Assessment of a patient’s kidney function is central to proper patient care in many settings (1). One of the most commonly measured analytes in routine blood testing is serum (or plasma) creatinine, which is available in almost all clinical laboratories. The analytical accuracy of serum creatinine measurement has been a great success story of clinical laboratory test standardization (2, 3). Glomerular filtration rate (GFR) is considered the best overall diagnostic for assessing kidney function. However, directly measuring GFR (mGFR) with exogenous glomerular filtration agents (e.g., inulin, iohexol, iothalamate) is complex and beyond the capabilities of most hospitals and clinics. Therefore, equations were developed using creatinine and other blood biomarkers that have differing non-GFR determinants producing a mathematical estimated GFR (eGFR). At present, the most widely accepted equation for this purpose was developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) that uses serum creatinine concentration for GFR estimation (i.e., eGFRcreatinine). Two additional CKD-EPI equations are also available: one incorporating both serum creatinine and cystatin C (eGFRcreatinine+cystatin) and one with only cystatin C (eGFRcystatin) (4). Briefly, the CKD-EPI group used rather complex least-squares linear regression statistical methodology to develop the equations using 5353 patients to relate mGFR to serum creatinine and cystatin C concentrations. They found when including several demographic factors the accuracy of eGFR improved. They subsequently validated these 3 equations in 1117 additional patients. For 2 of the CKD-EPI eGFR equations, beyond age and sex, self-identified race was found to be an important variable to include for accurate eGFR results. These CKD-EPI equations are presently recommended nationally [e.g., the United States National Institute of Health’s National Institute of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK), National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI)] and internationally by numerous kidney organizations [e.g., Kidney Disease: Improving Global Outcomes (KDIGO)] (5, 6). Nevertheless, one must keep in mind that these eGFR equations generate only estimates of GFR, with at best 80 to 90% of the computed eGFR values within ±30% of a patient’s mGFR.
A major issue with the CKD-EPI creatinine-based eGFR equations under recent increased scrutiny is inclusion of the race-related factor (i.e., non-Black vs Black) (7). Unlike more well-defined demographic factors of age and (in most cases) sex, race is primarily a social or psychological construct that can be unknown by many individuals. Individuals in the United States who self-identify as Black or African-American have genome-wide markers consistent with 73.2% African ancestry on average; however, there is regional variation in African ancestry ranging from <70% to >90% (8). Furthermore, creatinine-based CKD-EPI eGFR equations are simply not available for large segments of North American populations (e.g., Asian, Hispanics, Native Americans, and mixed-race patients). In addition, the CKD-EPI team used only Blacks from North America in development of the equations rather than Blacks from outside of North America. Although skeletal muscle mass and normal endogenous conversion of muscle creatine into creatinine is perceived to be the main factor influencing circulating serum creatinine concentrations, other non-GFR determinants may be important, such renal tubular or extra-renal handling of creatinine and perhaps even diet (9).
We anticipate many questions will be posed to clinical laboratories and their directors surrounding the use of the race-related factors in eGFR equations. While most laboratories have little direct information on a patient’s racial background, we believe how the clinical laboratories respond to clinician and patient inquiries regarding the inclusion of the race-related factor in eGFR calculations could have a substantial impact on whether to use or to abandon the race-related factor as Eneanya and colleagues have suggested (7). Thus, we believe laboratory directors need to understand the background for incorporating and implications of dropping the race-related factor in eGFR equations. Laboratory directors must also be cognizant that racism has existed in medicine with documented reports dating back to the 1800s and continues into the present day (10).
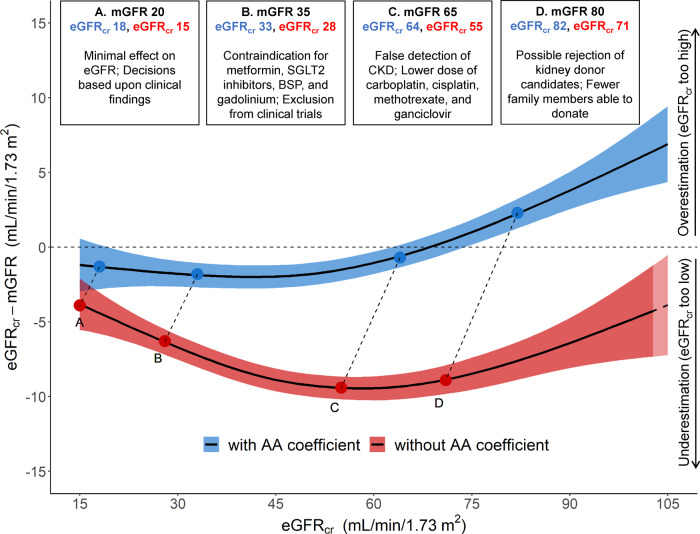
While we agree with Eneanya and colleagues that the use of a race-related factor in eGFR computations is problematic in many cases, we believe all potential implications of eliminating a race-related factor from eGFR computation need to be carefully assessed before simply abandoning its use. The Black race-related factor increases eGFR for any given serum creatinine by 15.9% compared to a non-Black with the same age, sex, and serum creatinine. It is because of this increase in eGFR, Eneanya and colleagues suggest that use of the race-related factor is inappropriate since it may place Blacks at a disadvantage for early treatment of advanced chronic kidney disease and for kidney transplantation. However, using a non-Black equation for Black patients means use of an equation that was not derived from Black patients’ mGFR data, presenting a potential ethical dilemma for providers. A recent report from Levey and colleagues illustrates differences in the CKD-EPI eGFRcreatinine equation with or without use of the race-related factor and its performance with respect to mGFR (1). That report clearly shows the eGFRcreatinine is more accurate and better correlated with mGFR when the race-related factor remains in the equation. Eliminating use of the race-related factor clearly would introduce a systematic bias in a Black patient’s eGFR relative to their mGFR, such that Black patients would have a lower eGFR relative to their mGFR. Many laboratories, including ours, report an eGFRcreatinine, calculated for both Black and non-Black patients with each serum creatinine result as recommended by the NIDDK; this allows the clinician and patient to discuss benefits and drawbacks of using each estimating equation (5). Levey and colleagues point out several potential adverse clinical consequences of ignoring the race-related factor (see Fig. 1) (1). Examples of specific negative consequences of using the non-Black eGFR equation for Blacks include rejection of potentially eligible kidney donors, false indications of lower than actual GFR resulting in lower and less effective doses of chemotherapeutics, avoidance of potentially effective diabetic medication and exclusion from clinical trials. Thus, we believe that great care needs to be taken with input from all stakeholders, before deciding simply to eliminate the race-related factor in the eGFRcreatinine equation as several medical centers appear to be doing. We believe this is not simply a racial equality issue, but an issue regarding what is the most scientifically accurate estimate of a GFR to benefit the patient. Clearly, ambiguities of whether or not to include the race-related factor in the eGFR computation when racial background is unknown are very problematic. Levey and colleagues also recommend alternative confirmatory methods such as cystatin C-based eGFR and mGFR in ambiguous cases (1).
Fig. 1.
Clinical decisions can be affected by accuracy of GFR assessment among Black people. Data from 2601 Black participants from the Chronic Kidney Disease Epidemiology Collaboration development and internal validation sample were used in this plot (4). We computed eGFR from serum creatinine (eGFRcr) values with (blue) and without (red) the application of the Black (AA) coefficient and removed values below and above the 2.5 and 97.5 percentiles of these distributions, leaving 2463 participants for the analysis. The model plotted represents generalized additive models for eGFRcr (mL per minute per 1.73 m2) on the difference between eGFRcr and measured GFR (mGFR) (mL per minute per 1.73 m2). We truncated the horizontal axis to eGFRcr values from 15 to 105 mL/min per 1.73 m2, excluding 190 participants from the plot. The colored areas along the line represent 95% confidence intervals of the estimate. Upper boxes represent hypothetical situations of eGFRcr values for the same mGFR, with numbers in blue representing the eGFRcr with the Black coefficient and the numbers in red representing the eGFRcr without the Black coefficient. AA, Black; BSP, bisphosphonate; SGLT2, sodium-glucose transport protein 2. Reproduced with permission from Levey et al. (1).
We believe this issue warrants very careful and thoughtful scrutiny for all the potential consequences before eliminating a race-related term in computing eGFR. For this very reason, the National Kidney Foundation and the American Society of Nephrology, which are the largest group of kidney disease health care providers and patients, have formed a joint task force to reassess the positive vs negative impacts of race-related factors in all eGFR equations for diagnosing kidney diseases (11). The task force hopes to issue initial recommendations in the fall of 2020. While the best solution is presently unclear for this challenging issue, we believe that sound scientifically based recommendations will be forthcoming.
With all these issues surrounding creatinine-based eGFR equations and race, it is important to recognize that alternative approaches already exist in assessing GFR. The cystatin C-based CKD-EPI equation (eGFRcystatin) has no racial factor (4). Besides being far more costly to measure, the accurate measurement of cystatin C concentration has been problematic (12). However, more recent College of American Pathologists Survey data suggest laboratories’ analytical accuracy is improving (13). Another option is to measure GFR directly with an exogenous filtration agent (14, 15). While directly measuring GFR requires careful attention to detail and is likely not available to providers at many institutions (16), should an accurate assessment of kidney function be required for a very important clinical decision, obtaining a mGFR may be the best course of action. Creatinine clearance has been proposed as an alternative to creatinine-based eGFR. However, with modern serum creatinine measurement procedures, creatinine clearance systematically overestimates mGFR by 10 to 20% in the normal through moderately impaired renal dysfunction ranges, and becomes >50% higher than mGFR as a patient approaches end stage renal disease (17). Unfortunately, we believe that the magnitude of bias in creatinine clearance is not appreciated by many practicing clinicians.
In summary, use of serum creatinine-based eGFR equations has numerous advantages. Serum creatinine measurement is one of the best standardized, most widely available, and lowest cost laboratory tests. On the other hand, we completely agree that incorporation of a race-related factor in creatinine-based eGFR computations can be very problematic in many cases. We think that creatinine-based eGFR results should be reported with calculations for both Black and non-Black patients. This additional information will enable clinicians and patients to have an open discussion surrounding implications of race on eGFR. It should be noted that the KDIGO task force recommends the CKD-EPI eGFRcystatin equation when eGFRcreatinine is ambiguous or borderline (6). Although relatively few clinical laboratories, at least in the US, offer cystatin C measurements, essentially all larger clinical reference laboratories now offer this test. Recommendations should be forthcoming quite soon from the task force that has been formed by the National Kidney Foundation and the American Society of Nephrology regarding the use of race in the diagnosis of chronic kidney disease. This task force’s recommendation should provide further guidance for laboratory directors, clinicians, and other healthcare professionals around the use of race-related factors in eGFR computations. In the meantime, we believe it is not appropriate simply to eliminate the race-related factor in creatinine-based eGFR equations, as there may be unintended negative consequences for proper patient care.
Nonstandard Abbreviations
- GFR
glomerular filtration rate
- mGFR
measured glomerular filtration rate
- eGFR
estimated glomerular filtration rate
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFRcreatinine
glomerular filtration rate estimating equation using only serum creatinine
- eGFRcreatinine+cystatin
glomerular filtration rate estimating equation using both serum creatinine and serum cystatin C
- eGFRcystatin
glomerular filtration rate estimating equation using only cystatin C
- NIH-NIDDK
United States National Institute of Health’s National Institute of Diabetes and Digestive and Kidney Diseases
- NKF-KDOQI
National Kidney Foundation-Kidney Disease Outcomes Quality Initiative
- AA
Black
- SGLT2
sodium-glucose transport protein 2
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
J.H. Eckfeldt, Gentian, a manufacturer of cystatin C reagents in Moss, Norway.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
J.C. Seegmiller’s and J.H. Eckfeldt’s laboratory has received NIH funding for work from the CKD-EPI collaboration, and J.H. Eckfeldt was Principal Investigator of the CKD-EPI Central Laboratory from approximately 2008 to 2018.
Expert Testimony
None declared.
Patents
None declared.
References
- 1. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA.. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol 2020;15:1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al. Recommendations for improving serum creatinine measurement: a report from The Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006;52:5–18. [DOI] [PubMed] [Google Scholar]
- 3. Killeen AA, Ashwood ER, Ventura CB, Styer P.. Recent trends in performance and current state of creatinine assays. Arch Pathol Lab Med 2013;137:496–502. [DOI] [PubMed] [Google Scholar]
- 4. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NIDDK. Reporting glomerular filtration rate. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate/reporting#:∼:text=In%20general%2C%20NIDDK%20recommends%20reporting,%2F1.73%20m2%22 (Accessed August 2020).
- 6. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney DIs 2014;63:713–35. [DOI] [PubMed] [Google Scholar]
- 7. Eneanya ND, Yang W, Reese PP.. Reconsidering the consequences of using race to estimate kidney function. Jama 2019;322:113–4. [DOI] [PubMed] [Google Scholar]
- 8. Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL.. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet 2015;96:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powe NR. Black kidney function matters: use or misuse of race? Jama 2020;324:737. [DOI] [PubMed] [Google Scholar]
- 10. Grubbs V. Precision in GFR reporting: let’s stop playing the race card. Clin J Am Soc Nephrol 2020;15:1201–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. Establishing a task force to reassess the inclusion of race in diagnosing kidney diseases. https://www.kidney.org/news/establishing-task-force-to-reassess-inclusion-race-diagnosing-kidney-diseases (Accessed August 2020).
- 12. Eckfeldt JH, Karger AB, Miller WG, Rynders GP, Inker LA.. Performance in measurement of serum cystatin C by laboratories participating in the College of American Pathologists 2014 CYS survey. Arch Pathol Lab Med 2015;139:888–93. [DOI] [PubMed] [Google Scholar]
- 13. Eckfeldt JH, Karger AB. CAP CYS A-2019 Participant Summary Report Discussion. https://documents.cap.org/documents/CYS-A-2019-discussion.pdf (Accessed August 2020).
- 14. Seegmiller JC, Burns BE, Fauq AH, Mukhtar N, Lieske JC, Larson TS.. Iothalamate quantification by tandem mass spectrometry to measure glomerular filtration rate. Clin Chem 2010;56:568–74. [DOI] [PubMed] [Google Scholar]
- 15. Schmit DJ, Carroll LJ, Eckfeldt JH, Seegmiller JC.. Verification of separate measurement procedures where analytical determinations influence the clinical interpretation of GFR: iohexol quantitation by HPLC and LC-MS/MS. Clin Biochem 2019;67:16–23. [DOI] [PubMed] [Google Scholar]
- 16. Seegmiller JC, Eckfeldt JH, Lieske JC.. Challenges in measuring glomerular filtration rate: a clinical laboratory perspective. Adv Chronic Kidney Dis 2018;25:84–92. [DOI] [PubMed] [Google Scholar]
- 17. Inker LA, Perrone RD.. Calculation of creatinine clearance In: Post TW, editor. UpToDate Waltham, (MA: ); Wolters Kluwer; 2019. [Google Scholar]