Abstract
To prevent the more severe spread of COVID-19 infections, sensitive, rapid, low-cost, and multiplexed detection is critical. Recently, Gao et al. reported a laser-engraved graphene-based wireless device to monitor multiple biomarkers from human biofluids, allowing for high-frequency self-testing of COVID-19 with high accuracy and low cost.
To prevent the more severe spread of COVID-19 infections, sensitive, rapid, low-cost, and multiplexed detection is critical. Recently, Gao et al. reported a laser-engraved graphene-based wireless device to monitor multiple biomarkers from human biofluids, allowing for high-frequency self-testing of COVID-19 with high accuracy and low cost.
Main Text
According to the data collected by the WHO, SARS-CoV-2 induced a higher case fatality rate (around 3.3%) than that of the previous influenza pandemics in 1918 and 1957,1 and its spread rate is even 40-fold higher than that of SARS-CoV.2 Until now, the collaboration of multidisciplinary scientists has promoted coordinated treatment and vaccination strategies, but risks associated with COVID-19 are not mitigated. To achieve a safe reopening of society, the economy, and college campuses, accurate, rapid, and low-cost detection strategies are critical to hinder the transmission of SARS-CoV-2.3 , 4
While it is readily clear that symptomatic individuals require identification in order to reduce community spread, asymptomatic persons should also be monitored. Real-time polymerase chain reaction (RT-PCR) on the virus nucleic acid is regarded as the current golden standard testing approach, though its drawbacks include expensive equipment, the need for professional technicians, being time-consuming, and as-known false negatives. These drawbacks restrict its potential use in daily self-testing.5 , 6 Besides, determinations of an individual’s serologic status regarding antibodies specific to the virus antigens and circulating inflammatory biomarkers are equally important on identifying convalescent persons and evaluating COVID-19 severities.3 Therefore, a highly sensitive, rapid, low-cost, and multiplexed COVID-19 test is still highly demanded.
Emerging two-dimensional (2D) monoelemental materials (Xenes) have shown a great potential in multiple biomedical applications including monitoring and detection of various diseases.7 As one of the most representative Xenes, the use of graphene in this specific field is very attractive. For example, in previous works, Gao et al. provided a wearable, inexpensive, and possibly scalable biosensing strategy based on mesoporous graphene electrodes made by CO2 laser engraving.8 , 9 The graphene-based biosensor enabled rapid, accurate, multiplexed, and wireless monitoring of tyrosine (Tyr) as well as uric acid (UA) at low concentrations in human perspiration.8 The satisfied carrier mobility, significant electron transfer rate, and large surface area endow graphene with outstanding electrochemical properties, particularly appropriate for constructing biosensors to probe electroactive analytes at ultra-low concentrations in body fluids.8, 9, 10 The detection limits of the biosensors to UA and Tyr were down to 0.74 μM and 3.6 μM, respectively.8 It is believed that the laser-engraved graphene-based biosensing device with high sensitivity, low-cost, and rapid detection ability to probe biomarkers from human biofluids will promote its potential clinical application.
With these solid foundations, Gao et al. recently demonstrated a wireless multiplexed electrochemical device to monitor COVID-19 with the features of low in price, high sensitivity, and rapidity, as named SARS-CoV-2 RapidPlex (Figure 1 ).3 The device could detect not only the viral antigen, immunoglobulins, but also the inflammatory biomarker, which represents three main COVID-19 aspects including the infection of virus, immunoreaction, and severity of clinical symptoms. This device contained four working electrodes and one counter electrode that were made of graphene, together with one Ag/AgCl reference electrode. All the electrodes were patterned by laser engraving on a polymeric substrate, which enabled its mass, low-cost production for wide application in the community. Instead of direct functionalization on graphene that generally requires a defected surface structure, 1-pyrenebutyric acid (PBA) was conjugated on the graphene sheet surface via π-π stacking and hydrophobic interaction, which was further utilized for binding the capturing receptors on the working electrodes. Immobilization of specific receptors including nucleocapsid protein (NP), immunoglobulins against spike protein of the virus (S1-IgG, S1-IgM), and C-reactive protein (CRP) relied on covalently bonding their amino groups with carboxylic groups in PBA. In this way, the graphene maintained its initial structure and functionalities, ensuring good stability of the sensing layer. Besides, the presence of PBA together with an optimal blocking process by bovine serum albumin (BSA) also contributed to preventing non-specific adsorptions of large biomolecules on the graphene surface. The detection of each target biomolecules was visualized through comparing the amperometric signals in the absence and after binding of target analytes on each graphene working electrode, and the data could be wirelessly transmitted to a remote device over Bluetooth.
Figure 1.
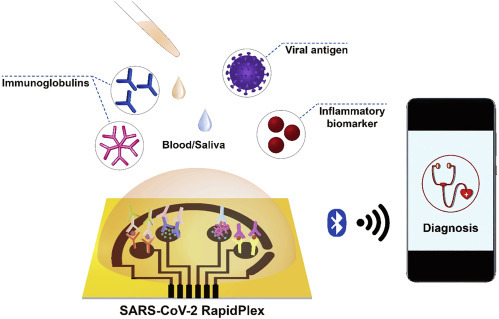
Sensitive, Rapid, Low-Cost and Multiplexed COVID-19 Monitoring by the Wireless Telemedicine Platform
Aiming for an urgently needed fast-detection platform for COVID-19, the target binding time greatly determines its implementation potential. By comparing the amperometric signals from each sensing unit after incubating with blank and target samples (low to 500 pg/mL), a significant change was quickly observed within 1 min of incubation. Notably, to ensure reliable sensitivity for detecting each target molecule at an ultra-low concentration, the incubation was recommended to perform for 10 min. Unlike other determination approaches based on either ELISA test, nucleic acid amplification, or mass spectrometry that is mostly hindered by complex sample preparation, high-cost, or bulky systems, the short-to-answer time endowed the as-developed device more implementation potential as a point-of-care (POC) system.3 Relative standard deviation (RSD) analysis on the response of different batch biosensors presented good reproducibility in terms of the fabrication process and signal transduction. Even after 5-days storage at 4°C, the detection performance showed negligible fluctuation. In the selectivity evaluation of the SARS-CoV-2 RapidPlex platform, no significant cross-reaction between the SARS-CoV-2 biomarker and that of interferents (non-target molecules) such as SARS and MERS coronaviruses was realized. More importantly, Gao et al. verified that there’s no mutual interference between the readout from neighboring working electrodes when buffered solutions containing all the targets were applied, i.e., 1 ng/mL of NP antigen, 250 ng/mL of S1 specific IgG and IgM, and 50 ng/mL of CRP.
To evaluate the clinical potential of this multiplexed platform, Gao et al. performed detections on both COVID-19-positive and COVID-19-negative blood/saliva samples from healthy individuals and confirmed patients. The device was able to simultaneously provide a positive readout for each individual target molecule after 1 min incubation with the COVID-19 positive serum samples. The signal-to-blank ratios representing the signal changes were 10.53 for NP, 11.62 for S1-IgG, 10.67 for S1-IgM, and 12.39 for CRP in serum samples, respectively, while the corresponding values were 2.81, 3.24, 1.62, and 1.76 in saliva samples.3 In the test on patient biospecimens, concentrations of the four biomarkers detected by the device were in the range of submicro- to micro-gram per milliliter in serum samples, and a range down to nanogram per milliliter in saliva samples. The positive readings of the biomarkers in patients’ saliva supported the non-invasive diagnosis approach of SARS-CoV-2 infection from this biofluid. Moreover, through CRP concentration evaluation, various COVID-19 symptom severity grades could be determined, which would benefit the efficient allocation of medical attention and precious resources.
Overall, this study presents a novel wireless electrochemical platform of SARS-CoV-2 RapidPlex, aiming to accurately, rapidly, and inexpensively detect COVID-19 through the simultaneously multiplex test of NP antigen, S1-specific IgG and IgM, and CRP in serum/saliva. Further studies concerning large-scale clinical tests are still needed to verify the reliability of the biosensors before its real implementation as an efficient POC system contributing to fighting this COVID-19 pandemic. Further technological improvement may rely on the integration of an automated microfluidic module for handling samples, and probably wearable biosensors for continuously monitoring an individual’s health status against the COVID-19 infection.
Acknowledgments
This work is supported by Harvard Medical School/Brigham and Women’s Hospital Department of Anesthesiology-Basic Scientist Grant (no. 2420 BPA075, W.T.), and Center for Nanomedicine Research Fund (no. 2019A014810, W.T.). W.T. is a recipient of the Khoury Innovation Award (no. 2020A003219) and American Heart Association (AHA) Collaborative Sciences Award (no. 2018A004190). W.T. also received a start-up package (for three years) from the Department of Anesthesiology, Perioperative, and Pain Medicine to establish his independent research laboratory at Harvard Medical School and Brigham and Women’s Hospital. We thank our department for this generous support. We also appreciate the support from the National Natural Science Foundation of China (no. 21877049), Major Program for Tackling Key Problems of Industrial Technology in Guangzhou (no. 201902020013), and Guangdong Provincial Key Laboratory Fund of Functional Supramolecular Coordination Materials.
References
- 1.Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 2.Gates B. Responding to Covid-19 - A Once-in-a-Century Pandemic? N. Engl. J. Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 3.Torrente-Rodríguez R.M., Lukas H., Tu J., Min J., Yang Y., Xu C., Rossiter H.B., Gao W. SARS-CoV-2 RapidPlex: A Graphene-based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter. 2020;3:1981–1998. doi: 10.1016/j.matt.2020.09.027. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paltiel A.D., Zheng A., Walensky R.P. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw. Open. 2020;3:e2016818. doi: 10.1001/jamanetworkopen.2020.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y., Yang M., Shen C., Wang F., Yuan J., Li J., Zhang M., Wang Z., Xing L., Wei J. Laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv. 2020 doi: 10.1101/2020.02.11.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao W., Kong N., Ji X., Zhang Y., Sharma A., Ouyang J., Qi B., Wang J., Xie N., Kang C. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019;48:2891–2912. doi: 10.1039/c8cs00823j. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Song Y., Bo X., Min J., Pak O.S., Zhu L., Wang M., Tu J., Kogan A., Zhang H. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020;38:217–224. doi: 10.1038/s41587-019-0321-x. [DOI] [PubMed] [Google Scholar]
- 9.Torrente-Rodríguez R.M., Tu J., Yang Y., Min J., Wang M., Song Y., Yu Y., Xu C., Ye C. IsHak, W. W., Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless mHealth System. Matter. 2020;2:921–927. doi: 10.1016/j.matt.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Dong X., Chen P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012;41:2283–2307. doi: 10.1039/c1cs15270j. [DOI] [PubMed] [Google Scholar]


