1998) | Macaque | Platinum/tungsten, seven microelectrodes | Behavioral task (flutter discrimination) | 65–100 μA | 50–200 | 1–1.5 MΩ | No |
| Rousche and Normann (1999) | Feline | Platinum, 96 electrode Utah Electrode Array | Behavioral task (auditory) | 100 μA | 25–2000 | 30–149 kΩ | No |
| Torab et al. (2011) | Macaque | SIROF, 96 electrode Utah Electrode Array | Behavioral task (visual) | 0–96 μA | 200 | 40 kΩ−2 MΩ | No |
| Bradley (2004) | Macaque | Iridium, 192 electrode array | Behavioral task (visual) | 12–20 μA | 200 | 80 kΩ−1.6 MΩ | No |
| Bak et al. (1990) | Human | Iridium, arrays of 1–3 electrodes | Verbal report (visual percepts) | 20 μA-2 μA | 100 | Not reported | No |
| Schmidt et al. (1996) | Human | Iridium, 12 single electrodes, 13 paired electrodes | Verbal report (visual percepts) | 1–80 μA | 200 | Not reported | No |
The stimulus amplitudes and parameters applied in this study were similar to those used in other microstimulation experiments which evoked sensory percepts. Shown are sample studies for comparison.
All-channel stimulation
Stimulation was periodically applied to the feline on all 96 electrodes in sequence in rounds of each indicated amplitude in order to determine whether stimulation across all channels affected functionality. In none of these sessions did the feline respond in any manner (e.g., vocalizations or movements) that would indicate an adverse reaction to stimulation while awake (listed as all-channel sessions in Table 2). All-channel stimulation was also performed with the feline anesthetized in order to perform EMG (listed as all-channel EMG sessions in Table 2). Microstimulating current was increased in rounds over the course of the session to determine the threshold current required to evoke EMG responses on each electrode.
Table 2.
Summary of microstimulation sessions
| Feline | Figure label (Fig. 5a or c) | Date | Description | Stimulus parameters | Amplitudes | Electrodes |
|---|---|---|---|---|---|---|
| 3 | 1 | 14/Aug/2009 | All-electrode (EMG efficacy test) | 5 trains, 50 pulses | 5–50 μA in steps of 5 μA | All |
| 3 | 2 | 29/Aug/2009 | All-electrode (EMG efficacy test) | 5 trains, 50 pulses | 5–35 μA, steps of 1-μA | 43,78,84 |
| 3 | 3 | 01/Sep/2009 02/Sep/2009 |
All-electrode stimulation | 2 rounds, 5 trains, 25 pulses | 20/30, 30/35 μA | All |
| 3 | 4 | 07/Sep/2009 | Test of parallel stimulation | 5 trains, 25 pulses | 25 μA | Distributed pattern of 72 electrodes |
| 3 | 5 | 14/Sep/2009 | All-electrode stimulation | 5 trains, 25 pulses | 20–35 μA in steps of 5 μA | All |
| 3 | 6 | 30/Oct/2009 | All-electrode (EMG efficacy test) | 10 trains, 25 pulses | 20–40 μA in steps of 5 μA | All |
| 3 | 7 | 31/Oct/2009–09/Nov/2009 | 15/20 μA on seven electrodes | 100 trains of 25 pulses | 15/20 μA | 88,89,90,91/79,80,81 |
| 3 | 15/20 μA (Teal bars) | 11/Oct/2009–03/Dec/2009 (23 sessions) | 15/20 μA paradigm | 200 trains (two rounds), 25 pulses | 15 or 20 μA | 20 μA—Electrodes 79,80,81 15 μA—Electrodes 26,28,29,31,39,40,41,43,89,90,91,92 |
| 3 | 8 | 04/Dec/2009 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 20–40 μA in steps of 5 μA | All |
| 3 | 20/25/30/25 μA (Blue bars) | 05/Dec/2009–13/Jan/2009 (20 sessions) | 20/25/30/25 μA paradigm | 200/electrode (two rounds), 25 pulses | 20, 25, 30, or 35 μA | 20 μA—Electrodes 39,40,41,43 25 μA—Electrodes 79,80,81 30 μA—Electrodes 89,90,91,92 35 μA—Electrodes 26,28,29,31 |
| 3 | 9 | 14/Jan/2010 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 100 μA | All |
| 4 | 1 | 17/Sep/2010 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 5–50 μA in steps of 5 μA | All |
| 4 | 2 | 28/Sep/2010 | Test for feline response | 5 trains of 25 pulses | 5–50 μA in steps of 5 μA | All |
| 4 | 3 | 01/Oct/2010 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 50–100 μA in steps of 10 μA | All |
| 4 | 4 | 28/Oct/2010 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 20–60 μA in steps of 10 μA | All |
| 4 | 5–7 | 24/Nov/2010–30/Nov/2010 | 25 μA paradigm | 100 trains of 25 pulses | 25 μA | 1,10,11,32,33,42,43,66,67,75,76,79,80,88,89 |
| 4 | 8 | 01/Dec/2010 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 40–100 μA in steps of 10 μA | All |
| 4 | 9 | 22/Dec/2010 | All-electrode (EMG efficacy test) | 5 trains of 25 pulses | 50–100 μA in steps of 10 μA | All |
Microstimulation was applied to Felines 3 and 4 according to several different stimulus paradigms depending on the goals of microstimulation, for example, to evaluate efficacy or to determine the effects of periodic stimulation on device functionality.
Test of parallel stimulation
To test the effects of synchronous stimulation on multiple electrodes, such as might be applied during a failure of patterned microstimulation, stimulation was applied simultaneously at 25 μA via 72 electrodes. The animal was disconnected from the experimental apparatus following five trains of stimulation.
Chronic stimulation
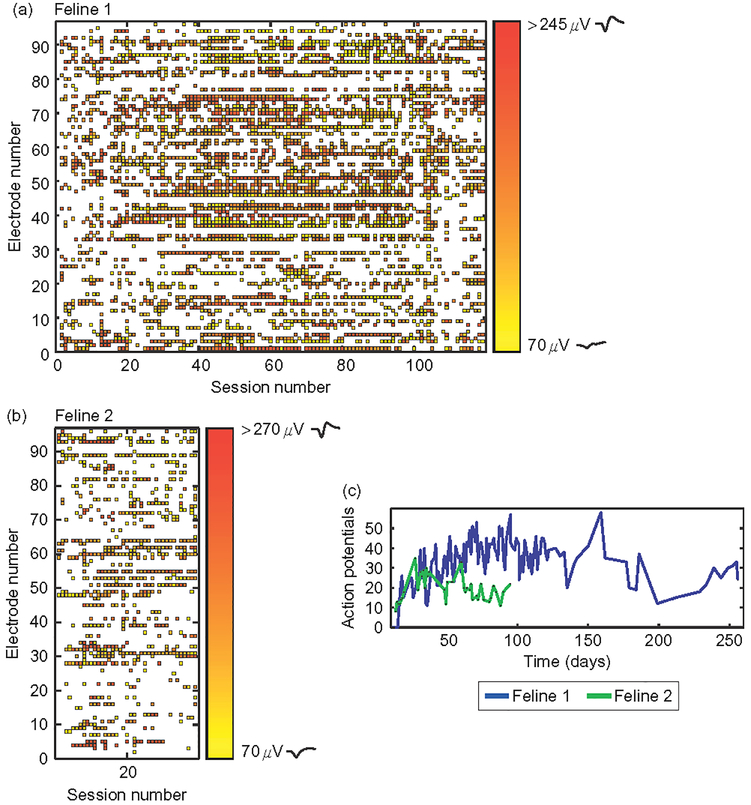
Chronic stimulation sessions were applied in two paradigms, low (15/20 μA) and high (20/25/30/25 μA), on 15 selected electrodes of the MEA in Felines 3 and 4 (Table 2). These specific 15 electrodes were chosen for microstimulation based on two factors: (1) Spatial distribution of charge application. (2) To stimulate on some electrodes which recorded action potentials and some electrodes which did not (Figs. 5a and 5c). Chronic microstimulation was applied at amplitudes ranging from 15 to 35 μA over the course of several months (Table 2). Stimulation levels were adjusted based on the results of EMG sessions.
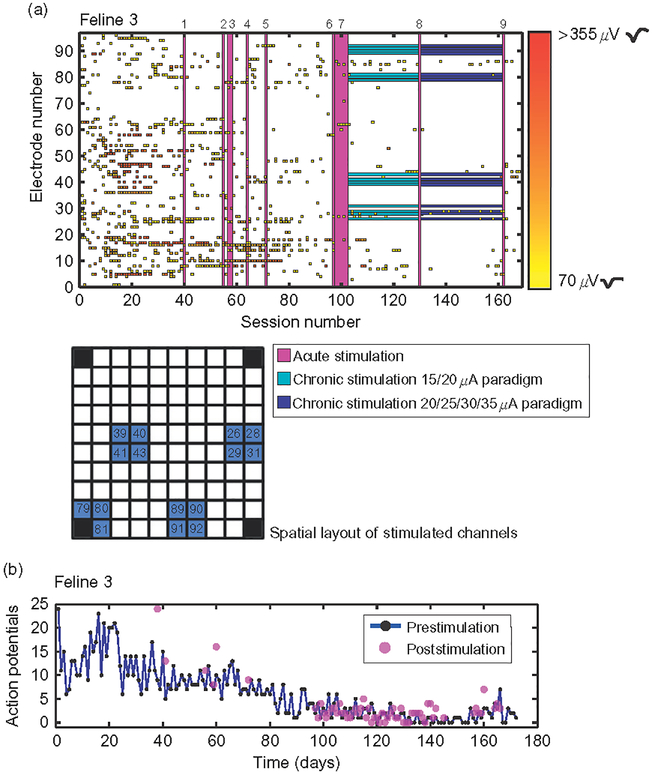
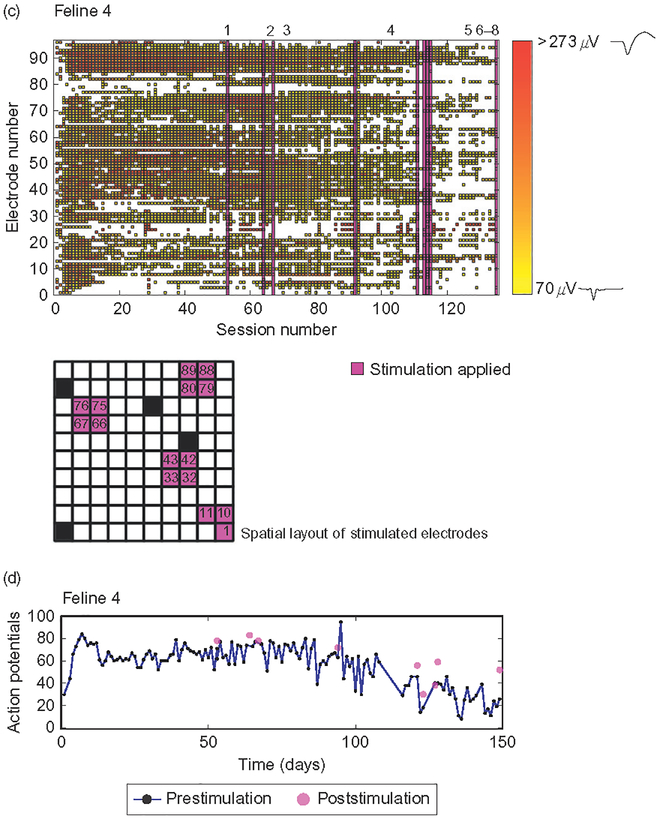
Fig. 5.
Microstimulation did not have a clear effect on the number or distribution of action potentials across the array over chronic timescales. (a and c) Raster of action potentials in Felines 3 and 4, color coded by amplitude over recording sessions. Purple bars represent an acute stimulation session, detailed by number in Table 2. Teal bars in Feline 3 represent chronic stimulation at the low stimulation paradigm listed in Table 2, while blue bars represent the high stimulation paradigm applied to the 15 electrodes highlighted. Grids show the spatial layout of electrodes stimulated, blue in Feline 3 and purple in Feline 4. Waveforms shown on color bar are samples of action potential shapes isolated at low and high amplitudes. (b, d) Number of action potentials recorded both over time before (blue) and after (purple) microstimulation in Felines 3 and 4. While the number of action potentials overall did decrease significantly by the end of the experiment in both felines (p<0.01, Student’s t-test), the number of action potentials isolated acutely, that is, before and after individual stimulation sessions, did not decrease (p<0.05, Student’s t-test).
Data analysis
All analyses and statistical tests were performed using custom Matlab code.
Impedance data
Electrodes which recorded 1 kHz impedance values over 2 MΩ on all days were considered to be out-of-specification and not included in any further analyses. Impedance values over 2 MΩ on a single day were replaced with a 2 MΩ ceiling value. Z-scored impedances were computed by electrode in each feline. The value for an impedance reading of a single electrode in a single dataset was compared to mean value for that electrode across all datasets, and then divided by the standard deviation of all values recorded on that particular electrode. Z-scores were averaged across all electrodes for each dataset. For pre- and poststimulation session impedance readings, median impedances and standard errors were computed. Wilcoxon’s signed rank test was applied to demonstrate significant drops in median impedance values immediately pre- and post-all-channel-stimulation in Feline 3. The Komolgorov–Smirnov test was applied in Feline 4 due to the small number of pre/post-stimulation datasets. Wilcoxon’s signed rank test was used to demonstrate the significance of acute impedance drops on 15 stimulated channels in both Felines 3 and 4. In Feline 3, the median impedance drops between the first and second chronic stimulation paradigms were compared using Wilcoxon’s signed rank test on the last 20 datasets of the first paradigm and all 20 datasets of the second paradigm. Wilcoxon’s signed rank test was also used to determine significance of drops in impedance over time. Impedances for the first and last 30 datasets were compared in Felines 1, 3, and 4, and the first and last 10 datasets were compared in Feline 2 due to the lower number of recording sessions.
Electrophysiological recording data
Action potential recordings were sorted using a PCA-based t-distribution algorithm (Shoham, 2003). A threshold for action potentials was subsequently imposed at 70 μV. t-tests were performed to quantify changes in the number of electrodes which recorded well-isolated action potentials over time (using the first and last 30 datasets in Felines 1, 3, and 4 and the first and last 10 datasets in Feline 2). Student’s t-test was also applied to acute pre- and poststimulation number of electrodes which recorded action potentials. The distribution of number of electrodes which recorded action potentials across all microstimulation sessions during pre-stimulation recordings was compared to the immediate post-stimulation distribution for Felines 3 and 4.
Electromyographic data
EMG data was rectified, and a boxcar filter (size 50 ms, stepped per sample) was applied across all recorded twitches evoked by a given amplitude of stimulus via a given electrode on EMG response traces to demonstrate the population-level response to a train of stimulation. The Komolgorov–Smirnov test was applied to the rectified averaged EMG data at −400 to −100 ms prior, and the +100 to +400 ms following, the application of microstimulation.
Results
Chronic 1 kHz impedance
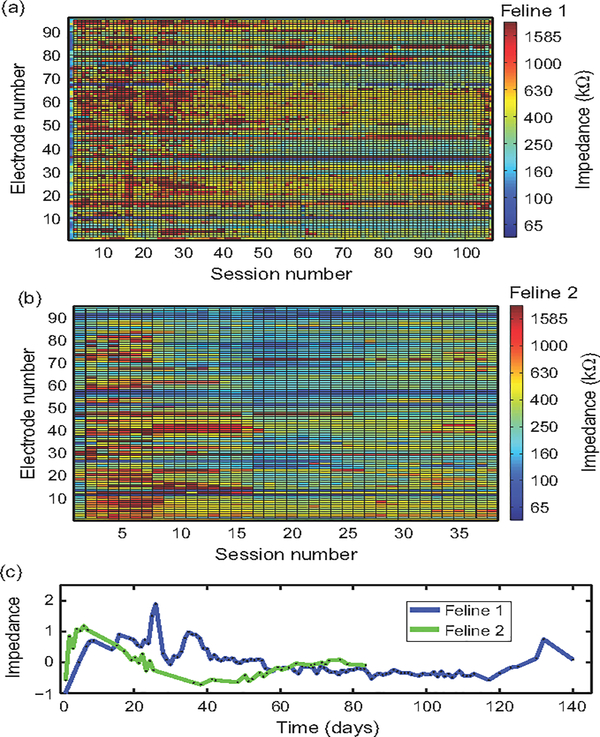
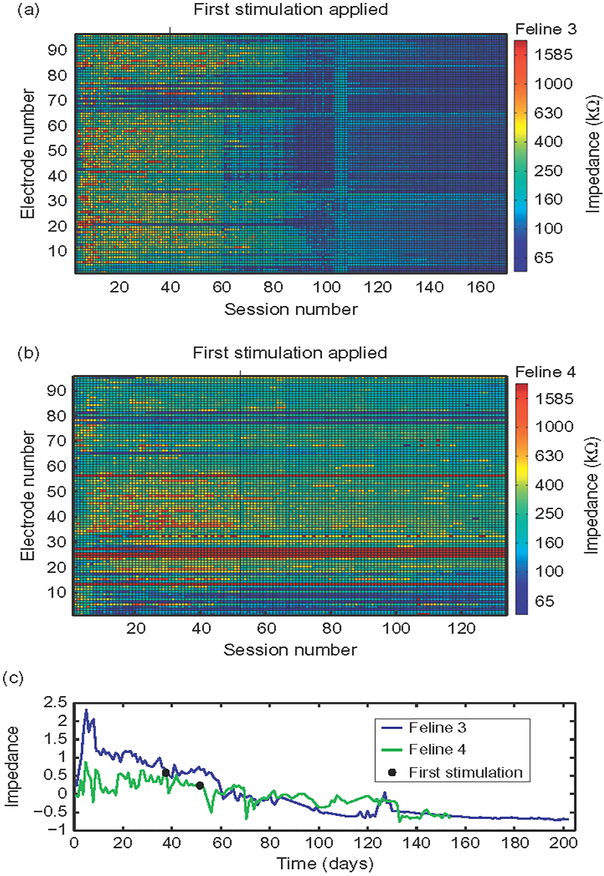
Impedances followed a pattern of increasing after implantation to a peak within the first month in all felines (Figs. 1 and 2). This increase was followed by a decrease over time. The first 30 impedance measurement datasets had higher mean impedance than the last 30 datasets in Felines 1, 3, and 4 (p<0.001, Wilcoxon’s signed rank test). Due to the smaller number of datasets in Feline 2, only the 10 first and last datasets were compared, with the same result (p<0.05, Wilcoxon’s signed rank test). A summary of impedances is included in Table 3. Impedances over 2 MΩ were considered to be out-of-specification. In Feline 1, three electrodes were out-of-specification. In Feline 4, seven electrodes were out-of-specification. Felines 2 and 3 did not have any out-of-specification electrodes.
Fig. 1.
1 kHz impedance is variable over chronic timescales. (a, b) 1 kHz impedance measurements were made in the two nonstimulated felines over 8 and 3 months of implantation, respectively. Shown are impedance measurements colored by value. (c) Mean Z-scored impedance over time, in days, for both felines. Both felines exhibited a pattern of increase in impedance following implant that peaked within the first month, followed by a decrease toward preimplantation values (p<0.01, Wilcoxon’s signed rank test).
Fig. 2.
Microstimulation did not change 1 kHz impedance patterns. (a, b) 1 kHz impedance measurements, colored by value, across all 96 electrodes of the MEA in Felines 3 and 4. Black lines and circles indicate the first application of stimulation. (c) Mean Z-scored impedance over time (prestimulation values shown) over time in days. In both Feline 3 (blue) and Feline 4 (green), impedances dropped toward baseline over time (p<0.01, Wilcoxon’s signed rank test).
Table 3.
Summary of impedances during implantation
| Feline | Mean (kΩ) | Median (kΩ) | Standard deviation (kΩ) | Recording sessions | Electrodes |
|---|---|---|---|---|---|
| 1 | 462 | 353 | 144 | 107 | 93 |
| 2 | 358 | 285 | 161 | 39 | 96 |
| 3 | 157 | 91 | 78 | 168 | 96 |
| 4 | 244 | 99 | 73 | 134 | 89 |
Impedance values were used as a measure of device performance over the course of implantation and microstimulation. Shown here is summary data on impedances from all four MEAs used in this study.
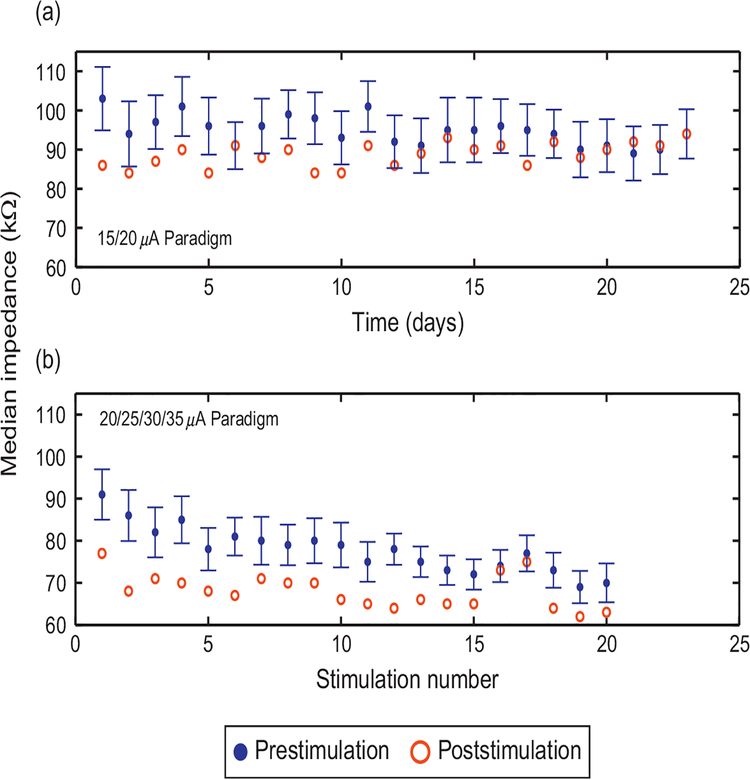
Pre- and post-stimulation impedance
Median 1 kHz impedance decreased acutely on 19 of 23 days with the application of daily microstimulation (low paradigm) on 15 electrodes in Feline 3 (p<0.01, Wilcoxon’s signed rank test, n=23 sessions; Fig. 3a), as well as acutely on 20 out of 20 days when stimulation (high paradigm) was not consistently applied on consecutive days (p<0.01, Wilcoxon’s signed rank test, n = 20 sessions; Fig. 3b). Median acute impedance drops were larger during the high chronic stimulation paradigm than the low paradigm (p<0.01, Wilcoxon’s signed rank test). Parallel microstimulation on 72 channels of the MEA in Feline 3 also led to an acute decrease in median impedance (p<0.001, Wilcoxon’s signed rank test, n = 96 electrodes) on all electrodes of the MEA. Median impedance across the 96 electrodes of the MEA decreased when stimulation was applied simultaneously on 72 electrodes in Feline 3 (Feline 3, p<0.05, n = 8 sessions, Wilcoxon’s signed rank test). In Feline 4, only five impedance readings were made following all-electrode stimulation sessions. Median impedance decreased following all-electrode stimulation (Komolgorov–Smirnov test, p<0.01, n = 5 sessions). Median impedance also decreased significantly on the 15 electrodes that passed current during the three applications of the 25 μA stimulation paradigm in Feline 4 (p<0.001, Wilcoxon’s signed rank test, 15 electrodes over three sessions, n = 45).
Fig. 3.
Microstimulation leads to acute drops in 1 kHz impedance. Median impedance before (blue, with standard error bars) and after (red) microstimulation on the 15 electrodes that passed current into tissue is shown for (a) the low stimulation paradigm, applied daily for 23 days and (b) the high stimulation paradigm, applied 20 times over a 6-week course. Impedance decreased significantly following microstimulation for both stimulus paradigms (p<0.01, Wilcoxon’s signed rank test). Impedances drops were larger for the second paradigm (p<0.01, Wilcoxon’s signed rank test).
Action potential recordings
In both nonstimulated and microstimulated felines (Figs. 4 and 5), the number of action potentials recorded from motor cortex followed a previously observed pattern of initially increasing followed by a decrease in numbers over the course of months (Suner et al., 2005). This chronic pattern of action potential recordings was similar between microstimulated and non-microstimulated felines. In nonstimulated Feline 1, the number of action potentials recorded during the plateau period (sessions 30–60) was greater than that recorded during the fade-out period (last 30 datasets, p<0.05, Student’s two-sample t-test). This same observation was made in the microstimulated Felines 3 and 4 (p<0.05, Student’s two-sample t-test). In non-microstimulated Feline 2, in which only 39 data acquisition sessions were performed, there was no significant change in the number of action potentials recorded between the first and last 10 datasets. All-electrode microstimulation, for example, for feline response tests and EMG sessions, did not lead to an acute decrease in the number of well-isolated action potentials recorded in either microstimulated feline (p<0.05, onetailed Student’s t-test; Feline 3, prestimulation mean=5 action potentials, poststimulation =8 action potentials, n=8 sessions; Feline 4, prestimulation mean=60 action potentials, poststimulation=70 action potentials, n =6 sessions). The number of action potentials recorded prior to multielectrode synchronous stimulation in Feline 3 was 12, while the number recorded poststimulation was 13, demonstrating that acute parallel synchronous stimulation did not preclude recording ability. A summary of action potential recording data in all four felines is included in Table 4.
Fig. 4.
Action potential amplitudes over chronic timescales. (a and b) Raster plots of thresholded, sorted action potential recordings across the array for the duration of the study for Felines 1 and 2. Each square represents the mean of the furthest cluster from noise as isolated by principle component analysis. Waveforms shown on color bar are samples of action potential shapes isolated at low and high amplitudes. (c) Number of isolated action potentials recorded over time in days. There was an initial increase in the number of action potentials recorded, followed by a significant decrease in Feline 1 by the end of the 8 months of implantation (p<0.01, Student’s t-test).
Table 4.
Summary of thresholded action potential amplitudes
| Feline | Number of recording sessions | Mean number of electrodes which recorded action potentials, all sessions | Mean number of electrodes which recorded action potentials, first 30 sessions | Mean number of electrodes which recorded action potentials, last 30 sessions |
|---|---|---|---|---|
| 1 | 123 | 34 | 38 | 33 |
| 2 | 39 | 20 | 18 (first 10 sessions) | 17 (last 10 sessions) |
| 3 | 168 | 6 | 9 | 2 |
| 4 | 135 | 57 | 73 | 40 |
Electrophysiological data recorded from each MEA was used as a chronic measure of device performance. Shown are summary statistics for all four MEAs used in this study.
Microstimulation in awake felines
For awake stimulation sessions in both Felines 3 and 4, microstimulation was applied to one electrode at a time without anesthesia. No adverse behavioral or physiological responses to such stimulation were observed. For multichannel parallel stimulation in Feline 3, stimulation was synchronously appliedto72electrodes of the MEA (Table 2;07/Sep/2009). A bilateral, tonic seizure of <1 min duration resulted from this microstimulation paradigm. Full ambulatory recovery occurred within 5 min of ictus. The animal exhibited neither behavioral deficits nor spontaneous seizures in the 5 months between seizure induction and termination of the experiment.
Microstimulation to effect
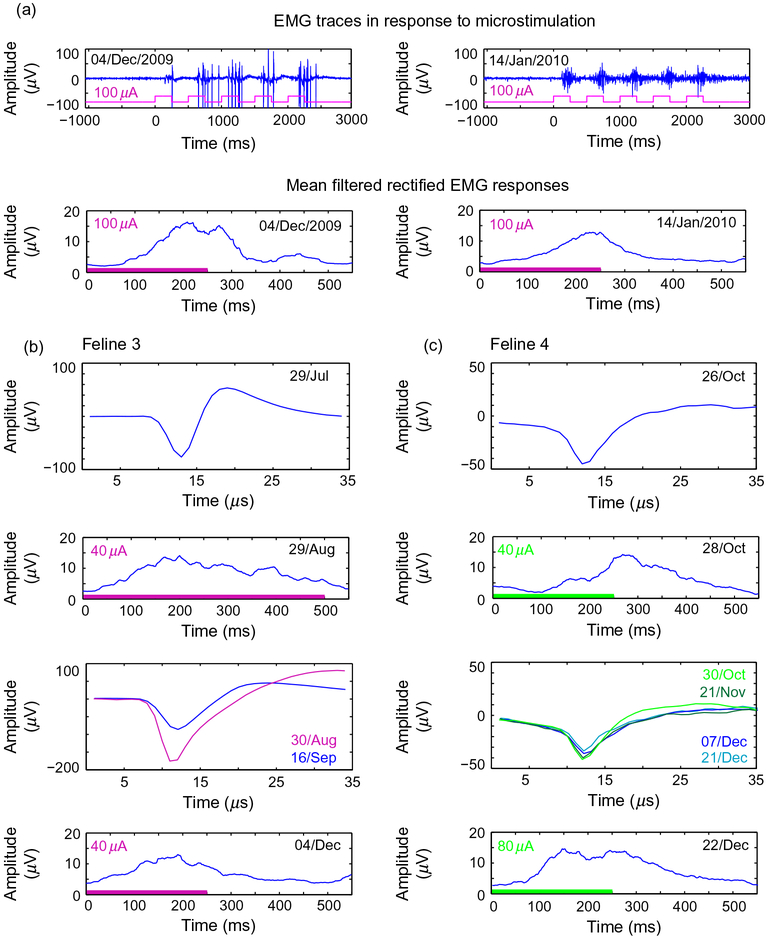
Chronic stimulation was applied on the same 15 electrodes (shown in Fig. 5a) for a total of 43 days in Feline 3 (Table 2). It was possible to evoke EMG responses via an electrode that performed chronic microstimulation, both after it delivered 23 sessions of stimulation at 15 μA, and after 20 sessions at 30 μA (Fig. 6a), though this electrode did not record action potentials between EMG sessions. Of the 15 electrodes used for chronic stimulation in Feline 3, four recorded action potentials during the time that chronic stimulation was applied. EMG responses could also be evoked several months apart in time via electrodes that recorded action potentials in both microstimulated felines (Fig. 6b and c), though these electrodes did not deliver chronic microstimulation (three electrodes in Feline 3, two electrodes in Feline 4). In Feline 3, 13 electrodes evoked electromyographic responses at 10–35 μA on 14/Aug/2009, seven electrodes evoked responses between 20 and 40 μA on 04/Dec/2009, and 11 electrodes evoked responses at 100 μA on 14/Jan/2009 (p<0.05, Komolgorov–Smirnov test). While responses were evoked at currents of <40 μA during the second month of implantation in Feline 3, 100 μA current was required to evoke responses during the sixth month of implantation. Currents higher than 100 μA were not tested due to the voltage excursion limitation of the stimulator. Of the electrodes which evoked responses in Feline 3, two consistently evoked responses throughout all EMG sessions. In Feline 4, eight electrodes evoked EMG responses at 60–80 μA on 01/Oct/2010, two electrodes evoked responses at 60 μA on 28/Oct/2010, and eight electrodes evoked responses at 60–100 μA on 22/Dec/2010. Of the electrodes that evoked EMG responses in Feline 4, two retained the ability to evoke responses throughout the experiments performed.
Fig. 6.
Chronically stimulated electrodes maintained the ability to stimulate to effect for multiple months. (a) Sample EMG recordings from both 04/Dec/2009 and 14/Jan/2009 evoked by stimulation on and electrode which applied current at 15 and 30 μA, according to the chronic paradigms detailed in Table 2, with the respective filtered mean rectified EMG responses below. Blue represents the recorded EMG response, while purple represents the pulses applied. (b) Sample action potentials recorded before and between electromyographic sessions during which responses were evoked at 40 μA in Feline 3. Colored bars along the time axis mark the application of microstimulation. (c) Sample action potentials recorded before and between EMG sessions in Feline 4. Dates of action potential recordings or stimulation and amplitude of stimulation applied are noted in each panel.
Discussion
Many studies have shown that intracortical microstimulation to effect can be performed in nonhuman primate, feline, and other model systems for many sensory modalities (see Table 5). In most of these studies, stimulation was performed acutely on a single electrode in order to evaluate behavioral responses to stimulation. Few of these studies examined the chronic response to stimulation, and still fewer evaluated the long-term consequences of stimulation in a functional context. In this study, we found that chronic, intracortically implanted MEAs could stimulate to effect on multiple electrodes over the course of several months. By evaluating device performance using electrophysiological data, stimulation ability, and 1 kHz impedance, we found that effective stimulation via chronically implanted MEAs did not appear to destroy either the device or underlying cortical tissue.
The stimulation applied in this study was in the 5–100 μA range. While this exceeds the stimulus amplitudes that have been used in many in vitro studies that reported electrode damage with long-term pulsing, and several rodent studies that evoked behavioral response (Cogan, 2004; Houweling and Brecht, 2007; McCreery et al., 2010; Tehovnik, 1996), it is equivalent to the stimulus amplitudes that have been used to evoke perceptual or other effects in felines, macaques, and humans (see Table 5), as well as in other rodent studies (Otto et al., 2005b; Tehovnik, 1996). Some of these studies have noted damage to the tissue surrounding the electrodes using histological markers. While these histological markers indicate that tissue surrounding the electrodes reacted to stimulation, they cannot demonstrate whether or not stimulation affected device performance. By using electrophysiological markers such as recorded action potentials and ability to evoke physiological responses, we were able to demonstrate sustained functionality, including action potential recording, which implies tissue viability in the recording radius of the microelectrode tips.
The chronic changes in impedance observed in our experiments followed a pattern which has been previously noted in the literature. Mean impedance of passively implanted microelectrodes tends to increase over the first weeks of implantation, followed by a decrease over time (Williams et al., 2007). The causes of this pattern remain unclear, though it could result from ongoing processes of the tissue response to implanted devices. We observed this trend during the first weeks of implantation, followed by a continued decrease in impedance throughout the duration of the experiments in both passively implanted felines. The same phenomenon has also been observed in deep brain stimulation studies, where current was applied via chronically implanted macroelectrodes over time (Lempka et al., 2009). Important to note is that the impedances of both microstimulated and nonstimulated felines followed the same general pattern over time. The application of microstimulation did not drive electrode impedances out-of-specification, that is, >2 MΩ, as might be expected in the case of device failure or catastrophic tissue damage over time.
We also observed short-term decreases in impedance on stimulating electrodes with the application of microstimulation, which are reported to occur both in vivo and in vitro (Otto et al., 2006). These changes in impedance could reflect tissue response, for example, disruption of the glial scar by microstimulation. Impedance changes may also reflect processes of device damage known to occur with stimulation, such as dissolution of metallization or damage to electrode insulation. Finally, decreases could also indicate processes of electrochemical activation, which would change the valence state of the stimulating SIROF. The repeatability of the short-term impedance drops, with subsequent recovery, suggests that reversible electrochemical activation rather than cumulative damage may be reflected by these short-term changes in impedance values. The second chronic microstimulation paradigm in Feline 3 yielded larger acute impedance drops. This could be a result of either the increased current used to stimulate, or the increased time between stimulation sessions which would allow impedances to return to baseline. This further supports the idea that reversible electrochemical processes, rather than damage, contribute to observed impedance drops. Further, the maintained ability to stimulate and record indicates that any damage that may have occurred with microstimulation did not preclude device functionality. Importantly, catastrophic changes in impedance, which might indicate device damage or tissue death, did not occur with the application of stimulation.
The microstimulation amplitudes used in this study never evoked seizure-like or aberrant electrical activity when performed on a single electrode. Further, no adverse behavioral responses occurred with the application of microstimulation in awake animals at 50 μA. However, a seizure did result from multielectrode simultaneous stimulation (Table 2; 07/Sep/2009) at 25 μA. Though no long-term device performance or physiological deficits were noted following this simultaneous multielectrode microstimulation, clearly the induction of a seizure event is unacceptable for any neural prosthetic application. In order to evoke complex spatiotemporal sensory percepts, interleaved multielectrode stimulation will need to be performed; it remains unknown how many electrodes can be simultaneously used without adverse physiological consequences. Patterns of stimulation can be sparsely distributed in both space and time, but must also be able to convey useful sensory information. An acceptable, safe balance between spatiotemporal patterns of microstimulation which convey useful sensory information and those which result in seizure must be found. Additionally, mechanisms to prevent unacceptably dense microstimulation will need to be implemented in the stimulation control electronics for human sensory prostheses to ensure that this failure mode does not occur.
It is unclear if complex spatiotemporal percepts can be evoked by patterned intracortical microstimulation. Testing patterned microstimulation in nonhuman primates is challenging (Bradley, 2004; Torab et al., 2011). It will be more efficiently addressed by means of psychophysical experiments conducted in human volunteers. Our ability to stimulate to effect and record electrophysiological data over multiple months demonstrated that tissue in the recording radius of the MEA remained viable after many months, and that the device maintained functionality over this time. These results suggest that microstimulation is ready for the next step in the development of sensory prosthetics, namely subchronic clinical trials in human subjects. Such trials will allow researchers to optimize stimulation parameters that are best at evoking sensory percepts, and will greatly speed the development of devices for the benefit of human patients.
Acknowledgments
Supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah.
Additional support from NIH Training Grant 5T32DC008553-02 and NIH R01 EY019363-01.
The authors would also like to thank Rick VanWagenen and Blackrock Microsystems for their ongoing support.
Abbreviations
- EMG
electromyogram
- MEA
multielectrode array
- SIROF
sputtered iridium oxide film
References
- Aggarwal V, Tenore F, Acharya S, Schieber MH, & Thakor NV (2009). Cortical decoding of individual finger and wrist kinematics for an upper-limb neuroprosthesis. Conference Proceedings—IEEE Engineering in Medicine and Biology Society, 2009, 4535–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, & Schmidt EM (1990). Visual sensations produced by intracortical microstimulation of the human occipital cortex. Medical & Biological Engineering & Computing, 28, 257–259. [DOI] [PubMed] [Google Scholar]
- Bradley DC (2004). Visuotopic mapping through a multichannel stimulating implant in primate V1. Journal of Neurophysiology, 93, 1659–1670. [DOI] [PubMed] [Google Scholar]
- Cogan S (2004). Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. Journal of Neuroscience Methods, 137, 141–150. [DOI] [PubMed] [Google Scholar]
- Cogan SF (2008). Neural stimulation and recording electrodes. Annual Review of Biomedical Engineering, 10, 275–309. [DOI] [PubMed] [Google Scholar]
- Cogan S, Ehrlich J, Plante T, Smirnov A, Shire D,Gingerich M, et al. (2009). Sputtered iridium oxide films for neural stimulation electrodes. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 89, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF (2003). Complex movements evoked by microstimulation of the ventral intraparietal area. Proceedings of the National Academy of Sciences of the United States of America, 100, 6163–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE, et al. (2006). Deep brain stimulation: Postoperative issues. Movement Disorders, 21, S219–S237. [DOI] [PubMed] [Google Scholar]
- Deyoe EA (2005). Laminar variation in threshold for detection of electrical excitation of striate cortex by macaques. Journal of Neurophysiology, 94, 3443–3450. [DOI] [PubMed] [Google Scholar]
- Dickey AS, Suminski A, Amit Y, & Hatsopoulos NG (2009). Single-unit stability using chronically implanted multielectrode arrays. Journal of Neurophysiology, 102, 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons NA, Drake W, Hanson TL, Lebedev MA, & Nicolelis MAL (2007). Primate reaching cued by multichannel spatiotemporal cortical microstimulation. The Journal of Neuroscience, 27, 5593–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GW, Chase SM, Whitford A, & Schwartz AB (2009). Control of a brain-computer interface without spike sorting. Journal of Neural Engineering, 6, 055004. [DOI] [PubMed] [Google Scholar]
- Ghosh S (1997). Cytoarchitecture sensorimotor areas cat cerebral cortex. Journal of Comparative Neurology, 388, 354–370. [DOI] [PubMed] [Google Scholar]
- Graziano MSA (2005). Arm movements evoked by electrical stimulation in the motor cortex of monkeys. Journal of Neurophysiology, 94, 4209–4223. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CS, & Moore T (2002). Complex movements evoke microstimulation precentral cortex. Neuron, 34, 841–851. [DOI] [PubMed] [Google Scholar]
- Hochberg L, & Donoghue J (2006). Sensors for brain-computer interfaces. IEEE Engineering in Medicine and Biology Magazine: The Quarterly Magazine of the Engineering in Medicine & Biology Society, 25, 32–38. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. (2006). Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature, 442, 164–171. [DOI] [PubMed] [Google Scholar]
- Houweling AR, & Brecht M (2007). Behavioural report of single neuron stimulation in somatosensory cortex. Nature, 451, 65–68. [DOI] [PubMed] [Google Scholar]
- Jones KE, Campbell PK, & Normann RA (1992). A glass/silicon composite intracortical electrode array. Annals of Biomedical Engineering, 20, 423–437. [DOI] [PubMed] [Google Scholar]
- Lempka SF, Miocinovic S, Johnson MD, Vitek JL, & McIntyre CC (2009). In vivoimpedance spectroscopy of deep brain stimulation electrodes. Journal of Neural Engineering, 6, 046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderman MD, Gilja V, Santhanam G, Afshar A, Ryu S, Meng TH, et al. (2006). Neural recording stability of chronic electrode arrays in freely behaving primates. Conference Proceedings—IEEE Engineering in Medicine and Biology Society, 1, 4387–4391. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, & Bullara LA (2002). The effects of prolonged intracortical microstimulation on the excitability of pyramidal tract neurons in the cat. Annals of Biomedical Engineering, 30, 107–119. [DOI] [PubMed] [Google Scholar]
- McCreery D, Pikov V, & Troyk PR (2010). Neuronal loss due to prolonged controlled-current stimulation with chronically implanted microelectrodes in the cat cerebral cortex. Journal of Neural Engineering, 7, 036005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, & Jefferys JGR (2005). Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. Journal of Neuroscience Methods, 141, 171–198. [DOI] [PubMed] [Google Scholar]
- Moss J (2004). Electron microscopy of tissue adherent to explanted electrodes in dystonia and Parkinson’s disease. Brain, 127, 2755–2763. [DOI] [PubMed] [Google Scholar]
- Murphey DK, & Maunsell JHR (2007). Behavioral detection of electrical microstimulation in different cortical visual areas. Current Biology, 17, 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey DK, & Maunsell JHR (2008). Electrical microstimulation thresholds for behavioral detection and saccades in monkey frontal eye fields. Proceedings of the National Academy of Sciences of the United States of America, 105, 7315–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam S (2004). Cognitive control signals for neural prosthetics. Science, 305, 258–262. [DOI] [PubMed] [Google Scholar]
- Negi S, Bhandari R, Rieth L, van Wagenen R, & Solzbacher F (2010). Neural electrode degradation from continuous electrical stimulation: Comparison of sputtered and activated iridium oxide. Journal of Neuroscience Methods, 186, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL (2003). Chronic, multisite, multielectrode recordings in macaque monkeys. Proceedings of the National Academy of Sciences of the United States of America, 100, 11041–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto KJ, Johnson MD, & Kipke DR (2006). Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Transactions on Biomedical Engineering, 53, 333–340. [DOI] [PubMed] [Google Scholar]
- Otto K, Rousche P, & Kipke D (2005a). Microstimulation in auditory cortex provides a substrate for detailed behaviors. Hearing Research, 210, 112–117. [DOI] [PubMed] [Google Scholar]
- Otto KJ, Rousche PJ, & Kipke DR (2005b). Cortical microstimulation in auditory cortex of rat elicits best-frequency dependent behaviors. Journal of Neural Engineering, 2, 42–51. [DOI] [PubMed] [Google Scholar]
- Rivera-Alvidrez Z, Kalmar RS, Ryu SI, & Shenoy KV (2010). Low-dimensional neural features predict muscle EMG signals. Conference Proceedings—IEEE Engineering in Medicine and Biology Society, 1, 6027–6033. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Brody CD, & Lemus L (2000). Sensing without touching: Psychophysical performance based on cortical microstimulation. Neuron, 26, 273–278. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, & Salinas E (1998). Somatosensory discrimination based on cortical microstimulation. Nature, 392, 387–390. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, & Normann RA (1992). A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Annals of Biomedical Engineering, 20, 413–422. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, & Normann RA (1999). Chronic intracortical microstimulation (ICMS) of cat sensory cortex using the Utah Intracortical Electrode Array. IEEE Transactions on Rehabilitation Engineering, 7, 56–68. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Otto KJ, Reilly MP, & Kipke DR (2003). Single electrode micro-stimulation of rat auditory cortex: An evaluation of behavioral performance. Hearing Research, 179, 62–71. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, & Newsome WT (1990). Cortical microstimulation influences perceptual judgements of motion direction. Nature, 346, 174–177. [DOI] [PubMed] [Google Scholar]
- Santhanam G, Ryu SI, Yu BM, Afshar A, & Shenoy KV (2006). A high-performance brain–computer interface. Nature, 442, 195–198. [DOI] [PubMed] [Google Scholar]
- Schmidt EM, Bak MJ, Hambrecht FT, Kufta CV, O’Rourke DK, & Vallabhanath P (1996). Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain, 119(Pt. 2), 507–522. [DOI] [PubMed] [Google Scholar]
- Schmidt EM, & McIntosh JS (1990). Microstimulation mapping of precentral cortex during trained movements. Journal of Neurophysiology, 64, 1668–1682. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Cui X, Weber D, & Moran D (2006). Braincontrolled interfaces: Movement restoration with neural prosthetics. Neuron, 52, 205–220. [DOI] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L,Fellows MR, & Donoghue JP (2002). Instant neural control of a movement signal. Nature, 416, 141–142. [DOI] [PubMed] [Google Scholar]
- Shoham S (2003). Robust, automatic spike sorting using mixtures of multivariate t-distributions. Journal of Neuroscience Methods, 127, 111–122. [DOI] [PubMed] [Google Scholar]
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, &Donoghue JP, (2005). Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 13, 524–541. [DOI] [PubMed] [Google Scholar]
- Taylor DM (2002). Direct cortical control of 3D neuroprosthetic devices. Science, 296, 1829–1832. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ (1996). Electrical stimulation of neural tissue to evoke behavioral responses. Journal of Neuroscience Methods, 65, 1–17. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ (2006). Direct and indirect activation of cortical neurons by electrical microstimulation. Journal of Neurophysiology, 96, 512–521. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, & Slocum WM (2009). Background luminance affects the detection of microampere currents delivered to macaque striate cortex. The European Journal of Neuroscience, 30, 263–271. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, & Schiller PH (2002). Differential effects of laminar stimulation of V1 cortex on target selection by macaque monkeys. The European Journal of Neuroscience, 16, 751–760. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, & Schiller PH (2003). Saccadic eye movements evoked by microstimulation of striate cortex. The European Journal of Neuroscience, 17, 870–878. [DOI] [PubMed] [Google Scholar]
- Torab K, Davis TS, Warren DJ, House PA, Normann RA, & Greger B (2011). Multiple factors may influence the performance of a visual prosthesis based on intracortical microstimulation: nonhuman primate behavioral experimentation. Journal of Neural Engineering, 8, 035001 (13 pp). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyk P, Detlefsen D, Cogan S, Ehrlich J, Bak M, McCreery D, et al. (2004). “Safe” charge-injection waveforms for iridium oxide (AIROF) microelectrodes. Conference Proceedings—IEEE Engineering in Medicine and Biology Society, 6, 4141–4144. [DOI] [PubMed] [Google Scholar]
- Vanwagenen R (2004). Micro-electrode impedance model. Salt Lake City, UT: Cyberkinetics, Inc. [Google Scholar]
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, et al. (2000). Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature, 408(6810), 361–365. [DOI] [PubMed] [Google Scholar]
- Williams JC, Hippensteel JA, Dilgen J, Shain W, & Kipke DR (2007). Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. Journal of Neural Engineering, 4, 410–423. [DOI] [PubMed] [Google Scholar]