Abstract
Objective
COVID-19 patients may develop coagulopathy, which is associated with poor prognosis and high risk of thrombosis. The main objective of this work was to evaluate the prevalence of deep venous thrombosis of lower limbs (DVT) through ultrasonography in patients infected with COVID-19 admitted to conventional units at our hospital with 5 days of monitoring. The secondary objective was to determine if D-dimer levels, body mass index, and C-reactive protein were associated with DVT.
Methods
A total of 72 patients, with a mean age of 65 ± 12.3 years, infected with COVID-19 were admitted to three conventional units at our institution; 28 patients were women. A COVID-19 diagnosis was made by a transcriptase polymerase chain reaction by means of nasopharyngeal swab or by chest computer tomography without iodine contrast media. Demographics, comorbidities, and laboratory parameters were collected. A preventive anticoagulation treatment was established on admission with low-molecular-weight heparin. A complete venous duplex ultrasound (DU) test of lower limbs was performed on day (D) 0 and D5. A pulmonary computer tomography angiogram with iodine contrast media was required when pulmonary embolism was suspected.
Results
On D0, the DU showed acute DVT in seven patients (9.75%). A pulmonary computer tomography angiogram was performed in 12 patients (16.65%), 3 (25%) of whom had an acute pulmonary embolism. On D0, acute DVT was not significantly associated with C-reactive protein (mean 101 ± 98.6 in the group without DVT vs 67.6 ± 58.4 mg/L, P = .43) or body mass index (27.7 ± 5.04 vs 28.1 ± 2.65 kg/m2, P = .54). However, we found a significant association between acute DVT and D-dimer levels (1536 ± 2347 vs 9652 ± 10,205 ng/mL, P < .01). Among the patients included on D0, only 32 had a DU on D5. Forty of them (55.55%) were not examined for the following reasons: 7 (9.7%) were previously diagnosed with venous thromboembolism on D0 and therefore were excluded on D5, 8 (11%) were transferred to the intensive care unit, 10 (14%) were discharged from the hospital, 5 (7%) died, and 10 (13.9%) were excluded because of technical issues. On D5, five (15.6%) patients had acute DVT in addition to those found on D0; three were distal and two proximal despite preventive anticoagulation with low-molecular-weight heparin.
Conclusions
Hospitalized non-intensive care unit patients with COVID-19 pneumonia have a high frequency of venous thrombotic events justifying screening with DU.
Keywords: COVID-19, Duplex ultrasound, Deep venous thrombosis
Article Highlights.
-
•
Type of Research: Single-center, retrospective cohort study
-
•
Key Findings: Of 72 patients admitted to standard hospital wards with COVID-19 and examined with duplex ultrasound, 9.7% had acute deep venous thrombosis of lower limbs (DVT) on day 0, and 5 (15.6%) of 32 initially without DVT with follow-up duplex at hospital day 5 developed DVT despite preventive anticoagulation.
-
•
Take Home Message: Hospitalized non-intensive care unit patients with COVID-19 pneumonia have a high frequency of venous thrombotic events justifying screening with duplex ultrasound of non-intensive care unit patients admitted with COVID-19 pneumonia.
From March 2 to April 26, 2020, 1249 COVID-19 patients were admitted to our institution. Among them, 278 were hospitalized in intensive care units (ICU).1 COVID-19 patients may develop venous thromboembolism (VTE) events as pulmonary embolism (PE) and/or deep venous thrombosis of lower limbs (DVT) despite antithrombotic prophylaxis.2 This prothrombotic state prompted us to perform a duplex ultrasound (DU) test as standard care for patients admitted to conventional units for COVID-19 pneumonia. The main objective of this study was to establish the prevalence of DVT through ultrasonography in patients infected with COVID-19 admitted to three conventional units at our hospital with a follow-up of 5 days. The secondary objective was to assess if D-dimer levels, body mass index (BMI), and C-reactive protein (CRP) were associated with DVT.
Methods
Our local ethical committee approved this single-center retrospective study. An oral consent was obtained from the patients for this study based on the principles outlined in the Declaration of Helsinki.
From March 25 to April 8, 72 patients, with a mean age 65 ± 12.3 years (range, 38-89 years), infected with COVID-19 were admitted to three conventional units of our institution: Internal Medicine, Cardiology, and Vascular Medicine. Twenty-eight patients were women (39%). A transcriptase polymerase chain reaction (RT-PCR) by means of nasopharyngeal swab was performed for the diagnosis of SARS CoV-2. The chest computed tomography (CT) images without iodine contrast media reviewed by a senior chest radiologist associated with the clinical signs of the infection were the criterion of the diagnosis of SARS CoV-2 for the patients who had a negative RT-PCR result. The diagnosis the lung lesions on CT was based on the recommendation of the French Society of Thoracic Imaging3 (Table I ). A pulmonary CT angiogram with iodine contrast media was required when PE was suspected. RT-PCR COVID-19 false-negative test results can be seen in patients infected.4 Demographics, comorbidities, and laboratory parameters were collected. No standard protocol was fixed for the D-dimer test; it was obtained based on individual physicians' choice. The HemosiL D-Dimer HS 500 reagent was used. We established on admission a common anticoagulation standard protocol for the three units. We used low-molecular-weight heparin (LMWH) with subcutaneous enoxaparin: preventive dose of 4000 IU/24 hours for patients weighing less than 100 kg or 6000 IU for those weighing over100 kg. A therapeutic dose of 100 IU/kg/12 hours was administered to patients who had a history of VTE events or atrial fibrillation.
Table I.
Lung parenchyma lesions on chest computed tomography (CT) (Recommendation of the French Society of Thoracic Imaging)
| Chest CT severity | Extent of lesions % of lung parenchyma |
|---|---|
| Stage 1: minimal | <10 |
| Stage 2: moderate | 10-25 |
| Stage 3: extended | 25-50 |
| Stage 4: severe | 50-75 |
| Stage 5: critical | >75 |
CT without concentration iodine contrast. In patients with COVID-19, CT showed characteristic lung parenchyma lesions: peripheral, bilateral ground-glass opacities. These lesions were classified into five stages based on the recommendation of the French Society of Thoracic Imaging.
The Vascular Medicine team performed venous DU on day (D) 0 and D5 for examination of deep and superficial veins of lower limbs using a Samsung HS50 ultrasound machine. The criteria for a positive diagnosis of venous thrombosis were venous incompressibility and the direct visualization of the thrombus for the proximal DVT (popliteal, femoral, and iliac veins). For the distal DVT (below the popliteal vein), the criterion was the absence of the Doppler flow and venous incompressibility. We followed a strict protocol to protect the examiner: complete infection-control training and wearing FFP3 (filtering facepiece) were mandatory for all sonographers. This work is an observational study; therefore, no power calculations were used. Qualitative and quantitative variables were analyzed using means or medians, percentages, and standard deviations. Quantitative variables were analyzed by the Mann-Whitney-Wilcoxon test and qualitative variables by the Fisher test. Only univariate analysis was performed because of the small sample size of the study. P values less than .05 were considered to indicate statistical significance. Statistical analysis was carried out using R Statistical Software and packages “shiny and simplestats”: Medistica, pvalue.io, a graphic user interface to the R statistical analysis software for scientific medical publications, 2019. Available at: https://www.pvalue.io, Paris, France.
Results
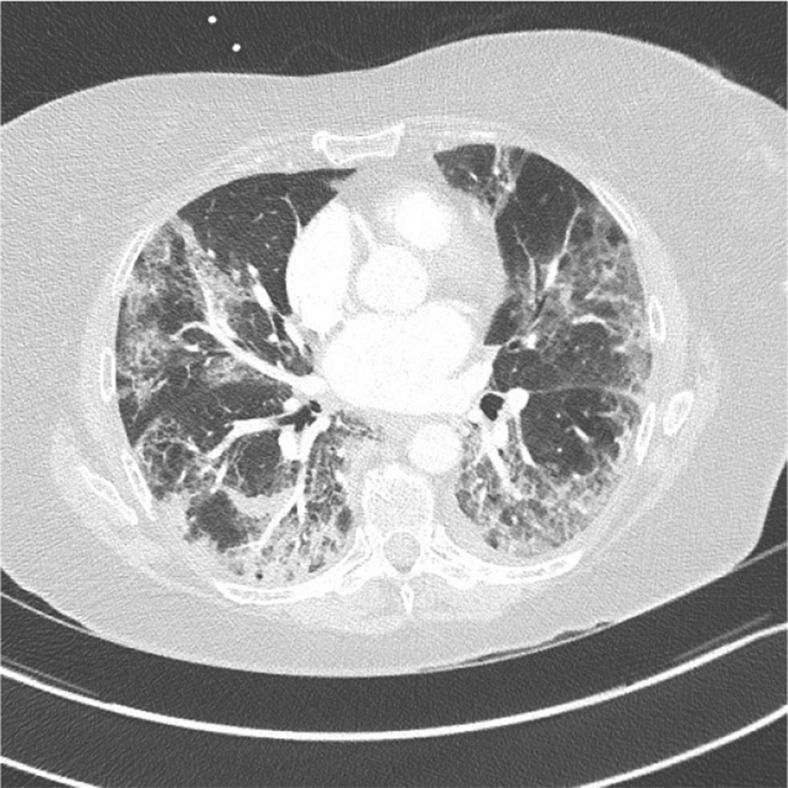
In our cohort, 50 patients (69.50%) had positive RT-PCR results. In 30.50%, the diagnosis of COVID-19 infection was confirmed by the clinical signs of the infection and by the chest CT images without iodine contrast media, which showed characteristic lung parenchyma lesions: bilateral, peripheral ground-glass opacities (Fig ). Four patients (5.5%) received therapeutic anticoagulation treatment on admission with a direct oral anticoagulant (DOAC), two of whom for atrial fibrillation and two due to history of VTE. In three patients, the DOAC (rivaroxaban) was stopped because of elevated transaminases; a therapeutic anticoagulant with subcutaneous enoxaparin was initiated. Acute moderate renal failure was observed in seven patients (9.70%). Fifty-five patients (76.5%) had lymphopenia and 44 (61%) elevated aminotransferasis (EA). Four patients (5.6%) with chronic renal failure (glomerular filtration rate [GFR] <20 mL/min) were treated with antithrombotic prophylaxis using subcutaneous unfractionated heparin with Calciparin. A DU test showed acute DVT in seven patients (9.75%) on D0; four were proximal and three distal. None of them had anticoagulant treatment before hospital admission; five (71%) did not show any signs or symptoms. Two had pain and edema leg. No acute arterial thrombosis and no superficial venous thrombosis were detected.
Fig.
Lung parenchyma lesions in COVID-19 infection. Chest computed tomography (CT) without iodine contrast shows bilateral, peripheral ground-glass opacity stage 4 (extended lesions 50%-75% of lung parenchyma): Classification of the French Society of Thoracic Imaging.
A pulmonary CT angiogram was performed in 12 patients (16.6%), 3 (25%) of whom had an acute PE. In two patients, the PE was associated with proximal DVT; the third patient had a primary PE.
On D0, acute DVT was not significantly associated with CRP (mean 101 ± 98.6 in the group without DVT vs 67.6 ± 58.4 mg/L, P = .43) or BMI (mean 27.7 ± 5.04 vs 28.1 ± 2.65 kg/m2, P = .54). However, we found a significant association between acute DVT and D-dimer levels (mean 1536 ± 2347 vs 9652 ± 10,205 ng/mL, P < .01) among 64 patients who had D-dimer sampling on D0. Patient characteristics on D0 are summarized in Table II .
Table II.
Patient characteristics on day 0 (D0): demographics, comorbidities, laboratory parameters, and history
| Total (N = 72) | DVT-free (n = 65) | DVT (n = 7) | P value | |
|---|---|---|---|---|
| Age | 66.3 (±11.9) | 65.9 (±12.4) | 69.3 (±6.80) | .55 |
| Sex | ||||
| Female | 28 (39) | 27 (41.5) | 1 (14.3) | .091 |
| Male | 44 (61) | 38 (58.5) | 6 (85.7) | |
| D-dimer <500 ng/mL | 2289 (±4312) N = 64 | 1622 (±2408) N = 58 | 9652 (±10,205) N = 6 | <.01 |
| CRP 0-3 mg/L | 181 (±76.3) | 96.1 (±75.0) | 67.6 (±58.4) | .37 |
| BMI (kg/m2) | 28.9 (±11.2) n = 63 | 27.7 (±5.04) n = 56 | 28.1 (±2.65) n = 7 | .94 |
| GFR >90 mL/min | 80.5 (±28.5) | 78.6 (±28.2) | 95.7 (±30.2) | .26 |
| Symptoms onset to D0 | 10.6 (±5.49) | 10.2 (±5.49) | 13.1 (±5.52) | .095 |
| Hypertension | 35 (53.8) | 31 (47.7) | 4 (57) | .71 |
| Diabetes | 13 (20) | 11 (17) | 2 (28.6) | 1 |
| Smoking | 8 (11) | 7 (10.8) | 1 (14.3) | .18 |
| Hypercholesterolemia | 24 (33.3) | 23 (35.4) | 1 (14.3) | .42 |
| Lymphopenia (<1.2 × 10.9/L) | 55 (76.4) | 51 (78.5) | 4 (57) | 1 |
| Sleep apnea syndrome | 5 (7) | 2 (3) | 3 (42.8) | .01 |
| Pulmonary embolism | 3 (4.16) | 1 (1.53) | 2 (28.6) | <.01 |
| Proximal DVT | 3 (4.2) | 0 (0) | 3 (43) | – |
| Distal DVT | 4 (5.5) | 0 (0) | 4 (57) | – |
| Signs and symptoms of DVT | – | – | 2 (28.6) | – |
| No signs and symptoms of DVT | – | – | 5 (71.5) | – |
| Medical history of VTE | 2 (2.8) | 1 (1.5) | 1 (14.3 | – |
| Atrial fibrillation | 2 (2.8) | 1 (1.5) | 1 (14.3) | – |
| Medical history of PAD | 5 (7) | 4 (6) | 1 (14.3) | – |
| Medical history of coronary diseases | 11 (15.3) | 10 (15.4) | 1 (14.3) | – |
| Antiplatelets | 12 (16.7) | 10 (15.4) | 2 (28.6) | – |
| DOACs | 4 (5.6) | 3 (5.6) | 1 (14.3) | – |
| Vit K antagonist | 1 (1.4) | 1 (1.5) | 0 (0) | – |
| Active cancer | 0 (0) | 0 (0) | 0 (0) | – |
| History of cancer | 3 (4.2) | 3 (4.6) | 0 (0) | – |
| COPD | 2 (2.8) | 1 (1.5) | 1 (14.3) | – |
| Leg ulcer | 1 (1.4) | 1 (1.5) | 0 (0) | – |
| Medical history of thrombophilia | 2 (2.8) | 1 (1.5) | 1 (14.3) | – |
| Medical history of immunologic diseases | 1 (1.4) | 0 (0) | 0 (0) | – |
| Venous insufficiency | 5 (7) | 4 (6.15) | 1 (14.3) | – |
| MARF | 7 (9.7) | 7 (10.8) | 0 (0) | – |
| Treatment (Sartans) | 5 (7) | 4 (6) | 1 (14.3) | – |
| Chronic renal failure | 4 (5.6) | 4 (6) | 0 (0) | – |
BMI, Body mass index; COPD, chronic obstructive pulmonary diseases; CRP, C-reactive protein; D0, day 0; DOAC, direct oral anticoagulant; DVT, deep venous thrombosis; GFR, glomerular filtration rate; IQR, interquartile range; M, median; MARF, moderate acute renal failure; PAD, peripheral arterial disease; Vit, vitamin; VTE, venous thromboembolism.
Continuous data are expressed as the mean ± standard deviation, and categorical data are expressed as number (%).
Among the patients included on D0, only 32 had a DU on D5. Forty (55.55%) were not examined for the following reasons: 7 (9.7%), who were previously diagnosed with VTE on D0, were excluded on D5; 8 (11%) were transferred to the ICU; 10 (14%) were discharged from the hospital; 5 (7%) died; and 10 (13.9%) were excluded because of technical issues. CRP, BMI, D-dimer levels, GFR, and VTE were not associated with transfer to the ICU; however, D-dimer levels (P ≤ .01) and GFR (P = .046) were significantly associated with death.
On D5, five (15.6%) patients had acute DVT in addition to those found on D0; three were distal and two proximal despite anticoagulation treatment with LMWH (four with a prophylactic dose and the fifth one with a therapeutic dose). Only two patients (40%) were symptomatic with leg pain and edema. Of the 32 patients, only 23 had a D-dimer sampling on D5. D-dimer levels were not significantly associated with DVT, P = .62 (Table III ). With a follow-up of 5 days, 13 of 72 (18%) patients who had DU developed VTE (on D0: 7 DVT and 1 primary PE and 5 DVT on D5). All were treated with a therapeutic dose of enoxaparin (100 IU/kg/12 hours); however, no bleeding complications were noted (no bruises, hematomas, digestive bleeding, or epistaxis was detected). Two of the 13 died because of acute respiratory failure. Of the 11 survivors, 5 were checked 30 days after the first episode. In three patients, DU did not show vein reflux or persistent thrombus. All the five patients had normal GFR and aminotransferases; enoxaparin was stopped, and a therapeutic dose of DOCAs (apixaban) was prescribed.
Table III.
Patients characteristics on day 5: demographics and laboratory parameters
| Total (N = 32), M (IQR) | DVT-free (n = 27), M (IQR) | DVT (n = 5), M (IQR) | P value | |
|---|---|---|---|---|
| Age | 65.6 (±10.7) | 64.6 (±10.5) | 71.4 (±10.9) | .25 |
| Sex | ||||
| Female | 15 | 13 | 2 | – |
| Male | 17 | 14 | 3 | – |
| BMI (kg/m2) | 27.6 (±4.91) | 27.5 (±3.52) | 27.0 (±5.00) | 1 |
| CRP 0-3 mg/L | 112 (±71.2) | 105 (±72.7) | 150 (±53.9) | .15 |
| GFR >90 mL/min | 80.5 (±28.5) | 81.4 (±26.1) | 68.8 (±23.3) | .23 |
| D-dimer <500 ng/mL | 1398 (±1370) N = 23 | 1200 (±821) N = 18 | 2162 (±2364) N = 5 | .62 |
| DVT proximal | – | – | 2 (40) | – |
| DVT distal | – | – | 3 (60) | – |
| Preventive anticoagulation | – | – | 4 (80) | – |
| Therapeutic anticoagulation | – | – | 1 (20) | – |
| With signs and symptoms | – | – | 2 (40) | – |
| No signs and symptoms | – | – | 3 (60) | – |
BMI, Body mass index; CRP, C-reactive protein; DVT, deep venous thrombosis; GFR, glomerular filtration rate; IQR, interquartile range; M, median.
Data are presented as n (%) unless otherwise specified.
Discussion
Viral infections are associated with coagulation disorders.5 Justo et al6 showed that the incidence of thrombosis among acute cytomegalovirus infections in hospitalized patients was 1.9%-9.1%. Paran et al7 reported increased risk for VTE in patients who had been tested positive for cytomegalovirus during the follow-up of 6 months. In our cohort, the prevalence of DVT on D0 was 9.7%.
Middeldorp et al8 reported 20% of VTE events in 198 patients with COVID-19 pneumonia (38% in ICU) during the follow-up of 7 days. Marone and Rinaldi9 observed DVT in 16 of 30 non-ICU-hospitalized patients using ultrasound scans. With 5 days' follow-up, we observed 13 patients (18%) with VTE events and 12 (16.7%) with acute DVT. The prevalence (15.6%) was significant mainly on D5 despite antithrombotic prophylaxis.
Obesity appears to be associated with an increased risk of VTE in general population.10 For this reason, we treated our patients weighing over than 100 kg with the preventive dose of enoxaparin of 6000 IU/24 hours. Grimnes et al11 found that acute inflammation, assessed by CRP, was a marker for VTE. However, elevated CRP levels12 and obesity13 can be a marker for severity of COVID-19 infection. In our study, BMI and CRP were not significantly associated with DVT on D0 and D5 or with the transfer to ICU and death. Elevated CRP in our study could be related to the inflammatory response; the patients infected with COVID-19 had excessive cytokine storm.14
Elevated D-dimer levels may be a marker of asymptomatic DVT15 and for disease severity and mortality in COVID-19 patients.16 In our series, 8 of the 12 patients (66.7%) with DVT on D0 and D5 were asymptomatic and D-dimer levels were elevated and significantly associated in patients developing DVT on D0 and the same goes for the 5 patients who passed away (P < .01).
On D5, only 23 had a D-dimer sampling, so probably for this reason D-dimer levels were not significantly associated with DVT, P = .62.
COVID-19 pneumonia can provoke aminotransferase disorders with EA.17 Forty-four patients (61%) of our cohort had EA; for this reason, DOAC was not prescribed. Our patients were treated with preventive and therapeutic LMWH.
Conclusions
Hospitalized non-ICU patients with COVID-19 pneumonia have a high frequency of VTE. These patients require an early administration of preventive anticoagulation with LMWH. They also require a follow-up with DU.
Author contributions
Conception and design: JT, MT, BD, TN, NB, PM, ML-H, NB, GO, LJ, OH
Analysis and interpretation: AH, DS, BW
Data collection: AH, LJ, JT
Writing the article: AH, DS, BW
Critical revision of the article: LJ, JT, MT, BD, TN, NB, PM, ML-H, NB, GO, LJ, OH
Final approval of the article: AH, LJ, JT, MT, BD, TN, NB, PM, ML-H, NB, GO, LJ, OH, DS, BW
Statistical analysis: AH, LJ, DS, BW
Obtained funding: Not applicable
Overall responsibility: AH
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kuteifan K., Pasquier P., Meyer C., Escarment J., Theissen O. The outbreak of COVID-19 in Mulhouse: hospital crisis management and deployment of military hospital during the outbreak of COVID-19 in Mulhouse, France. Ann Intensive Care. 2020;10:59. doi: 10.1186/s13613-020-00677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La société d’Imagerie Thoracique propose un compte-rendu structuré de scanner thoracique pour les patients suspects de COVID-19. https://ebulletin.radiologie.fr/actualites-covid-19/societe- dimagerie-thoracique-propose-compte-rendu-structure-scanner-thoracique [in French]. SFR e-Bulletin; 2020. Available at:
- 4.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;172:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goeienbier M., van Wissen M., van de Weg C., Jong E., Gerders V.E.A., Meijers J.C.M. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justo D., Finn T., Atzmony L., Guy N., Steinvil A. Thrombosis associated with acute cytomegalovirus infection: a meta-analysis. Eur J Intern Med. 2011;22:195–199. doi: 10.1016/j.ejim.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Paran Y., Shalev V., Steinvil A., Justo D., Zimmerman O., Finn T. Thrombosis following acute cytomegalovirus infection: a community prospective study. Ann Hematol. 2013;92:969–974. doi: 10.1007/s00277-013-1715-3. [DOI] [PubMed] [Google Scholar]
- 8.Middeldorp S., Coppen M., van Haaps T., Foppen M., Vlaar A., Müller M.C. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thrombo Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8:694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang G., De Staercke C., Hooper W.C. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499–509. doi: 10.4236/ojpm.2012.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimnes G., Isaksen T., Tichelaar Y.I.G.V., Brox J., Brækkan S.K., Hansen J.-B. C-reactive protein and risk of venous thromboembolism: results from a population-based case- crossover study. Haematologica. 2018;103:1245–1250. doi: 10.3324/haematol.2017.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W., Zheng K., Liu S., Yan Z., Xu C., Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbio Antimicrob. 2020;19:18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng K., Gao F., Wang X.-B., Sun Q.-F., Pan K.-H., Wang T.-Y. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macias M., Toledo-Samaniego N. Incidence of asymptomatic deep venous thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamadé A., Woehl B., Talbot M., Bensalah N., Michel P., Obringer O. Aminotransferases disorders associated with venous thromboembolic events in patients infected with COVID-19. Ann Hepatol. 2020 Oct 29 doi: 10.1016/j.aohep.2020.10.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]