Abstract
Objective
To study non-enhanced computer tomographic features of chest imaging in 302 patients with Corona Virus Disease 2019 (COVID-19) in Jordan.
Material and method
A retrospective analysis of non-enhanced computer tomographic scans has been performed in the main center for patients diagnosed with COVID-19 in Prince Hamzah Hospital for those scanned from 13th of March 2020 to 13th of May 2020. Included scans were routinely performed during 24-hs of admission apart from having respiratory complaint. CT protocol included non-enhanced 1 mm slice thickness by Philips Brilliance Big Bore scanner (Philips; Amsterdam, Netherlands).
All computer tomographic scans were reviewed by two senior radiologists with more than 8 years of experience each and senior registrar.
Several factors have been thoroughly studied including patient age, gender, positive versus negative pulmonary findings, laterality of lung involvement, lobar distribution, pattern of pulmonary changes on initial and follow-up scans.
Results
The total number of patients evaluated was 302. There were 188 men and 114 women studied. Among the totally studied 302 cases; 181 cases (59.9 %) showed no pulmonary changes.
Positive findings were present in 121 patients with a total number of 191 computer tomographic scans including initial and follow-up scans. Positive findings were present in 51 female and 70 male patients (age range, 12–87 years; mean age ± standard deviation, 46.1 ± 16.5).
Bilateral disease was more frequently encountered presented in 86 cases (71.1 %), while unilateral disease showed two times more predilection for the right lung compared to the left. The incidence of lobar involvement in descending order: right lower (75.2 %), left lower (71.9 %), right upper (62.8 %), left upper (60.3 %) and right middle (50.4 %). The incidence of the affected lobes on the initial scans were as follow: one lobe (24 %), two lobes (10.7 %), three lobes (9.1 %), four lobes (16.5 %) five lobes (36.4 %). In cases with single lobar involvement (24 %); the left upper and right middle lobes showed lowest incidence of involvement accounting for 10.3 % & 13.8 %, respectively; on the other hand, in cases with four lobar involvement (16.5 %); the right middle lobe was most commonly spared in two third of cases (63.2 %).
Several initial patterns of the pulmonary changes resulting from Corona Virus Disease 2019 (COVID-19) were present with a descending order; ground-glass pattern (96.7 %), lenticular pattern (32.2 %), Halo sign (15.7 % %), rounded (14.9 %), nodular (10.7 %), ground-glass with consolidation (8.3 %), tree-in-bud (1.7 %) and pleural effusion (1.7 %). Pathologically-enlarged lymph node was not a feature of COVID-19.
The total number of patients with positive findings having follow-up scans was 57 including single (45 patients) versus two (12 patients) follow-up scans. Initial follow up scans showed regression and progression of the pulmonary changes in 35 and 22 patients, respectively.
A remarkable pattern was seen in almost all regressed cases that showed patchy reticular pattern changes with septal thickening which was referred to “pulmonary synapses” (34 patients) with only one patient showed complete resolution of the parenchymal changes. Patterns seen in progressed cases were lenticular ground-glass (63.6 %) vs patchy ground-glass (36.4 %) patterns.
Conclusion
Computer tomographic scan of the chest is a principal diagnostic measure for Corona Virus Disease 2019 (COVID-19). The pulmonary changes showed more propensity being bilateral disease and affecting the lower lobes, while the right middle lobe was the least likely involvement. Several pattern of pulmonary changes can be seen on initial scans including ground glass, consolidation, Halo sign, lenticular, nodular, pleural effusion and tree-in-bud patterns. The tree-in-bud is first-time described pattern of COVID-19 in the current article and thought to be an excluding criterion. In follow-up scans; the lenticular and patchy ground-glass patterns were present in cases with disease progression, compared with “pulmonary synapses” pattern encountered in cases with disease regression.
Keywords: Covid-19, Chest CT scan, Tree-in-bud
1. Introduction
The outbreak of Corona Virus Disease 2019 (COVID-19) infection was first reported in December 2019 in Wuhan city in China [1,2]. Infected numbers of patient has been reported as of May 17, 2020 in 4,713,603. The World Health Organization (WHO) declared a global health emergency on January 30, 2020 [3]. The described clinical features include fever and cough along with non-specific symptoms for example dyspnea, headache, muscle soreness, and fatigue [4]. In Jordan; the total number of cases has reached 607 with reported 9 deaths and 404 recovered cases as of May 17, 2020.
COVID-19 is considered the seventh known coronavirus to infect humans [1]. Six coronavirus species are known to cause human disease; four viruses include 229E, OC43, NL63, and HKU1 typically cause generalized common cold symptoms [5], while the two other viruses include Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) can result in fatal illness [6]. The severe acute respiratory syndrome coronavirus (SARS-CoV) began in China and resulted in 774 deaths out of 8098 infected individuals from November 2002 to July 2003 while the Middle East respiratory syndrome coronavirus (MERS-CoV) began in Saudi Arabia and resulted in 848 deaths among 2458 individuals through July 2019 [7,8].
Thoracic imaging evaluation is crucial in assessment of the COVID-19 infection aiding in prompt recognition of disease, which is considered crucial to ensure timely treatment and implementing of active measures to prevent disease dissemination among suspected patients as well as assessing the degree of respiratory morbidity. The pattern of lung changes has been initially described to include bilateral involvement in 98 % with typical findings in ICU patients on admission included bilateral multiple lobular and subsegmental areas of consolidation findings [9]. Other described features of chest CT scan include multi-lobar involvement (71 %), ground-glass opacities (57 %), rounded morphology- opacities (33 %), peripheral distribution, consolidation with ground-glass opacities (29 %), crazy-paving pattern (19 %) [10,11].
In the current analysis we described and characterized the CT findings in 121 patients infected with COVID-19 in the main center of COVID-19 in Jordan, with the goal of highlighting the CT imaging findings for radiologists and clinicians regarding the current outbreak. A newly-described pattern of pulmonary involvement in COVID-19 was reported; the tree-in-bud pattern, which was thought to represent a criteria of exclusion of COVID-19.
2. Material and method
A retrospective analysis of the chest CT scans has been performed for all infected cases of COVID-19, which was conducted at the main center for COVID-19 in Jordan (Prince Hamzah Hospital) between the period from 13th of March 2020 to 13th of May 2020. No written informed consent was obtained for the current retrospective study that involved no potential risk to patients nor breach of patient confidentiality.
All included patients were confirmed COVID-2019 infection by positive rRT-PCR test of respiratory secretions obtained by nasopharyngeal or oropharyngeal swab and underwent chest CT within 24 h of admission.
The rRT-PCR test kits used were manufactured by DAAN Gene Co., Ltd. Of Sun Yat-sen University (Guangzhuo, China) and KogeneBiotech Co., Ltd. (Korea).
The total number of patients evaluated was (302). There were 188 men and 114 women studied.
Positive findings were present in 121 patients with a total number of 201 computer tomographic scans including initial and follow-up scans. Positive findings were present in 51 female and 70 male patients (age range, 12–87 years; mean age ± standard deviation, 46.1 ± 16.5).
All patients were scanned onsite with 1 mm slice thickness by Philips Brilliance Big Bore scanner (Philips; Amsterdam, Netherlands). No intravenous contrast media was administered.
Exclusion criteria include imaging artifacts interfering with proper evaluation of the computer tomographic scans and patients scanned after 24 h of admission.
CT scans were reviewed by two senior radiologists with more than 8 years of experience each and senior registrar. A reporting consensus was made prior to analyzing cases and final agreement was made for cases with discordance.
Initial CT scans was assessed for the following characteristics: (1) laterality, (2) lobar distribution of the lung abnormality, (3) initial patterns of the pulmonary changes: (a) ground-glass, (b) ground glass-opacities with consolidation, (c) lenticular, (d) rounded, (e) Halo sign, (f) pulmonary nodules, (g) tree-in-bud, (4) pleural effusion, (5) pathologically-enlarged thoracic lymph nodes, and (6) follow-up patterns of pulmonary changes: (a) patchy vs lenticular ground-glass in cases with progression, (b) reticulation/pulmonary synapses in cases with disease regression.
The described pulmonary patterns were agreed according to the definitions by Fleischner society as follow: (1) ground-glass pattern: area of hazy increased opacity of lung, with preservation of bronchial and vascular margins, (2) consolidation: homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls, (3) halo sign: ground-glass opacity surrounding a nodule, (4) nodule: a rounded or irregular opacity, well or poorly defined less than 3 cm, (5) tree-in-bud: represents centrilobular branching structures that resemble a budding tree (6) reticular pattern: a collection of innumerable small linear opacities that, by summation, produce an appearance resembling a net [12].
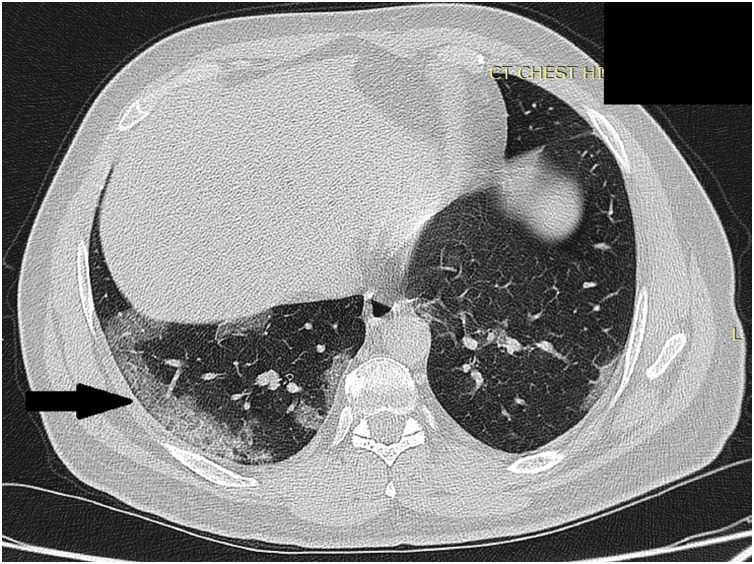
We suggested the use of descriptive terms including: (a) lenticular pattern to describe the peripherally-located conglomerate of pulmonary changes forming lens-shaped opacity seen in cases with progression (Fig. 1 ) and (b) “pulmonary synapses” pattern was used to describe reticulations at the pre-affected lung parenchyma with inter-connecting thickened septae seen with disease regression (Fig. 2 ).
Fig. 1.
High resolution chest CT scan axial slice shows conglomerate of pulmonary changes at the right lower lobe (Black arrow) referred as lenticular pattern.
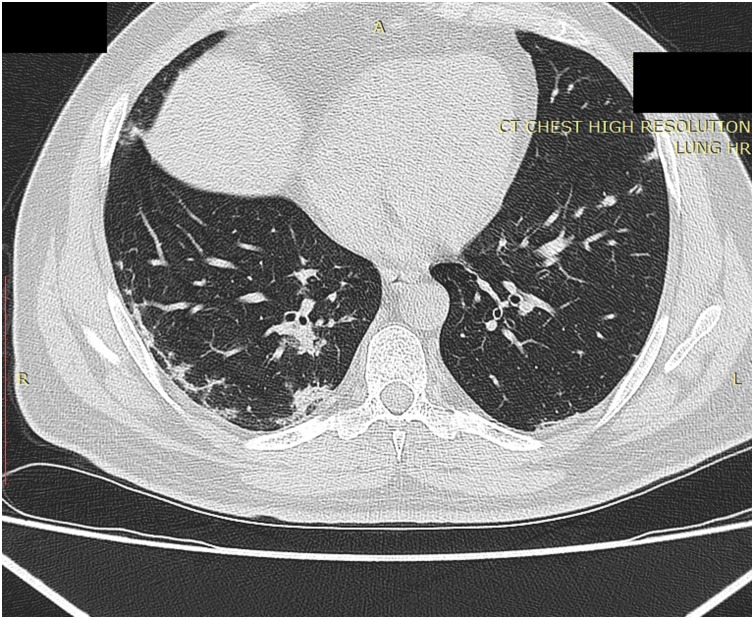
Fig. 2.
High resolution chest CT scan axial slice in the same case in Fig. 1 on follow-up imaging shows reticulations at the pre-affected lung parenchyma at the right lower lobe with inter-connecting thickened septae referred as “pulmonary synapses”.
The total number of patients with followed-up scans was 57 patients including single (45 cases) versus two (12 cases) scans.
3. Results
The total number of patients evaluated in the current analysis was 302. There were 188 men and 114 women studied. Negative imaging findings were defined as normal imaging findings of the initial and subsequent scans seen in 181 cases (59.9 %).
Positive findings were present in 121 patients (60.1 %) with a total number of 201 computer tomographic scans including initial and follow-up scans. Positive findings were present in 51 female and 70 male patients (age range, 12–87 years; mean age ± standard deviation, 46.1 ± 16.5). Only four cases (3.3 %) of the total 121 positive cases showed no initial CT findings, making early appearances of pulmonary changes in most COVID-19 patients in those who will develop pulmonary changes in the future.
3.1. Lobar distribution (Table 1, Table 2, Table 3)
Table 1.
Lung involvement in COVID-19 showing the unilateral vs bilateral distribution.
| Initial lung involvement (%) | Number of cases | |
|---|---|---|
| None (3.3 %) | 4 | |
| Unilateral (25.6 %) | Right (17.4 %) | 21 |
| Left (08.3 %) | 10 | |
| Bilateral (71.1 %) | 86 | |
| Total | 121 | |
Table 2.
The lobar distribution of COVID-19.
| Lobar distribution | Incidence | Incidence of lobar involvement in uni-lobar disease | Incidence of lobar sparing in four-lobar disease |
|---|---|---|---|
| Right upper lobe | 62.8 % | 17.2 % | 15.0 % |
| Right lower lobe | 75.2 % | 38.0 % | 00.0 % |
| Right middle lobe | 50.4 % | 13.8 % | 65.0 % |
| Left upper lobe | 60.3 % | 10.3 % | 10.3 % |
| Left lower lobe | 71.9 % | 20.7 % | 05.0 % |
Table 3.
Number and incidence of involved lobes in COVID-19.
| Number of lobes involved on the initial CT scans | Number of involved cases | Incidence (%) |
|---|---|---|
| None | 4 | 3.30 % |
| One | 29 | 24.0 % |
| Two | 13 | 10.7 % |
| Three | 11 | 9.1 % |
| Four | 20 | 16.5 % |
| Five | 44 | 36.4 % |
| Total | 121 |
Lung involvement was bilaterally present in 86 cases (71.1 %) compared to unilateral disease in 31 cases (25.6 %), showing more predilection for the right lung compared to the left one, 21 cases (17.4 %) vs 10 cases (8.8 %) (Table 1 ).
The distribution of the pulmonary changes was assessed according to the involved lobes with a descending order: right lower (75.2 %), left lower (71.9 %), right upper (62.8 %), left upper (60.3 %) and right middle (50.4 %). As can be seen from these results; the lower lobes show higher propensity for pulmonary changes compared to other lobes, while the right middle lobe was the least commonly involved (Table 2).
In cases with single lobar involvement (24 %); the lower lobes are more likely affected, while the left upper and right middle lobes showed least incidence of involvement in 10.3 % & 13.8 %, respectively, while the right lower lobe was the most commonly involved (38 %). In cases with one lobe spared/ four lobar involvement; the right middle lobe was spared in most cases accounting for 65 %, while the right lower lobe was always involved. (Table 2)
The number of the affected lobes on the initial scans was assessed; one lobe (24 %), two (10.7 %), three (9.1 %), four (16.5 %) and five (36.4 %). Making the disease more likely to have an initial multi-lobar involvement in more than two third of cases. (Table 3)
3.2. Initial CT patterns (Table 4)
Table 4.
Lung pattern on initial scans in COVID-19.
| Lung pattern in initial scans | Number of cases (%) |
|---|---|
| Ground-glass opacities | 117 (96.7 %) |
| Ground glass-opacities with consolidation | 10 (8.3 %) |
| Lenticular pattern | 39 (32.2 %) |
| Rounded pattern | 18 (14.9 %) |
| Halo sign | 19 (15.7 %) |
| Lung nodules | 13 (10.7 %) |
| Tree-in-bud | 2 (1.7 %) |
| Pleural effusion | 2 (1.7 %) |
| Pathologically-enlarged thoracic lymph nodes | 0 (0 %) |
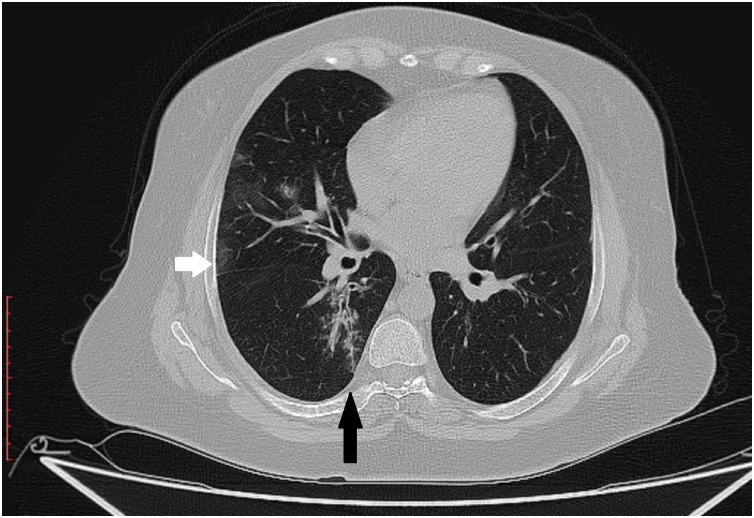
Several patterns of the lung involvement were looked for in the initial scans. Four cases (3.3 %) of positive cases were free of pulmonary changes in initial scans. Ground-glass pattern was the most commonly encountered pattern seen in 117 cases (96.7 %), while a coincident consolidation was present in 10 cases 8.3 %. The peripheral conglomerates of pulmonary changes giving a lenticular pattern were present in 39 cases (32.2 %). The presence of zoning phenomena surrounding the denser central pulmonary changes were described as Halo sign was present in 19 cases (15.7 %) (Fig. 3 ). Rounded-shaped pulmonary changes seen in 18 cases (14.9 %) (Fig. 4 ). Pulmonary nodules were identified in 13 cases (10.7 %) (Fig. 3). The first-time reported tree-in-bud pattern was present in two cases (1.7 %) (Fig. 5 ). Pleural effusion was seen in two cases (1.7 %) which was resolved parallel to resolution of parenchymal disease
Fig. 3.
High resolution chest CT scan axial slice shows ground glass changes surrounding a denser central lung nodule at the right lower lobe (Black arrow).
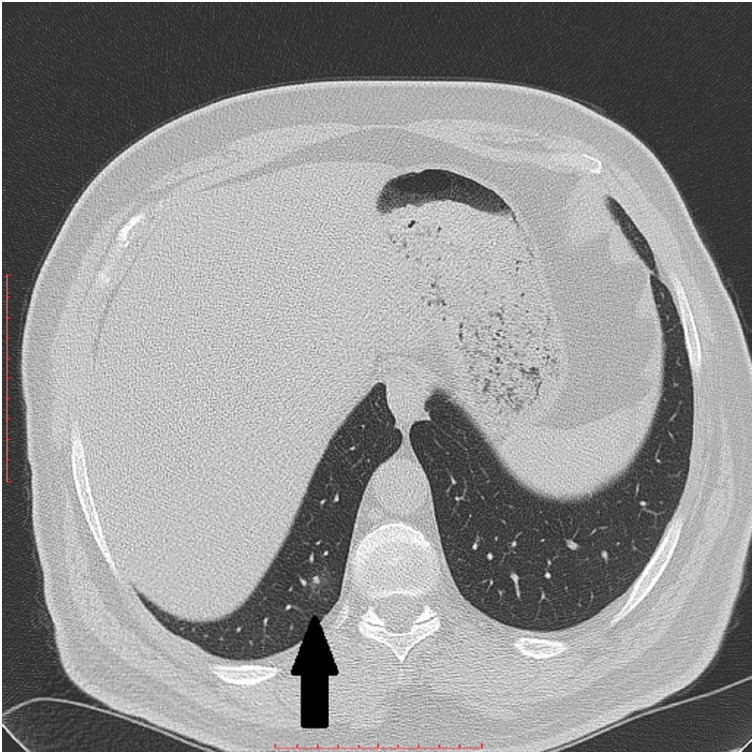
Fig. 4.
High resolution chest CT scan axial slice shows a rounded-shape consolidation at the right lower lobe (Black arrow).
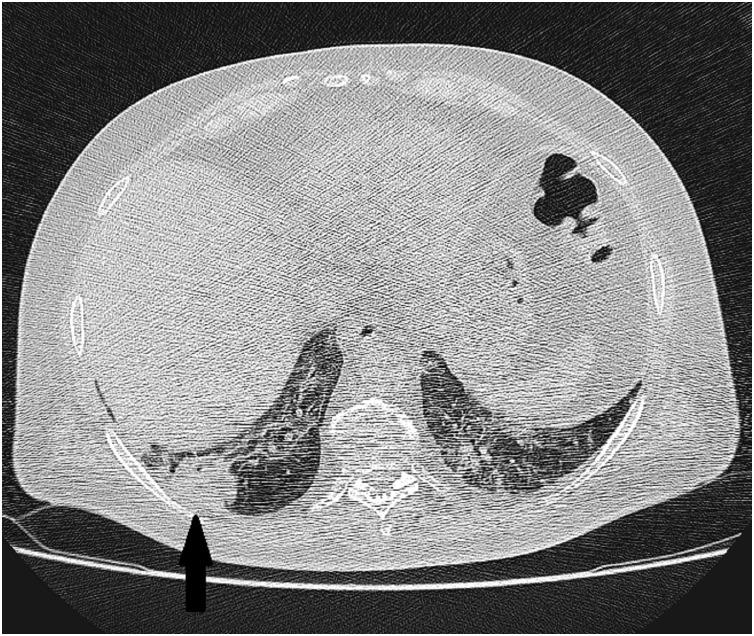
Fig. 5.
High resolution chest CT scan axial slice shows tree-in-bud pattern of pulmonary changes at the right lower lobe (Black arrow) and patchy ground glass pattern (White arrow).
The two cases with tree-in-bud pattern were negative regarding tuberculosis and one showed marked improvement of the tree-in-bud along with the ground glass changes on follow up scans). The two cases with mild bilateral pleural effusion was free regarding other medical conditions, which can result in similar findings, and showed complete resolution parallel to the parenchymal disease-regression. Pathologically-enlarged thoracic lymph nodes was not a feature of COVID-19 in the studied cases.
3.3. Follow-up CT patterns (Table 5)
Table 5.
Lung pattern on follow-up scans in COVID-19.
| Outcome of 1st follow-up scans | Number of cases 57 cases | Pulmonary Pattern | Number of cases (%) |
|---|---|---|---|
| Regression | 35 (62.5 %) | Reticular/pulmonary synapses | 34 (97.1 %) |
| Healed | 1 (2.8 %) | ||
| Progression | 22 (37.5 %) | Patchy ground-glass | 8 (36.4 %) |
| Lenticular ground-glass | 14 (63.6 %) | ||
| Outcome of 2nd follow-up scans | 12 cases | ||
| Regression | 10 (83.3 %) | Reticular/pulmonary synapses | 9 (90 %) |
| Healed | 1 (10 %) | ||
| Progression | 2 (16.7 %) | Lenticular | 2 (100 %) |
The total number of patients with pulmonary changes having follow-up scans was 57 including single (45 patients) versus two (12 patients) scans. Initial follow-up scans were classified into regression vs progression of the pulmonary changes seen in 35 (62.5 %) and 22 patients (37.5 %), respectively. No cases showed stabilization of pulmonary changes making COVID-19 a dynamic pulmonary condition in all patients.
A unique pattern of regression was seen in 34 cases out of 35 regressed cases, which was
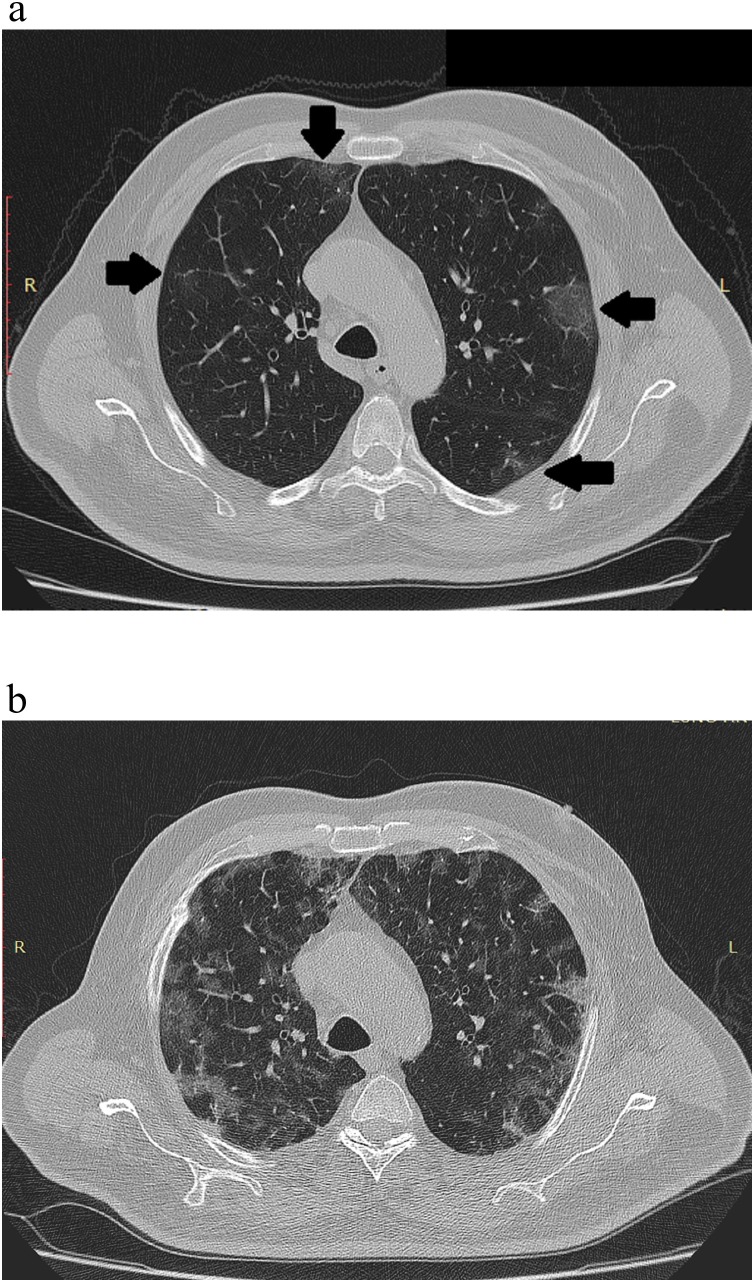
described as reticulations/“pulmonary synapses” due to similarities to the neuronal synapses, while one patient showed complete resolution of the parenchymal changes on initial follow up scans. 22 cases (37.5 %) showed progression on the initial follow-up scans presented as a peripheral conglomerates of the ground-glass opacities/consolidation parallel to the pleural lining, which was referred as lenticular pattern in 14 cases (63.6 %) (Fig. 1), and progressed patchy ground-glass pattern seen in 8 cases (36.4 %) (Fig. 6 ).
Fig. 6.
High resolution chest CT scan axial slices shows patchy ground glass opacities (a, black arrows), with progression on follow up scan (b).
Twelve cases had second follow-up scans; ten cases (83.3 %) showed regression including nine cases with reticulations/ “pulmonary synapses” pattern and one complete healing while two cases (16.7 %) showed progression with lenticular pattern.
Four patients including one female and three males (age range, 63–84 years; mean age 72.5 years) were died with one case showed ground glass changes and died due to chronic medical illness complicated by lung aspiration, while other three cases showed lenticular patterns of pulmonary changes. Based on pulmonary changes on these few cases; there was no remarkable radiological changes compared to some cases who had more severe initial changes and survived.
4. Discussion
CT is considered a crucial and routinely performed modality for evaluation of chest infection including viral pneumonia. In the current COVID-19 outbreak chest CT scan has been routinely used to assess the degree of lung involvement in rRT-PCR positive patients as well as in negative rRT-PCR cases with clinical suspicion of corona viral infection. It is also believed that CT chest cannot be used as a screening method, and rRT-PCR remains the reference diagnostic test for COVID-19 [13]. In our analysis CT scan has shown particular regional lung distribution along with several pattern of parenchymal changes. Multimodal imaging of COVID-19 infection was recently studied and showed disease manifestation upon admission including B-lines and consolidations on ultrasound, consolidations and hazy increased opacities on radiography, and multifocal ground glass opacities with consolidations on CT scan [14].
Among the studied 302 patients, negative CT findings was observed in 59.9 % of cases. The lung changes tend to affect more than one lobe (72.7 %) with particular involvement of the lower lobes (Right 75.2 % and left 71.9 %). Bilateral disease was more frequently encountered presented in 86 cases (71.1 %), compared to unilaterality in 31 cases (25.6 %) with about two folds more predilection for the right lung compared to the left one. The relationship between the lung changes and the duration of infection has been studied and showed increase from 28 % in early course of illness to 88 % in late patients [15].
Five lobar involvement was observed in 36.4 % similar to a recent report of 38 % involvement [10]. In case with uni-lobar involvement the left upper and the right middle lobes were least likely involved (10.3 % & 13.8 %, respectively); while the right middle lobe was the most frequently spared lobe in those with four lobar involvement (65 %).
Several patterns of the lung involvement were observed in the initial scans including: ground-glass pattern (96.7 %), ground-glass pattern with coincident consolidation (8.3 %), lenticular pattern (32.2 %), Halo sign (15.7 %), rounded-shaped pattern (14.9 %), pulmonary nodules (10.7 %), tree-in-bud (1.7 %) and bilateral pleural effusion (1.7 %). The presence of nodular and the first-time encountered tree-in bud pattern in the current analysis is in contradistinction to a recent assumption for being a differentiating features of influenzas than COVID-19 infection [16]. The presence of pleural effusion in two cases in our study is first time observed, which was concluded to represent an excluding criteria [17].
Follow up scans were available 69 cases. Regression was observed in 62.5 %. A unique pattern of regression was observed in 97.1 % of cases that showed reticular/“pulmonary synapses” pattern, while cases with progression showed lenticular pattern (63.6 %) and ground-glass pattern (36.4 %).
Bilateral pleural effusion was observed in two cases (1.7 %) with no other comorbidities that can results in pleural fluid, which was resolved parallel to the regressed pulmonary changes. The current finding reported on corona virus infection in contradistinction to the recent analysis done on 21 cases from china [10]. A case report has documented small bilateral pleural effusion in asymptomatic patient [18,19]. Tree-in-bud pattern was observed in two cases (1.7 %) which was not described before [19].
In other corona virus infection, a similar initial pattern of ground-glass opacities and consolidation and late reticular patterns were observed a 30 cases analysis severe acute respiratory syndrome (SARS), though pleural effusion was not a documented feature [20]. It was concluded that bilateral lung involvement on initial imaging is more likely to be seen with COVID-19, while unilateral lung involvement is more likely to be seen in SARS and MERS [21].
The limitations of the current analysis include: lack of clinical correlation as our study was concentrated on CT scan findings for all cases with positive rRT-PCR as well as the precise time of symptom onset was unknown, radiographic evaluation was not involved as CT is superior in delineating the parenchymal changes. No selection bias was involved in the current analysis as CT scan was routinely done for all positive cases apart from being severely symptomatic.
In the current analysis we analyzed the chest CT findings in 302 patients infected with COVID-19 in the main center of COVID-19 in Jordan, with the goal of highlighting the pulmonary CT findings encountered in the current COVID-19 pandemic. A newly-described pattern of pulmonary involvement in COVID-19 is reported “tree-in-bud pattern”, which was thought to represent an exclusion criterion.
Ethical
The article was approved by the ethical committee.
Funding
No funding/financial support was given during the research, nor agreed post publication.
CRediT authorship contribution statement
Omar M. Albtoush: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Supervision. Rawan B. Al-Shdefat: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft. Alabed Al-Akaileh: Conceptualization, Methodology, Validation, Investigation, Resources, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. January 24 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan W.J., Zhao X., Ma X.J. A novel coronavirus genome identified in a cluster of pneumonia cases - Wuhan, China 2019-2020. China CDC Weekly. 2020;2:61–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. China coronavirus: WHO declares international emergency as death toll exceeds 200. BMJ. 2020 doi: 10.1136/bmj.m408. January 31 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25689. January 29 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su S., Wong G., Shi W. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. Crossref. opens in new tab Web of Science. opens in new tab Medline. opens in new tab Google Scholar. opens in new tab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam C.W., Chan M.H., Wong C.K. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin. Biochem. Rev. 2004;25:121–132. [PMC free article] [PubMed] [Google Scholar]
- 8.Hui Azhar E.I., Memish D.S.C., Drosten Z.A., Zumla C. A the Middle East respiratory syndrome (MERS) Infect. Dis. Clin. North Am. 2019;33:891–905. doi: 10.1016/j.idc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. January 24 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung M., Bernheim A., Mei X. CT imaging features of 2019 novel coronavirus (2019nCoV) Radiology. 2020 doi: 10.1148/radiol.2020200230. February 4 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jajodia A., Ebner L., Heidinger B., Chaturvedi A., Prosch H. Imaging in corona virus disease 2019 (COVID-19)-A scoping review. Eur. J. Radiol. Open. 2020;7 doi: 10.1016/j.ejro.2020.100237. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 13.El Homsi M., Chung M., Bernheim A., Jacobi A., King M.J., Lewis S., Taouli B. Review of chest CT manifestations of COVID-19 infection. Eur. J. Radiol. Open. 2020;7 doi: 10.1016/j.ejro.2020.100239. Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomoro P., Verde F., Zerboni F., Simonetti I., Borghi C., Fachinetti C., Natalizi A., Martegani A. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur. J. Radiol. Open. 2020;7 doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. Feb 20, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M., Zeng W., Wen Y., Zheng Y., Lv F Xiao K. COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06928-0. May 12, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Fang X., Bian Y., Lu J. Comparison of chest CT findings between COVID-19 pneumonia and other types of viral pneumonia: a two-center retrospective study. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06925-3. May 12, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C., Ding Y., Xie B., Sun Z., Li X., Chen Z., Niu M. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin. Imaging. 2020;63:7–9. doi: 10.1016/j.clinimag.2020.02.008. Jul, Epub 2020 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20(April (4)):425–434. doi: 10.1016/S1473-3099(20)30086-4. Epub 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ooi G.C., Khong P.L., Müller N.L. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 21.Kheiralla O.A.M., Tajaldeen A.A., Bakheet A.O. Imaging differences between coronavirus disease 2019, severe acute respiratory syndrome, and Middle East respiratory syndrome. Eur. J. Radiol. Open. 2020;7 doi: 10.1016/j.ejro.2020.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]