Highlights
-
•
The immediate morbidity from COVID-19 is high in UK thoracic cancer patients.
-
•
Mortality, hospitalisation and treatment interruption rates were high.
-
•
All patients who died were current or ex-smokers.
Keywords: Covid-19;LUng cancer, Thoracic cancer
Abbreviations: ARB, Angiotensin-II receptor blockers; ARDS, Acute respiratory distress syndrome; CFR, Case fatality rate; COVID-19, Severe acute respiratory syndrome coronavirus 2; CT, Computerised tomography; GGO, Ground-glass opacities; HDU, High dependency unit; ICU, Intensive care unit; MERS-CoV, Middle East respiratory syndrome coronavirus; NHS, National Health Service; NIV, Non-invasive ventilation; PET, Positron Emission Tomography; RMH, Royal Marsden Hospital; RT-PCR, Reverse transcriptase polymerase chain reaction; SARS-CoV, Severe acute respiratory syndrome; TERAVOLT, Thoracic Cancers International COVID-19 Collaboration; TKI, Tyrosine kinase inhibitor; UK, United Kingdom; UKCCMP, UK Coronavirus Cancer Monitoring Project; US, United States; WHO, World Health Organisation
Abstract
Background
UK COVID-19 mortality rates are amongst the highest globally. Controversy exists on the vulnerability of thoracic cancer patients. We describe the characteristics and sequelae of patients with thoracic cancer treated at a UK cancer centre infected with COVID-19.
Methods
Patients undergoing care for thoracic cancer diagnosed with COVID-19 (RT-PCR/radiology/clinically) between March-June 2020 were included. Data were extracted from patient records.
Results
Thirty-two patients were included: 14 (43%) diagnosed by RT-PCR, 18 (57%) by radiology and/or convincing symptoms. 88% had advanced thoracic malignancies. Eleven of 14 (79%) patients diagnosed by RT-PCR and 12 of 18 (56%) patients diagnosed by radiology/clinically were hospitalised, of which four (29%) and 2 (11%) patients required high-dependency/intensive care respectively. Three (21%) patients diagnosed by RT-PCR and 2 (11%) patients diagnosed by radiology/clinically required non-invasive ventilation; none were intubated. Complications included pneumonia and sepsis (43% and 14% respectively in patients diagnosed by RT-PCR; 17% and 11% respectively in patients diagnosed by radiology/clinically). In patients receiving active cancer treatment, therapy was delayed/ceased in 10/12 (83%) and 7/11 (64%) patients diagnosed by RT-PCR and radiology/clinically respectively. Nine (28%) patients died; all were smokers. Median time from symptom onset to death was 7 days (range 3–37).
Conclusions
The immediate morbidity from COVID-19 is high in thoracic cancer patients. Hospitalisation and treatment interruption rates were high. Improved risk-stratification models for UK cancer patients are urgently needed to guide safe cancer-care delivery without compromising efficacy.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (COVID-19) was first reported in Wuhan China in December 2019 and has rapidly spread worldwide [1]. On 11th March 2020, the World Health Organisation (WHO) characterised COVID-19 as a pandemic [1]. Globally, the United Kingdom (UK) has the second highest overall mortality rate from COVID-19 [1,2]; and the highest rate of excess mortality in Europe [3]. Despite initial suppression of COVID-19 infection after a nationwide lockdown, the COVID-19 case rate is again rapidly rising, resulting in a second peak of COVID-19 infection in the UK. Over 989,000 COVID-19 cases have been confirmed in the UK by 31st October 2020, resulting in over 58,000 deaths [2].
The WHO estimates the global case fatality rate (CFR) from COVID-19 is 2.6% [1], but CFR varies significantly between countries. The UK's CFR is currently 2.1 per 100,000 resident population, however the CFR was as high as 26% in hospitalised patients during the first COVID-19 peak [2,4]. Whilst interpretation is limited by differences in COVID-19 testing, data collection, and definitions of COVID-19-related mortality between countries, this indicates that variation in ethnicity and medical practice between countries may be associated with differential outcomes, and thus assessment of region-specific patient populations are important to guide local guidelines and recommendations.
Patients with cancer are susceptible to infection due to the immunosuppressed state caused by local and systemic anti-neoplastic treatments and presence of malignancy [5,6]. Population-based studies have demonstrated that patients with cancer have a higher risk of severe events and death from COVID-19 compared with patients without cancer; and patients with haematological and lung malignancies are particularly at risk [4,6,7,8,9,10]. The global Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) published the first report of outcomes in thoracic cancer patients with COVID-19 in July 2020. This was followed by a single institution United States (US) study describing COVID-19 outcomes in lung cancer patients. Both demonstrated a high mortality rate (33% and 25% respectively), supporting prior observations that this is a high-risk patient population [5,11].
Access to healthcare in the UK is different to other geographies given the control placed on emergency access from the National Health Service (NHS) Gold command since declaration of a major incident, and may have impacted on UK-specific outcomes not easily distinguishable from pooled international datasets. Additionally, UK-specific cancer guidelines have resulted in treatment modifications including short radiotherapy fractionation, extended duration between immunotherapy doses and use of non-chemotherapy-based regimens [12].
Given the rising COVID-19 case rate in the UK and the high mortality rate from COVID-19 in thoracic cancer patients, factors associated with mortality need to be specifically explored for a UK cohort of patients with thoracic cancer, so clinicians can modify treatment advice to reduce morbidity and mortality in this at-risk patient population. Therefore, we describe the clinical characteristics and sequelae of UK patients with thoracic malignancy infected with COVID-19.
2. Materials and methods
2.1. Population
Patients undergoing care for thoracic malignancies (as per the TERAVOLT inclusion criteria: small cell lung cancer, non-small cell lung cancer, mesothelioma, thymic epithelial tumours) at the Royal Marsden Hospital (RMH), a large academic cancer referral centre, and diagnosed with COVID-19 between 1 March and 30 June 2020 were included.
Patients were diagnosed using one or more of the following methods: reverse transcriptase polymerase chain reaction (RT-PCR) test (defined as a positive RT-PCR assay result from a nose and/or throat swab as reported by local laboratory), computerised tomography (CT) scan of the chest (defined as reported to have radiological findings consistent with COVID-19 infection by radiologist and documented by treating clinician to have suspected COVID-19 infection on patient electronic medical records) and/or clinical symptoms (defined as having symptoms consistent with COVID-19 and documented by treating clinician to have clinically suspected COVID-19 infection on patient electronic medical records).
2.2. Data collection
COVID-19 positive patients were identified through the RMH microbiology list of patients who received RT-PCR testing, and through chart review of all patients booked into RMH lung unit clinics between March–June 2020 using electronic clinic lists. Additional chart review was performed on patients who made contact with the thoracic cancer nurse with symptoms suggestive of COVID-19 infection and/or were admitted to hospital.
Data was prospectively collected from the electronic medical records, radiology reports and microbiology reports. The following data points were collected: demographics (age, sex, ethnicity, smoking status, performance status, height, weight, comorbidities, concomitant medications); cancer history (stage, histology, molecular status, sites of metastatic disease, cancer treatment); COVID-19 infection (diagnosis, symptoms, imaging, blood and RT-PCR results, hospitalisation, ventilation, complications, treatment received); and outcomes (death, cancer treatment interruption, home oxygen requirement, post COVID-19 imaging). Never-smokers were defined as those who had smoked less than 100 cigarettes in their lifetime. Fever was defined as a temperature of 38.0 °C or higher. Active treatment was defined as receiving treatment within 3 months before COVID-19 diagnosis.
2.3. Outcomes
The objectives were to describe the clinical characteristics, management and outcomes of patients with thoracic malignancy infected with COVID-19, and to describe factors associated with mortality.
2.4. Statistical analysis
Descriptive analyses were performed. Categorical variables were reported as frequencies (percentage) and continuous variables were reported as median (range). Outcomes for patients diagnosed by RT-PCR and those diagnosed by radiology and/or clinical symptoms only are described separately.
Approval for this study was obtained through the RMH committee for clinical research (service evaluation number SE936). Patient consent was not required for this service evaluation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
3. Results
3.1. Patient characteristics
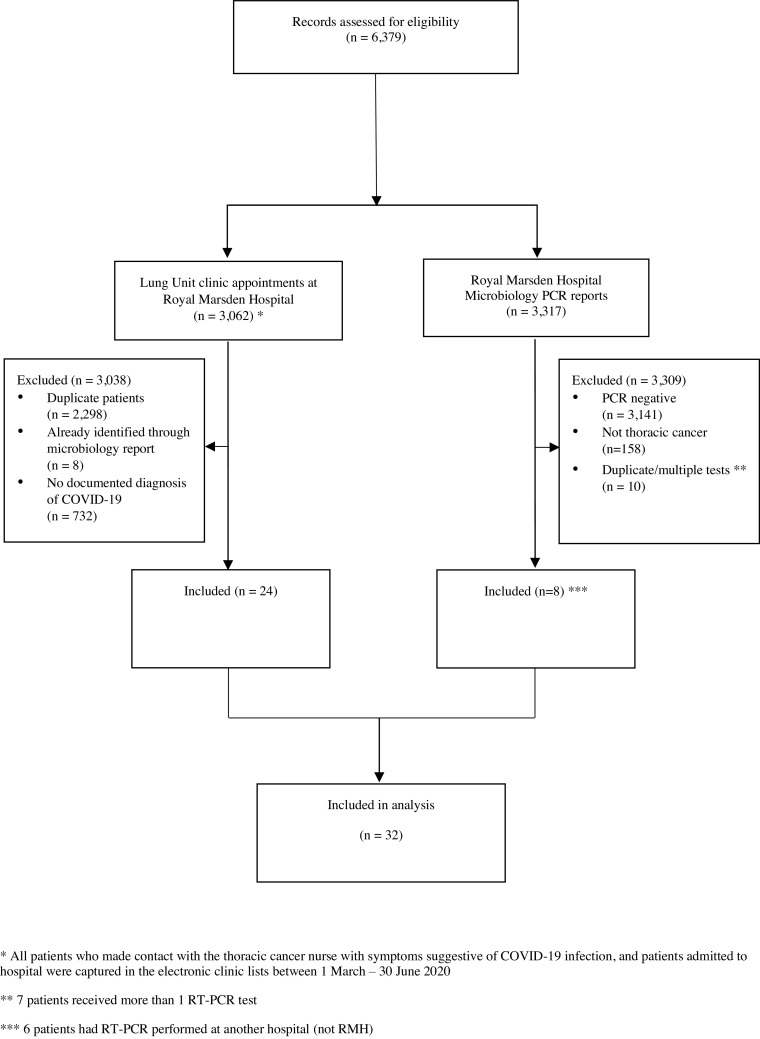
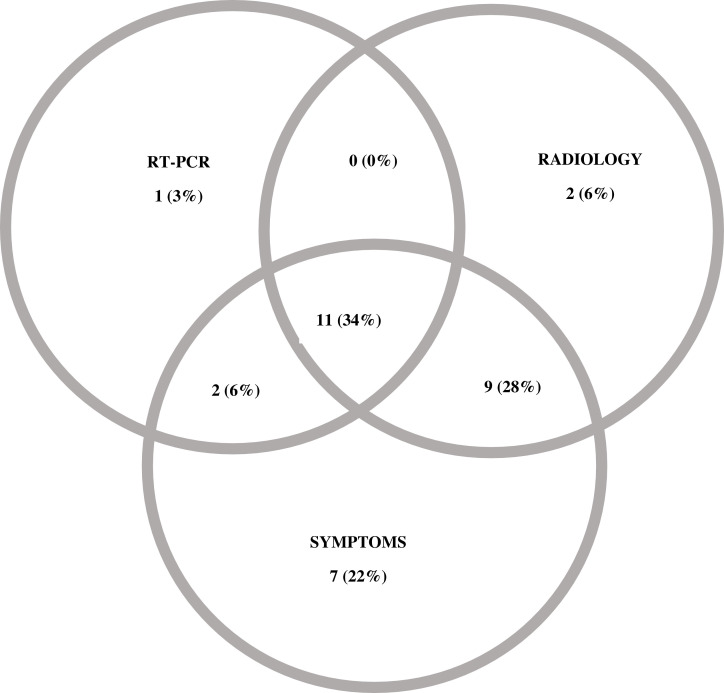
Of 6379 records assessed, 32 patients with COVID-19 infection were included: 14 patients were diagnosed by RT-PCR, 18 patients were diagnosed by radiology and/or clinical symptoms only (Fig. 1, Fig. 2 ). Three of 18 eligible patients who had clinical symptoms and radiological findings suspicious for COVID-19 tested negative on RT-PCR.
Fig. 1.
Eligible patients. * All patients who made contact with the thoracic cancer nurse with symptoms suggestive of COVID-19 infection, and patients admitted to hospital were captured in the electronic clinic lists between 1 March – 30 June 2020. ⁎⁎ 7 patients received more than 1 RT-PCR test. ⁎⁎⁎ 6 patients had RT-PCR performed at another hospital (not RMH).
Fig. 2.
Methods of COVID-19 diagnosis.
Demographics are shown in Table 1 . The median age was 70 years (range 40–84 years). Most were active or ex-smokers (75%); 31% had underlying chronic obstructive pulmonary disease and 19% had cardiac comorbidities. The majority had advanced thoracic malignancies (88%), and were on active treatment (72%). One patient included in the study was operated on for suspected lung cancer, but was found to have histoplasmosis.
Table 1.
Demographics of included patients.
| Total | Diagnosed by RT-PCR | Diagnosed by clinical symptoms / radiological findings | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| N = 32 | N = 14 | N = 18 | |
| Age (years) | |||
| Median (range) | 70 (40 – 84) | 63 (40 - 84) | 70 (42 – 83) |
| Sex | |||
| Male | 19 (59%) | 8 (57%) | 11 (61%) |
| Female | 13 (41%) | 6 (43%) | 7 (39%) |
| Ethnicity | |||
| White | 28 (88%) | 12 (86%) | 16 (89%) |
| Other | 4 (12%) | 2 (14%) | 2 (11%) |
| Smoking | |||
| Never | 7 (22%) | 6 (43%) | 1 (6%) |
| Former / Current | 24 (75%) | 8 (57%) | 16 (89%) |
| NA | 1 (3%) | 0 (0%) | 1 (6%) |
| Pack year history | |||
| Median (range) | 27.5 (0 – 120) | 10 (0 - 70) | 30 (0 - 120) |
| Body Mass Index | |||
| Median (range) | 24.2 (20.9 – 41.4) | 23.2 (20.9 - 34.5) | 24.8 (21.2 - 41.4) |
| ECOG* | |||
| 0 | 5 (16%) | 3 (21%) | 2 (11%) |
| 1 | 21 (65%) | 10 (71%) | 11 (61%) |
| 2 | 6 (19%) | 1 (7%) | 5 (28%) |
| >2 | 0 (0%) | 0 (0%) | 0 (0%) |
| Comorbidities | |||
| COPD | 10 (31%) | 2 (14%) | 8 (44%) |
| Cardiac disease⁎⁎ | 6 (19%) | 2 (14%) | 4 (22%) |
| Diabetes | 3 (9%) | 0 (0%) | 3 (17%) |
| Cerebrovascular disease | 4 (12%) | 1 (4%) | 3 (17%) |
| Chronic kidney disease | 3 (9%) | 1 (4%) | 2 (11%) |
| Regular medications | |||
| Steroids⁎⁎⁎ | 7 (22%) | 2 (14%) | 5 (28%) |
| ACE inhibitor | 3 (9%) | 1 (4%) | 2 (11%) |
| ARB inhibitor | 3 (9%) | 1 (4%) | 2 (11%) |
| Aspirin | 7 (22%) | 2 (14%) | 5 (28%) |
| NSAID | 5 (16%) | 5 (36%) | 0 (0%) |
| Cancer type | |||
| NSCLC | 23 (72%) | 9 (64%) | 14 (78%) |
| SCLC | 3 (9%) | 1 (4%) | 2 (11%) |
| Mesothelioma | 3 (9%) | 2 (14%) | 1 (6%) |
| Thymoma/Thymic | 2 (6%) | 1 (4%) | 1 (6%) |
| NA⁎⁎⁎⁎ | 1 (3%) | 1 (4%) | 0 (0%) |
| Current stage | |||
| 1 to 2 | 3 (9%) | 2 (14%) | 1 (6%) |
| 3 to 4 | 28 (88%) | 11 (79%) | 17 (94%) |
| NA | 1 (3%) | 1 (7%) | 0 (0%) |
| Cancer treatment | |||
| None | 9 (28%) | 2 (14%) | 7 (39%) |
| Active treatment | 23 (72%) | 12 (86%) | 11 (61%) |
| • Immunotherapy | 2 (6%) | 0 (0%) | 2 (11%) |
| • Chemotherapy | 6 (19%) | 3 (21%) | 3 (17%) |
| • Chemoimmunotherapy | 7 (22%) | 5 (35%) | 2 (11%) |
| • TKI | 5 (16%) | 3 (21%) | 2 (11%) |
| • Radiation | 2 (6%) | 0 (0%) | 2 (11%) |
| • Surgery | 1 (3%) | 1 (7%) | 0 (0%) |
Abbreviations: ACE: Angiotensin-converting enzyme; ARB: Angiotensin II receptor blockers; COPD: chronic obstructive pulmonary disease; ECOG: Eastern Cooperative Oncology Group performance status; NA: not applicable; NSAID: non-steroidal anti-inflammatory; NSCLC: non-small cell lung cancer; RT-PCR: reverse transcriptase polymerase chain reaction; SCLC: small cell lung cancer; TKI: tyrosine kinase inhibitor.
ECOG at last review prior to diagnosis of COVID-19.
Cardiac comorbidities: 4/6 had ischaemic heart disease and 2/6 had arrhythmias requiring permanent pacemaker.
Steroids ≥ 10 mg prednisolone equivalent.
Patient had suspected lung cancer based on imaging at time of COVID-19 diagnosis, but surgical resection specimen showed necrotising granuloma only.
3.2. Presentation
Twenty-seven patients (84%) were symptomatic at diagnosis. Appendix table A.1 shows the clinical characteristics of the 14 patients diagnosed by RT-PCR. Common symptoms were fever (57%), cough (50%) and dyspnoea (50%). Appendix Table A.2 shows the clinical characteristics of the 18 patients diagnosed by clinical symptoms and/or radiological findings. Of these 18 patients, 14 (78%) experienced cough, 10 (56%) experienced dyspnoea and 9 (50%) experienced fever; 6 (33%) patients experienced cough, dyspnoea and fever. One of the 2 patients who were asymptomatic at COVID-19 diagnosis later developed symptoms consistent with COVID-19 (fever and dyspnoea) and died from respiratory failure.
Twenty-three patients had chest imaging, 22 (96%) of which reported radiological changes suggestive of viral infection. Of the 14 patients diagnosed by RT-PCR, 11/14 had radiological findings on chest imaging: 6/11 (55%) on CT imaging, 5/11 (45%) on CXR (Appendix table A.1). The most common radiological findings were ground-glass change (7/11, 64%) and consolidation (5/11, 45%). Of the 18 patients diagnosed by clinical symptoms and/or radiological findings, 11/18 had radiological findings on chest imaging: 7/11 (64%) on CT imaging, 4/11 (36%) on CXR (Appendix table A.2). The most common radiological findings were ground-glass change (7/11, 64%) and consolidation (5/11, 45%).
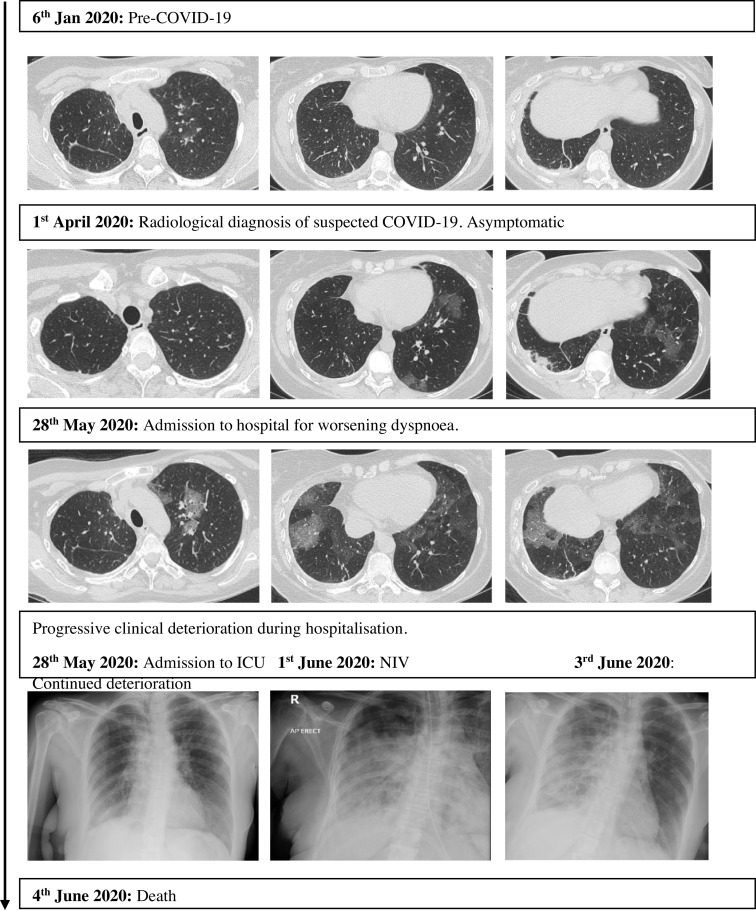
Thirteen patients had a subsequent scan after recovery from COVID-19 infection, of which 9 (69%) reported persistent radiological changes. The median time between the initial radiological scan at time of COVID-19 diagnosis and the subsequent radiological scan after recovery from COVID-19 infection was 60 days (range 34–91 days). Fig. 3 shows an example of one patient with COVID-19 who demonstrated radiological changes on routine response evaluation chest imaging, two months before development of symptoms. After development of symptomatic COVID-19 infection, she deteriorated rapidly, requiring hospitalisation and non-invasive ventilation for respiratory failure. She died one week after hospitalisation (Appendix Figure A.1: patient 2).
Fig. 3.
Radiographic changes of COVID-19 seen in one patient.
3.3. Management and outcomes
Outcomes are described in Table 2 . Of the 14 patients diagnosed by RT-PCR, 11 (79%) patients were hospitalised (median duration of hospitalisation 7 days; range 2–31 days). Four of 14 patients (29%) were admitted to the high dependency unit (HDU), and 4 (29%) died. Three of the 4 patients (75%) admitted to HDU died. Seven patients (50%) patients required oxygen, 3 (21%) required non-invasive ventilation (NIV) but no patients were intubated. Acute complications included pneumonia (43%) and sepsis (14%). Acute treatment received for COVID-19 infection included antibiotics (9/14, 64%), corticosteroids (3/14, 21%) and anti-viral therapy (1/14, 7%). One patient required domiciliary oxygen at home after hospital discharge, and later died at home. All 3 patients requiring NIV received antibiotics, 1 received corticosteroids (prednisolone 30 mg daily), and none received anti-viral or anti-fungal therapy. Cancer therapy was delayed or ceased in 10 of 12 (83%) patients on active treatment.
Table 2.
Consequences of COVID-19.
| Totaln (%)N = 32 | Diagnosed by RT-PCRn (%)N = 14 | Diagnosed by clinical symptoms / radiological findingsn (%)N = 18 | |
|---|---|---|---|
| Died | |||
| Yes | 9 (28%) | 4 (29%) | 5 (28%) |
| No | 23 (72%) | 10 (71%) | 13 (72%) |
| Hospitalised | |||
| Yes | 19 (59%) | 11 (79%) | 8 (44%) |
| No | 13 (41%) | 3 (21%) | 10 (56%) |
| Required oxygen | |||
| Yes | 13 (41%) | 7 (50%) | 6 (33%) |
| No | 19 (59%) | 7 (50%) | 12 (67%) |
| Required NIV | |||
| Yes | 5 (16%) | 3 (21%) | 2 (11%) |
| No | 27 (84%) | 11 (79%) | 16 (89%) |
| HDU/ICU admission | |||
| No | 26 (81%) | 10 (71%) | 17 (94%) |
| Yes | 6 (19%) | 4 (29%) | 2 (12%) |
| • ICU • HDU |
1 (3%) 5 (16%) |
0 (0%) 4 (29%) |
1 (6%) 1 (6%) |
| Complications | |||
| Pneumonia | 9 (28%) | 6 (43%) | 3 (17%) |
| ARDS | 3 (9%) | 1 (7%) | 2 (11%) |
| Sepsis | 4 (12%) | 2 (14%) | 2 (11%) |
| Arrhythmia | 3 (9%) | 1 (7%) | 2 (11%) |
| Treatment received | |||
| Antibiotics | 21 (66%) | 9 (64%) | 12 (67%) |
| Antivirals | 1 (3%) | 1 (7%) | 0 (0%) |
| Antifungals | 2 (6%) | 0 (0%) | 2 (11%) |
| Steroids | 10 (31%) | 3 (21%) | 7 (39%) |
| Home oxygen on discharge | |||
| Yes | 2 (6%) | 1 (7%) | 1 (6%) |
| No | 30 (94%) | 13 (93%) | 17 (94%) |
| Treatment interruption | |||
| No | 15 (47%) | 4 (29%) | 11 (61%) |
| Yes | 17 (53%) | 10 (71%) | 7 (39%) |
| • Delay • Cessation |
9 (28%) 8 (25%) |
6 (43%) 4 (29%) |
3 (17%) 4 (22%) |
Abbreviations: ARDS: acute respiratory distress syndrome; HDU: high dependency unit; ICU: intensive care unit; NIV: non-invasive ventilation; RT-PCR: reverse transcriptase polymerase chain reaction.
Of the 18 patients diagnosed by clinical symptoms and/or radiological findings, 12 (56%) patients were hospitalised (median duration of hospitalisation 3 days; range 2–9 days). Six (33%) patients required oxygen, 2 (11%) required NIV and 5 (28%) patients died. One patient was admitted to HDU, and one patient was admitted to the intensive care unit (ICU); both patients died. Patients were treated with antibiotics (12/18, 67%), corticosteroids (7/18, 39%) and anti-fungal therapy (2/18, 11%). Acute complications included pneumonia (17%), acute respiratory distress syndrome (ARDS) (11%), sepsis (11%) and arrhythmias (11%). Cancer therapy was delayed or ceased in 7 of 11 (64%) patients on active treatment.
Of the 9 patients who died, all were active or ex-smokers (Appendix Table A.3). Timelines for these patients are shown in Appendix Figure A.1. Half (55%) of the deceased patients were receiving chemotherapy at time of COVID-19 infection (Appendix Table A.3). The median time from symptom onset to death was 7 days (range 3–37 days). Seven patients died in hospital.
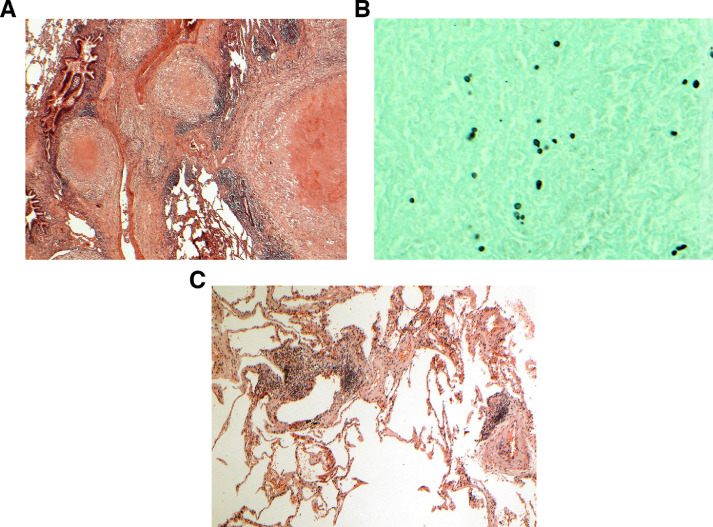
One patient with a fluorodeoxyglucose (FDG) avid lung nodule on positron emission tomography (PET)/CT was incidentally diagnosed with COVID-19 infection on a routine pre-operative screening COVID-19 RT-PCR prior to her elective lung surgery. She did not undergo a preoperative biopsy due to COVID-19 resource limitations. She underwent a left lower lobe wedge excision and frozen section with a view to proceed to lobectomy, 23 days after COVID-19 diagnosis. The final pathological diagnosis of the resected lung mass on the wedge resection was fungal infection, likely histoplasmosis. Patchy mild interstitial chronic inflammation was noted in the lung away from the nodule (Fig. 4 ). She did not proceed to lobectomy, and did no experience complications from her surgery or COVID-19 infection.
Fig. 4.
Histoplasmosis mimicking lung cancer. A) The mass comprises a large necrotising granuloma with adjacent satellite granulomas. B) A Grocott stain shows small focally budding fungal spores, characteristic of histoplasmosis. C) Lung away from the nodules shows a patchy lymphoid infiltrate, consistent but not specific with COVID-19 infection.
4. Discussion
This study of 32 thoracic cancer patients treated at a single academic UK cancer centre confirms the high mortality rate (28%) from COVID-19 infection in patients with thoracic malignancies, which is higher than the CFR of the UK general population [2]. The mortality rate in our study is similar to the global TERAVOLT study of thoracic cancer patients (33%) and the US study of lung cancer patients with COVID-19 (25%) [5,11]. Likewise, the UK Coronavirus Cancer Monitoring Project (UKCCMP) study of 800 UK cancer patients diagnosed with COVID-19, reported an overall mortality rate of 28% (226/800) and a calculated mortality rate of 36% (32/90) in patients with respiratory and intrathoracic cancers (odds ratio for death 1.50; 95% CI, 0.91–2.45; p = 0.12) [6].
Despite the high mortality rate, only 1 patient (6%) in our study was admitted to ICU, and no patients received invasive ventilation. This proportion is lower than that seen in the general UK population (17% and 10% of hospitalised patients respectively) [4], and US lung cancer patients (21% and 18% respectively) [11]. However, it is similar to the UK general cancer population and the predominantly European thoracic cancer patient cohort enroled on the TERAVOLT registry [5,6]. It is unclear if this is related to patient wishes, cultural and/or institutional expectations or prioritisation of limited ICU resources to patients without cancer, especially in a cohort of patients with predominately advanced-stage thoracic malignancy.
Patient characteristics in our study were similar to the TERAVOLT study (predominantly white, current/ex-smokers, advanced-stage, NSCLC, on active therapy), however most patients included in TERAVOLT were enroled from Italy, Spain and France (90%); only 2 patients from the UK were included in the preliminary analysis [5]. Due to the established high false-negative rate with nasopharyngeal swab RT-PCR [13,14], and the low rates of COVID-19 testing in the UK early in the pandemic (199 tests per million people) [15,16], half of the patients included in our study were diagnosed by clinical symptoms and/or radiological findings with unknown or negative RT-PCR results, higher than that reported by TERAVOLT [5]. Demographics were similar between those who were diagnosed by RT-PCR versus clinically; and patients in our study experienced similar symptoms (most common: cough, dyspnoea, fever) compared to previous studies with cancer and non-cancer patients, and similar rates of hospitalisation and death compared to previous studies with thoracic and non-thoracic cancer patients [4,5,6,7,9,10,17,18,19,20].
While chest imaging features, such as peripheral bilateral ground-glass opacities (GGOs) with or without consolidation, are sensitive for COVID-19 infection, they are not specific [6,14]. This is especially a challenge in thoracic cancer patients, as radiotherapy and drug-related pneumonitis are common mimics of COVID-19. The Radiological Society of North America and the British Society of Thoracic Imaging have published guidance on reporting chest CT findings related to COVID-19, to help delineate COVID-19 changes from other aetiologies [21,22]. In our study, we found that almost all patients who had chest imaging had reported COVID-19 changes. One patient with chest imaging shown in Fig. 3 demonstrated radiologic changes typical of COVID-19. Before onset of symptoms, she demonstrated typical early stage changes of small subpleural GGOs mainly in the lower lobes. After symptom development, she developed progressive stage changes of peripheral bilateral multifocal GGOs, which then rapidly developed into areas of consolidation.
To reduce the risk of COVID-19 transmission and mortality in patients with cancer, significant changes to patient care have occurred internationally, with fewer face-to-face patient visits, modifications to local and systemic cancer therapy, and alterations to response evaluation [23]. Furthermore, the UK government implemented interim shielding measures for vulnerable populations, including patients with thoracic malignancies, which further complicate care delivery in UK cancer patients [2]. However, to balance cancer-related morbidity and mortality with that of COVID-19, it is crucial to identify predictors of poor outcomes, to thereby better inform appropriate treatment modifications. Albeit small sample size, only 1 of 5 (20%) patients on tyrosine kinase inhibitor (TKI) therapy died, while 5 of 8 (63%) patients on chemotherapy died. While small studies have previously suggested an increased risk of mortality in patients who recently received cytotoxic chemotherapy [19,24], this observation has not been reinforced by larger cancer database studies [5,6,10]. All patients who died in our study were current or ex-smokers, a risk factor observed in other studies with thoracic cancer patients [5,11]. A systematic review showed that 22% of current smokers and 46% of ex-smokers had study-defined severe complications from COVID-19 infection. Current smokers had a higher risk of severe complication (relative risk 1.45; 95% CI, 1.03–2.04) compared to former and never smokers, and a higher mortality rate (38.5%) [25]. Tobacco smoking could therefore be an important modifiable risk factor in the thoracic cancer population.
In addition to risk-minimising care delivery changes, treatment strategies for COVID-19 remains a rapidly changing paradigm. Use of corticosteroids for management of COVID-19 was until recently controversial, with previous metanalysis from the Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome (SARS-CoV) and H1N1 influenza pandemics suggesting harm in patients treated with corticosteroids; and small early Chinese studies reported increased mortality in patients with COVID-19 treated with corticosteroids [24,26]. Recently, the RECOVERY study, which randomised 6425 patients to receive dexamethasone 6 mg once daily or usual care, demonstrated lower 28-day mortality rates in patients treated with dexamethasone compared to usual care (22.9% versus 25.7%; rate ratio 0.83; 95% CI, 0.75–0.93; p<0.001) [27]. The benefit was mostly seen in those who required mechanical ventilation (rate ratio 0.64; 95% CI, 0.51–0.81) or oxygen therapy (rate ratio 0.82; 95% CI, 0.72–0.94), and not in patients who did not require ventilatory support (rate ratio 1.19; 95% CI, 0.91–1.55) [27]. Ten patients in our study received corticosteroids for COVID-19 management, of which 7 (70%) survived and 3 (30%) died. However, of these 10 patients, 5 (50%) developed pneumonia as a secondary complication of COVID-19 infection.
Another important treatment consideration is antimicrobial therapy. Evidence for anti-viral therapy has been disappointing [28]; only 1 patient in our study received anti-viral therapy. Co-infections with bacteria or fungi are common complications of influenza, and are major cause of mortality [29,30,31]. In patients with COVID-19, prevalence of reported secondary co-infection is low (6–15%), much lower than reported antimicrobial use (up to 70%) [20,32,33,34]. In the UK, a retrospective study of 836 hospitalised patients found a low frequency of bacterial infection (6.1%) and no evidence of fungal infection [34]. Twenty-eight percent of patients in our study developed pneumonia, higher than the general UK population; 66% received antibiotics and 6% received anti-fungal therapy. This finding may reflect the immunocompromised state of this patient cohort, and thus a lower threshold to implement antimicrobial therapy.
In addition, 1 patient in this study with radiologically suspected lung cancer was later proven to have histoplasmosis on surgical excision and no malignancy (Fig. 4). Of note, there was patchy interstitial chronic inflammation in the background lung, consistent with studies examining pathological changes in patients with COVID-19 infection which have also reported patchy inflammatory cellular infiltrates without hyaline membrane formation in early phase infection [35,36,37,38]. Severe COVID-19 lung pathology, such as diffuse alveolar damage and thrombotic changes which can progress to fibrosis weeks to months later, were not detected in this asymptomatic patient [38].
This patient also highlights the significant disruption to lung cancer pathways during the COVID-19 pandemic, in that biopsy was not undertaken prior to surgery due to resource prioritisation and reduced access to interventional procedures. Delays to diagnostic procedures is expected to lead to later presentation of more advanced malignancies in the months ahead. This is a hidden, grave consequence of the COVID-19 pandemic which is not captured in current datasets. In addition, the high rates of cancer treatment interruption in patients on active treatment (74% in this study) has serious potential implications on future survival and morbidity. Furthermore, long term respiratory complications of COVID infection itself in patients with underlying lung disease are unknown. Therefore, longitudinal follow-up will be crucial to understand the lasting implications of COVID-19 infection in survivors.
Our study has several limitations. It is a small single-centre study, and therefore our results may not be generalisable to specific subgroups, such as patients receiving radiotherapy, or thoracic cancer patients outside the UK. Due to UK-specific implementation of patient shielding and UK-specific risk modifying changes to treatment, this population may not be representative of the global thoracic cancer population. In addition, UK patients with non-severe symptoms were advised to self-isolate at home rather than present to hospital during the first peak of COVID-19 infections. Therefore, our study may under-report the total number of thoracic cancer patients with COVID-19, as patients shielding in the community with mild COVID-19 symptoms, who were not booked into outpatient clinic and did not notify the RMH of their symptoms, may not have been captured in this study. Due to low COVID-19 testing rates in the UK during the early phase of the pandemic, we included patients diagnosed clinically and/or radiographically. Although our patient demographics is consistent with previously published studies which only included patients with RT-PCR-confirmed COVID-19 infection, pathological confirmation is not available for all patients in this study. Lastly, data regarding long-term consequences of COVID-19 infection is lacking in our study, therefore we are not able to describe the true impact of this virus on lasting morbidity and subsequent mortality.
This study highlights that COVID-19 mortality rates are particularly high in patients with thoracic malignancies. However, it also raises many questions regarding identification of predictors of poor outcome in thoracic cancer patients, to thereby better inform treatment decisions, an area of crucially needed research. Ten percent of lung cancer patients are never-smokers, and are usually on TKI therapy due to the oncogene-addicted nature of never-smoking lung cancer [39]; hence theoretically have minimal excess mortality risk. Furthermore, we did not demonstrate increased mortality in patients receiving immunotherapy or radiotherapy. Treatment modifications, such as strict shielding measures, short radiotherapy fractionation and extended duration between immunotherapy treatments, may only achieve modest improvements in minimising COVID-19-related complications, but could compromise patient care and sense of wellbeing in this subgroup of thoracic patients. For clinicians to provide the best advice regarding cancer treatment, risk-stratification models to assess an individual's vulnerability to serious COVID-19 infection are urgently needed.
5. Conclusion
Our single institution study describes a high mortality rate from COVID-19 in patients with thoracic malignancies during the first COVID-19 infection peak in the UK. All patients who died were current or ex-smokers, supporting the observation that smoking is an important modifiable risk factor in the thoracic cancer population during COVID-19.
Uncertainty regarding balancing the need to modify practices to minimise COVID-19 risk, while not compromising cancer care and quality of life is difficult, particularly in patients with advanced cancer and limited prognosis. Whilst the UK government initially enforced interim shielding and social distancing measures for vulnerable patients and provided short-term guidelines regarding delivering cancer therapy, future recommendations in this at-risk population remains unclear. As COVID-19 cases are again increasing in Europe, and COVID-19 is predicted to persist long-term in the UK, development of more complex and nuanced models to assess an individual's vulnerability to serious COVID-19 infection are critically needed, that take into account not only the characteristics of the cancer but co-morbidities and other factors that influence risk. Treatment factors, such as the interaction of radiotherapy and COVID-19 infection, need to be further explored. Longitudinal follow-up is needed to better understand the chronic sequelae from COVID-19 infection, and its direct and indirect impacts on morbidity and subsequent mortality, and to guide chronic care recommendations for survivors of COVID-19.
Funding
Nil
Declaration of competing interest
Dr Cui reports research grant funding from the Breast Cancer Trials group and the Australian Government Research Training scholarship, outside of the submitted work. Dr Minchom has served on advisory boards and received fees from Merck, FARON, Novartis, Bayer, Janssen, all unrelated to this work. Dr McDonald reports speaker fees from Astra Zeneca, Elekta and Takeda; research grant funding from MSD and consulting fees from Astra Zeneca and Accuray. Dr Lee reports grants from the Royal Marsden Cancer Charity outside of the submitted work. Prof Nicholson has received compensation from Astra Zeneca, Pfizer, Merck, Bristol Myers Squib, Roche, Novartis, Abbvie and Oncologica for advisory boards, an educational grant from Pfizer, and for speaking at a symposium from Astra Zeneca, all unrelated directly to this study. Prof O'Brien reports advisory work for MSD, BI, Abbot, Pierre Fabre and Roche outside the submitted work. Prof Popat reports personal fees from BMS, Roche, Takeda, AstraZeneca, Pfizer, MSD, EMD Serono, Guardant Health, Abbvie, Boehringer Ingelheim, OncLive, Medscape, Incyte, Paradox Pharmaceuticals, Eli Lilly, outside the submitted work. Dr Yousaf, Dr Bhosle, Dr Ahmed and Dr Locke report no potential competing interests.
Acknowledgements
The authors would like to acknowledge the patients who included in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ctarc.2020.100261.
Appendix. Supplementary materials
References
- 1.World Health Organisation. WHO Coronavirus Disease (COVID-19) dashboard, Available from: https://covid19.who.int/. Accessed 31st October 2020.
- 2.Gov.UK. Coronavirus (COVID-19) in the UK 2020, Available from: https://coronavirus-staging.data.gov.uk/. Accessed 31st October 2020.
- 3.Office for National Statistics. Comparisons of all-cause mortality between European countries and regions: january to June 2020 2020, Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/comparisonsofallcausemortalitybetweeneuropeancountriesandregions/januarytojune2020#main-points. Accessed 5th August 2020.
- 4.Docherty A.B., Harrison E.M., Green C.A. Features of 20133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020 doi: 10.1136/bmj.m1985. 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garassino M.C., Whisenant J.G., Huang L.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee L.Y.W., Cazier J.B., Starkey T. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M., Liu D., Liu M. Patients with Cancer appear more vulnerable to SARS-CoV-2: a Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J., Ouyang W., Chua M.L.K. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J., Rizvi H., Preeshagul I.R. COVID-19 in patients with lung cancer. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.06.007. Published online first: 16 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Health and Care Excellence. COVID-19 rapid guideline: delivery of systemic anticancer treatments 2020. Available from: https://www.nice.org.uk/guidance/ng161. Accessed 6th August 2020. [PubMed]
- 13.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai T., Yang Z., Hou H. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: a Report of 1014 Cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gov.UK. Coronavirus cases in the UK: daily updated statistics 2020. Available from: https://www.gov.uk/guidance/coronavirus-covid-19-information-for-the-public#testing-capacity. Accessed 5 July 2020.
- 16.Roser M., Ritchie H., Ortiz-Ospina E., J H. Coronavirus pandemic (COVID-19) online 2020. Available from: https://ourworldindata.org/coronavirus. Accessed 15 July 2020.
- 17.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 18.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson S., Kay F.U., Abbara S. Radiological society of North America expert consensus statement on reporting chest ct findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - secondary publication. J Thorac Imaging. 2020;35(4):219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Society of Thoracic Imaging. Thoracic imaging in COVID-19 infection 2020. Available from: https://www.bsti.org.uk/media/resources/files/BSTI_COVID-19_Radiology_Guidance_version_2_16.03.20.pdf. Accessed 7th August 2020.
- 23.Dingemans A.C., Soo R.A., Jazieh A.R. Treatment guidance for patients with lung cancer during the Coronavirus 2019 Pandemic. J Thorac Oncol. 2020;15(7):1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K., Sheng Y., Huang C. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alqahtani J.S., Oyelade T., Aldhahir A.M. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: a Rapid Systematic Review and Meta-Analysis. PLoS ONE. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Recovery Collaborative Group. Horby P., Lim W.S. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Published online first: 17 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao B., Wang Y., Wen D. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic Influenza: implications for pandemic Influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metersky M.L., Masterton R.G., Lode H. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating Influenza. Int J Infect Dis. 2012;16(5):e321–e331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Klein E.Y., Monteforte B., Gupta A. The frequency of Influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawson T.M., Moore L.S.P., Zhu N. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. Published online first: 02 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes S., Troise O., Donaldson H. Bacterial and fungal coinfection among hospitalised patients with COVID-19: a retrospective cohort study in a UK secondary care setting. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.06.025. Published online first: 26 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian S., Hu W., Niu L. Pulmonary pathology of early-phase 2019 Novel Coronavirus (COVID-19) Pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polak S.B., Van Gool I.C., Cohen D. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020 doi: 10.1038/s41379-020-0603-3. Published online first: 22 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson A.G., Osborn M., Devaraj A. COVID-19 related lung pathology: old patterns in new clothing. Histopathology. 2020;77(2):169–172. doi: 10.1111/his.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.