Abstract
Objective:
In the primary analysis of the phase 2b VESTA study, oral fezolinetant reduced frequency and severity of menopausal vasomotor symptoms (VMS) compared with placebo. This secondary analysis evaluates effects of fezolinetant on responder rates and patient-reported outcomes (PROs).
Methods:
In this 12-week, double-blind study, postmenopausal women with moderate/severe VMS were randomized to fezolinetant 15, 30, 60, or 90 mg BID or 30, 60, or 120 mg QD or placebo. Proportion of responders was based on reductions in VMS from daily diary records. P values for comparisons between active treatment and placebo were calculated using logistic regression. Changes from baseline in PROs (Menopause-Specific Quality of Life questionnaire, Hot Flash-Related Daily Interference Scale, Greene Climacteric Scale) were conducted using a mixed model for repeated measurements and compared post hoc with published minimally important differences (MIDs).
Results:
Of 356 women randomized, 352 were treated and analyzed. A greater proportion of women receiving fezolinetant versus placebo met definitions of response at week 12. For all doses, mean changes from baseline in Menopause-Specific Quality of Life questionnaire VMS scores exceeded the MID (1.2) at weeks 4 (placebo: −1.8; fezolinetant: range, −1.9 to −3.6) and 12 (placebo: −2.3; fezolinetant: range, −2.9 to −4.4). Mean changes in Hot Flash-Related Daily Interference Scale at weeks 4 (placebo: −2.2; fezolinetant: range, −2.5 to −3.8) and 12 (placebo: −2.9; fezolinetant: range, −3.3 to −4.3) exceeded the MID (1.76). Greene Climacteric Scale-VMS domain scores improved for most fezolinetant doses versus placebo (week 4, placebo: −1.7; fezolinetant: range, −2.1 to −3.3; week 12, placebo: −2.1; fezolinetant: range, −2.7 to −3.6).
Conclusions:
Oral fezolinetant was associated with higher responder rates than placebo and larger improvements in QoL and other PRO measures, including a reduction in VMS-related interference with daily life.
Keywords: Hot flash, Hot flush, Health-related quality of life, Menopausal vasomotor symptoms, Night sweats, Nonhormone therapy
Vasomotor symptoms (VMS), characterized by hot flashes (also known as hot flushes) and/or night sweats, are experienced by about 80% of American women during the menopausal transition1,2 and are the most common menopause-associated symptoms for which women seek treatment.3 With a median duration of 7.4 years,4 these symptoms can have prolonged and deleterious effects on multiple aspects of women's lives. The occurrence of VMS has been shown to interfere with sleep, concentration, memory, work productivity, and personal relationships and has been linked to feelings of depression, irritability, anxiety, fatigue, and social embarrassment/isolation.5-9 All of these factors contribute to the observed negative influence of VMS on psychological well-being and health-related quality of life.8,10 Well-tolerated, safe, and effective nonhormone treatment options are limited.
The pathophysiology of VMS involves neurons that coexpress kisspeptin, neurokinin B (NKB), and dynorphin (KNDy neurons), which innervate the thermoregulatory center in the hypothalamic region of the brain. These KNDy neurons are inhibited by estrogen and stimulated by NKB.11-13 During menopause, estrogen levels decline, leading to increased NKB signaling.14 No longer counterbalanced by estrogen, this increased NKB signaling overstimulates KNDy neurons, thereby increasing activity in the temperature control center, resulting in VMS.11,15 Fezolinetant is an oral, nonhormone therapy in clinical development for the treatment of moderate/severe menopausal VMS. Fezolinetant is a neurokinin 3 receptor antagonist that blocks NKB signaling,16,17 thereby normalizing the activity of KNDy neurons in the thermoregulatory center of the brain and reducing VMS.
Fezolinetant 90 mg BID significantly reduced moderate/severe VMS frequency and VMS score that encompassed both frequency and severity in postmenopausal women in a phase 2a clinical proof-of-concept trial.18 Also, in a phase 2b, dose-ranging clinical trial (VESTA), fezolinetant significantly reduced moderate/severe VMS frequency and severity compared with placebo in postmenopausal women.19 The majority of women who received fezolinetant (81%-95%) experienced a 50% or greater reduction from baseline in moderate/severe VMS frequency. VMS symptoms improved as early as the first week on treatment and were maintained throughout the 12-week duration. This manuscript reflects the results of secondary endpoints from VESTA, including various prespecified definitions of treatment response and the corresponding patient-reported outcomes (PROs) used to evaluate the impact of fezolinetant on VMS-related interference with activities of daily living and health-related quality of life.
METHODS
Study design and participants
Methodology, including detailed inclusion/exclusion criteria of this phase 2b, randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study (NCT03192176), has been published, along with primary efficacy and safety outcomes.19 Briefly, healthy postmenopausal women >40-65 years of age with ≥50 moderate/severe VMS episodes per week during a 35-day screening period were randomized to treatment with one of seven fezolinetant dosing regimens (15, 30, 60, or 90 mg BID or 30, 60, or 120 mg QD) or placebo for 12 weeks. Participants recorded their VMS episodes daily in an e-diary and completed PRO measures consisting of the Menopause-Specific Quality of Life (MENQoL) questionnaire, the Hot Flash-Related Daily Interference Scale (HFRDIS), and the Greene Climacteric Scale (GCS) at baseline and weeks 4, 8, and 12.
The study protocol was approved by an institutional review board at each study site and was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines. All study participants provided written informed consent.
Responder analysis
Responders were the proportion of participants at each study week who either (1) experienced at least a 50%, 70%, 90%, or 100% reduction form baseline in the frequency of moderate or severe VMS; (2) experienced at least a 50%, 70%, 90%, or 100% reduction from baseline in the frequency of mild, moderate, or severe VMS; (3) had absolute reductions from baseline of 2, 3, 4, and 5 in the mean number of moderate or severe VMS per 24 hours; or (4) had absolute reductions from baseline of 2, 3, 4, and 5 in the mean number of mild, moderate, or severe VMS per 24 hours.
Patient-reported outcome measures
Menopause-specific quality of life questionnaire
The MENQoL questionnaire is self-administered and consists of 29 items within four domains of menopausal symptoms (vasomotor, psychosocial, physical, and sexual domains). Items are rated as present or not present in the previous month; when present, they are further graded on the degree to which they are bothersome on a Likert scale of 0 (not bothersome) to 6 (extremely bothersome).20,21 Absence of an item is scored a “1” and presence as a “2” to which the Likert scale score is added, yielding a possible score on any item of 1 to 8.22 Means were computed for each domain. The overall score was the mean of the domain mean scores. If any of the contributing items had missing values, the mean score for the associated domain was not calculated. If any of the domain mean scores were missing, the overall mean score was not calculated.
The threshold for a clinically important difference (CID) in MENQoL questionnaire domain and total scores was previously identified in an anchor-based analysis performed by Bushmakin et al,23 in which MENQoL scores were assessed relative to items in the menopause symptoms treatment satisfaction questionnaire used as anchors in a population of 332 postmenopausal women who reported ≥7 moderate/severe hot flashes per day at baseline in a VMS treatment clinical trial. Estimated CIDs were 1.2 for vasomotor function, 0.7 for psychosocial function, 0.5 for physical function, 0.8 for sexual function, and 0.7 for total score.
Hot flash-related daily interference scale
Perceived VMS interference with daily activities was evaluated using the HFRDIS. The HFRDIS is a 10-item scale that measures a woman's perceptions of the degree to which VMS interfere with nine daily life activities (work, social activities, leisure, sleep, mood, concentration, relations with others, sexuality, and enjoying life); the 10th item measures interference with overall quality of life.24 Participants were asked to rate the extent to which VMS interfered with each item during the previous 2-week time interval on a scale of 0 (do not interfere) to 10 (completely interfere). If any of the 10 items had missing values, the overall mean score was not calculated.
The threshold for a minimally important difference (MID) in HFRDIS was based on the analysis of Carpenter et al,25 who defined an MID as the change in HFRDIS that correlated with (1) a reduction from baseline of 40% to 60% in VMS frequency during clinical trials of VMS therapies and (2) a decrease of 0.5 to 1.5 in MENQoL total score. The mean MID in HFRDIS was 1.66. The MID for 40%-60% reduction in total VMS frequency was 1.76.
Greene climacteric scale
Changes in climacteric symptoms were evaluated using the GCS. The GCS is a 21-item scale that provides a measure of climacteric symptomatology.26 Each item is rated by the participant according to its severity using a 4-point rating scale from 0 (none) to 3 (severe). The first 20 individual items are grouped into domains: psychological symptoms (possible score: 0-33), physical symptoms (possible score: 0-21), and VMS (possible score: 0-6). A final item (item 21) is intended as a probe for issues related to sexual function. The total score can range from 0 to 63; higher scores indicate more severe symptoms. If any of the contributing items have missing values, scores for the affected domain were not calculated. If any of the domain scores were missing, the total score was not calculated. There are no published data establishing a CID or an MID for change in GCS.
Leeds sleep evaluation questionnaire
The study protocol also included assessment of sleep quality using the Leeds sleep evaluation questionnaire, a 10-item self-rated questionnaire. However, a number of operational issues arose with the conduct of this assessment; hence, the results were considered invalid and are not reported.
Statistical analyses
Efficacy analyses were performed using the full analysis set, which included all participants who received at least one dose of study drug and who had a baseline and at least one postbaseline efficacy evaluation.
Responder analyses are reported for the last on-treatment week, which was defined as the last 7 days of treatment. A missing value for last on-treatment week was imputed as a nonresponder. P values for comparisons between active treatment and placebo were calculated using a logistic regression model, with responder as the dependent variable, treatment group and smoking status (current vs former/never) as factors, and baseline measurement (mean frequency of VMS) as a covariate. No adjustments were performed for multiple comparisons. Smoking status was included as a factor because smoking has been associated with an increased prevalence of VMS27 and because it was included as a stratification factor at baseline.
For each of the PRO endpoints, change from baseline analyses were conducted using a mixed model for repeated measurements. This analysis used a restricted maximum likelihood-based repeated-measures approach. The analyses included the treatment group, week and smoking status as factors and baseline measurement as a covariate, as well as an interaction of treatment by week and an interaction of baseline measurement by week. An unstructured covariance structure shared across treatment groups was used to model within-participant errors. The Kenward-Roger approximation was used to estimate denominator degrees of freedom and adjust standard errors.
Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC).
RESULTS
Of the 356 postmenopausal women randomized to study treatment, 352 received at least one dose of study drug and 287 (81%) completed the 12-week study. Baseline characteristics and demographics were generally balanced across treatment groups. Participants had a mean age of 54.6 (range: 41-65) years. The majority of women (73.0%) self-identified as white; 24.7% black, 0.9% Asian, and 0.9% other. In addition, 28.7% of all participants identified as Hispanic/Latino ethnicity. Approximately one third of the women were current or former smokers. At baseline, participants experienced an average of 9.3-11.2 moderate or severe VMS episodes per 24-hour period. Baseline scores for MENQoL, HFRDIS, GCS, and Leeds sleep evaluation questionnaire were similar between treatment groups (Table 1).
TABLE 1.
Patient-reported outcome scale scores at baseline
| Fezolinetant | ||||||||
| Parameters | Placebo (n = 43) | 15 mg BID (n = 45) | 30 mg BID (n = 43) | 60 mg BID (n = 45) | 90 mg BID (n = 44) | 30 mg QD (n = 43) | 60 mg QD (n = 45) | 120 mg QD (n = 44) |
| MENQoL, n | 43 | 45 | 43 | 45 | 42 | 42 | 44 | 44 |
| Overall score | 4.3 (1.6) | 4.1 (1.3) | 4.0 (1.3) | 4.1 (1.5) | 4.1 (1.3) | 4.4 (1.5) | 4.2 (1.5) | 4.1 (1.3) |
| HFRDIS, n | 43 | 45 | 43 | 45 | 42 | 42 | 44 | 44 |
| Overall mean score | 6.0 (2.3) | 5.4 (2.0) | 5.4 (2.1) | 5.6 (2.3) | 5.1 (2.3) | 6.0 (2.1) | 5.3 (2.3) | 5.7 (2.3) |
| GCS, n | 43 | 45 | 43 | 44 | 42 | 43 | 44 | 44 |
| Overall sum | 21.7 (10.3) | 19.6 (8.2) | 17.6 (10.1) | 19.8 (12.0) | 17.8 (10.3) | 20.5 (9.1) | 19.3 (9.3) | 19.4 (10.4) |
Data are means (standard deviations).
GCS, Greene climacteric scale; HFRDIS, hot flash related daily interference scale; MENQoL, menopause-specific quality of life.
Responder analyses
The proportion of participants who experienced at least a 50%, 70%, or 90% reduction in moderate or severe VMS frequency was higher with fezolinetant versus placebo, with the magnitude of the difference and level of significance varying across doses and responder definitions (Fig. 1). The mean number of days to achieve a 50% reduction in moderate or severe VMS frequency ranged from 8.4 days for fezolinetant 15 mg BID to 2.2 days for fezolinetant 90 mg BID (compared with 15.1 d in the placebo group). A similar pattern of results was observed for reductions in the frequency of mild, moderate, or severe VMS and responder rates based on absolute reductions in VMS frequency (data not shown).
FIG. 1.
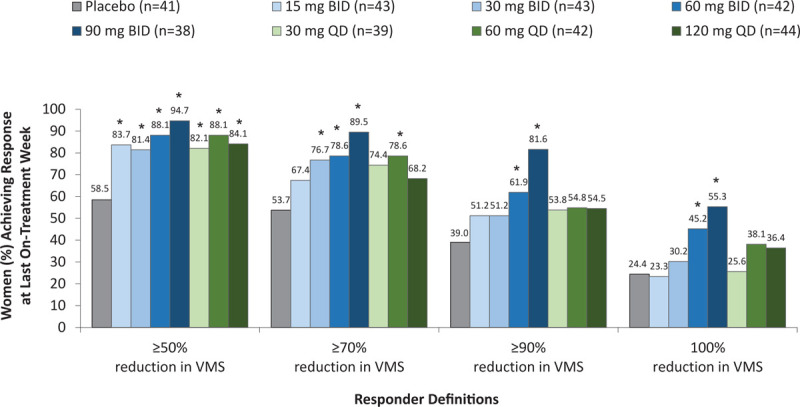
Responder analyses for reduction in moderate or severe VMS frequency at last on-treatment week. The last on-treatment week was defined as the last 7 days of treatment. ∗P < 0.05 for paired comparisons of fezolinetant versus placebo at last on-treatment week, with no adjustments for multiplicity. VMS, vasomotor symptoms.
MENQoL questionnaire
Improvements in overall mean MENQoL score, as indicated by decreases from baseline, were observed in all treatment groups at weeks 4 and 12. The reduction in overall mean score was numerically greater with fezolinetant versus placebo for the majority of dose groups and time points (Supplemental Table 1).
Among MENQoL domains, changes from baseline in the vasomotor function domain score demonstrated the greatest numerical differences from baseline compared with the other domains with fezolinetant versus placebo (Supplemental Table 1). Higher doses in the fezolinetant BID and QD dosing groups were associated with a greater improvement in vasomotor function domain score. The threshold for a CID (1.2 for vasomotor function)23 was exceeded in all fezolinetant treatment groups and the placebo group at all measurement time points (Fig. 2).
FIG. 2.
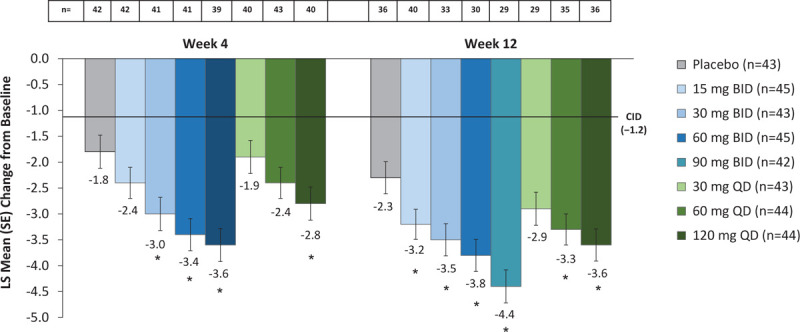
Change from baseline in MENQoL vasomotor function domain score. LS means and SEs are from a mixed model for repeated measurements with change from baseline as the dependent variable and the treatment group, visit, and smoking status as factors and baseline measurement as a covariate as well as interaction of treatment by week and an interaction of baseline measurement by week. Baseline values on the VMS function domain ranged from 1 to 8 with a mean score of 6.9 (SD 1.1). Reductions from baseline indicate improvement. The CID was derived by Bushmakin et al.23∗P < 0.05 for paired comparisons of fezolinetant versus placebo at last on-treatment week, with no adjustments for multiplicity. CID, clinically important difference; LS, least squares; MENQoL, menopause-specific quality of life questionnaire; SE, standard error; VMS, vasomotor symptoms.
Few differences were observed in the other domains (Supplemental Table 1), particularly at week 12, with the exception of the sexual function domain, in which participants taking fezolinetant 30 mg BID showed improvement relative to placebo at both week 4 (mean change vs placebo: −1.1; 95% CI: −1.8 to −0.4) and week 12 (mean change vs placebo: −1.0; 95% CI: −1.8 to −0.3).
HFRDIS
A decrease (improvement) from baseline in mean HFRDIS score that exceeded the MID (1.76)25 was seen with all fezolinetant doses and placebo at weeks 4 and 12 (Fig. 3). The magnitude of this decrease was numerically larger with all doses of fezolinetant than with placebo.
FIG. 3.
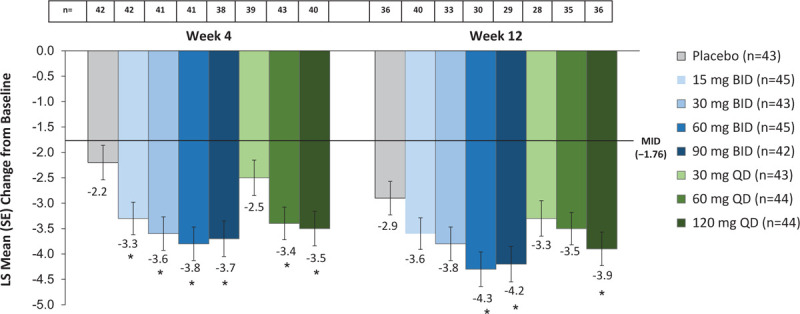
Change from baseline in HFRDIS score. LS means and SEs are from a mixed model for repeated measurements with change from baseline as the dependent variable, treatment group, visit, and smoking status as factors, and baseline measurement as a covariate, as well as interaction of treatment by week and an interaction of baseline measurement by week. Baseline values ranged from 0 to 10 with a mean score of 5.6 (SD 2.2). Reductions from baseline indicate improvement. The MID was derived by Carpenter et al.25∗P < 0.05 for paired comparisons of fezolinetant versus placebo at last on-treatment week, with no adjustments for multiplicity. HFRDIS, hot flash related daily interference scale; LS, Least squares; MID, minimally important difference; SE, standard error.
GCS
The GCS total and domain scores showed a decrease from baseline (improvement) in all treatment groups at weeks 4 and 12. For the majority of dose groups and time points, improvements were numerically greater with fezolinetant versus placebo (Supplemental Table 2).
Notable differences with fezolinetant versus placebo were observed in the VMS domain of the GCS (Fig. 4). Improvements in the VMS domain were numerically greater in all fezolinetant dose groups than those observed in the placebo group.
FIG. 4.
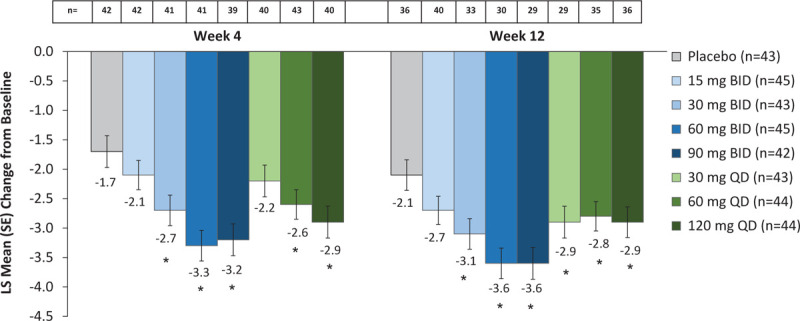
Change from baseline in GCS vasomotor symptom score. LS means and SEs are from a mixed model for repeated measurements with change from baseline as the dependent variable and the treatment group, visit and smoking status as factors and baseline measurement as a covariate as well as interaction of treatment by week and an interaction of baseline measurement by week. Baseline values on the VMS function domain ranged from 0 to 6 with a mean score of 4.8 (SD 1.4). Reductions from baseline indicate improvement. ∗P < 0.05 for paired comparisons of fezolinetant versus placebo at last on-treatment week, with no adjustments for multiplicity. GCS, Greene climacteric scale; LS, least squares; SE, standard error, VMS, vasomotor symptom.
Safety
Primary safety results have been reported.19 Rates of reported adverse events were similar across treatment groups, with no major dose-related events that would potentially skew results on PROs.
DISCUSSION
In this phase 2b, dose-ranging study, treatment with fezolinetant not only reduced the frequency and severity of VMS on the previously reported primary endpoints,19 it resulted in higher responder rates and improvements in PROs in a population of postmenopausal women with a considerable baseline burden of moderate/severe VMS. Across all fezolinetant dose groups, more than 80% of the participants experienced a reduction in moderate or severe VMS of ≥ 50%, and more than half of the women achieved a ≥ 90% reduction. These reductions in VMS were accompanied by improvements in PROs (MENQoL, HFRDIS, and GCS), with marked changes from baseline observed at the first measurement time point; improvements were maintained throughout the 12-week treatment period. Consistent with the reported efficacy of fezolinetant to reduce the frequency and severity of VMS (ie, the occurrence of hot flashes and/or night sweats),18,19 this manuscript reflects notable improvements in PRO measure domains that have a strong association with VMS, including the MENQoL vasomotor function domain, HFRDIS total score, and GCS VMS score. Improvements on the MENQoL and HFRDIS exceeded previously published MID/CID values for all fezolinetant groups and placebo.
A strength of the study was the inclusion of multiple PRO measures that quantified different aspects of the impact VMS have on women's lives, including impairment in menopause-related quality of life (MENQoL) and interference with daily life activities (HFRDIS), as well as the psychological and physical symptoms associated with VMS (GCS). A strong placebo effect was observed; however, numerically greater improvements were observed across these measures in one or more domains with fezolinetant. Considerable placebo effects are a recognized phenomenon in clinical trials of treatments for VMS.28 A Cochrane review of clinical trials of estrogen/progestogen treatment for VMS reported that placebo therapy was associated with a 58% reduction in VMS frequency.29 Placebo responder rates (≥50% reduction in hot flash score or VMS frequency) from trials of nonhormone therapies for VMS have ranged from 21% to 57%.30,31 In the current study, changes from baseline in PROs in the placebo group align with improvements in ratings of VMS frequency and severity.19 The clear trend for numerically greater improvement in the fezolinetant treatment group relative to the placebo group, coupled with the magnitude with which fezolinetant treatment groups exceeded the previously published CID or MID thresholds relative to placebo, suggest a potential benefit of therapy that should be evaluated further in larger trials.
These results are subject to the inherent limitations of the study design, including the 12-week study duration, which precluded assessment of longer term benefits, and the relatively small sample size within each active treatment group, which limited statistical power to detect smaller treatment effects (eg, incremental improvements over placebo that were less than approximately 1 point on MENQoL domains). The inability to evaluate the impact on sleep was also a limitation of this study.
CONCLUSIONS
In this study of postmenopausal women experiencing moderate/severe VMS, treatment with fezolinetant significantly improved responder rates and PRO measures of daily life interference, associated symptoms, and health-related quality of life. The body of clinical data accrued for fezolinetant warrant proceeding to further studies of longer treatment duration and a larger treated population. Such studies should include comprehensive evaluation of PROs to fully assess the potential benefits of this nonhormone therapy for menopause-related VMS.
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank the women who participated in the study. The authors also thank Marci English, Misun Lee, and Emad Siddiqui for their contributions to the data analysis. Medical writing and editorial support were provided by Diane M. Sloan, PharmD, of Echelon Brand Communications, LLC (Parsippany, NJ), an OPEN Health company, and funded by Astellas Pharma, Inc.
Footnotes
Funding/support: This study was sponsored by Astellas Pharma Inc. Medical writing and editorial support were provided by Diane M. Sloan, PharmD, of Echelon Brand Communications, LLC (Parsippany, NJ), an OPEN Health company, and funded by Astellas Pharma Inc.
Financial disclosure/conflicts of interest: N. Santoro: Member of the Scientific Advisory Board for Astellas Pharma, Inc., and Menogenix, Inc., and may own stock/options in Menogenix, Inc. Coinvestigator on a Small Business Innovative Research grant on a compound patented by Menogenix, Inc. A. Waldbaum: Astellas, Abbvie, Obseva, Chemo, Sebela, Mithra, Menogenix, Myovant, Ferring, Pharmavite, Viveve, Endoceutics, TherapeuticsMD. S. Lederman: Research grants from GSK, Astellas, Lallemand, Skynexis, Mitsubishi, Endoceutics, AbbVie, and Myovant R. Kroll: Research grants; Astellas, OGEDA, Mitsubishi, Endoceutics, Therapeutics MD, Mithra, AbbVie, Myovant. Consultant for Astellas and speakers bureau for AbbVie G.L. Fraser: Former employee of OGEDA SA and consultant for Astellas Pharma, Inc. C. Lademacher: Employee of Astellas Pharma US, Inc. L. Skillern: Former employee of Astellas Pharma Europe Ltd. J. Young: Employee of Astellas Pharma US, Inc. S. Ramael: Former employee of OGEDA SA and consultant for Astellas Pharma, Inc.
Data presentation: Data from this study were presented at The North American Menopause Society 2019 Annual Meeting on September 25-28, 2019, in Chicago, Illinois.
Author contributions: Study design: GF, SR, LS; Study investigator: SL, AW; Enrolled participants: SL, AW; Collection and assembly of data, including data management: JY; Data analysis: JY; Data interpretation: JY, NS, CL, AW, LS; Manuscript preparation: NS, GF, SR, CL, AW; Manuscript review and revisions: All authors; Final approval of manuscript: All authors.
DATA SHARING STATEMENT: Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
REFERENCES
- 1.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health 2006; 96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn ovarian aging study cohort. Menopause 2014; 21:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas 2007; 58:348–358. [DOI] [PubMed] [Google Scholar]
- 4.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savolainen-Peltonen H, Hautamaki H, Tuomikoski P, Ylikorkala O, Mikkola TS. Health-related quality of life in women with or without hot flashes: a randomized placebo-controlled trial with hormone therapy. Menopause 2014; 21:732–739. [DOI] [PubMed] [Google Scholar]
- 6.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinkerton JV, Abraham L, Bushmakin AG, Cappelleri JC, Komm BS. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause 2016; 23:1060–1066. [DOI] [PubMed] [Google Scholar]
- 8.Whiteley J, DiBonaventura M, Wagner JS, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health 2013; 22:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiBonaventura MD, Chandran A, Hsu MA, Bushmakin A. Burden of vasomotor symptoms in France, Germany, Italy, Spain, and the United Kingdom. Int J Womens Health 2013; 5:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartoulla P, Bell RJ, Worsley R, Davis SR. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas 2015; 81:487–492. [DOI] [PubMed] [Google Scholar]
- 11.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A 2012; 109:19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology 2011; 152:4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep 2015; 5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991; 128:2239–2247. [DOI] [PubMed] [Google Scholar]
- 15.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 2013; 34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoveyda HR, Fraser GL, Dutheuil G, et al. Optimization of novel antagonists to the neurokinin-3 receptor for the treatment of sex-hormone disorders (part II). ACS Med Chem Lett 2015; 6:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser GL, Ramael S, Hoveyda HR, Gheyle L, Combalbert J. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J Clin Endocrinol Metab 2016; 101:417–426. [DOI] [PubMed] [Google Scholar]
- 18.Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab 2019; 104:5893–5905. [DOI] [PubMed] [Google Scholar]
- 19.Fraser GL, Lederman S, Waldbaum A, et al. A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause 2020; 27:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996; 24:161–175. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the menopause-specific quality of life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas 2005; 50:209–221. [DOI] [PubMed] [Google Scholar]
- 22.Radtke JV, Terhorst L, Cohen SM. The menopause-specific quality of life questionnaire: psychometric evaluation among breast cancer survivors. Menopause 2011; 18:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushmakin AG, Abraham L, Pinkerton JV, Cappelleri JC, Mirkin S. Evaluation of the measurement model and clinically important differences for menopause-specific quality of life associated with bazedoxifene/conjugated estrogens. Menopause 2014; 21:815–822. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter JS. The hot flash related daily interference scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage 2001; 22:979–989. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter JS, Bakoyannis G, Otte JL, et al. Validity, cut-points, and minimally important differences for two hot flash-related daily interference scales. Menopause 2017; 24:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene JG. Constructing a standard climacteric scale. Maturitas 1998; 29:25–31. [DOI] [PubMed] [Google Scholar]
- 27.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol 2000; 152:463–473. [DOI] [PubMed] [Google Scholar]
- 28.Freeman EW, Ensrud KE, Larson JC, et al. Placebo improvement in pharmacologic treatment of menopausal hot flashes: time course, duration, and predictors. Psychosom Med 2015; 77:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev 2004; CD002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J Clin Oncol 2009; 27:2831–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause 2013; 20:1027–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


