Abstract
Introduction
Radiation therapy (RT), commonly used in cancer management, has been considered as one of the potential treatments for COVID-19 pneumonia. Here, we present the results of the pilot trial evaluating low-dose whole-lung irradiation (LD-WLI) in patients with COVID-19 pneumonia.
Methods
Ten patients with moderate COVID-19 pneumonia were treated with LD-WLI in a single fraction of 0.5 or 1.0 Gy along with the national protocol. The primary endpoint was an improvement in Spo2. The secondary endpoints were the number of days of hospital/intensive care unit stay, the number of intubations after RT, 28-day mortality, and changes in biomarkers. The response rate (RR) was defined as an increase in Spo2 upon RT with a rising or constant trend in the next 2 days, clinical recovery (CR) including patients who were discharged or acquired Spo2 ≥93% on room air, and 28-day mortality rate defined based on days of RT.
Results
The median age was 75 years (80% male). Five, 1, and 4 patients received single-dose 0.5 Gy, two-dose 0.5 Gy, and single-dose 1.0 Gy LD-WLI, respectively. The mean improvement in Spo2 at days 1 and 2 after RT was 2.4% (±4.8%) and 3.6% (±6.1%), respectively, with improvement in 9 patients after 1 day. Five, 1, and 4 patients were discharged, opted out of the trial, and died in the hospital, respectively. Two of 5 discharged patients died within 3 days at home. Among discharged patients, the Spo2 at discharge was 81% to 88% in 3 patients and 93% in the other 2 patients. Overall, the RR and CR were 63.6% and 55.5%, respectively. The RR, CR, and 28-day mortality of the single 0.5 Gy and 1.0 Gy WLI groups were 71.4% versus 50% (P = .57), 60% versus 50% (P = .64), and 50% versus 75% (P = .57), respectively.
Conclusion
LD-WLI with a single fraction of 0.5 Gy or 1 Gy is feasible. A randomized trial with patients who do not receive radiation is required to assess the efficacy of LD-WLI for COVID-19.
Introduction
Since December 2019, the novel COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to global catastrophe. Based on historical evidence using radiation therapy (RT) for viral pneumonia,1 lung irradiation has been proposed for COVID-19 pneumonia.2 , 3 The preliminary results in 5 patients were previously reported.4 Herein, we report the results of all 10 patients treated with LD-WLI and compare the clinical outcomes of 2 applied RT regimens.
Methods and Materials
Study population and eligibility
Patients aged >60 years with COVID-19 (based on real-time polymerase chain reaction of SARS-CoV-2 RNA, antibody tests, or radiographic pneumonic consolidations) with moderate pulmonary involvement (defined as blood oxygen saturation level [Spo 2] ≤93% on room air or respiratory rate >30/min) were eligible. Details of evaluating the eligibility criteria have been previously described.4 Briefly, peripheral blood Spo 2 was measured on room air within 1 hour before RT and in subsequent mornings using a pulse oximeter. The body temperature of patients was measured every morning using tympanic membrane thermometry. Likewise, C-reactive peptide (CRP), interleukin 6 (IL-6), ferritin, procalcitonin, and D-dimer were evaluated as the prognostic biomarkers of COVID-19.5 The exclusion criteria were presented in the preliminary report.4
Study design and treatment
Eligible patients were enrolled in a single-arm pilot trial (Clinical Trial Registration Number NCT04390412). The protocol was approved by the Institutional Review Board and Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.RETECH.REC.1399.073), and all patients provided written and verbal informed consent. The study design and details of the protocol for transportation of patients to the RT department and details of LD-WLI have been described previously.4 Briefly, in conjunction with the national protocol for COVID-19 pneumonia,6 irradiation was delivered in a single fraction of 0.5 Gy to both lungs via 2 opposed anteroposterior and posteroanterior open portals. The irradiation was extended to another 0.5 Gy, after at least 3 days, based on the physician’s discretion. Treatment with a single fraction of 1.0 Gy WLI was planned for 4 patients in the second phase of the pilot study. This decision originated from the preliminary results of an ongoing study evaluating single-dose 1.5 Gy WLI.7 Incidentally, no patient received dexamethasone, remdesivir, (hydroxy)chloroquine, or macrolides.
Study endpoints and assessments
The primary endpoint was an improvement in Spo 2. The secondary endpoints were the number days of hospital/intensive care unit stay, the number of intubations after RT, 28-day mortality rate, and changes in CRP, IL-6, ferritin, procalcitonin, and D-dimer. To evaluate 28-day mortality, participants were contacted by telephone to confirm vital status at 28 days after lung radiation. The applied ferritin test kit could not measure amounts >1600 ng/mL. Response rate (RR) was defined as improvement in Spo 2 on the first day after RT, with an increasing or constant trend for the next 2 days. Clinical recovery (CR) was defined as discharge from the hospital or weaning off the supplemental oxygen with Spo 2 ≥93% on room air.
Statistical analysis
To examine and compare the effects of radiation dose on Spo 2, CRP, and IL-6, mixed-design analysis of variance with time as the between-subjects factor was performed. We tested the normality, homogeneity, and sphericity using the Shapiro-Wilk, Levene, and Mauchly tests. The comparison of RR, CR, and 28-day mortality rate between the radiation doses was examined using Fisher exact test. The statistical significance level was set at .05. All analyses were performed using IBM SPSS Statistics (version 26.0, IBM Corp, Armonk, NY).
Results
Between May 21, 2020, and July 2, 2020, 10 patients with moderate COVID-19 pneumonia received LD-WLI at the Clinical Oncology Department of Imam Hossein Hospital, Tehran, Iran. Patients’ demographic and baseline disease characteristics are summarized in Table 1 . The median age was 75 years (range, 60-87 years), and 80% were male. All except 2 patients had comorbidities. Dyspnea was the predominant symptom (70%). All participants, except for 1 (patient #3), were positive for real-time polymerase chain reaction of SARS-CoV-2 RNA; patient #3 had the typical computed tomography (CT) features of COVID-19 pneumonia and elevated CRP. Initial physical examination revealed stable vital signs for all patients with a median Spo 2 of 80.5% (range, 70%-89%). All patients received O2 supplement mainly (60%) via facial mask with reservoir bag.
Table 1.
Patient demographics, clinical characteristics, and clinical outcomes
| Patients | Sex/age (y) | Comorbidity | Presenting symptom | Dx of COVID-19 | Presenting V/S | O2 supply | Radiation dose | Hospital stay after RT to discharge or death | Intubation after RT | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Male/60 | CHF∗ | Dyspnea | Clinical findings RT-PCR | PR/min: 75 RR/min: 12 SBP (mm Hg): 110 T (°C): 37.5 Spo2 (%): 87 |
Facial mask | Single 0.5 Gy | 7 d | None | Discharged Spo2 on room air at day of discharge: 93% Change in Spo2 from day of RT delivery to day of discharge: 6% |
| #2 | Male/69 | HTN-IHD | Fever-cough | Clinical findings RT-PCR | PR/min: 88 RR/min: 16 SBP (mm Hg): 130 T (°C): 38.1 Spo2 (%): 86 |
Nasal cannula | Single 0.5 Gy | 5 d | None | Discharged Spo2 on room air at day of discharge: 93% Change in Spo2 from day of RT delivery to day of discharge: 7% |
| #3 | Female/82 | IHD | LOC | Clinical findings Imaging |
PR/min: 90 RR/min: 20 SBP (mm Hg): 110 T (°C): 37.6 Spo2 (%): 75 |
Facial mask with reservoir bag | Single 0.5 Gy | 3 d | None | Opted out of trial |
| #4 | Male/84 | HTN | Cough | Clinical findings RT-PCR | PR/min: 82 RR/min: 12 SBP (mm Hg): 140 T (°C): 37.0 Spo2 (%): 89 |
Facial mask | Single 0.5 Gy | 3 d | None | Died |
| #5 | Male/64 | HTN | Dyspnea, cough, fever | Clinical findings Imaging RT-PCR |
PR/min: 90 RR/min: 15 SBP (mm Hg): 120 T (°C): 39.0 Spo2 (%): 74 |
Facial mask with reservoir bag | Single 0.5 Gy | 6 d | None | Discharged, but died at home after 3 d Spo2 on room air at day of discharge: 83% Change in Spo2 from day of RT delivery to day of discharge: 9% |
| #6 | Male/71 | DM-HTN | Dyspnea | Clinical findings RT-PCR | PR/min: 80 RR/min: 20 SBP (mm Hg): 110 T (°C): 37.0 Spo2 (%): 70 |
BiPAP | Double 0.5 Gy (7-d interval) | 10 d | Nome | Died |
| #7 | Male/80 | None | Dyspnea, fever | Clinical findings RT-PCR | PR/min: 90 RR/min: 18 SBP (mm Hg): 130 T (°C): 37.0 Spo2 (%): 81 |
Facial mask with reservoir bag | Single 1.0 Gy | 2 d | None | Discharged, but died at home after 1 d Spo2 on room air at day of discharge: 81% Change in Spo2 from day of RT delivery to day of discharge: 0% |
| #8 | Male/87 | HTN | Dyspnea | Clinical findings RT-PCR | PR/min: 60 RR/min: 18 SBP (mm Hg): 110 T (°C): 37.1 Spo2 (%): 80 |
Facial mask with reservoir bag | Single 1.0 Gy | 4 d | None | Died |
| #9 | Male/68 | None | Dyspnea | Clinical findings RT-PCR | PR/min: 100 RR/min: 16 SBP (mm Hg): 130 T (°C): 37.4 Spo2 (%): 87 |
Facial mask with reservoir bag | Single 1.0 Gy | 14 d | None | Discharged Spo2 on room air at day of discharge: 87% Change in Spo2 from day of RT delivery to day of discharge: 0% |
| #10 | Female/79 | HTN | Dyspnea | Clinical findings RT-PCR | PR/min: 86 RR/min: 16 SBP (mm Hg): 120 T (°C): 37.0 Spo2 (%): 80 |
Facial mask with reservoir bag | Single 1.0 Gy | 10 d | For 7 d | Died |
Abbreviations: BiPAP = bilevel positive airway pressure; CHF = congestive heart failure; DM = diabetes mellitus; Dx = diagnosis; HTN = hypertension; IHD = ischemic heart disease; LOC = loss of consciousness; PR = pulse rate; RR = respiratory rate; RT = radiation therapy; RT-PCR = real-time polymerase chain reaction; SBP = systolic blood pressure; Spo2 = blood oxygenation; T = temperature; V/S = vital signs.
Diagnosed after radiation, during hospitalization.
Patients were allocated to receive WLI in 2 plans: (1) LD-WLI with a single 0.5 Gy fraction (patients #1-5), and (2) LD-WLI with a single 1.0 Gy fraction (patients #7, 8, 9, and 10). Patient #6 experienced Spo 2 improvement within a day after 0.5 Gy LD-WLI. After 6 days he developed clinical deterioration and received the second 0.5 Gy. Patients were followed for 2 to 155 days (median, 10 days). Within a day after RT, 9 (90%) and 6 (60%) patients demonstrated initial improvement in Spo 2 (median, 2.5%, range, 1%-10%) and body temperature (median, –0.45°C; range, –0.1 to –2.0°C). Despite clinical improvement, patient #3 opted out of the trial on the third day after RT. Based on telephone follow-up, patient #3 has fully recovered and is alive.
Overall, the RR and CR were 63.6% and 55.5%, respectively. When including patient #3, overall CR improves to 60%. The mean magnitude of the improvement in Spo 2 at days 1 and 2 after RT was 2.4% (±4.8%) and 3.6% (±6.1%), respectively. In the 0.5 and 1.0 Gy groups, the mean improvement in Spo 2 within 2 days was 6.1 versus 0.25% (P = .95), respectively. The RR of single-fraction 0.5 Gy and 1.0 Gy WLI was 71.4% versus 50% (P = .57), and CR rates of single-fraction 0.5 Gy and 1.0 Gy WLI were 60% versus 50%, respectively (P = .64) (including patient #6, who responded well to 2 × 0.5 Gy fractions of WLI, twice in the RR and not including patient #3, who left the trial, in CR). Including patient #3, the CR of the 0.5 Gy group improves to 66.7% (P = .54).
The clinical course and outcomes are summarized in Tables 1 and 2 . The median time to discharge was 6 days (range, 2-14 days) for 5 patients. The remaining 4 patients experienced a decline in Spo 2 at a median of 2.5 days (range, 2-3 days) and died at median of 7 days (range, 3-10 days) (Fig. 1 A). Patients #5, 7, and 9 experienced Spo 2 ≥94% with supplementary O2 and 81% to 88% on room air at discharge day. They were discharged on the sixth, second, and 15th days after irradiation with medical advice to receive O2 supply at home due to hospital bed shortage. The remaining 2 (of 5 discharged) patients had an Spo 2 of 93% at discharge. Patient #10 was the only one who experienced intubation with mechanical ventilation, done on the fourth day after RT.
Table 2.
Hospital stay, intensive care unit admission, oxygen supplementation, and clinical outcome
 |
Fig. 1.
Evolution in time of (A) O2 saturation, (B) body temperature, (C) C-reactive peptide, and (D) IL-6 in patients with COVID-19 pneumonia after whole-lung irradiation.
Laboratory test results after irradiation had diverse patterns (Figs. 1 and 2 ). Three of 5 discharged patients had a decrease in CRP for 1 to 2 days after RT, and all patients who had died experienced an increase in CRP during the same period (Fig. 1C). The mean magnitude (±SD) of the change in CRP over hospitalization in discharged versus patients who died was –20.2 mg/L (±40.7) versus 40.5 mg/L (±87.8) (P = .23). The changes in CRP (in mg/L) 1 day after RT in discharged patients and those who died were 11.1 (±25.3) versus 23.5 (±43.3) (P = .58); after 2 days, they were 24.4 (±59.2) versus 82.1 (±29.2) (P = .10), respectively. After RT, IL-6 levels decreased in all discharged patients after 1 to 3 days, and increased within 2 days in 2 of 4 participants who died of COVID-19 (Fig. 1D). The mean magnitude (±SD) of the change in IL-6 over hospitalization in discharged versus patients who died was 24.6 pg/mL (±29.0) versus 2.7 pg/mL (±67.9) (P = .39). The changes in IL-6 (in pg/mL) 1 day after RT in discharged and deceased patients were –25.3 (±53.4) versus –20.3 (±15.2) (P = .63); after 2 days, they were –28.7 (±37.8) versus 32.4 (±51.0) (P = .99), respectively.
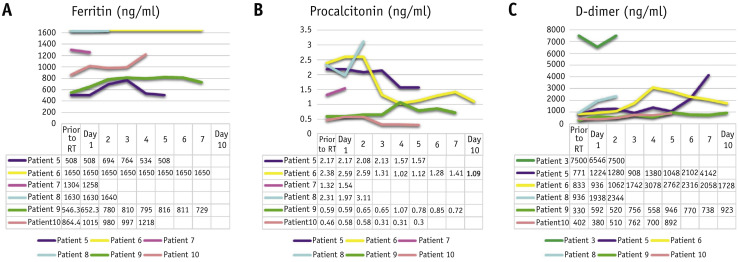
Fig. 2.
Evolution in time of (A) ferritin, (B) procalcitonin, and (C) D-dimer in patients with COVID-19 pneumonia after whole-lung irradiation.
In the 0.5 and 1.0 Gy groups, the mean change in CRP within 3 days was –8.1 versus 56.45% (P = .46), and the mean change in IL-6 within 1 day was –29.2 versus –14.7% (P = .13), respectively (the timepoints were set based on the half-life of biomarkers [CRP ∼19 hours, IL-6 ∼10 minutes]). Ferritin, procalcitonin, and D-dimer tests were available in 6 patients, and their trends are demonstrated in Figure 2. During observation, no acute toxicity was detected. Based on telephone follow-up, patients #5 and #7 died at home on the third and first day after discharge, respectively. The 28-day mortality of patients who received 0.5 Gy and 1.0 Gy WLI was 50% (3 of 6 patients) and 75% (3 of 4 patients), respectively (P = .57).
Discussion
Herein, we have reported the updated results of LD-WLI in moderate COVID-19 pneumonia. Within 1 day after 1.0 Gy WLI, the Spo 2 increased in 4 of the 4 patients; however, it decreased later in 2 patients, who eventually died. Both patients who were discharged had no underlying medical condition, whereas others who died had at least 1 of the comorbidities known as risk factors for COVID-19 mortality.8 Within 1 to 2 days after RT, CRP and IL-6 changes were in agreement with Spo 2 in 66.7% and 77.8% of all cases, consistent with previous studies.9 , 10 Given the plasma half-life of IL-6 (10 minutes) and CRP (19 hours), they declined in 80% and 100% of patients after 1 and 3 days, respectively (CRP was evaluable in 7 patients on the third day after RT).11 , 12 This highlights the anti-inflammatory effect of LD-WLI in patients with COVID-19 pneumonia. However, the number of cases was not large enough to statistically reveal the difference with pre-radiation status. Therefore, well-designed larger trials are essential to reveal the potential use of these biomarkers to predict outcome after radiation. The magnitude of change (from baseline) in IL-6 and CRP was maximal in the first and third day after RT, respectively. These amounts in discharged versus patients who died were –25.3 pg/mL (±53.4) versus –20.3 pg/mL (±15.2) for IL-6 and 6.5 mg/L (±55.0) versus 21.5 mg/L (±26.1) for CRP, respectively. The mixed analysis of variance demonstrated no significant time-by-group interaction effects (between-groups) for changes in Spo 2, CRP, and IL-6.
Considering RR of 80% and CR of 75%, the initial results of this trial addressed the potential efficacy of 0.5 Gy WLI in moderate COVID-19 pneumonia.4 In this updated report, however, both RR and CR of 1.0 Gy WLI were 50%. In addition, the 28-day mortality rate of the 0.5 Gy WLI group was less than that of the 1.0 Gy group (50% vs 75%). These findings may reflect the concept that doses <1.0 Gy have an anti-inflammatory effect, whereas higher doses may exacerbate the proinflammatory response.13 Wunderlich et al found that the inflammatory responses to a single fraction of radiation between 0.1 and 2.0 Gy are different in an ex vivo analysis. They demonstrated that transmigration of activated macrophages decreased between 0.1 and 0.5 Gy, secretion of IL-1β (a proinflammatory cytokine) decreased between 0.5 and 2.0 Gy, TGF-β release (an anti-inflammatory cytokine) increased between 0.1 and 0.5 Gy, and expression of NF-kB p65 (a proinflammatory mediator) decreased between 0.5 and 2.0 Gy. They concluded that a maximal anti-inflammatory effect occurs at a single dose of 0.5 Gy.14
Several factors may interfere with our results. First, the mean age of the 1.0 Gy group was approximately 7 years older (71.6 vs 78.5 years), which may influence the RR and CR. Overall, 4 of 5 patients older than age 70 years who received LD-WLI died; one, who had no underlying medical condition, surviving to discharge. This finding suggests that the efficacy of LD-WLI in patients >70 years is limited, but a randomized trial of LD-WLI would be needed to define whether there is any benefit. All 3 patients who were discharged in the 0.5 Gy group were aged 60 to 70 years, and 1 patient who recovered from COVID-19 in the 1.0 Gy group was in this range of age. The effect of age on radiosensitivity and inflammation has been demonstrated.15 , 16 Second, data on body mass index and smoking were not evaluated. The effect of these 2 factors on the prognosis of COVID-19, inflammation, and radiosensitivity has been demonstrated.17, 18, 19 Third, according to the national guideline, patients who had Spo 2 <90% under noninvasive ventilation with Fio 2 >50% were indicated for intensive care unit admission, intubation, and mechanical ventilation.6 Owing to limited ventilation facilities, 1 of the 8 indicated patients was intubated. This limitation may affect the clinical results of patients #4, 6, and 8, as well as patients #5 and 7, who died after discharge. Fourth, patients in the 1.0 Gy group were recruited on average 22 days later in the study. This may have led to SARS-CoV-2 with more genetic mutations with possibly worse prognosis.20 Although these limitations are important, this study is the first that has compared 2 radiation doses of WLI in patients with COVID-19 pneumonia.
Conclusion
The results of this trial may signal the feasibility of LD-WLI in patients with moderate COVID-19 pneumonia but require further study in randomized controlled trials.
Acknowledgments
Special thanks are due to Dr Mosavi Jarrahi, PhD, Mr Jabbari, MSc, Dr Azadeh, MD, Dr Motlagh, MD, Dr Manafi, MD, Dr Haghighi, MD, and Mrs Khoshbakht for their kind support. The authors would like to express their gratitude to the staff of Imam Hossein Hospital, Tehran, and to all physicians and nurses around the world who are doing their best to treat patients with COVID-19.
Footnotes
Disclosures: The authors have no relevant relationships to disclose.
Clinical trial registration number: NCT04390412
Data sharing: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Powell E.V. Roentgen therapy of lobar pneumonia. JAMA. 1938;110:19–22. [Google Scholar]
- 2.Kirkby C., Mackenzie M. Is low dose radiation therapy a potential treatment for COVID-19 pneumonia? Radiother Oncol. 2020;147:221. doi: 10.1016/j.radonc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kefayat A., Ghahremani F. Low dose radiation therapy for COVID-19 pneumonia: A double-edged sword. Radiother Oncol. 2020;147:224–225. doi: 10.1016/j.radonc.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ameri A., Rahnama N., Bozorgmehr R., et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: Short course results. Int J Radiat Oncol Biol Physics. 2020;108:1134–1139. doi: 10.1016/j.ijrobp.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponti G., Maccaferri M., Ruini C., et al. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health. The flowchart of approach to patients with COVID-19, seventh edition [in Persian]. Available at: http://treatment.sbmu.ac.ir/uploads/7__dastoor_flochart_treatment_covid_19.pdf. Accessed August 8, 2020.
- 7.Hess C.B., Buchwald Z.S., Stokes W., et al. Low-dose whole-lung radiation for COVID-19 pneumonia: Planned day-7 interim analysis of a registered clinical trial [abstract]. In: Proceedings of the AACR Virtual Meeting: COVID-19 and Cancer; 2020 July 20-22. Philadelphia (PA): AACR. Clin Cancer Res. 2020;26(18_Suppl) doi: 10.1002/cncr.33130. Abstract nr PO-093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albitar O., Ballouze R., Ooi J.P., et al. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z., Li J., Chen D., et al. Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19. Front Pharmacol. 2020;11:1093. doi: 10.3389/fphar.2020.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahu B.R., Kampa R.K., Padhi A., et al. C-reactive protein: A promising biomarker for poor prognosis in COVID-19 infection. Clin Chim Acta. 2020;509:91–94. doi: 10.1016/j.cca.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endler G., Marsik C., Joukhadar C., et al. The interleukin-6 G (-174) C promoter polymorphism does not determine plasma interleukin-6 concentrations in experimental endotoxemia in humans. Clin Chem. 2004;50:195–200. doi: 10.1373/clinchem.2003.022459. [DOI] [PubMed] [Google Scholar]
- 12.Pepys M.B., Hirschfield G.M. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royo L.T., Redondo G.A., Pianetta M.Á., et al. Low-dose radiation therapy for benign pathologies. Rep Pract Oncol Radiother. 2020;25:250–254. doi: 10.1016/j.rpor.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunderlich R., Ernst A., Rödel F., et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179:50–61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández L., Terradas M., Camps J., et al. Aging and radiation: Bad companions. Aging Cell. 2015;14:153–161. doi: 10.1111/acel.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh T., Newman A.B. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taghizadeh-Hesary F., Akbari H. The powerful immune system against powerful COVID-19: A hypothesis. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batatinha H.A.P., Rosa Neto J.C., Krüger K. Inflammatory features of obesity and smoke exposure and the immunologic effects of exercise. Exerc Immunol Rev. 2019;25:96–111. [PubMed] [Google Scholar]
- 19.Ginot A., Doyen J., Hannoun-Lévi J., et al. [Normal tissue tolerance to external beam radiation therapy: Skin] Cancer Radiother. 2010;14:379–385. doi: 10.1016/j.canrad.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Toyoshima Y., Nemoto K., Matsumoto S., et al. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65:1075–1082. doi: 10.1038/s10038-020-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]