Abstract
Hepatocellular carcinoma (HCC) is one of the most prevalent types of cancer worldwide. Long non-coding RNAs (lncRNAs) have been reported to frequently participate in the carcinogenesis and development of various types of cancer, including HCC. However, the molecular mechanisms of lncRNA ST8SIA6-AS1 in HCC remain poorly understood. The present study performed bioinformatics analysis, in addition to using reverse transcription-quantitative PCR (RT-qPCR), nuclear-cytoplasmic fractionation, RNA immunoprecipitation, and Transwell, wound healing, and dual-luciferase reporter assays, to determine the biological role and regulatory mechanisms of ST8SIA6-AS1 in HCC. The results revealed that the expression levels of ST8SIA6-AS1 were upregulated in HCC tissues and cell lines, which were associated with a poor prognosis. Moreover, the genetic knockdown of ST8SIA6-AS1 inhibited the hypoxia-induced HCC cell migration and invasion. Additionally, microRNA (miR)-338, which exhibited downregulated expression levels in HCC tissues and cell lines, was discovered to bind with ST8SIA6-AS1. The inhibition of miR-338 partially reversed the inhibitory effects of ST8SIA6-AS1-knockdown on the migration and invasion of HCC cells under hypoxia. Subsequently, methylphosphate capping enzyme (MEPCE) was identified to be targeted and negatively regulated by miR-338. Notably, the overexpression of MEPCE recovered the inhibitory influence over the migratory and invasive abilities of hypoxia-treated HCC cells promoted by ST8SIA6-AS1 inhibition. In conclusion, the findings of the present study suggest that lncRNA ST8SIA6-AS1 may promote the migration and invasion of hypoxia-induced HCC cells via the miR-338/MEPCE axis, indicating a potential diagnostic or therapeutic marker for HCC treatment.
Keywords: hepatocellular carcinoma, hypoxia, ST8SIA6-AS1, miR-338, MEPCE
Introduction
Hepatocellular carcinoma (HCC) is listed as one of the top 10 malignant tumors by the World Health Organization (1). With an increasing morbidity rate, HCC contributes to ~600,000 deaths annually worldwide (2). Despite the significant progress in the diagnosis and surgical treatment of the disease, patients with HCC have a low overall survival rate, with dismal prognosis (2,3). Therefore, an improved understanding and further determination of the regulatory mechanisms underlying HCC initiation and progression are required to identify potential diagnostic markers for the treatment of patients with HCC.
According to the results of genomic and transcriptomic sequencing, a large proportion of the genome is transcribed into non-coding RNAs (ncRNAs), while only a small percentage of the human genome is transcribed into protein-coding mRNAs (4,5). Long ncRNAs (lncRNAs) are members of the ncRNA family, which are characterized by being >200 nucleotides in length (6). Increasing evidence has identified that lncRNAs are frequently aberrantly expressed in numerous types of disease, particularly in several types of cancer (7,8). Despite lacking the ability to encode proteins, lncRNAs are capable of regulating gene expression via several different mechanisms, including mRNA transportation, and both transcriptional and post-transcriptional modulation (9–11). Notably, emerging studies have proposed a competitive endogenous RNA (ceRNA) mechanism, by which lncRNAs sponge microRNAs (miRNAs/miRs) to modulate gene expression at a post-transcriptional level (12,13). For instance, the lncRNA LOXL1-AS1 was discovered to upregulate USF1 expression levels by sponging miR-708-5p in gastric carcinoma (14). In addition, lncRNA NONHSAT101069 was found to promote breast cancer by downregulating the expression levels of miR-129-5p and upregulating the expression levels of Twist1 (15). Nevertheless, the mechanism of action of ST8SIA6-AS1 in HCC remains poorly understood.
miRNAs are another category of ncRNAs, which are short, single-stranded ncRNAs of ~22 nucleotides in length (16). miRNAs bind with the 3′ untranslated region (3′UTR) of mRNAs to degrade mRNA and/or inhibit the translation of target genes, which has been identified to regulate tumor occurrence and development (16,17). For example, miR-98-5p was found to regulate the cell cycle via targeting CDC25A in osteosarcoma (18), while the overexpression of miR-367-3p was found to inhibit cell proliferation and invasion through the Wnt/β-catenin signaling pathway by targeting SPAG5 in cervical cancer (19). However, although miR-338 has been reported to regulate HCC progression, the interaction between ST8SIA6-AS1 and miR-338 remains to be fully elucidated in HCC.
The present study aimed to investigate the function and mechanism of action of ST8SIA6-AS1 in HCC. The results revealed that ST8SIA6-AS1 promoted the migration and invasion of hypoxia-induced HCC cells via the miR-338/MEPCE axis, providing a potential diagnostic or therapeutic marker for the treatment of HCC.
Materials and methods
Patient studies
HCC tissues (n=22) and adjacent normal tissues (n=22) were obtained from HCC patients with a mean age of 58 years (range, 32–76 years) between September 2015 and November 2017 at The Affiliated Hospital of Xuzhou Medical University (Xuzhou, China); all tissue specimens were maintained at −80°C. The patients had not received any other anticancer treatment before surgery. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University and written informed consent was obtained from all patients.
Cell culture and treatment
HCC cell lines (HCCLM6, Hep3B and Huh-7) and a human normal liver cell line (THLE-3) were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. All cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS and 1% penicillin/streptomycin stock solution (Sigma-Aldrich; Merck KGaA). The cell lines were maintained in a humidified atmosphere containing 5% CO2 at 37°C. Oxygen concentrations were maintained at 1% to induce hypoxia and hypoxic conditions were established using a humidified variable aerobic workstation with 5% CO2 at 37°C.
Cell transfection
The miR-338 mimic and negative control (NC) mimic, and the miR-338 inhibitor and NC inhibitor, were used to overexpress and inhibit miR-338, respectively. Short hairpin RNA (shRNA/sh) targeting ST8SIA6-AS1 (sh-ST8SIA6-AS1#1 and sh-ST8SIA6-AS1#2) and the NC (sh-NC) were used to knock down the expression levels of ST8SIA6-AS1. Full length methylphosphate capping enzyme (MEPCE) was subcloned into the pcDNA3.1 vector to overexpress MEPCE; an empty pcDNA3.1 vector was used as the control. All the plasmids were purchased from Shanghai GenePharma Co., Ltd. and transfected into Hep3B or Huh-7 cells using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from HCC tissues or cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). An miRNeasy Mini kit (Qiagen GmbH) and the TaqMan® MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to isolate miRNAs and reverse transcribe miRNA into cDNA, respectively. For the quantification of mRNAs, a Prime Script™ RT reagent kit (Takara Bio, Inc.) was used for RT. qPCR was subsequently performed using a TaqMan® Universal PCR Master mix II (Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative expression levels of RNAs (normalized to GAPDH or U6) were quantified using the 2−ΔΔCq method.
Nuclear-cytoplasmic fractionation
The distribution of ST8SIA6-AS1 in the cytoplasm or nucleus of HCC cells was determined using a PARIS kit (Thermo Fisher Scientific, Inc.). Briefly, Hep3B or Huh-7 cells were harvested and lysed on ice, and the supernatant was collected following centrifugation. The extracted RNAs were analyzed using RT-qPCR, and GAPDH and U6 were used as the cytoplasmic and nucleic controls, respectively.
Wound healing assay
A total of 8×104 transfected Hep3B or Huh-7 cells/ml were cultured in RPMI-1640 medium, supplemented with 10% FBS, until 100% confluence. A sterile pipette tip was used to generate single-line scratches and the cell monolayer was subsequently washed twice with PBS. The wound closure was imaged at 0 and 24 h using a light microscope (magnification, ×200).
Transwell Matrigel assay
A total of 1×105 Hep3B or Huh-7 cells suspended in 500 µl serum-free medium were plated into the upper champers of Transwell plates (Corning Inc.). The upper chambers were precoated with Matrigel. Medium supplemented with 5% FBS was plated into the lower chambers. Following incubation for 24 h, the invasive Hep3B or Huh-7 cells were fixed with methanol and stained with 0.1% crystal violet for 20 min at room temperature. The invasive cells were visualized under a light microscope (magnification, ×200; Olympus Corporation).
Dual-luciferase reporter assay
Full-length MEPCE 3′UTR and ST8SIA6-AS1 were amplified and cloned into pmirGLO vectors (Promega Corp.) to construct the pmirGLO-MEPCE-WT reporter gene plasmid (5′-CAGCAAGGCUGGCUGGUGCUGGA-3′) and pmirGLO-ST8SIA6-AS1-WT reporter gene plasmid (5′-GAACAGAAUCGCUAAUAUGCUGG-3′), respectively. Mutations within the miR-338 binding site were created using the QuikChange II Site-Directed Mutagenesis kit (Stratagene; Agilent Technologies, Inc.), to generate the pmirGLO-MEPCE-MUT (5′-CAGCAAGGCUGGCUGGCAACAAC-3′) reporter gene plasmid and pmirGLO-ST8SIA6-AS1-MUT (5′-GAACAGAAUCGCUAAACCAACAA-3′) reporter gene plasmid. Hep3B and Huh-7 cells were co-transfected with miR-338 mimics or NC mimics and ST8SIA6-AS1-WT (or MEPCE-WT) or ST8SIA6-AS1- Mut (or MEPCE-Mut) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Following 48 h of transfection, the relative luciferase activity was analyzed using a Dual-Luciferase Reporter assay system. Firefly luciferase activity was normalized to Renilla luciferase activity (Promega Corp.).
RNA immunoprecipitation (RIP) assay
A RIP assay was performed using the Magna RNA-binding protein immunoprecipitation kit (EMD Millipore). Briefly, the cell lysate (Hep3B or Huh-7 cells) was incubated in RIP buffer containing magnetic beads conjugated to human anti-Ago2 antibody. IgG served as the endogenous control. The immunoprecipitated RNA was isolated, purified and analyzed using RT-qPCR.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used for statistical analysis and data are presented as the mean ± SD of three independent repeats. Clinicopathological characteristics were evaluated using the χ2 test. Comparison between HCC and adjacent normal tissues was performed using a paired Student's t-test, while comparisons between the experimental and control groups was performed using an unpaired Student's t-test. Comparison among multiple groups was performed using one-way ANOVA, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
ST8SIA6-AS1 expression levels are upregulated in HCC and are associated with a poor prognosis
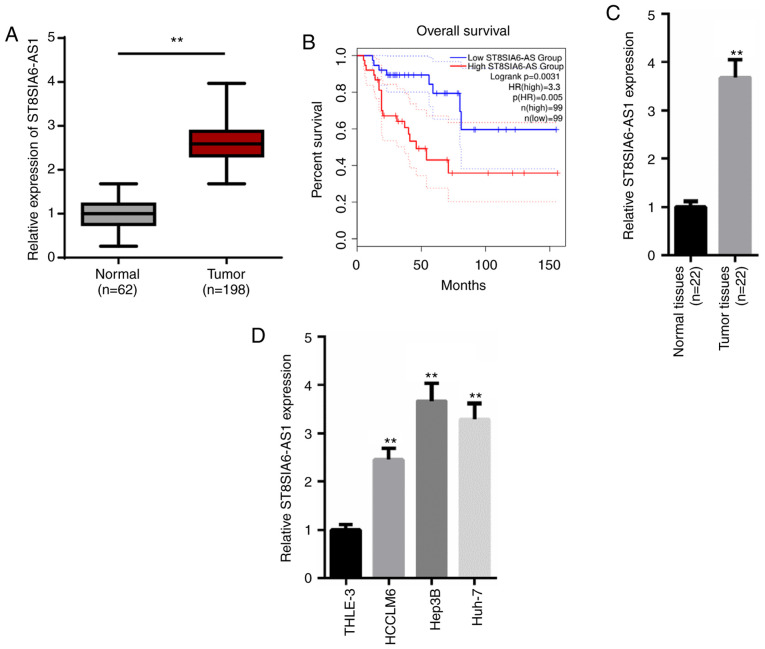
According to the TCGA database (https://tcga-data.nci.nih.gov/tcga/), ST8SIA6-AS1 expression levels were significantly upregulated in liver hepatocellular carcinoma (LIHC) tissues (n=198) compared with normal tissues (n=62; Fig. 1A). Additionally, patients with HCC with higher levels of ST8SIA6-AS1 had a lower survival rate compared with patients with low expression levels of ST8SIA6-AS1 (Fig. 1B). Similarly, RT-qPCR analysis revealed that expression levels of ST8SIA6-AS1 were significantly upregulated in HCC tissues (n=22) or cell lines (HCCLM6, Hep3B and Huh-7) compared with the adjacent normal tissues (n=22) or the human normal liver cell line (THLE-3; Fig. 1C and D). Moreover, the clinical data indicated that the ST8SIA6-AS1 expression levels were positively correlated with the histological grade, TNM stage and vein invasion (Table I). Altogether, these results suggest that ST8SIA6-AS1 expression levels may be upregulated in HCC tissues and cell lines, and that the upregulation of ST8SIA6-AS1 expression levels may predict the poor prognosis of HCC.
Figure 1.
ST8SIA6-AS1 expression levels are upregulated in HCC and associated with a poor prognosis. (A) Data from TCGA demonstrated that ST8SIA6-AS1 expression levels are upregulated in liver hepatocellular carcinoma tissues. **P<0.01. (B) Kaplan-Meier survival analysis showed that the upregulation of ST8SIA6-AS1 predicted a poor prognosis in HCC patients. (C and D) RT-qPCR was used to analyze ST8SIA6-AS1 expression levels in HCC tissues and cell lines. **P<0.01, compared with the normal tissues or THLE-3 cell line. HCC, hepatocellular carcinoma; TCGA, The Cancer Genome Atlas.
Table I.
Association between ST8SIA6-AS1 expression and clinicopathologic features in patients with HCC.
| ST8SIA6-AS1 | |||||
|---|---|---|---|---|---|
| Parameters | Group | Total | Low | High | P-value |
| Sex | Male | 13 | 6 | 7 | 0.496 |
| Female | 9 | 5 | 4 | ||
| Age (years) | <50 | 10 | 4 | 6 | 0.272 |
| ≥50 | 12 | 7 | 5 | ||
| Tumor size (cm) | <5 | 8 | 4 | 4 | 0.807 |
| ≥5 | 14 | 8 | 6 | ||
| Histological grade | Low | 7 | 5 | 2 | <0.001 |
| High | 15 | 4 | 11 | ||
| TNM stage | I–II | 8 | 5 | 3 | 0.006 |
| III–IV | 14 | 5 | 9 | ||
| Vein invasion | Absence | 7 | 6 | 1 | <0.001 |
| Presence | 15 | 5 | 10 | ||
| Cirrhosis | Negative | 16 | 8 | 8 | 0.805 |
| Positive | 6 | 3 | 3 | ||
| Hepatitis B | Negative | 7 | 4 | 3 | 0.818 |
| Positive | 15 | 7 | 8 | ||
Knockdown of ST8SIA6-AS1 inhibits hypoxia-induced HCC cell progression
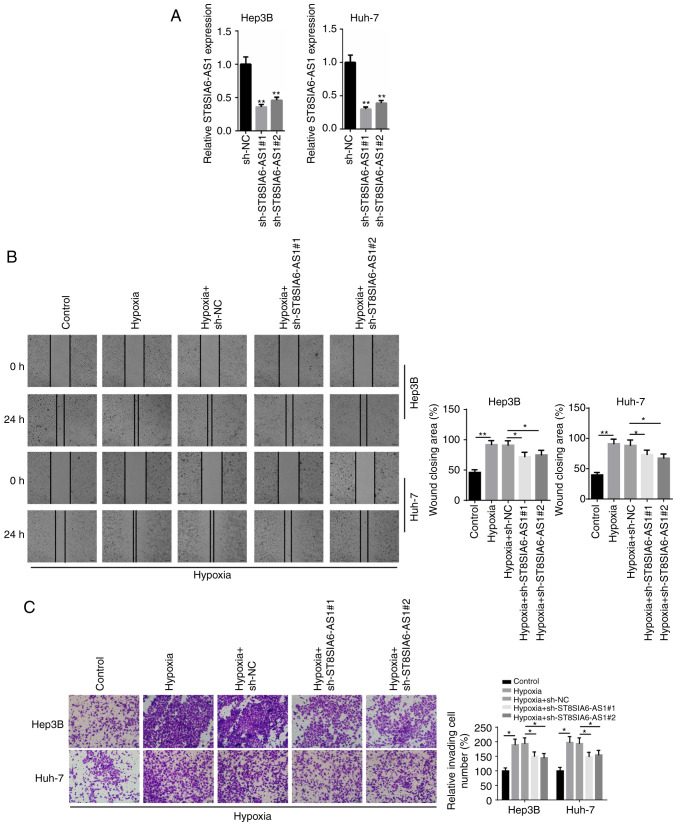
To investigate the biological role of ST8SIA6-AS1 in HCC, loss-of-function assays were performed. The expression levels of ST8SIA6-AS1 were markedly downregulated following the transfection of sh-ST8SIA6-AS1#1 or sh-ST8SIA6-AS1#2 into Hep3B and Huh-7 cells (Fig. 2A). Subsequently, a wound healing assay demonstrated that the migratory ability of HCC cells was significantly increased following hypoxia induction compared with the control group, while the migratory rate was significantly hindered following the transfection with sh-ST8SIA6-AS1#1 or sh-ST8SIA6-AS1#2 (Fig. 2B). Similarly, the Transwell Matrigel assay revealed that the invasive capacity of HCC cells was enhanced by hypoxia treatment, while the invasive capacity was significantly inhibited by the knockdown of ST8SIA6-AS1 (Fig. 2C). Together, these results indicated that the genetic knockdown of ST8SIA6-AS1 may inhibit the migration and invasion of hypoxia-induced HCC cells.
Figure 2.
Knockdown of ST8SIA6-AS1 inhibits hypoxia-induced HCC cell progression. (A) RT-qPCR showed the relative expression levels of ST8SIA6-AS1 in Hep3B and Huh-7 cells transfected with sh-NC, sh-ST8SIA6-AS1#1 and sh-ST8SIA6-AS1#2. **P<0.01 compared with the sh-NC group. (B) Wound healing and (C) Transwell assays were used to determine the migration and invasion ability of the Hep3B and Huh-7 cells. *P<0.05, **P<0.01. HCC, hepatocellular carcinoma.
ST8SIA6-AS1 directly binds with miR-338 to inhibit its expression levels
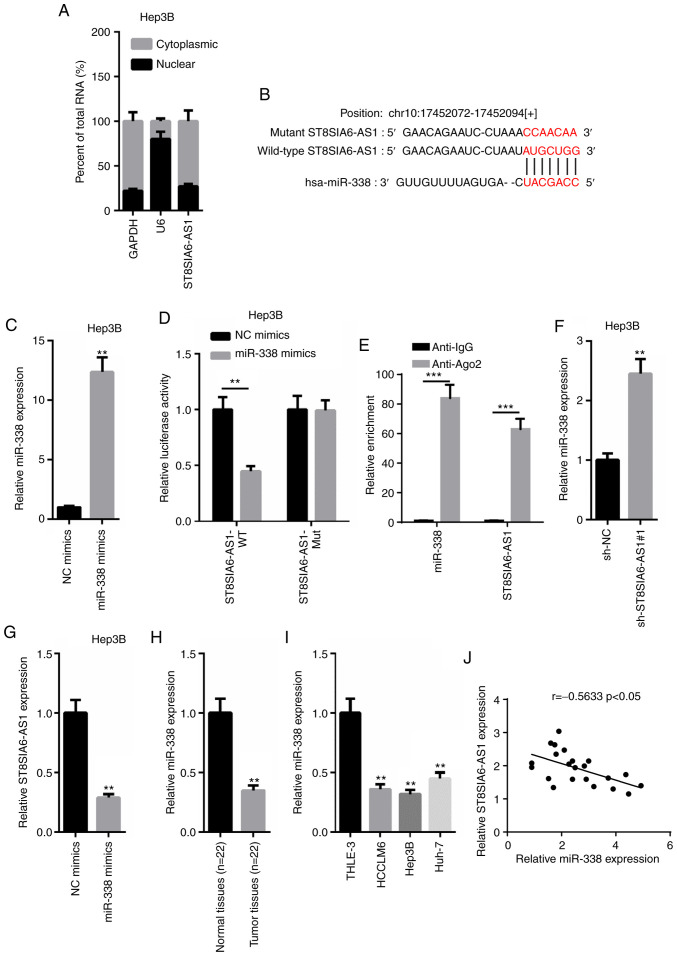
According to the results obtained from nuclear-cytoplasmic fractionation, the majority of ST8SIA6-AS1 was observed to be distributed in the cytoplasm of Hep3B cells (Fig. 3A). Thus, it was hypothesized that ST8SIA6-AS1 may form a ceRNA network in HCC. miR-338 was predicted to contain a binding site on ST8SIA6-AS1 using starBase (http://starbase.sysu.edu.cn; Fig. 3B). Thus, the expression levels of miR-338 were overexpressed by transfecting miR-338 mimics into Hep3B cells (Fig. 3C). A dual-luciferase reporter assay revealed that miR-338 overexpression induced a marked decrease in pmirGLO-ST8SIA6-AS1-WT relative luciferase activity in Hep3B cells, whereas no differences were observed in pmirGLO-ST8SIA6-AS1-Mut-transfected cells (Fig. 3D). Subsequently, a RIP assay revealed that both ST8SIA6-AS1 and miR-338 expression levels were enriched in anti-Ago2 cells, suggesting that ST8SIA6-AS1 and miR-338 may co-exist in RNA-induced silencing complexes (RISC; Fig. 3E). In addition, ST8SIA6-AS1 knockdown significantly upregulated miR-338 expression levels, while miR-338 overexpression significantly downregulated ST8SIA6-AS1 expression levels (Fig. 3F and G). Furthermore, miR-338 expression levels were revealed to be downregulated in HCC tissues or cells compared with the adjacent normal tissues or THLE-3 cells (Fig. 3H and I). Moreover, the expression levels of ST8SIA6-AS1 and miR-338 in HCC tissues were negatively correlated with other (Fig. 3J). In conclusion, these findings suggest that ST8SIA6-AS1 directly interacts with miR-338 to suppress its expression levels in HCC.
Figure 3.
ST8SIA6-AS1 directly binds with miR-338 to inhibit its expression levels. (A) Nuclear-cytoplasmic fractionation assay was performed to locate ST8SIA6-AS1. (B) Binding sequences were predicted using starBase. (C) Overexpression transfection efficiency of miR-338 was determined using RT-qPCR. **P<0.01 compared with the NC mimics group. (D and E) Dual-luciferase reporter and RIP assays were performed to validate the binding ability between ST8SIA6-AS1 and miR-338. **P<0.01, ***P<0.005. (F) RT-qPCR showed the relative expression of miR-338 in Hep3B cells transfected with sh-NC and sh-ST8SIA6-AS1#1. **P<0.01 compared with the sh-NC group. (G) RT-qPCR showed the relative expression of ST8SIA6-AS1 in Hep3B cells transfected with NC mimics and miR-338 mimics. **P<0.01 compared with the sh-NC group. (H and I) The expression levels of miR-338 in HCC tissues and cell lines were analyzed by RT-qPCR. **P<0.01 compared with the normal tissues or THLE-3 cell line. (J) Pearson's correlation analysis was performed to analyze the correlation between ST8SIA6-AS1 and miR-338. HCC, hepatocellular carcinoma.
miR-338 partially counteracts the effect of ST8SIA6-AS1 in hypoxia-treated HCC cell progression
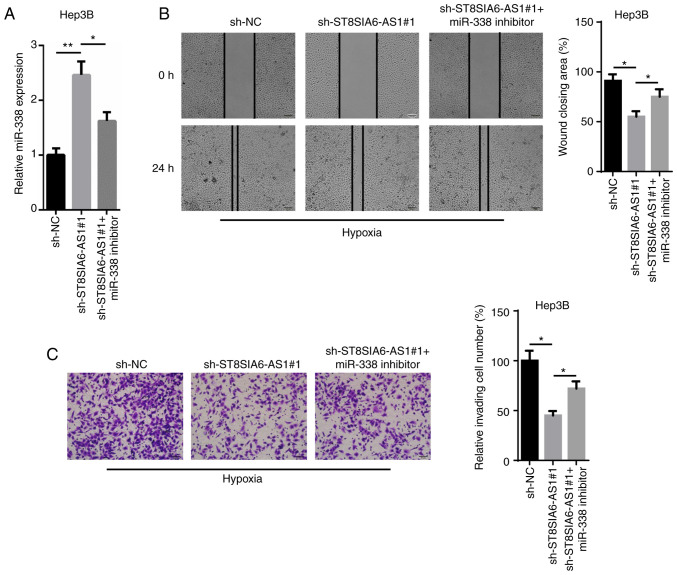
To determine whether miR-338 is an important effector for ST8SIA6-AS1-mediated carcinogenesis and the development of hypoxia-treated HCC cells, an miR-338 inhibitor was transfected into sh-ST8SIA6-AS1-expressing Hep3B cells. The upregulation of miR-338 mediated by ST8SIA6-AS1 silencing was reversed following the transfection of the miR-338 inhibitor (Fig. 4A). Moreover, the wound healing and Transwell assays indicated that the inhibitory effects caused by the genetic knockdown of ST8SIA6-AS1 on cell migration and invasion were abolished following miR-338 inhibition (Fig. 4B and C). Taken together, these findings suggest that miR-338 may partially counteract the effects of ST8SIA6-AS1 in regards to hypoxia-induced HCC progression.
Figure 4.
miR-338 partially reverses the effect of ST8SIA6-AS1 on HCC progression. (A) RT-qPCR showed the expression levels of miR-338 in hypoxia-treated Hep3B cells transfected with sh-NC, sh-ST8SIA6-AS1#1 and sh-ST8SIA6-AS1#1+miR-338 inhibitor. (B) Wound healing assay showed the migration ability in hypoxia-treated Hep3B cells transfected with sh-NC, sh-ST8SIA6-AS1#1 and sh-ST8SIA6-AS1#1+miR-338 inhibitor. (C) Transwell assay analyzed the cell invasion in hypoxia-treated Hep3B transfected with sh-NC, sh-ST8SIA6-AS1#1 and sh-ST8SIA6-AS1#1+miR-338 inhibitor. *P<0.05, **P<0.01. HCC, hepatocellular carcinoma.
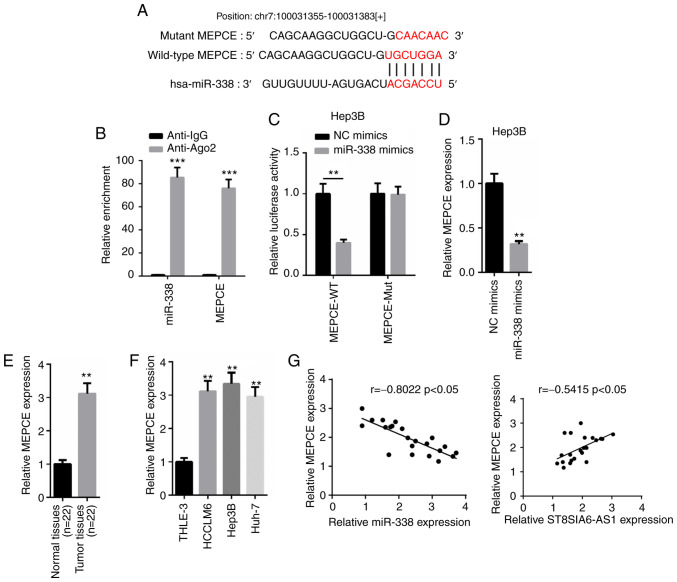
MEPCE is directly targeted and downregulated by miR-338
According to the prediction using the TargetScan website (http://www.targetscan.org), MEPCE was discovered to contain a complementary binding site for miR-338 (Fig. 5A). The RIP assay revealed that both miR-338 and MEPCE were abundantly expressed in the Ago2 group, but not in the IgG group (Fig. 5B). A dual-luciferase reporter assay also discovered that the overexpression of miR-338 decreased the relative luciferase activity of the MEPCE-WT reporter in Hep3B cells, while the relative luciferase activity in cells transfected with the MEPCE-Mut reporter remained the same (Fig. 5C). Moreover, MEPCE mRNA expression levels were suppressed following the transfection of miR-338 mimics into Hep3B cells (Fig. 5D). RT-qPCR analysis demonstrated that the expression levels of MEPCE were significantly upregulated in HCC tissues and cells compared with normal tissues and cells (Fig. 5E and F). In addition, a negative correlation was identified between the expression levels of MEPCE and miR-338 in HCC tissues, whereas a positive correlation was revealed between the expression levels of MEPCE and ST8SIA6-AS1 (Fig. 5G). Altogether, these findings indicate that MEPCE expression levels may be upregulated in HCC and may directly interact with miR-338.
Figure 5.
MEPCE is directly targeted and downregulated by miR-338. (A) Binding sequence between miR-338 and MEPCE was predicted by TargetScan. (B and C) RIP and dual-luciferase reporter assays were performed to validate the interaction between MEPCE and miR-338. **P<0.01, ***P<0.005 compared with the anti-IgG group or NC mimics group. (D) The relative expression of MEPCE in Hep3B cells transfected with NC mimics and miR-338 mimics was analyzed using RT-qPCR. **P<0.01 compared with the NC mimics group. (E and F) MEPCE expression levels in HCC tissues and cell lines were analyzed using RT-qPCR. **P<0.01 compared with the normal tissues or THLE-3 cell line. (G) Pearson's correlation analysis was performed to analyze the correlation between MEPCE and miR-338 or ST8SIA6-AS1.
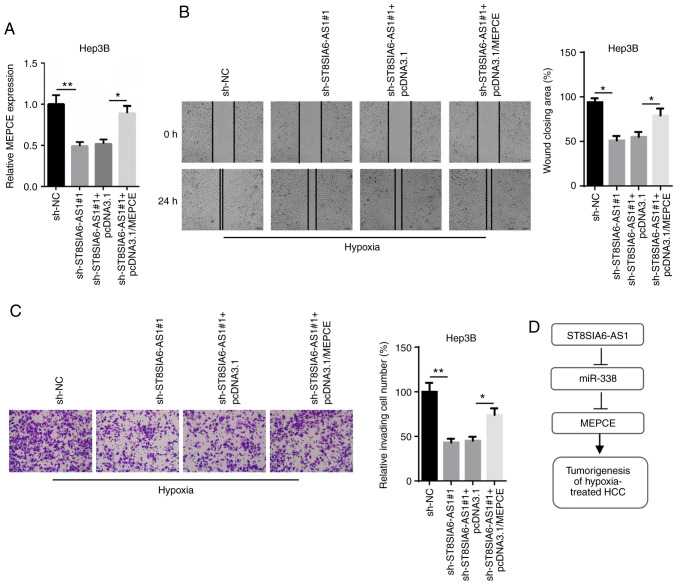
ST8SIA6-AS1 regulates the migration and invasion of hypoxia-treated HCC cells by targeting MEPCE
To validate that MEPCE mediates the oncogenic role of ST8SIA6-AS1, rescue assays were performed. The downregulation of MEPCE mRNA expression levels induced by ST8SIA6-AS1 inhibition were reversed following the transfection with pcDNA3.1/MEPCE (Fig. 6A). Additionally, both wound healing and Transwell Matrigel assays revealed that the suppressive effect of ST8SIA6-AS1 knockdown on cell migration and invasion was reversed following MEPCE overexpression in hypoxia-treated Hep3B cells (Fig. 6B and C). These findings suggest that ST8SIA6-AS1 may affect the migration and invasion of HCC cells under hypoxia by targeting MEPCE.
Figure 6.
ST8SIA6-AS1 regulates the migration and invasion of HCC cells by targeting MEPCE. (A) RT-qPCR showed the expression levels of MEPCE in hypoxia-treated Hep3B cells. (B and C) Cell migration and invasion capacities in hypoxia-treated Hep3B cells were analyzed using wound healing and Transwell assays. *P<0.05, **P<0.01. (D) A schematic diagram illustrating the involvement of ST8SIA6-AS1, miR-338 and MEPCE in HCC. HCC, hepatocellular carcinoma; MEPCE, Methylphosphate capping enzyme.
Discussion
Hepatocellular carcinoma (HCC) has contributed to considerable mortality rates globally, demonstrating a high incidence rate and an unfavorable prognosis (20). Modern medical treatments, such as surgical resection, radiotherapy, biotherapy and chemotherapy have been adopted to treat HCC; however, the overall survival rate of patients with HCC remains low due to the high recurrence rates and distant metastasis (21,22). Previous studies have identified several molecular mechanisms of different long non-coding RNAs (lncRNAs), including SNHG16, MIAT and HANR, in HCC (23–25). However, the role of ST8SIA6-AS1 in HCC remains to be determined.
lncRNAs have been discovered to modulate several cellular processes, including cell proliferation, apoptosis, differentiation, migration and invasion in numerous types of cancer. For instance, the lncRNA C5orf66-AS1 was found to accelerate the proliferation and epithelial-mesenchymal transition (EMT) of cervical cancer cells by regulating the miR-637/RING1 axis (26), while the lncRNA ZEB2-AS1 was found to promote cell proliferation, but prevent cell apoptosis, by targeting the miR-143-5p/HIF-1α axis in gastric cancer (27). Similarly, in the present study, ST8SIA6-AS1 expression levels were revealed to be upregulated in HCC tissues and cell lines, and the upregulated expression levels of ST8SIA6-AS1 were closely associated with a poor prognosis. Additionally, hypoxia has been demonstrated to be strongly related with malignant progression, tumor metastasis and drug resistance (28). However, although hypoxia has been identified to be closely associated with cellular processes in HCC (21), the mechanism by which this occurs remains unknown. In the present study, the migratory and invasive abilities of HCC cells were promoted following hypoxia induction, while these effects were hindered following the genetic knockdown of ST8SIA6-AS1.
Mechanistically, it has been widely established that lncRNAs form a competitive endogenous RNA (ceRNA) network to regulate the downstream target genes of miRNAs (29). For example, SPRY4-IT1 was proposed to function as a ceRNA to sponge miR-101-3p and regulate EZH2 expression levels in bladder cancer (30). In addition, the lncRNA H19 was found to modulate gastric cancer cell growth and metastasis through the miR-22-3p/Snail1 axis (31). In the present study, the majority of ST8SIA6-AS1 was observed to be distributed in the cytoplasm of HCC cells, which may resemble the ceRNA pattern of lncRNAs. miR-338, of which its expression levels were found to be downregulated in HCC cells and tissues, was predicted and revealed to bind with ST8SIA6-AS1, and the inhibition of miR-338 partially counteracted the suppressive influence of ST8SIA6-AS1 silencing on cell migration and invasion.
Methylphosphate capping enzyme (MEPCE) is an RNA methyltransferase that methylates and stabilizes 7SK in the nucleosol (32). Several previous studies have reported that MEPCE is often overexpressed in human breast cancer cells, and the upregulation of MEPCE is associated with the tumorigenic phenotype and a poor prognosis in breast cancer (33,34). However, to the best of our knowledge, the biological functions of MEPCE remain unknown in HCC. In the present study, MEPCE was revealed to be directly targeted by miR-338. Additionally, MEPCE expression levels were upregulated and negatively correlated with miR-338 expression levels in HCC. Finally, rescue assays revealed that the overexpression of MEPCE reversed the inhibitory effects of ST8SIA6-AS1 inhibition on cellular processes.
In conclusion, the results of the present study suggest that ST8SIA6-AS1 may promote the migration and invasion of hypoxia-induced HCC cells via the miR-338/MEPCE axis (Fig. 6D). Thus, these findings suggest that ST8SIA6-AS1 may represent a potential therapeutic target for patients with HCC.
Acknowledgements
Not applicable.
Funding
This work was supported by the foundations of Jiangsu Provincial Commission of Health and Family Planning (H2018037, BJ18010); the Basic Research Program of Jiangsu Province (BK20191153); and the Foundation of Xuzhou Institute of Technology (KC19035).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
BZ and RW designed the present study. ZL, KC and JL performed all of the experiments. WS and QW analyzed the data and prepared the figures. BZ and RW drafted the initial manuscript. BZ reviewed and revised the manuscript. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University and written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Banaudha KK, Verma M. Epigenetic biomarkers in liver cancer. Methods Mol Biol. 2015;1238:65–76. doi: 10.1007/978-1-4939-1804-1_4. [DOI] [PubMed] [Google Scholar]
- 2.Raza A, Sood GK. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Jeong H, Kim EY, Kim JF, Lee SY, Yoon SH. Genomic and transcriptomic landscape of Escherichia coli BL21(DE3) Nucleic Acids Res. 2017;45:5285–5293. doi: 10.1093/nar/gkx228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Bai J, Zhang X, Lv Y, Gong Y, Liu L, Zhao H, Yu F, Ping Y, Zhang G, et al. A comprehensive overview of lncRNA annotation resources. Brief Bioinform. 2017;18:236–249. doi: 10.1093/bib/bbw015. [DOI] [PubMed] [Google Scholar]
- 7.Tian JB, Cao L, Dong GL. Long noncoding RNA DDX11-AS1 induced by YY1 accelerates colorectal cancer progression through targeting miR-873/CLDN7 axis. Eur Rev Med Pharmacol Sci. 2019;23:5714–5729. doi: 10.26355/eurrev_201907_18309. [DOI] [PubMed] [Google Scholar]
- 8.Xie Q, Lin S, Zheng M, Cai Q, Tu Y. Long noncoding RNA NEAT1 promotes the growth of cervical cancer cells via sponging miR-9-5p. Biochem Cell Biol. 2019;97:100–108. doi: 10.1139/bcb-2018-0111. [DOI] [PubMed] [Google Scholar]
- 9.Geisler S, Coller J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez-Dominguez JR, Lodish HF. Emerging mechanisms of long noncoding RNA function during normal and malignant hematopoiesis. Blood. 2017;130:1965–1975. doi: 10.1182/blood-2017-06-788695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Fu H, Wu Y, Zheng X. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci. 2013;56:876–885. doi: 10.1007/s11427-013-4553-6. [DOI] [PubMed] [Google Scholar]
- 12.Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: Possible functions and clinical implications. J Med Genet. 2015;52:710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 13.Chiu HS, Rodríguez Martínez M, Bansal M, Subramanian A, Golub TR, Yang X, Sumazin P, Califano A. High-throughput validation of ceRNA regulatory networks. BMC Genomics. 2017;18:418. doi: 10.1186/s12864-017-3790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Li J, Li F, Li H, Bei S, Zhang X, Feng L. lncRNA LOXL1-AS1 facilitates the tumorigenesis and stemness of gastric carcinoma via regulation of miR-708-5p/USF1 pathway. Cell Prolif. 2019;52:e12687. doi: 10.1111/cpr.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao N, Fu Y, Chen L, Liu Z, He J, Zhu Y, Xia T, Wang S. Long non-coding RNA NONHSAT101069 promotes epirubicin resistance, migration, and invasion of breast cancer cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene. 2019;38:7216–7233. doi: 10.1038/s41388-019-0904-5. [DOI] [PubMed] [Google Scholar]
- 16.Wojczakowski W, Kobylarek D, Lindner J, Limphaibool N, Kaczmarek M. MicroRNAs-novel biomarkers for malignant pleural effusions. Contemp Oncol (Pozn) 2019;23:133–140. doi: 10.5114/wo.2019.89241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chhatriya B, Mukherjee M, Ray S, Sarkar P, Chatterjee S, Nath D, Das K, Goswami S. Comparison of tumour and serum specific microRNA changes dissecting their role in pancreatic ductal adenocarcinoma: A meta-analysis. BMC Cancer. 2019;19:1175. doi: 10.1186/s12885-019-6380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Cui M. miRNA-98-5p inhibits the progression of osteosarcoma by regulating cell cycle via targeting CDC25A expression. Eur Rev Med Pharmacol Sci. 2019;23:9793–9802. doi: 10.26355/eurrev_201911_19542. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Tian S, Wang L, Wang Y, Zhao J. MicroRNA-367-3p overexpression represses the proliferation and invasion of cervical cancer cells through downregulation of SPAG5-mediated Wnt/β-catenin signaling. Clin Exp Pharmacol Physiol. 2019;47:687–695. doi: 10.1111/1440-1681.13222. [DOI] [PubMed] [Google Scholar]
- 20.Chen DH, Wu QW, Li XD, Wang SJ, Zhang ZM. SYPL1 overexpression predicts poor prognosis of hepatocellular carcinoma and associates with epithelial-mesenchymal transition. Oncol Rep. 2017;38:1533–1542. doi: 10.3892/or.2017.5843. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZB, Chen F, Bai XF. Long noncoding RNA MALAT1 regulates hepatocellular carcinoma growth under hypoxia via sponging microRNA-200a. Yonsei Med J. 2019;60:727–734. doi: 10.3349/ymj.2019.60.8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang R, Lin Y, Ye JZ, Yan XX, Liu ZH, Li YQ, Luo XL, Ye HH. High expression of RBM8A predicts poor patient prognosis and promotes tumor progression in hepatocellular carcinoma. Oncol Rep. 2017;37:2167–2176. doi: 10.3892/or.2017.5457. [DOI] [PubMed] [Google Scholar]
- 23.Xiao J, Lv Y, Jin F, Liu Y, Ma Y, Xiong Y, Liu L, Zhang S, Sun Y, Tipoe GL, et al. lncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell Physiol Biochem. 2017;43:1926–1938. doi: 10.1159/000484116. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Gao Y, Qin J, Lu S. lncRNA MIAT promotes proliferation and invasion of HCC cells via sponging miR-214. Am J Physiol Gastrointest Liver Physiol. 2018;314:G559–G565. doi: 10.1152/ajpgi.00242.2017. [DOI] [PubMed] [Google Scholar]
- 25.Xu F, Zha G, Wu Y, Cai W, Ao J. Overexpressing lncRNA SNHG16 inhibited HCC proliferation and chemoresistance by functionally sponging hsa-miR-93. Onco Targets Ther. 2018;11:8855–8863. doi: 10.2147/OTT.S182005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rui X, Xu Y, Jiang X, Ye W, Huang Y, Jiang J. Long non-coding RNA C5orf66-AS1 promotes cell proliferation in cervical cancer by targeting miR-637/RING1 axis. Cell Death Dis. 2018;9:1175. doi: 10.1038/s41419-018-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu F, Gao H, Liu K, Gao B, Ren H, Li Z, Liu F. The lncRNA ZEB2-AS1 is upregulated in gastric cancer and affects cell proliferation and invasion via miR-143-5p/HIF-1α axis. Onco Targets Ther. 2019;12:657–667. doi: 10.2147/OTT.S175521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Hamblin MH, Yin KJ. The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biol. 2017;14:1705–1714. doi: 10.1080/15476286.2017.1358347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X, Wang M, Huang C, Wang L, Zeng F, Jiang G. lncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017;388:281–291. doi: 10.1016/j.canlet.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Gan L, Lv L, Liao S. Long noncoding RNA H19 regulates cell growth and metastasis via the miR223p/Snail1 axis in gastric cancer. Int J Oncol. 2019;54:2157–2168. doi: 10.3892/ijo.2019.4773. [DOI] [PubMed] [Google Scholar]
- 32.Shelton SB, Shah NM, Abell NS, Devanathan SK, Mercado M, Xhemalçe B. Crosstalk between the RNA methylation and histone-binding activities of MePCE regulates P-TEFb activation on chromatin. Cell Rep. 2018;22:1374–1383. doi: 10.1016/j.celrep.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Chi Y, Hu X, Li S, Qiao F, Wu J, Shao ZM. Elevated expression of RNA methyltransferase BCDIN3D predicts poor prognosis in breast cancer. Oncotarget. 2016;7:53895–53902. doi: 10.18632/oncotarget.9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.