Abstract
Natural products remain an important source of new therapeutics for emerging drug-resistant pathogens like Candida albicans, which particularly affects immunocompromised patients. A bioactive 3-decalinoyltetramic acid, pyrrolocin A, was isolated from extracts of a novel Amazonian fungal endophyte, E6927E, of the Diaporthales family. The structure of the natural product was solved using NMR and CD spectroscopy and it is structurally related to the fungal setins, equisetin and phomasetin, which are well-characterized tetramic acid antibiotics specific for Gram-positive organisms. We show that the compound inhibits growth of Staphylococcus aureus and Enterococcus faecalis. It shows selective and potent bioactivity against fungal strains, with an MIC of 4 μg/mL for C. albicans, 100 μg/mL for Aspergillus sp. and greater than 100 μg/mL for Saccharomyces cerevisiae. Further, the compound is less toxic to mammalian cells (IC50 = 150 μg/mL), with an inhibitory concentration greater than forty times that for C. albicans. Pyrrolocin A retained potent activity against eight out of seventeen strains of clinical Candida sp. isolates tested.
Keywords: Diaporthales, Fungal Endophyte, Candida albicans, Tetramic Acid, Antifungal Activity
A significant percentage of approved therapeutic agents are derived from natural products [1]. In recent decades, however, pharmaceutical approaches have favored a high-throughput screening approach of synthetic compounds due to the ease and relatively low cost of chemical libraries, structure-based design, and parallel synthesis strategies [2]. One concern in the pursuit of natural products is that efforts to identify novel compounds face diminishing returns because of the increasing re-discovery rate of known compounds [3]. Despite such investment concerns, microbes represent a rich source for natural products [4]. Notably, only a small fraction of existing microorganisms have been studied, suggesting that an abundance of opportunities remain to be investigated [5].
Our group is dedicated to locating new microbial sources of bioactive compounds. Neotropical endophytic fungi are richly diverse and may provide a source for unique bioactive and structurally diverse natural products. Screening of diverse endophytic fungi revealed that 74% of them exhibited antimicrobial activity, prompting us to pursue research projects to investigate them by bioactive-guided fractionation [6]. Here we report the isolation of 3-decalinoyltetramic acid (pyrrolocin A) from a novel Amazonian endophyte of the order Diaporthales. Pyrrolocin A is a bioactive natural product that was first isolated from Fusarium heterosporum last year by a separate research group [7]. However, the Fusarium strain ceased production of the metabolite before the authors were able to finish characterizing the compound and its antimicrobial activities. Pyrrolocin A is structurally related to equisetin and more closely to phomasetin. The compound exhibits unique antimicrobial activity, distinguishing it from previous equisetin derivatives [8].
Growth of E6927E in potato dextrose broth consistently led to the robust production of pyrrolocin A (Figure 1) at approximately 10 mg L−1, with some cultures producing less material, associated with a lightening of the mycelium. Purification was accomplished by bioactivity guided fractionation, testing aliquots of the extracts and fractions against Staphylococcus aureus and Candida albicans (Figure 2). An oleoresin was formed by concentrating dichloromethane, ethyl acetate, and methanol extractions of dried E6927E cultures. A methanol back-extraction from the oleoresin was then purified by preparative MPLC followed by preparative HPLC. Evaporation of the chromophoric fractions yielded pure pyrrolocin A in a thin transparent film, which yielded the pure off-white solid through iterative evaporation with chloroform.
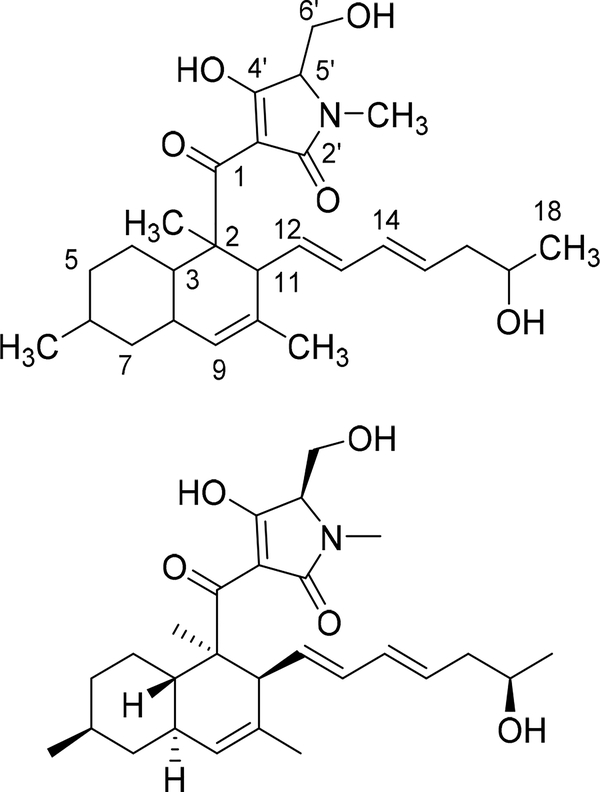
Figure 1:
The planar and stereo structures of pyrrolocin A.
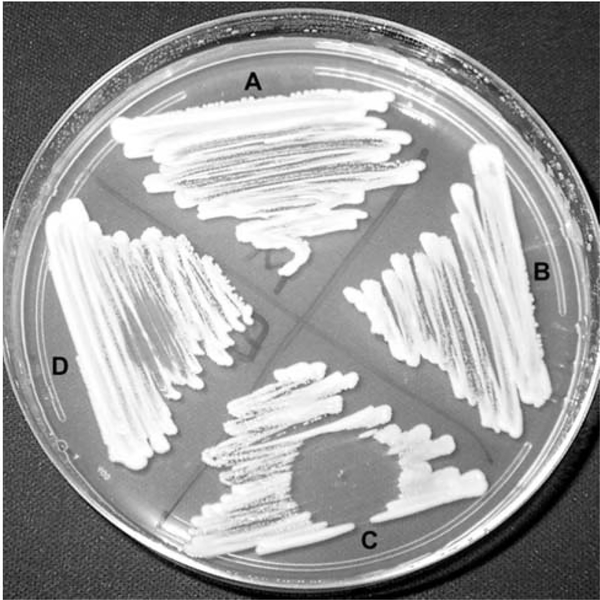
Figure 2:
Growth inhibition of Candida albicans. A) Methanol control, B) untreated, C) dichloromethane extract of E6927E dissolved in methanol, and D) ethyl acetate extract of E6927E dissolved in methanol. Following 12 h treatment with dichloromethane extract, inoculum was taken from the zone of inhibition and re-plated, but no cells could be recovered.
High resolution mass spectrometry indicated a molecular formula of C27H39NO5, with the [M + H]+ peak at m/z 458.3084 and the [M + Na]+ peak at m/z 480.2739. The 1H NMR spectrum revealed keto-enol tautomerization of the tetramic acid portion of the compound, and the spectrum, which clarified in CD3OD but not CDCl3, was used to indicate purity and to visualize proton splitting. The 13C and 2D Heteronuclear Single Quantum Coherence (HSQC) spectra revealed 27 carbon signals with 6 quaternary carbons. These spectra, along with 1H-1H COrrelation SpectroscopY (COSY), 2D Heteronuclear Multiple Bond Correlation (HMBC), and 1H-1H TOtal Correlated SpectroscopY (TOCSY) were used to assign and link structural fragments, yielding the planar structure in Figure 1. The Rotating frame Overhauser Effect SpectroscopY (ROESY) NMR data were used to determine relative stereochemistry. The NMR data and assignments are shown in Table 1 with ROESY correlations illustrated in Figure 3. The CD spectrum of the material was virtually identical to the published CD spectrum for pyrrolocin A [7]; therefore, we have tentatively assigned the same absolute configuration. The optical rotation was opposite to the configuration of commercially available equisetin (Santa Cruz Biotechnology), which supports this assignment.
Table 1:
NMR data and assignments for Pyrrolocin A, collected in CD3OD.
| Pos. | δC, Type | δH | COSY | HMBC | ROESY |
|---|---|---|---|---|---|
| 1 | 198.3, C | ||||
| 2 | 50.5, C | ||||
| 2-Me | 14.3, CH3 | 1.44 | 1, 2, 3 | 8, 11 | |
| 3 | 41.2, CH | 1.69 | 8 | 4 | 12 |
| 4 | 29.4, CH2 | 1.09 | 3, 5 | 6, 8 | |
| 2.00 | |||||
| 5 | 37.0, CH2 | 1.09 | 4, 6 | 3, 6, 7, 6-Me | |
| 1.77 | |||||
| 6 | 34.8, CH | 1.53 | 5, 7, 6-Me | 7, 8, 6-Me | |
| 6-Me | 22.9, CH3 | 0.94 | 6 | 5, 6, 7 | 6 |
| 7 | 43.9, CH2 | 0.87 | 5, 6, 8, 9, 6-Me | ||
| 1.83 | 6 | ||||
| 8 | 40.5, CH | 1.87 | 3, 7, 9 | 3, 9, 10 | 6, 9, 2-Me |
| 9 | 127.2, CH | 5.22 | 3, 7, 11, 10-Me | 8 | |
| 10 | 132.8, C | ||||
| 10-Me | 22.6, CH3 | 1.58 | 9, 10, 11 | 12 | |
| 11 | 50.8, CH | 3.23 | 12 | 2 | 2-Me |
| 12 | 132.7, CH | 5.27 | 11, 13 | 11, 14 | 3, 14, 10-Me |
| 13 | 133.9, CH | 5.81 | 12 | 11, 14 | |
| 14 | 133.4, CH | 5.93 | 15 | 13, 16 | 12 |
| 15 | 130.7, CH | 5.53 | 14, 16 | 14, 16, 17 | |
| 16 | 43.3, CH2 | 2.13 | 15, 17 | 14, 15, 17, 18 | |
| 2.17 | |||||
| 17 | 68.5, CH | 3.72 | 16, 18 | 15, 16, 18 | |
| 18 | 23.0, CH3 | 1.12 | 17 | 16, 17 | |
| 2′ | 178.1, C | ||||
| 3′ | 102.1, C | ||||
| 4′ | 192.6, C | ||||
| 5′ | 69.4, CH | 3.68 | 6′ | 4′ | |
| 6′ | 59.9, CH2 | 3.89 | 4′,5′ | ||
| 3.95 | |||||
| N-Me | 27.3, CH3 | 3.03 | 2′, 5′ |
Recorded at 150 MHz for 13C and 600 MHz for 1H.
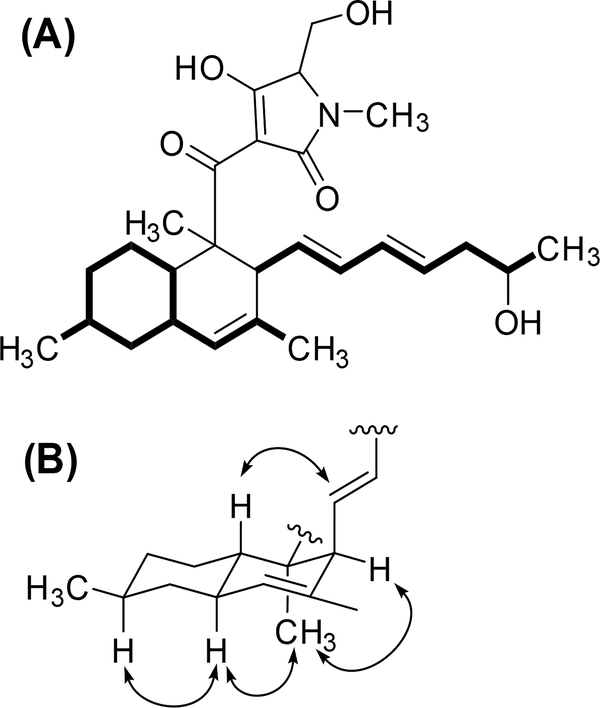
Figure 3:
(A) COSY ( ) and (B) ROESY (
) and (B) ROESY ( ) correlations for pyrrolocin A.
) correlations for pyrrolocin A.
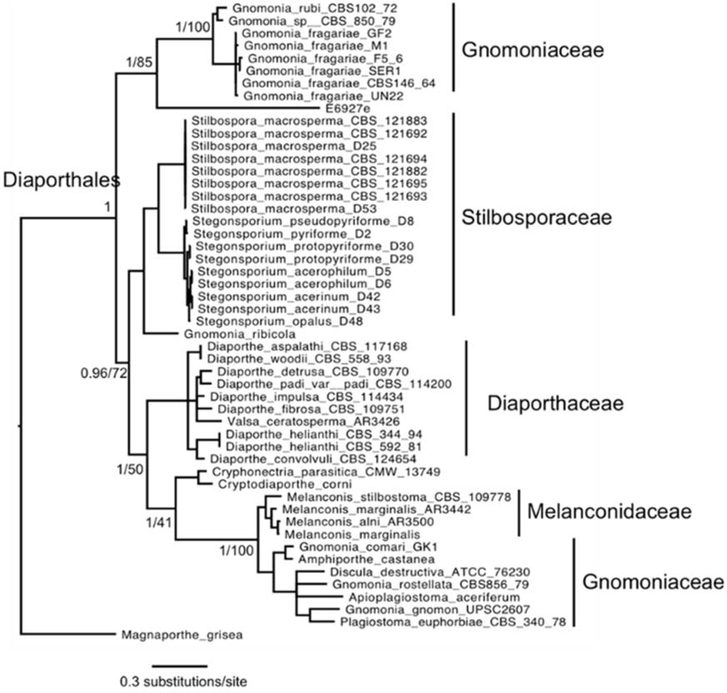
Molecular phylogeny, using four nuclear loci, showed E6927E clusters most closely to members of the family Gnomoniaceae, with strong support from both Bayesian and Maximum Likelihood methods (Figure 5) [9]. However, Gnomoniaceae appears paraphyletic in this analysis, which was not observed in previous studies of either Diaporthales or Gnomoniaceae systematics [10]. The paraphyly is driven by Gnomonia fragariae and G. rubi [11], neither of which were included in the previous studies of the order or family. Members of the genus Gnomonia are identifiable by erumpent perithecia and septate ascospores, which were not observed in E6927E (Figure 6) [12]. Moreover, the branch length from isolate E6927E to its closest neighbor is longer than that between any two families in this analysis, suggesting that E6927E may be a distinct family. Though members of this family have been known to be endophytic, we choose to place E6927E in Diaporthales incertae sedis until such time that the isolate can be more clearly placed within a family by molecular means [12b, 13].
Figure 5:
Phylogentic tree placing E6927E in Diaporthales incertae sedis.
Figure 6:
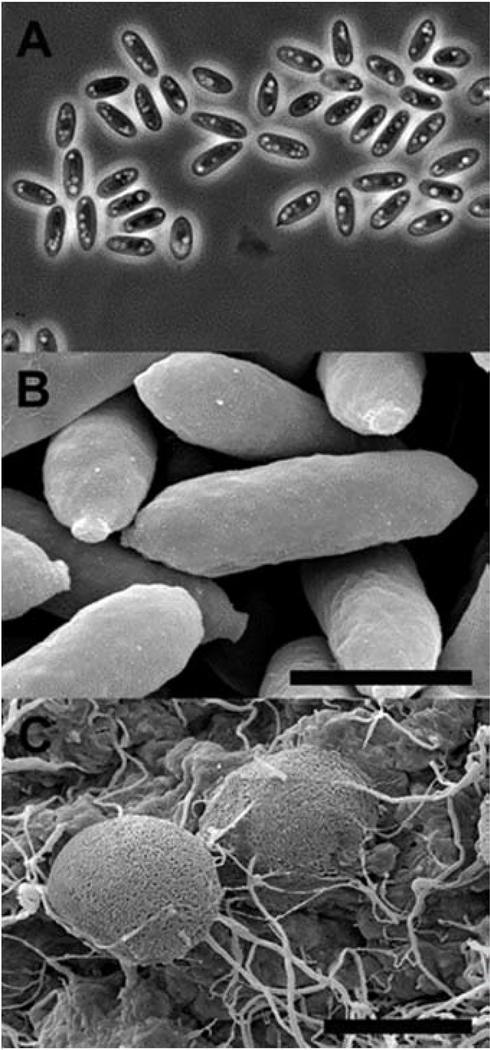
E6927E microscopy. A) Light microscope image of ascospores at 100x magnification. B) Scanning electron microscope image of ascospores with scale bar equal to 2 μm. C) Scanning electron microscope image of ascospore structures with scale bar equal to 50 μm.
Several setin compounds have been identified previously, including equisetin, phomasetin, ophiosetin (and N-demethyl-ophiosetin), trichosetin (and epi-trichosetin), methiosetin, coniosetin, hymenosetin, paecilosetin, pallidorosetin A (and B), and altersetin [8a, 14]. In addition to these, pyrrolocins A thru C were identified in Fusarium heterosporum last year [7]. Our cultivated strain and methods enabled us to purify several hundred milligrams of the compound for antifungal and antibacterial evaluation, and our characterization of the compound demonstrates selective activity that distinguishes it from all previous ‘setin’ compounds. All of the characterized setins exhibit strong antibiotic activity against Gram-positive bacteria, with MICs ranging from 0.5 to 10 μg/mL, but only coniosetin has shown activity against yeast, and we have confirmed the lack of activity for equisetin in Table 2. Thus, pyrrolocin A is the second to demonstrate significant and selective activity with yeast [8a, 14b, f, h, i]. Notably, coniosetin and pyrrolocin have structural similarities and highlight the alkenyl chain as an area for additional medicinal chemistry studies.
Table 2:
Minimum inhibitory concentrations.
| Pyrrolocin A | Equisetin | |
|---|---|---|
| Candida albicans | 4 μg/mL | > 10 μg/mL |
| Enterococcus faecalis | 5 μg/mL | – |
| Staphylococcus aureus | 4 μg/mL | – |
| Aspergillus flavus | 100 μg/mL | > 100 μg/mL |
| Aspergillus parasiticus | 100 μg/mL | > 100 μg/mL |
| Saccharomyces cerevisiae | > 100 μg/mL | – |
| Neurospora sp | > 100 μg/mL | 100 μg/mL |
| Escherichia coli | > 1000 μg/mL | – |
| Mammalian 293TT cells | 150 μg/mLa | – |
Value that inhibits growth >50% of cells (IC50), rather MIC.
Candida typically exhibit robust growth in potato dextrose broth, but we show that Candida do not recover after 20–24 hour drug treatment, indicating that pyrrolocin has cidal inhibition at 4 μg/mL, whereas Saccharomyces cerevisiae were unaffected by pyrrolocin A up to 100 μg/mL (Table 2). Candida treated with pyrrolocin A and grown in either buffered or unbuffered RPMI gave similar MIC values depending on starting cell density, but RPMI is not an ideal medium for yeast and Candida growth was uniformly poor for controls as well as drug treatment conditions. To assess selectivity further, pyrrolocin A was tested for inhibition of three filamentous fungi that are plant pathogens. One hundred μg/mL inhibited growth of Aspergillus flavus and A. parasiticus, but not Neurospora sp. The Aspergillus cultures did not recover leading us to conclude that inhibition is cidal.
As seen in Table 3, seventeen strains of Candida sp. isolated by researchers in the Division of Infectious Disease at Cincinnati Children’s Hospital were used to assess whether pyrrolocin A, purified from E6927E, can inhibit a diverse range of infectious yeasts. The strains tested represent drug-resistant and rare or emerging strains of Candida sp. Half of the strains tested showed growth inhibition at the concentrations tested (MIC of 5 or 10 μg/mL) and three have an MIC of 5 μg/mL, which is comparable with wild-type C. albicans (ATCC MYA-2876) sensitivity to pyrrolocin A.
Table 3:
Clinical isolates of Candida sp. tested with 0, 1, 5 and 10 μg/mL
| Isolate Name | MIC (μg/mL) |
|---|---|
| 3300 | 5 |
| F5 | 5 |
| S2 | 5 |
| 5674 | 10 |
| CGUI | 10 |
| CLUSB | 10 |
| CTROB | 10 |
| S1 | 10 |
| 4380 | >10 |
| 35176 | >10 |
| 35177 | >10 |
| CKRUA | >10 |
| CKRUB | >10 |
| CTROA | >10 |
| F2 | >10 |
| SM1 | >10 |
| SM3 | >10 |
Mammalian cells, strains 293T and HeLa, were treated with 10 to 160 μg/mL pyrrolocin A for 16 hours (data not shown). At a concentration of 160 μg/mL, the compound killed 44% of cells and full viability was observed for treatment with 40 μg/mL and less. Fitting a curve to these data indicates that the IC50 of pyrrolocin A for mammalian cell culture is approximately 150 μg/mL.
Although the mechanism of antibiotic activity is not known for any tetramic acid, the results obtained indicate that pyrrolocin A, a newly discovered 3-decalinoyltetramic acid isolated from the novel endophyte Diaporthales sp. E6927E, is selective. Whereas most tetramic acid antibiotics have not demonstrated anti-fungal activity, pyrrolocin A is one of only two tetramic acids with anti-Candida activity. This study is the first to show that pyrrolocin A exhibits selectivity between yeast strains, with no activity against Saccharomyces cerevisiae up to 100 μg/mL and potent anti-Candida activity against wild-type (MIC = 4 μg/mL) and clinical isolates.
Experimental
Isolation of endophytes from plant samples:
Endophyte Diaporthales sp. E6927E was isolated from the inner tissue of a clipping from a Ficus sphenophyllum (fig) tree, collected from an Ecuadorean dry forest near the Napo River. A plant voucher specimen is archived in the Yale Herbarium with accession number E6927, and the endophyte is archived with accession number E6927E. After surface sterilization of the outer bark by brief ethanol immersion and flaming, the inner stem tissues were dissected onto 1:10 potato dextrose agar (PDA). The endophyte was selected through outgrowth on PDA, employing the previously described hyphal tipping procedure [15]. E6927E produced ascospores after greater than 2 weeks growth on corn meal (CM) agar grown at 30°C.
Endophyte fermentation and crude material preparation:
Starter cultures of 100 mL Potato Dextrose Broth (PDB) were inoculated using a plug from the PDA plate and grown for 7 days (standing) at room temperature. One L of PDB was inoculated using a 100 mL starter culture. These standing cultures were grown for 14 days at room temperature, and they were then shaken (160 rpm) for 19 days at 30°C. The fermented whole broth was harvested by filtration through cheesecloth, separating fungal mycelium from culture broth. The broth partition was evaporated to 200 mL using a GeneVac Rocket Evaporator 4D at 30°C, and a rotovap dried the broth partition. The mycelium partition was dried using a high vacuum, and both partitions were frozen for short term storage.
Extraction and bioactive-guided fractionation:
The first extract was derived by extracting both the mycelium and broth partitions with ethyl acetate followed by dichloromethane and then methanol; these were each tested, and they were then combined and evaporated to an oleoresin. Bioactivity was examined by spotting 5–10 μL of resuspended oleoresin in methanol onto PDA (Figure 2). Aliquots and methanol control spots were evaporated, and C. albicans was streaked across the plate and allowed to grow at 28°C. After 24 h, a zone of inhibition was qualitatively assessed, indicating fungicidal anti-Candida activity. The second extract was partitioned from the oleoresin with methanol, and this was then evaporated to dryness and tested for activity. The refined extract was resuspended in 1:1 DMSO-methanol and separated on a C18 column by MPLC using a linear gradient (water/acetonitrile). The active fractions eluted near 80% acetonitrile, correlating with a chromatographic peak (λ= 254 nm). These fractions were pooled, evaporated to dryness, resuspended in methanol, and re-purified by HPLC using the same conditions; the active fractions eluted near 70% acetonitrile. These fractions were pooled, evaporated to a cloudy solution, and dried to a film, which ranged from opaque to transparent. The NMR and IR spectra of the purified pyrrolocin A were collected in both deuterated chloroform and deuterated methanol.
Pyrrolocin A
Off-white solid
[α]D: +340 (c 0.34, CHCl3); +451 (c 0.10, MeOH).
IR νmax film: 3488, 2917, 1686, 1567, 1495, 1455, 1378, 1260, 1201, 1084, 993, 939, 920 cm−1
1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz): Table 1.
HR-ESI-MS/MS: m/z 458.3084 [M+H]+, m/z 480.2739 [M+Na]+, calcd for C27H40NO5: 458.2906.
Bioactivity:
For determination of the compound’s Minimum Inhibitory Concentration (MIC), pure compound was deprotonated in 1 mM sodium bicarbonate (pH 8.0), followed by titration with 1 N NaOH, to a final concentration 100 μM. Compound stocks were maintained at 100 mg/mL prior to treatments. MIC values were determined with several tester organisms: Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Candida albicans (ATCC MYA-2876) and Saccharomyces cerevisiae (BY4741 derived from S288C), Aspergillus parasiticus (A1243 Delta ku70), A. flavus (A1421 CA14 deltaKu70 delta PyrG), Neurospora sp. (sequenced environmental isolate), as well as 17 clinical isolates of drug-resistant Candida sp. (Table 3). Bacterial test organisms were grown in either Mueller Hinton Broth (E. coli and S. aureus) or Brain Heart Infusion (BHI) (E. faecalis) media for 8–20 h, shaking at 30°C, and growth was measured continuously by monitoring at OD600. S. cerevisiae was grown in yeast extract peptone dextrose (YPD) media for 20–24 h shaking at 30°C and growth was measured continuously by monitoring OD600. C. albicans was grown in either Potato Dextrose (PD) broth or RPMI 1640 (buffered and not buffered) media for 20–24 h shaking at 30°C and growth was measured continuously by monitoring OD600. Filamentous fungi (Aspergillus and Neurospora) were grown on solid PD media containing compound at known final concentrations for 96 h at 25°C. 10,000 spores were plated for each condition. Amphotericin B (fungicide) at either 2.5 μg/mL or ciprofloxacin (antibacterial) at 50 μg/mL was used as positive controls in growth assays. The minimal inhibitory concentrations (MICs) reported represent the lowest compound concentration for which no organism growth is detectable up to the growth endpoint reported.
Cytotoxicity:
For MICs with the human embryonic kidney 293TT cell line, cells were plated at 20,000 cells per well in 96 well plates, 100 μL per well. All cells were cultured in standard Dulbecco’s Modified Essential Medium containing 10% fetal bovine serum (FBS) (DMEM10). Twenty four h later, cells were treated with 1 μL of each dilution of compound in triplicate (10, 20, 40, 80, 160 μg/mL). Data were normalized using a DMSO negative control experiment, in which cells were treated with 1 μL of DMSO. Cell viability was determined by using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) after 18 h of treatment. The assay was performed by adding 20 μL of the CellTiter 96® AQueous One Solution Reagent to 100 μL of culture media and incubating the plate for 4 h. To measure the conversion of tertrazolium to formazan product, the absorbance was recorded at 490 nm using a 96-well microplate reader.
Isolation of genomic DNA and PCR amplification of loci:
Genomic DNA was extracted from fungal mycelium scraped from PDA plates, using the Qiagen DNeasy Plant Mini Kit to process approximately 100 mg fresh mycelium.
The primers used to characterize this endophyte are listed in Supplemental Table 1. For DNA amplification, the primer annealing temperature was 55°C for 30 amplification cycles on a Bio-Rad Thermal Cycler. A small aliquot of the PCR product was visualized on an agarose gel with ethidium bromide stain, and a Qiagen QiaQuick PCR Purification Kit was used to purify the remainder of the PCR product. This DNA was sent with forward/reverse primer for sequencing at the W.M. Keck Sequencing Facility (Yale University).
Phylogenetic analysis:
Genomic DNA was isolated from an agar plate culture using a Plant DNeasy kit (Qiagen), as described previously [16]. The Internal Transcribed Spacer (ITS) rDNA, Small Subunit (SSU) rDNA, Large Subunit (LSU) rDNA and Translation Elongation Factor I (TEF1) nuclear gene regions were amplified (primers sequences in Supplemental Table 1). The PCR amplicons were cleaned and sequenced by the W.M. Keck Foundation, as described previously [16]. A subset of taxa in the order Diaporthales was assembled, comprising 50 organisms (See Supplemental Table 2). Sequences were aligned with MUSCLE v3.8.31 and gaps present in more than 20% of the sequences removed with Phyutility [17]. Jmodeltest v2.1.6 identified the substitution model GTR+I+G for all loci [18]. Alignments were interleaved using Mesquite v3.01 creating a matrix of 2833 characters [19]. Trees were assembled using MrBayes v3.2.2 using 2 runs of 4 chains and a burn-in of 50%, and RAxML v8.1.3 in rapid bootstrap mode [20]. The tree files are available through TreeBase (http://treebase.org/). (Reviewer access available at http://purl.org/phylo/treebase/phylows/study/TB2:S16887?x-access-code=764b28d8100d6b16c8c3d54f60dab315&format=html.)
Microscopy:
For light microscopy a Zeiss microscope with 100x objective lens was used. For scanning electron microscopy 5 mm diameter plugs obtained from axenic cultures were vapor fixed for 12 h with osmium tetroxide. Samples were subsequently fixed with 2% gluteraldehyde in a 0.1 M sodium cacodylate solution and then dehydrated with increasing concentrations of ethanol (25%, 50%, 75%, 95%, and 100% 3 times) for 10-min intervals prior to critical point drying with a Polaron E3000 CPD and gold sputter coating with ~15 nm of gold using an EMS 550x sputter coater. Images were collected using an ISI SS-40 scanning electron microscope operating at 10kV.
Supplementary Material
Figure 4:
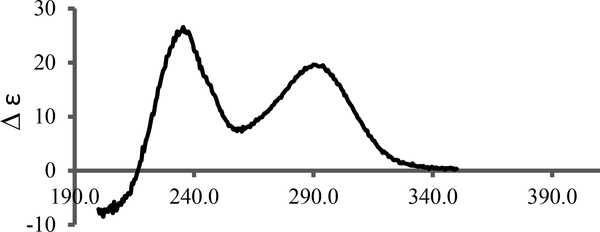
Experimental CD spectrum for purified pyrrolocin A in methanol.
Acknowledgments
Endophytes were isolated from plants collected with permission of the Ministerio del Ambiente of Ecuador. We thank the Biophysics Resource in the Structural Biophysics Laboratory, NCI at Frederick for assistance with CD-spectroscopy experiments. We would also like to thank Dr Kelly Caudle at the University of Cincinnati Children’s Hospital for providing Candida sp. clinical isolates, and the Endophyte Collection Quito Catolica (CEQCA) for cataloguing the microorganisms that produced the extracts tested here. This project was supported by an HHMI Professor’s grant and NSF grant OISE 853408 to S.A.S. This research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and with Federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Supplementary data: The primers and organisms used to phylogenetically characterize E6927E are also available.
References
- [1].Brown DG, Lister T, May-Dracka TL. (2014) New natural products as new leads for antibacterial drug discovery. Bioorganic & Medicinal Chemistry Letters, 24, 413–418. [DOI] [PubMed] [Google Scholar]
- [2].(a) Lam KS. (2007) New aspects of natural products in drug discovery. Trends in Microbiology, 15, 279–289; [DOI] [PubMed] [Google Scholar]; (b) Salemme FR, Spurlino J, Bone R. (1997) Serendipity meets precision: the integration of structure-based drug design and combinatorial chemistry for efficient drug discovery. Structure, 5, 319–324; [DOI] [PubMed] [Google Scholar]; (c) Shu YZ. (1998) Recent natural products based drug development: a pharmaceutical industry perspective. Journal of Natural Products, 61, 1053–1071. [DOI] [PubMed] [Google Scholar]
- [3].Cordell GA, Shin YG. (1999) Finding the needle in the haystack. The dereplication of natural product extracts. Pure and Applied Chemistry, 71, 1089–1094. [Google Scholar]
- [4].Watve MG, Tickoo R, Jog MM, Bhole BD. (2001) How many antibiotics are produced by the genus Streptomyces? Archives of Microbiology, 176, 386–390. [DOI] [PubMed] [Google Scholar]
- [5].(a) Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, Arrieta JM, Herndl GJ. (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proceedings of the National Academy of Sciences of the United States of America, 103, 12115–12120; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ward DM, Weller R, Bateson MM. (1990) 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature, 345, 63–65; [DOI] [PubMed] [Google Scholar]; (c) Handelsman J, Rondon MR, Brady SF, Clardy J, Goodman RM. (1998) Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chemistry & Biology, 5, R245–249. [DOI] [PubMed] [Google Scholar]
- [6].Smith SA, Tank DC, Boulanger LA, Bascom-Slack CA, Eisenman K, Kingery D, Babbs B, Fenn K, Greene JS, Hann BD, Keehner J, Kelley-Swift EG, Kembaiyan V, Lee SJ, Li P, Light DY, Lin EH, Ma C, Moore E, Schorn MA, Vekhter D, Nunez PV, Strobel GA, Donoghue MJ, Strobel SA. (2008) Bioactive endophytes warrant intensified exploration and conservation. PLoS One, 3, e3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jadulco RC, Koch M, Kakule TB, Schmidt EW, Orendt A, He H, Janso JE, Carter GT, Larson EC, Pond C, Matainaho TK, Barrows LR. (2014) Isolation of pyrrolocins A-C: cis- and trans-decalin tetramic acid antibiotics from an endophytic fungal-derived pathway. Journal of Natural Products, 77, 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].(a) Burmeister HR. (1976) Antibiotic equisetin and method of production. U.S. Patent: 3,959,468 A.;; (b) Singh SB, Zink DL, Goetz MA, Dombrowski AW, Polishook JD, Hazuda DJ. (1998) Equisetin and a novel opposite stereochemical homolog phomasetin, two fungal metabolites as inhibitors of HIV-1 integrase. Tetrahedron Letters, 39, 2243–2246. [Google Scholar]
- [9].Winter G (1886) Die Pilze Deutschlands, Osterreichs und der Schweiz. II. Abteilung: Ascomyceten: Gymnoasceen. Rabenhorst’s Kryptogamen-Flora, 1(2), Verlag E. Kummer, Leipzig. [Google Scholar]
- [10].(a) Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN. (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia, 94, 1017–1031; [PubMed] [Google Scholar]; (b) Sogonov MV, Castlebury L, Rossman A, Mejía L, White J. (2008) Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology, 62, 1–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].(a) Klebahn H (1918) Haupt-und Nebenfruchtformen der Askomyzeten: Eine darstellung eigener und der in der literatur niedergelegten beobachtungen über die zusammenhänge zwischen schlauchfrüchten und konidienfruchtformen. Gebrüder Borntraeger, pp 395; [Google Scholar]; (b) Rehm H (1885) Ascomyceten: Sclerotinia baccarum. Fasc. XVI. Hedwigia, 24, 7–17. [Google Scholar]
- [12].(a) Monod M (1983) Monographie taxonomique des Gnomoniaceae. Beihefte zur Sydowia. Annales Mycologici, Ser, 2, 1–315; [Google Scholar]; (b) Rossman AY, Farr DF, Castlebury LA. (2007) A review of the phylogeny and biology of the Diaporthales. Mycoscience, 48, 135–144. [Google Scholar]
- [13].(a) Barengo N, Sieber TN, Holdenrieder O. (2000) Diversity of endophytic mycobiota in leaves and twigs of pubescent birch (Betula pubescens). Sydowia, 52, 305–320 [Google Scholar]; (b) Kaneko S, Kobayashi T. (1984) Fungi inhabiting fagaceous trees V. Three species of Diaporthaceae on evergreen oak leaves. Transactions of the Mycological Society of Japan, 25, 11–19. [Google Scholar]
- [14].(a) Hazuda D, Blau CU, Felock P, Hastings J, Pramanik B, Wolfe A, Bushman F, Farnet C, Goetz M, Williams M, Silverman K, Lingham R, Singh S. (1999) Isolation and characterization of novel human immunodeficiency virus integrase inhibitors from fungal metabolites. Antiviral Chemistry & Chemotherapy, 10, 63–70; [DOI] [PubMed] [Google Scholar]; (b) Marfori EC, Kajiyama S, Fukusaki E, Kobayashi A. (2002) Trichosetin, a novel tetramic acid antibiotic produced in dual culture of Trichoderma harzianum and Catharanthus roseus callus. Zeitschrift fur Naturforschung C, Journal of Biosciences, 57, 465–470; [DOI] [PubMed] [Google Scholar]; (c) Marfori EC, Bamba T, Kajiyama Si, Fukusaki E-i, Kobayashi A. (2002) Biosynthetic studies of the tetramic acid antibiotic trichosetin. Tetrahedron, 58, 6655–6658; [Google Scholar]; (d) Herath K, Jayasuriya H, Zink DL, Sigmund J, Vicente F, de la Cruz M, Basilio A, Bills GF, Polishook JD, Donald R, Phillips J, Goetz M, Singh SB. (2012) Isolation, structure elucidation, and antibacterial activity of methiosetin, a tetramic acid from a tropical sooty mold (Capnodium sp.). Journal of Natural Products, 75, 420–424; [DOI] [PubMed] [Google Scholar]; (e) Wheeler M, Stipanovic R, Puckhaber L. (1999) Phytotoxicity of equisetin and epi-equisetin isolated from Fusarium equiseti and F. pallidoroseum. Mycological Research, 103, 967–973; [Google Scholar]; (f) Halecker S, Surup F, Kuhnert E, Mohr KI, Brock NL, Dickschat JS, Junker C, Schulz B, Stadler M. (2014) Hymenosetin, a 3-decalinoyltetramic acid antibiotic from cultures of the ash dieback pathogen, Hymenoscyphus pseudoalbidus. Phytochemistry, 100, 86–91; [DOI] [PubMed] [Google Scholar]; (g) Whitt J, Shipley SM, Newman DJ, Zuck KM. (2014) Tetramic acid analogues produced by coculture of Saccharopolyspora erythraea with Fusarium pallidoroseum. Journal of Natural Products, 77, 173–177; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Inokoshi J, Shigeta N, Fukuda T, Uchida R, Nonaka K, Masuma R, Tomoda H. (2013) Epi-trichosetin, a new undecaprenyl pyrophosphate synthase inhibitor, produced by Fusarium oxysporum FKI-4553. The Journal of Antibiotics (Tokyo), 66, 549–554; [DOI] [PubMed] [Google Scholar]; (i) Vertesy L, Knauf M, Markus-Erb A, Toti L, Raynal-Wetzel M-C, Fassy F. (2003) Coniosetin and derivatives thereof, process for the preparation and the use thereof. U.S. Patent: 6,599,930 B2.
- [15].(a) Bascom-Slack CA, Arnold AE, Strobel SA. (2012) IBI series winner. Student-directed discovery of the plant microbiome and its products. Science, 338, 485–486; [DOI] [PubMed] [Google Scholar]; (b) Strobel G, Daisy B. (2003) Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews, 67, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Griffin MA, Spakowicz DJ, Gianoulis TA, Strobel SA. (2010) Volatile organic compound production by organisms in the genus Ascocoryne and a re-evaluation of myco-diesel production by NRRL 50072. Microbiology, 156, 3814–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].(a) Edgar RC. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith SA, Dunn CW. (2008) Phyutility: a phyloinformatics tool for trees, alignments and molecular data. Bioinformatics, 24, 715–716. [DOI] [PubMed] [Google Scholar]
- [18].(a) Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772–772; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guindon S, Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52, 696–704. [DOI] [PubMed] [Google Scholar]
- [19].Maddison WP, Maddison D. (2014) Mesquite: a modular system for evolutionary analysis. v.3.01
- [20].(a) Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574; [DOI] [PubMed] [Google Scholar]; (b) Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications, 18, 315–322. [Google Scholar]
- [22].Spatafora JW, Mitchell TG, Vilgalys R. (1995) Analysis of genes coding for small-subunit rRNA sequences in studying phylogenetics of dematiaceous fungal pathogens. Journal of Clinical Microbiology, 33, 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vilgalys R, Sun BL. (1994) Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of the National Academy of Sciences, 91, 4599–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172, 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rehner SA, Buckley E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia, 97, 84–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.