Abstract
Aim of the Study:
Elevation of the head and thorax (HUP) during cardiopulmonary resuscitation (CPR) has been shown to double brain blood flow with increased cerebral perfusion pressures (CerPP) after active compression-decompression (ACD) CPR with an impedance threshold device (ITD). However, the optimal angle for HUP CPR is unknown.
Methods:
In Study A, different angles were assessed (20°, 30°, 40°), each randomized over 5-min periods of ACD + ITD CPR, after 8 min of untreated ventricular fibrillation in an anesthetized swine model. Based upon Study A, Study B was performed, where animals were randomized to 1 of 2 sequences: 20°, 30°, 40° or 40°, 30°, 20° with a similar protocol. The primary endpoint was CerPP for both studies.
Results:
In Study A, no optimal HUP angle was observed in 18 pigs. CerPPs for 30° and 40° (mmHg, mean ± SD) were equivalent (44 ± 22 and 47 ± 26, p = 0.18). However, CerPP appeared higher when 40° HUP was performed during the last 5-min of CPR, suggestive of a sequence effect. For Study B, after 17 min of CPR, CerPP (mmHg) were higher with the 20°, 30°, 40° sequence: 60 ± 17 versus 33 ± 18 (p = 0.035).
Conclusions:
No optimal HUP CPR angle was observed. However, controlled progressive elevation of the head and thorax during CPR is more beneficial than an absolute angle or height to maximize CerPP. Further studies are needed to determine the optimal rate of rise during HUP ACD + ITD CPR.
Keywords: Active compression-decompression CPR, Cardiac arrest, Cardiopulmonary resuscitation, Cerebral perfusion, Head up CPR, Head and thorax elevation, Impedance threshold device, Mechanical CPR
Introduction
Elevation of the head and thorax (HUP) during cardiopulmonary resuscitation (CPR) is a recent advance in resuscitation, employing gravity to enhance venous return from the head and neck to the right side of the heart, mitigate transmission of pressures to the brain via central vasculature, and enhance transpulmonary circulation when used with circulatory1–4 adjuncts. HUP CPR has been shown to double brain blood1,3 flow with improved cerebral perfusion pressures (CerPP)1–3 and coronary1 perfusion pressures (CoPP) in animal and5 human cadaver models. The highest CerPP with HUP CPR has been observed with the combination of active compression-decompression (ACD) CPR and an impedance threshold device (ITD).2,3 Recently, an observational study of HUP CPR as part of a newly implemented bundle of pre-hospital cardiac arrest care reported improved rates of sustained return of spontaneous circulation (ROSC) in humans.6
As the concept of HUP CPR continues to develop, further understanding of physiologic mechanisms and refinements are needed in order to maximize its potential benefit. For example, the optimal HUP CPR angle and rate of elevation remain unknown. Prior animal work has shown a benefit of decreasing intracranial pressure (ICP) while steadily increasing the angle of elevation up to 60° during HUP CPR.1,7 However, as the angle increased, the mean arterial pressure (MAP) declined steadily. This inverse relationship between ICP and MAP must be considered to optimize circulation.
In this porcine model of cardiac arrest, the initial objective was to determine which angle of elevation during HUP CPR resulted in the highest CerPP, with a hypothesis that the highest angle, 40°, would result in the highest CerPP. Based on these results, a second protocol was then performed to compare two angle elevation sequences. The primary objective in the second protocol was to determine which progressive angle elevation sequence resulted in the highest CerPP after 17 min of CPR.
Materials and methods
Study ethics
The study was approved by the Institutional Animal Care and Use Committee of the Hennepin Healthcare Research Institute (HHRI). Care of swine was compliant with the National Research Council’s 2011 Guidelines for the Care and Use of Laboratory Animals. A certified and licensed HHRI veterinarian assured the study protocol was in compliance with these guidelines.
Study design and measurements
Study preparation and surgical techniques were performed as previously described.2,3,8 Female Yorkshire farm pigs weighing approximately 40 kg were acclimatized to the facility and fasted overnight prior to the study. Immediately before the study, pigs received intramuscular ketamine (1000 mg) in the holding pen and then transferred to the surgical suite. In the surgical suite, inhaled isoflurane at 5% was given, and pigs were intubated with a 7.5 mm cuffed endotracheal tube (ETT) and placed on a ventilator (Narkomed, North American Drager) with a tidal volume of 10 mL/kg. Isoflurane was continued for anesthesia at 1%–2.5% after intubation. End tidal carbon dioxide (ETCO2) and oxygen saturation measured via pulse oximetry were with a CO2SMO Plus (Novametrix Systems). Respiratory rate and FiO2 were titrated to keep oxygen saturation values > 92% and ETCO2 between 37–43 mmHg. Intravenous (IV) access via was then obtained and all animals received saline during the preparatory phase to maintain a right atrial pressure (RA) between 4 and 7 mmHg. Animals also received a 100 unit/kg bolus of IV heparin every hour. Temperature was measured via esophageal probe and maintained between 36.5 and 38 °C with a warming blanket. Proximal airway pressure was measured at the end of the ETT with a differential pressure transducer (TSD160C, Biopac Systems, Inc). Aortic blood pressure was measured with a micromanometer-tipped catheter (Mikro-Tip Transducer, Millar Instruments) placed in the femoral artery into the thoracic aorta at the diaphragm. Central venous access for continuous monitoring was obtained via catheter placement in the femoral vein with advancement to the level of the RA. Arterial and venous catheter placement were confirmed via fluoroscopy prior to study start. An intracranial bolt via burr hole in the skull was placed to measure ICP with a micromanometer-tipped catheter placed approximately 5 mm into brain tissue.
Data were continuously monitored during the study, including electrocardiographic monitoring, ETCO2, ICP, RA pressure, and aortic pressure using the BioPac data acquisition system (BioPac MP150; BioPac Systems Inc) and stored using the BioPac Acqknowledge 4.2 software. Arterial blood gases (ABG) were obtained and analyzed with a Gem Premier 3000 device (Instrumentation Laboratory).
Isoflurane was discontinued at the end of the preparation, and after 3 min, ventricular fibrillation (VF) was induced via direct current from a pacing wire placed in the right ventricle under fluoroscopy. ACD CPR was performed with an automatic piston device (Caztek Engineering). ACD CPR was performed at a rate of 100 compressions/minute, with a 50% duty cycle and depth of 22.5% antero-posterior chest diameter. Decompression was performed with a suction cup at a force of 10 kg. The ITD (ResQPOD 16, Zoll) was placed at the end of the ETT at the initiation of CPR. During CPR, manual positive pressure ventilation was provided with oxygen, with a target Sp02 value of ≥ 92% and tidal volume of 10 mL/kg. Succinylcholine 100 mg (~2.5 mg/kg) was given after 1 min of CPR to inhibit gasping.
After induction of VF, animals were placed on a customized elevation device (CED) capable of elevating the head and thorax to perform HUP CPR. This device was electrically-coupled to the CPR machine to maintain continuous chest compressions at a 90° angle to the mid-sternum. The CED has three support planes that move at different angles relative to one other. One plane is for the lower extremities, abdomen, and lower thorax, a middle plane for mid-thorax, and a third for the upper thorax and head. The CED can maximally elevate the head to 22 cm and heart 9 cm, corresponding roughly to 40° (Fig. 1).
Fig. 1 –
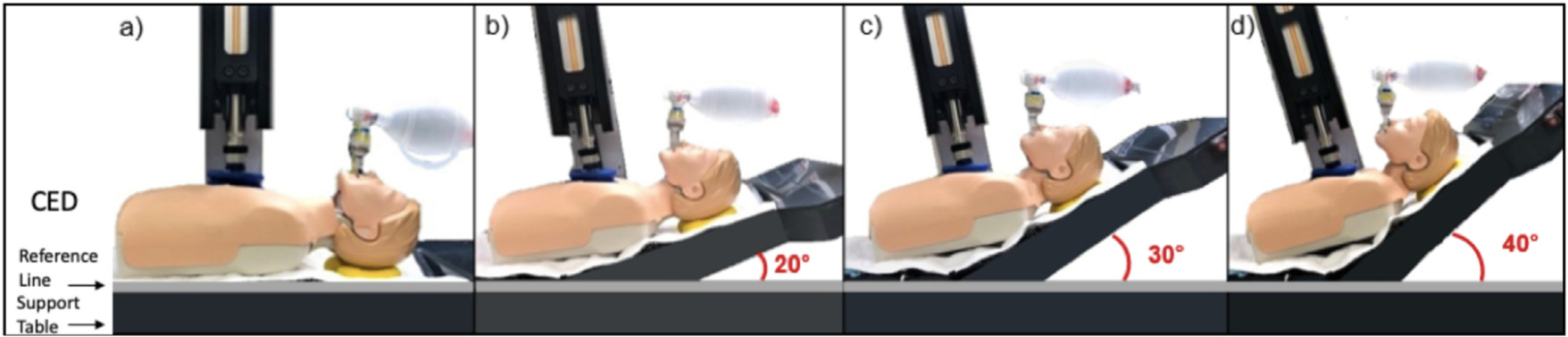
The customized elevation device (CED) has different support planes, one for the head and upper thorax, a second for the mid-thorax, and a third for the lower thorax, abdomen, and lower extremities. (a) 0°, (b) 20°, (c) 30°, (d) 40°.
Experimental protocol
Study A
After 8 min of untreated VF, ACD + ITD CPR was performed with a 30:2 compression: ventilation ratio, and manual positive pressure ventilation was performed without oxygen with the animal in the horizontal plane and supine position. This combination was performed to simulate basic life support (BLS)9 and also to prime the cardio-cerebral circuit prior to elevation. After 2 min of BLS ACD + ITD CPR, CPR was performed continuously with a 10:1 compression to ventilation ratio and animals were randomized to one of 6 combinations for 5-min CPR intervals; (a) 20°, 30°, 40° (b) 20°, 40°, 30° (c) 30°, 20°, 40° (d) 30°, 40°, 20° (e) 40°, 20°, 30° or (f) 40°, 30°, 20°. These combinations were performed to mitigate the effect of time on haemodynamics, and ensure each angle was equally studied at all the experimental intervals throughout the resuscitation. Transition from one angle to the next was performed over 10 (for elevation to 20°) to 24 s (for elevation to 40°). After 17 min of CPR, 0.5 mg of adrenaline was administered if the diastolic blood pressure was ≤ 25 mmHg, and 0.25 mg was given if the diastolic blood pressure was > 25 mmHg, followed by 25 mg of amiodarone. One minute later, animals were defibrillated with up to three 200 J biphasic shocks (X-series, Zoll Medical).If ROSC was not obtained, CPR was resumed, and a shock was given every 2 min with 0.5 mg adrenaline and 25 mg amiodarone. If ROSC was not obtained after three cycles of CPR, the resuscitation was stopped. If ROSC was obtained, animals were treated with isoflurane and 15 min later euthanized with an intravenous injection of KCl. The Study A protocol is outlined in Fig. 2.
Fig. 2 –
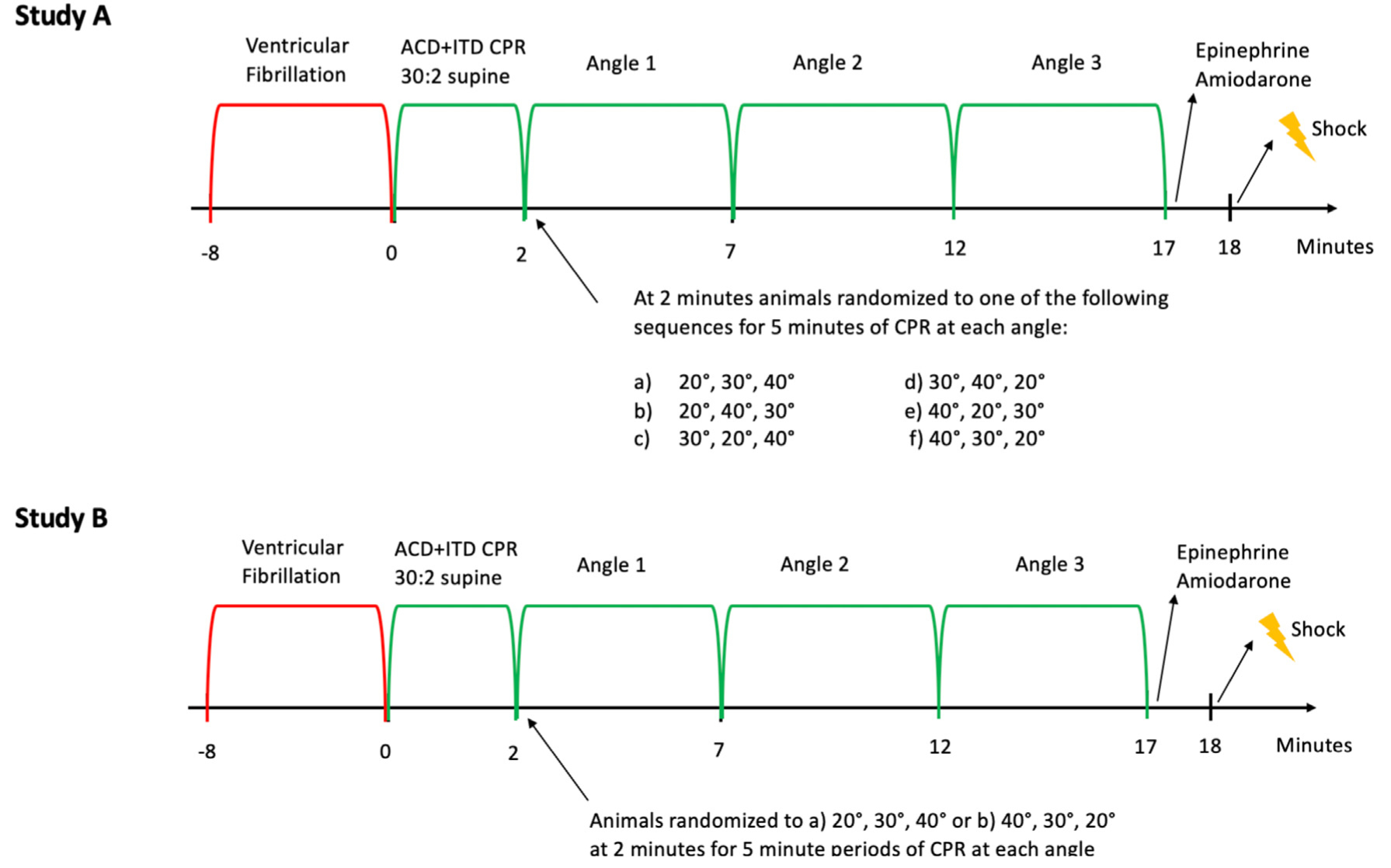
Study A and B protocols. Abbreviations: active compression-decompression (ACD) cardiopulmonary resuscitation (CPR), impedance threshold device (ITD).
Study B
After 8 min of untreated VF, BLS ACD + ITD CPR was again performed for the first 2 min of the resuscitation with the animal inthe same position asinStudyA.At2 min, continuous ACD + ITDCPR was performed asin Study A, and animals were randomized to one of 2 angle sequences for 5-min CPR intervals; a) 20°, 30°, 40° or b) 40°, 30°, 20°. Angle transition was again performed over a period of 10–24 seconds. After 17 total minutes of CPR, medications and defibrillation attempts were administered as described in Study A in order to obtain ROSC. If ROSC was obtained, the animal was euthanized as described above. The Study B protocol is outlined in Fig. 2.
Data analysis
Aortic, RA, ICP, and airway pressures were measured from 3sequential compression-decompression cycles between ventilations. These values were then averaged to represent one measurement for each time point. CoPP was calculated at the difference between the decompression phase aortic and RA pressures. CerPP was calculated as the difference between aortic pressure and ICP over a 15 s interval at each time point. Studies where technical difficulties were encountered due to catheter dislodgement, or inability to compress the chest 22.5% of the antero-posterior diameter were not included in the results.
For study A, it was assumed the 40° HUP CPR angle would result in a CerPP of 50 mmHg and the CerPP 20° HUP CPR would be 35 mmHg. Assuming an a value of 0.05, 90% power, and correlation of 0.6 with a crossover design, we calculated a total sample size of 12 would be needed.
Statistical analysis was performed using STATA (STATA 15). Data are presented as mean ± standard deviation (SD). A mixed effect model was used to compare the primary endpoint, CerPP, in Study A between the three angles, with angle and order as fixed effects and pig as a random effect. A similar model was used for CoPP. An unpaired t-test was used to compare the primary endpoint, CerPP, in Study B after 17 min of CPR, and also for secondary endpoints in Study B. Study B tests were two-sided and a p value of less than 0.05 was used to reject the null hypothesis. P values are presented unadjusted for secondary endpoints.
Results
Study A
Eighteen pigs were studied at each angle. The mean CerPP (mmHg) for each 5-min period of CPR were as follows: 20°, 38± 18, 30°, 44± 22, 40°, 47± 26.The30° and 40 °CerPPs were equivalent(p = 0.18),and the 40 °CerPP was higher than the 20 °CerPP (p = 0.002). The mean CoPP (mmHg) was similar between all groups: 20°, 35± 16, 30° 38± 19, 40°, 40± 24 (20° versus 40° p = 0.26) Baseline and study hemodynamic values are presented in Table 1. ROSC was obtained in 16 of 18 animals.
Table 1 –
Haemodynamic and physiologic parameters for Study A, comparing three separate angles with elevation of the head and thorax during CPR to a flat position (Baseline). All values are presented as mmHg ± SD.
| Study A (n =18) | Baseline | 20° | 30° | 40° |
|---|---|---|---|---|
| ITP Dec | 3.0 ±0.5 | −5.4 ±1.9 | −4.7 ±2.0 | −4.0 ±2.3 |
| AO Com/Dec | 85 ±11/70 ±10 | 66 ±19/41 ±15 | 72 ± 21/44 ± 19 | 74 ±27/45 ±23 |
| RA Com/Dec | 5.0 ± 1.4/6.1 ±1.3 | 62 ±9/5.9 ±2.4 | 71 ±11/6.3 ±2.0 | 81 ±22/5.4 ±1.7 |
| ICP Dec | 16.3 ±5.0 | 10.6 ±4.0 | 8.3 ±3.4 | 6.3 ±3.8 |
| CorPP Dec | 63.5 ±10.2 | 35.5 ±15.6 | 37.9 ±19.6 | 39.8 ±23.0 |
| CerPP Mean | 60.6 ±13.1 | 38.3 ±17.9 | 43.8 ±21.1 | 47.3 ±25.1 |
| EtCO2 Mean | 43.7 ±2.3 | 48.3 ±6.0 | 48.6 ±4.8 | 49.7 ±8.0 |
When the data was graphed by angle and intervention order, it was observed that the mean CerPP and CoPP of the 40° group were visually higher than any other group at any other time point when 40° was randomized to the last 5 min epoch of the resuscitation protocol (Fig. 1). Specifically, when 40° HUP was performed during the last 5 min of the resuscitation, CerPPs were 65 ± 33, versus 42 ± 21 for 20° HUP and 30° HUP combined.
Study B
Based on the results of Study A, 6 additional animals were evaluated to test the hypothesis that CerPP with a 20°, 30°, 40° sequence would be superior to a 40°, 30°, 20° sequence after 17 min of CPR. A total of 6 animals were studied in each sequence.
After 17 min of CPR, CerPP (mmHg) were higher with the 20°, 30°, 40° sequence: 60 ± 17 versus 33 ± 17 (p = 0.035, Fig. 2). CorPP (mmHg) were higher, but not significant with the 20°, 30°, 40° sequence: 50 ± 17 versus 33 ± 17 (p = 0.14, Fig. 3). ROSC rates were similar between groups: 100% (6/6) in the 20°, 30°, 40° sequence and 67% (4/6) in the 40°, 30°, 20° sequence. Baseline and study hemodynamic values are presented in Table 2 and graphically in Fig. 3. Arterial blood gas values are presented in Table 3.
Fig. 3 –
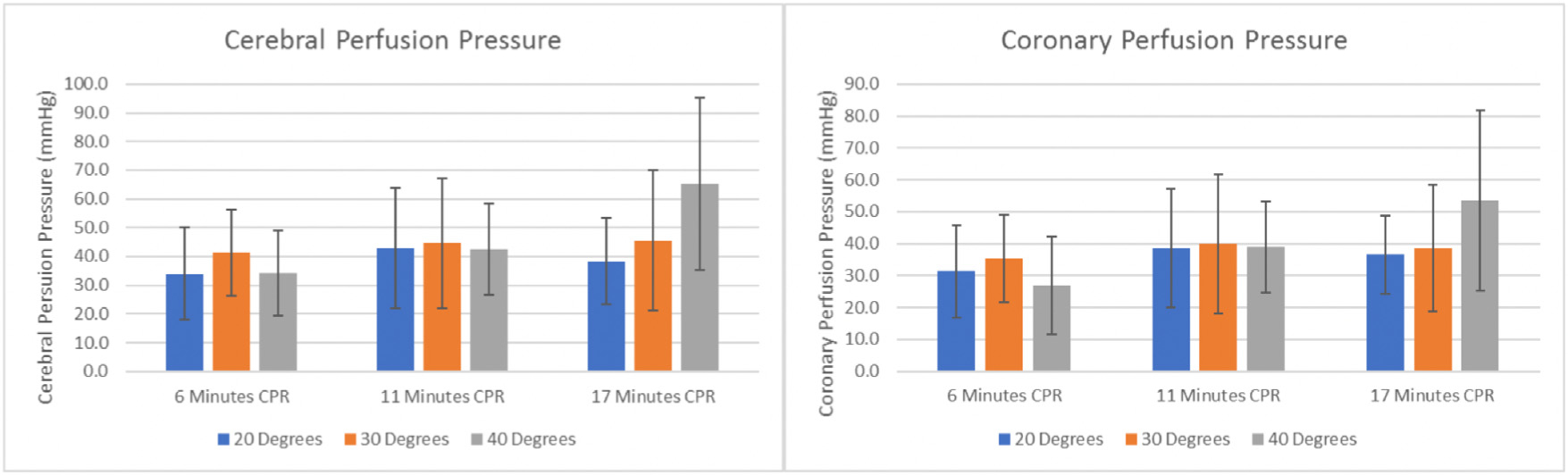
Cerebral perfusion pressures and coronary perfusion pressures for Study A, where three different angles of head and thorax elevation during cardiopulmonary resuscitation (CPR) were studied in the same animal (n = 18).
Table 2 –
Haemodynamic and physiologic parameters for Study B comparing two elevation of the head and thorax sequences during active compression-decompression cardiopulmonary resuscitation with an impedance threshold device. All values are presented as mmHg ± SD.
| Study B (n=12) | Baseline | 6 Minutes CPR | 11 Minutes CPR | 17 Minutes CPR | ||||
|---|---|---|---|---|---|---|---|---|
| 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | |
| ITP Dec | 2.8 ±0.6 | 3.5 ±0.7 | −5.9 ±1.0 | −3.7 ± 2.0 | −4.2 ±1.4 | −2.5 ±1.3 | 2.4 ± 1.5 | −4.0 ± 2.4 |
| AO Com/Dec | 84 ±9/70 ±9 | 79 ±10/64 ±7 | 69 ± #45 ± 8 | 60± 12/30± 10 | 77 ± 11/50 ±14 | 61 ±18/34 ±17 | 85 ± 18^56 ± 18 | 61 ± 16/38 ± 15 |
| RA ConVDec | 5.3 ± 1.9/62 ±1.8 | 5.4 ± 1.9/6.4 ±1.9 | 56 ± 5/7.6 ± 1.8 | 94 ±15/6.1 ± 2.6 | 65 ±7/6.1 ± 1.4 | 82 ±25/6.1 ± 1.4 | 79 ±14/6.0 ± 2.0 | 69 ± 13/5.0 ± 3.0 |
| I CP ConVDec | 152 ±8.8 | 16.7 ± 1.8 | 172 ±5.6 | 16.7 ± 3.4 | 14.8 ±4.7 | 15.6±2.5 | 11.3 ± 5.3 | 15.7 ± 3.6 |
| CorPP | 63.4 ±8.7 | 57.7 ±7.3 | 37.6 ±8.8 | 23.8 ±11.3 | 43.9 ± 14.6 | 28.4 ± 18.1 | 49.7 ± 17.1 | 32.5 ± 16.9 |
| CerPP Mean | 61.4 ±14.1 | 60.9 ±10.2 | 46.4 ± 9.7 | 34.5 ± 12.6 | 57.4 ± 11.3 | 39.7 ± 18.2 | 68.2 ±17.0 | 40.3±18.5 |
| EtC02 Mean | 44.4 ± 2.7 | 42.5 ±0.7 | 50.1 ± 6.4 | 48.5 ± 4.3 | 52.1 ± 6.6 | 49.3±3.6 | 52.5 ±4.6 | 48.7 ± 4.2 |
Table 3 –
Arterial blood gas values for Study B, comparing two elevation of the head and thorax sequences during active compression-decompression cardiopulmonary resuscitation with an impedance threshold device. All values are presented as mean ± SD.
| Study B (n = 12) | Baseline | CPR 6.5′ | CPR 11.5′ | CPR 16.5′ | ROSC 15′ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | 20, 30, 40 | 40, 30, 20 | |
| pH | 7.45 ± 0.01 | 7.46 ±0.04 | 7.19 ±0.05 | 7.23 ±0.02 | 7.16 ±0.05 | 7.20 ±0.03 | 7.10 ±0.05 | 7.17 ±0.03 | 7.17 ±0.05 | 7.24 ±0.04 |
| PaCO2 | 44.7 ±3.8 | 44.5 ±5.9 | 62.7 ± 7.6 | 58.3 ±4.7 | 64.0 ± 10.7 | 56.5 ±7.5 | 71.5 ±10.2 | 59.0 ±6.0 | 56.8 ±7.6 | 47.3 ±6.5 |
| PaO2 | 121.5 ±48.6 | 83.2 ±7.4 | 119.5 ± 43.7 | 136.2 ±62.0 | 119.8 ±49.3 | 130.2 ±62.4 | 106.8 ±92.2 | 134.5 ±70.7 | 89.3 ±20.6 | 96.0 ±7.6 |
| HCO3– | 30.7 ± 2.5 | 31.2 ±2.8 | 23.8 ±0.9 | 24.5 ±2.3 | 22.4 ± 1.7 | 22.1 ±2.8 | 22.0 ±2.0 | 21.7 ±2.2 | 20.3 ± 1.3 | 20.0 ±2.3 |
| BE | 6.6 ±2.5 | 7.3 ±2.9 | −4.4 ±0.9 | −3.1 ±2.5 | −6.3 ± 1.1 | −6.0 ±2.9 | −7.5 ± 1.7 | −6.8 ±2.4 | −8.3 ±1.3 | −7.5 ±2.6 |
| SaO2 | 98.0 ± 1.3 | 95.0 ±2.8 | 94.8 ±5.0 | 96.0 ±3.9 | 92.0 ± 9.8 | 95.5 ±4.3 | 82.7 ± 12.0 | 95.0 ±5.1 | 91.7 ±6.9 | 95.8 ± 1.5 |
Discussion
The physiologic understanding of HUP CPR continues to evolve. In the initial study, a 30° whole body tilt resulted in a higher CerPP versus the supine position.1 Subsequently, the body position changed from a whole-bodytilttoonlyheadandthoraxelevationtoreducebloodpooling in the lower extremities during prolonged resuscitation. Subsequent studies were performed with a device that elevated just the thorax and head 30°.2,3 These studies demonstrated improved CerPP and brain blood flow3 with HUP CPR. The current investigation was performed to the optimal HUP CPR angle to maximize cerebral perfusion using a more sophisticated CED device. Unexpectedly, the results from Study A failed to identify a single best angle to perform HUP CPR.
However, Study A suggested an intriguing sequence effect. Elevating the head and heart slowly appeared to be superior to a more rapid elevation sequence. CerPP and CoPP appeared visually higher when 40° was randomized to the last 5 min epoch of the study. Thus, Study B was performed, where two sequences were compared, specifically looking at CerPP after prolonged ACD + ITD CPR. CerPP was not only significantly higher in the 20°, 30°, 40° sequence, but CerPP values continued to increase during each 5-min epoch of CPR. Notably, after 17 min of CPR, CerPP was nearly 100% of the baseline value. This kind of physiological response has not been previously observed in our laboratory in over 25 years. By contrast, with the 40°, 30°, 20° sequence, CerPP remained constant throughout the study, at about 60% of the baseline value. By elevating slowly to a target angle of 40°, aortic pressure increased and ICP decreased. This maximized the CerPP. A more rapid initial elevation to 40°sequence resulted in a loss of the aortic driving pressure, as seen in Fig.3,where MAP and CoPP are significantly lower after 3 min,1 min after initial elevation, and then remain lower thereafter.
This newly discovered controlled slow elevation sequence effect in Study B was profound. Although not powered for significance, a variety of biophysical parameters were higher but not significant in the 20°, 30°, 40° group, suggestive of improved long-term perfusion. EtCO2 values were higher throughout the resuscitation with a slow elevation sequence. Furthermore, RA pressures were lower in the 20°, 30°, 40° group in the first 5-min CPR epoch (Table 2). There are multiple potential mechanisms for the observed benefits of the gradual elevation sequence which could include: increased flow and increased right to left pulmonary flow, increased arterial tone, increase responsivity to endogenous catecholamines and vasopressors as flow increases, and partial restoration of vascular autoregulation.
ACD + ITD CPR was used in the current study as this combination has been shown previously to improve neurological and haemodynamic outcomes in both human and animal studies.8,10,11 These circulatory adjuncts are needed to maximize the haemodynamics required to pump blood “uphill” during HUP CPR. In the initial HUP CPR study with a whole-body tilt, mean aortic pressure decreased when an ITD was not used in combination with automated CPR.1 In subsequent studies haemodynamics were further enhanced during HUP CPR with the combination of ACD + ITD CPR.2,3 The authors and others have not been able to achieve the same high CerPP values observed in the current study without use of automated CPR + ITD or ACD + ITD CPR.2,12,13 HUP CPR is able to further enhance blood flow during CPR with the use of gravity when circulatory adjuncts are used to establish a higher level of baseline perfusion.
These results build upon previous studies and refine HUP CPR to maximize hemodynamic and short-term survival outcomes by (1) elevation of the head and thorax only (2) priming of the cardio-cerebral circuit by first performing CPR in a relatively supine position (3) use of circulatory adjuncts (4) gradual elevation of the head and thorax.13 A recent animal study showed poor hemodynamics and survival outcomes with an immediate whole-body tilt upwards, without any priming prior to elevation. It is important to thoughtfully approach the performance of HUP CPR in order to avoid blood pooling in the extremities, with the establishment and maintenance of the ‘uphill’ aortic driving pressure during elevation.14
Further work is needed to determine the optimal rate of rise. In the current study, the animal achieved the 40° angle target after 12 min of CPR. It is unknown if the sequence rate of rise can be shortened yet achieve the same CerPP values.
There are limitations. This is an animal model of cardiac arrest with a small sample size. The animals were young, healthy pigs without cardiac disease. Pigs are not people, but this study cannot be performed in humans and the VF swine model is commonly used and accepted in animal models of cardiac arrest.15 The primary outcome of the study was CerPP, which is a calculation, and not a measure of blood flow or a clinical endpoint. However, previous studies have shown improved blood flow with HUP CPR as compared to flat CPR.1,3,16–18 Lastly, we assumed a 40% increase between the 20° angle and 40° angle based on previous work in the power calculation for this study. If there is a smaller difference between angle groups, the study may not be adequately powered to detect this.
Conclusions
No optimal HUP CPR angle was found in a comparison of 20°, 30°, and 40° angles. However, controlled progressive sequential elevation of the head and thorax during HUP CPR provided unexpectedly high and sustained CerPP. The elevation sequence appears to be more important than an absolute height or angle when performing HUP CPR. Further studies are needed to determine the optimal rate of rise of the head and thorax during ACD + ITD CPR.
Supplementary Material
Acknowledgements
This study was funded by a NIH NHBLI SBIR grant, contract number 1R43HL139184-01. The authors thank Dr. Dave Gilbertson for his statistical assistance.
Footnotes
Conflict of interest statement
Keith Lurie is an inventor of devices and methods to elevate the head and heart during CPR. He is the co-founder of Advanced CPR Solutions that makes head up CPR resuscitation devices.
The remainder of the authors have no conflict of interests to declare.
Institutional Protocol Number: 17-06. This institution has an Animal Welfare Assurance on file with the Public Health Service, through the Office for Laboratory Animal Welfare. The National Institute of Health Assurance No. is A-3875–01.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resuscitation.2020.02.023.
REFERENCES
- 1.Debaty G, Shin SD, Metzger A, et al. Tilting for perfusion: head-up position during cardiopulmonary resuscitation improves brain flow in a porcine model of cardiac arrest. Resuscitation 2015;87:38–43. [DOI] [PubMed] [Google Scholar]
- 2.Ryu HH, Moore JC, Yannopoulos D, et al. The effect of head up cardiopulmonary resuscitation on cerebral and systemic hemodynamics. Resuscitation 2016;102:29–34. [DOI] [PubMed] [Google Scholar]
- 3.Moore JC, Segal N, Lick MC, et al. Head and thorax elevation during active compression decompression cardiopulmonary resuscitation with an impedance threshold device improves cerebral perfusion in a swine model of prolonged cardiac arrest. Resuscitation 2017;121:195–200. [DOI] [PubMed] [Google Scholar]
- 4.Guerci AD, Shi AY, Levin H, Tsitlik J, Weisfeldt ML, Chandra N. Transmission of intrathoracic pressure to the intracranial space during cardiopulmonary resuscitation in dogs. Circ Res 1985;56:20 30. [DOI] [PubMed] [Google Scholar]
- 5.Moore JC, Holley J, Segal N, et al. Consistent head up cardiopulmonary resuscitation haemodynamics are observed across porcine and human cadaver translational models. Resuscitation 2018;132:133–9. [DOI] [PubMed] [Google Scholar]
- 6.Pepe PE, Scheppke KA, Antevy PM, et al. Confirming the clinical safety and feasibility of a bundled methodology to improve cardiopulmonary resuscitation involving a head-up/torso-up chest compression technique. Crit Care Med 2019;47:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T, Shin SD, Song KJ, et al. The effect of resuscitation position on cerebral and coronary perfusion pressure during mechanical cardiopulmonary resuscitation in porcine cardiac arrest model. Resuscitation 2017;113:101–7. [DOI] [PubMed] [Google Scholar]
- 8.Metzger AK, Herman M, McKnite S, Tang W, Yannopoulos D. Improved cerebral perfusion pressures and 24-hr neurological survival in a porcine model of cardiac arrest with active compression-decompression cardiopulmonary resuscitation and augmentation of negative intrathoracic pressure. Crit Care Med 2012;40:1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinman ME, Brennan EE, Goldberger ZD, et al. Part 5: adult basic life support and cardiopulmonary resuscitation quality: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S414–435. [DOI] [PubMed] [Google Scholar]
- 10.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet 2011;377:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolcke BB, Mauer DK, Schoefmann MF, et al. Prospective randomized controlled trial comparing standard CPR with active compression decompression cardiopulmonary resuscitation and an impedance threshold valve in patients with out of hospital cardiac arrest. Circulation 2002;106:538. [DOI] [PubMed] [Google Scholar]
- 12.Putzer G, Braun P, Martini J, et al. Effects of head-up vs. supine CPR on cerebral oxygenation and cerebral metabolism — a prospective, randomized porcine study. Resuscitation 2018;128:51–5. [DOI] [PubMed] [Google Scholar]
- 13.Moore JC, Segal N, Debaty G, Lurie KG. The “do’s and don’ts” of head up CPR: lessons learned from the animal laboratory. Resuscitation 2018;129:e6–7. [DOI] [PubMed] [Google Scholar]
- 14.Park YJ, Hong KJ, Shin SD, et al. Worsened survival in the head-up tilt position cardiopulmonary resuscitation in a porcine cardiac arrest model. Clin Exp Emerg Med 2019;6:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debaty G, Lurie K, Metzger A, et al. Reperfusion injury protection during Basic Life Support improves circulation and survival outcomes in a porcine model of prolonged cardiac arrest. Resuscitation 2016;105:29–35. [DOI] [PubMed] [Google Scholar]
- 16.Halperin HR, Lee K, Zviman M, et al. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med 2010;28:195–202. [DOI] [PubMed] [Google Scholar]
- 17.Pantazopoulos IN, Xanthos TT, Vlachos I, et al. Use of the impedance threshold device improves survival rate and neurological outcome in a swine model of asphyxial cardiac arrest. Crit Care Med 2012;40:861–8. [DOI] [PubMed] [Google Scholar]
- 18.Xanthos T, Iacovidou N, Pantazopoulos I, et al. Ischaemia-modified albumin predicts the outcome of cardiopulmonary resuscitation: an experimental study. Resuscitation 2010;81:591–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


