Abstract
Cytochrome P4501A (CYP1A) enzymes play important roles in xenobiotic and endobiotic metabolism. Due to uncoupling reactions during the enzymatic cycle, CYP1A enzymes can release reactive oxidative species (ROS) in the form of superoxide radical, hydrogen peroxide, hydroxyl radical etc. An imbalance between production of free radicals and the ability of antioxidants to detoxify the free radicals can lead to accumulation of ROS, which in turn can lead to oxidative stress. Oxidative stress can lead to inflammation and toxicity, which in turn can cause human diseases such as bronchopulmonary disease (BPD), ARDS, renal hypertension, etc. CYP1A enzymes, depending on the organ system, they either contribute or protect against oxidative injury. Thus, they have dual roles in regard to oxidative stress. This review presents an overview of the mechanistic relationship between CYP1A enzymes and oxidative stress in relation to various diseases in different organs (e.g., liver, lungs, heart, kidneys, and reproductive organs).
Keywords: Cytochrome P450 1A (CYP1A) enzymes, oxidative stress, reactive oxygen species (ROS), reaction uncoupling, epoxyeicosatrienoic acid (EETs), hydroxyeicosatetraenoic acid (HETEs)
1. INTRODUCTION
The cytochrome P450 (CYP) enzymes consist of numerous isoforms that are known to play important roles in the metabolism of xenobiotic as well as endobiotic compounds [1]. The CYP1A enzymes belong to a versatile subfamily of enzymes that consist of CYP1A1 and CYP1A2 enzymes. CYP1A2 is liver-specific, while CYP1A1 is extrahepatic but inducible in the liver [2]. CYP1A enzymes are monooxygenases that metabolize drugs and xenobiotic molecules and also play a role in physiological signaling and endogenous molecular metabolism and synthesis [3]. CYP1A enzymes contribute to the metabolic activation of pro-carcinogens such as polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines/amides (HAAs), to reactive metabolites which can bind to DNA abd proteins, leading to cancer and toxicity [4, 5]. A significant number of studies have examined the role that CYP1A enzymes play in contributing to or protecting against oxidative stress in different areas of the body.
The impact of oxidative stress in physiology has been studied extensively. Oxidative stress occurs when anti-oxidant systems are unable to overcome the action of oxidants, resulting in an imbalance between the oxidative and antioxidant systems in the cell [6]. When an imbalance occurs, reactive oxygen species (ROS) accumulate and cause detrimental effects to biomolecular components and physiological pathways of the cell. Types of ROS include superoxide anion, hydroxyl radial, hydrogen peroxide, etc. [7]. Oxidative stress causes inflammation and toxicity, which can lead to pulmonary disease, reproductive toxicity, cardiovascular disease, neurodegenerative diseases, aging, cancer, etc. [8-12].
2. HOW CYP1A ENZYMES INDUCE OXIDATIVE STRESS
The breakdown of PAHs and aromatic amines by CYP1A enzymes produce pro-inflammatory ROS and reactive metabolites [13-15]. ROS react with endogenous molecules, such as lipids, proteins, and nucleic acids, which can in turn cause damage to the cell that interferes with normal cellular function and can even lead to cellular apoptosis [8, 15, and 16]. Despite these harmful effects, ROS also assist in normal physiological functioning through cell signaling and immunity [17, 18]. Because CYP1A enzymes facilitate the oxidation of molecules, blocking antioxidant enzymes or inducing CYP1A enzymes could result in an accumulation of H2O2 from reaction uncoupling and contribute to oxidative stress in the cell [19]. However, studies have also shown that H2O2 operates as a negative feedback loop for CYP1A transcription, resulting in the downregulation of CYP1A enzymes [1].
CYP1A enzymes have long been implicated in the metabolism of drug and xenobiotic molecules. This process is a major source of ROS generation. Additionally, the alternative role of these enzymes in the metabolism of endogenous molecules, such as arachidonic acid and estrogens, is a growing area of study with significant physiological implications. For example, CYP1A enzymes convert arachidonic acid (AA) to the epoxyeicosatrienoic acid (EETs), which are anti-inflammatory, and ω-terminal hydroxyeicosatetraenoic acid (HETEs), which are pro-inflammatory [20].
3. CONTRIBUTION OF CYP1A ENZYMES TO OXIDATIVE STRESS
The unregulated release of ROS contributes to the inability of the cell to maintain the oxidation-reduction balance, which ultimately leads to oxidative stress [21]. Upregulation of CYP1A enzyme expression stems from exposure to harmful PAHs, 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD), etc. Numerous studies have shown the mechanisms by which CYP1A enzymes contribute to oxidative stress. Figure 1 summarizes the contributory effects of CYP1A enzymes to oxidative stress.
Figure 1: Possible Mechanism of Contribution of CYP1A Enzymes to Oxidative Stress.
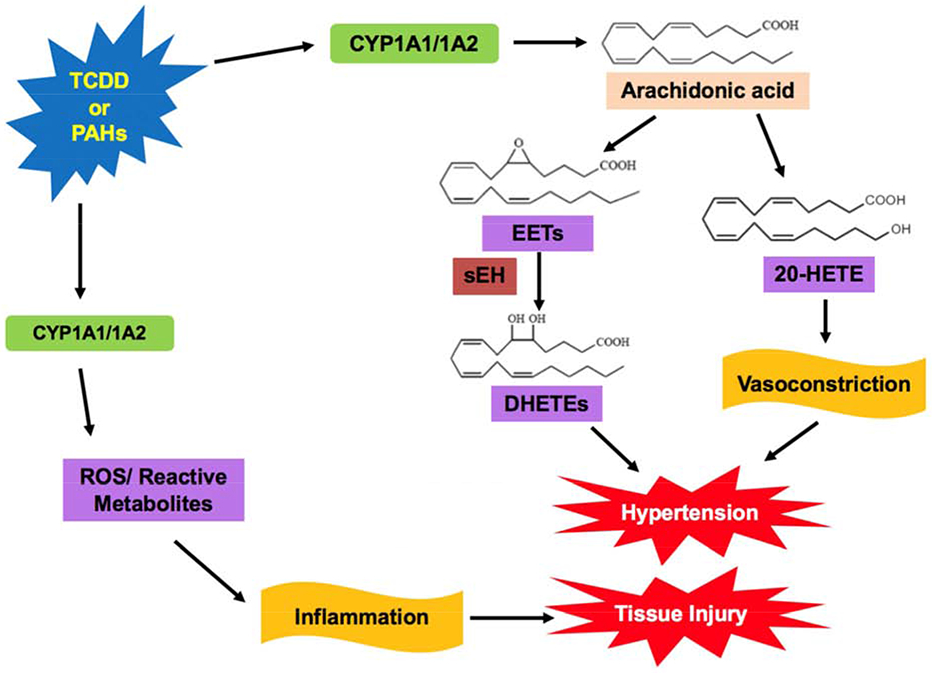
Polycyclic aromatic hydrocarbons (PAH) or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can induce CYP1A expression, which triggers metabolism of arachidonic acid (AA) to epoxyeicosatrienoic acid (EETs) and hydroxyeicosatetraenoic acid (20-HETEs) [24, 25]. Increased levels of 20-HETEs results in vasoconstriction and can contribute to renal and cardiovascular hypertension [25]. Production of EETs typically has beneficial cardiovascular properties; however, conversion of EETs to dihydroxyeicosatetraenoic acids (DHETEs) via soluble epoxide hydrolase (sEH) can result in hypertension [33]. CYP1A enzymes also produce reactive oxygen species (ROS) and reactive metabolites from reaction uncoupling [13, 14]. This contributes to oxidative stress, resulting in inflammation and cytotoxicity [21].
3.1. Contributive Effects of CYP1A Enzymes to Oxidative Stress in the Liver
Understanding the mechanism in which oxidative stress damages the liver is key in treating several liver diseases, including alcoholic liver disease, nonalcoholic fatty liver disease, hepatic encephalopathy, liver fibroproliferative diseases, and Hepatitis C virus [22]. Because of the substantial expression of CYP1A enzymes in the liver, particularly CYP1A2, significant research has been conducted to understand the role of these enzymes in oxidative stress. One recent study examined how pyrene contributed to oxidative damage in human liver Hep2G cells, and determined that pyrene upregulated CYP1A1, 1A2, and 1B1 enzymes while downregulating phase II antioxidant enzymes, resulting in increased ROS production and cytotoxicity [5].
The effect TCDD, an environmental toxin, on CYP1A enzyme regulation has also been extensively studied. TCDD is a high affinity aryl hydrocarbon receptor (AHR) ligand, which induces CYP1A enzyme production and disrupts the oxidation-reduction balance [23]. Labitzke et al. [24] have shown that TCDD induces CYP1A-enzyme in chick embryo liver mitochondria [24]. EET and 20-HETE are derived from CYP1A-mediated metabolism of AA, which could in turn lead to modulation of oxidative stress. Thus, TCDD-mediated induction of CYP1A enzymes could lead to oxidative stress within the liver mitochondria [24].
3.2. Contributive Effects of CYP1A Enzymes to Cardiovascular Diseases
Many studies underscore the impact of CYP1A enzymes on cardiovascular diseases. Aboutabl et al [25] examined AHR activation and its contribution to cardiac hypertrophy. AHR ligands, 3-methylcholanthrene (MC )- and benzo[a]pyrene [BP], induce CYP1A1 enzymes and cause increase in the 20-HETE: total EETs ratio, which may in turn contribute to cardiac hypertrophy [25]. Another component to consider when examining CYP1A enzymes with regards to disease pathways is its polymorphic nature. A study of coronary artery disease (CAD) in the Uygur and Han of China found that certain single nucleotide polymorphisms (SNPs) of CYP1A have significant associations with smokers who develop CAD [26]. In-depth studies are needed to understand the mechanism by which these SNPs influence the risk of CAD.
One significant consequence of CYP1A-mediated-ROS is the resulting lipid peroxidation. Aliwarga et. al. showed that ROS-induced lipid peroxidation of AA can result in the formation of both cis- and trans-EETs in red blood cells (RBC) of humans and mice, while AA oxidation via the CYP1A catalysis pathway results in only cis-EETs [27]. Therefore, the ROS generated from CYP1A enzymatic uncoupling can trigger free radical oxidation of AA and alter the ratio of cis- to trans-EETs. The distribution of cis- to trans-EETs within RBCs may affect the protective role EETs play against cardiovascular diseases. The regio- and geometric- isomers of EETs produced by ROS present another interesting dynamic to consider when evaluating the contribution of these molecules to cardiovascular disease pathways and warrant further research [28].
3.3. Contributive Effects of CYP1A Enzymes to Renal Hypertension
The multi-variable pathway of CYP1A enzymes is best exemplified in its effects on renal hypertension. Studies have shown that the inhibition of soluble epoxide hydrolase (sEH), which converts the CYP1A enzymatic product, EETs, to DHETEs, protects against renal hypertension [29, 30]. Xenobiotic-induced increase of EETs was initially thought to solely cause the antihypertensive effects arising from chronic sEH inhibition. Instead, a study examining the effects of sEH inhibition and NO synthase inhibition on transgenic mice with inducible hypertension found that the bioavailability of nitric oxide (NO) also contributes to the protection against hypertension [31, 32]. Although CYP1A enzymes appeat not to directly cause renal hypertension, these enzymes produce EETs and could offer alternative treatment options for this disease. For example, controlled induction of CYP1A enzymes in conjunction with NO bioavailability may stimulate EET production and, therefore, sEH inhibition, may result in protection against renal hypertension. This disease pathway reiterates the complex interplay of endogenous and xenobiotic molecules that affects the role of CYP enzymes.
3.4. Contributive Effects of CYP1A Enzymes to Reproductive Toxicity
Because of its role in xenobiotic and drug metabolism, CYP1A enzymes also play a significant role in pregnancy loss. Due to smoking or alcohol consumption, CYP1A1 enzymes are upregulated in the placenta in the phase I detoxification process. Generation of ROS due to this upregulation contributes to oxidative stress in the placenta and can lead to pregnancy loss, most commonly at the end of the first trimester due to a low presence of antioxidant enzymes [33]. Some SNPs of CYP1A enzymes have been positively correlated with an increased risk of recurrent pregnancy loss [33, 34]. Furthermore, a new study found an association between PAH exposure and preterm birth rates, further insinuating a connection between CYP1A enzyme metabolism and reproductive toxicity [35].
CYP1A enzyme also play a role in reproductive toxicity in males. Several factors may influence the way in which CYP1A enzymes contribute to male infertility via oxidative stress. One such factor is cigarette smoke. One study found that cigarette smoke condensate contains PAH, which activates AHR and upregulates the expression of CYP1A enzymes in mouse spermatocytes [36]. During CYP1A-enzyme metabolism of PAH, ROS are generated and may contribute to apoptosis of the spermatocytes [36]. Another factor that may influence susceptibility to male infertility is single nucleotide polymorphism (SNP) of CYP1A enzymes. Several studies have found associations between CYP1A SNPs and male infertility in certain ethnic groups [37, 38]. Further research is needed to investigate the mechanism by which these SNPs contribute to male infertility.
While CYP1A enzymes contribute to oxidative stress, as mentioned above, there are also a number of instances wherein these enzymes protect against oxidative stress, in relation to human diseases.
4. PROTECTION OF CYP1A ENZYMES AGAINST OXIDATIVE STRESS
One of the more fascinating areas of research is how CYP1A enzymes actually protect against oxidative stress. This protective effect of CYP1A enzymes is best exhibited in the case of hyperoxic lung injury (HLI). A representation of the protecting pathway of CYP1A enzymes on oxidative stress is shown in Figure 2.
Figure 2: Possible Mechanism of Protection of CYP1A Enzymes from Oxidative Stress.
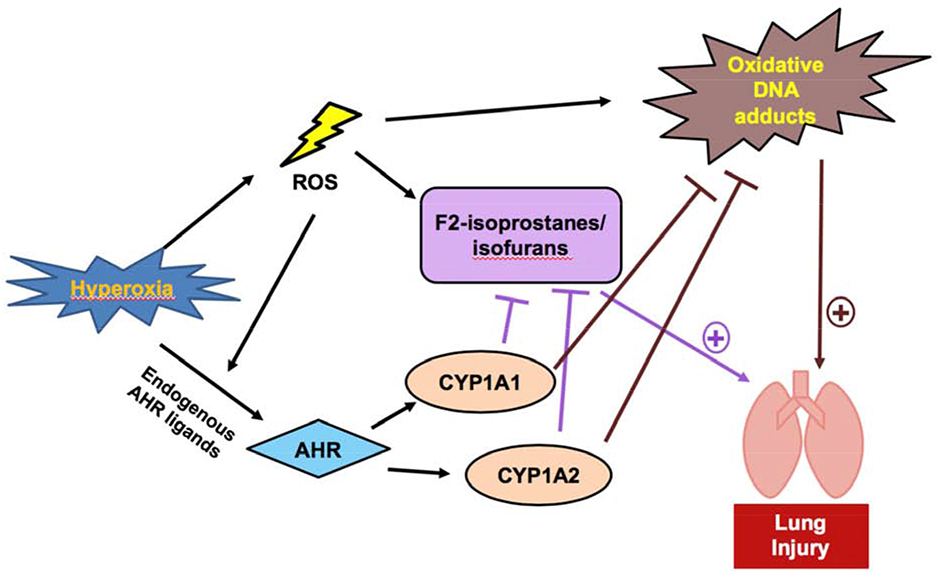
Hyperoxia results in an increase of ROS, which can cause increased levels of lipid hydroperoxide products, such as F2-isoprostanes and isofurans, and DNA adducts [57, 53]. These harmful effects are signs of oxidative stress and result in pulmonary injury [53]. Hyperoxia also upregulates CYP1A enzyme expression via AHR activation [52]. CYP1A enzymes metabolize lipid hydroperoxides and DNA-reactive metabolites, which protects against hyperoxic lung injury [56].
4.1. Protective Effects of CYP1A1 Enzymes in the Lungs against Hyperoxic Lung Injury (HLI)
The high expression of CYP1A1 enzyme in the lungs makes this enzyme a highly researched protein in pulmonary diseases, specifically HLI. Supplemental oxygen is often given to adults suffering from acute respiratory distress syndrome (ARDS) and premature infants who have pulmonary insufficiency, leading to bronchopulmonary dysplasia (BPD) [39-42]. ROS is known to contribute to these diseases [40-42]. Although it has been thought that increased CYP1A expression would lead to increased levels of H2O2 and, thereby, increased oxidative stress in the lungs, studies from our laboratory as well as others have shown that CYP1A1 enzymes protect against HLI [39, 43-49]. Because HLI affects infants and adults, studies have examined both newborn and adult mice and have found that in both cases, Cyp1a1−/− mice had higher incidence of HLI than that of wild type (WT) mice [49, 50].
A possible mechanism underlying increased CYP1A1 expression is the AHR pathway. Studies found that AHR induction via omeprazole (a proton pump inhibitor), increased levels of CYP1A1 expression and reduced HLI [51-53]. Furthermore, hyperoxia induced higher levels of CYP1A1 enzyme expression in AHR (+/+) mice but not in AHR (−/−) mice, further signifying that induction of CYP1A1 enzyme by hyperoxia is dependent upon the AHR pathway [49, 54].
Gender may be another factor that affects the role of CYP1A1 enzyme on HLI. A few studies have found that male mice had lower levels of CYP1A1 enzymes as well as higher levels of inflammatory and oxidative markers than female mice when exposed to hyperoxia [55]. Interestingly, Cyp1a1−/− female mice were more susceptible to HLI than male mice were, which may indicate that a female-specific mechanism exists that regulates CYP1A1 enzyme expression [56]. A possible explanation of this mechanism may be the 2-methoxyestradiol generation from 17-β estradiol via CYP1A enzymes, resulting in reduced inflammation [56].
Understanding the mechanism behind CYP1A-modulated HLI is important in developing better treatment options in the future. Upon analysis of the differences between WT and Cyp1a1−/− pulmonary transcriptome and proteome, Cyp1a1−/− mice had higher levels of lipid hydroperoxides, such as F2-isoprostanes/isofurans, than WT mice did, indicating increased oxidative stress in the mice lacking CYP1A1 enzymes [57, 58]. Therefore, CYP1A enzymes may protect against hypoxic lung injury by metabolizing lipid hydroperoxides and diminishing the oxidative damage caused by these molecules. CYP1a1−/− mice also showed a larger increase in DNA adducts than their WT counterparts did when exposed to hypoxia, which suggests that CYP1A enzymes contributes to the detoxification of DNA-reactive species, resulting in the attenuation of oxidative DNA adducts [57]. Further analysis of these mechanisms will potentially allow for the development of treatment that alters or controls these reaction pathways to prevent or treat HLI.
4.2. Protective Effects of CYP1A2 Enzymes in the Liver against Hyperoxic Lung Injury
One intriguing discovery was that hepatic CYP1A2 enzyme is protective against hyperoxic lung injury. We have shown that Cyp1a2−/− mice are more susceptible to HLI than wild type (WT) mice, suggesting that CYP1A2 enzyme protects against oxidative damage in the lungs [59]. The involvement of enzymes from extra-pulmonary organs in HLI has significant implications in this field of research and opens the possibility that oxidative stress from one organ can affect disease pathology in another organ.
Unlike CYP1A1 enzymes, CYP1A2 expression can be induced independently of the AHR pathway because studies showed that hyperoxia increased hepatic CYP1A2 expression in both WT and AHR(−/−) mice [49]. However, like CYP1A1, CYP1A2 enzyme displays sex-specific effects. Hyperoxia induces higher levels of CYP1A2 expression in WT females than in WT males [56]. Furthermore, Cyp1a2−/− female mice show higher incidence of HLI than their male counterparts [56]. In comparison to Cyp1a1−/− mice, Cyp1a2−/− mice showed more pronounced sex-specific differences in HLI, which signify the importance of CYP1A2 in protecting against oxidative stress [56].
The mechanism by which CYP1A2 enzymes protect against HLI is interesting because of their location in the liver. Because of the role of CYP1A2 enzymes in lipid hydroperoxide metabolism, accumulation of F2-isoprostanes in the liver of Cyp1a2−/− mice would result in systemic circulation of the lipid peroxides to the lungs and contribution to pulmonary oxidative stress [57, 59]. Similar to Cyp1a1−/− mice, Cyp1a2−/− mice showed decreased gene expression of DNA repair molecules, indicating that CYP1A2 enzymes may contribute to the detoxification of DNA adduct precursors [57]. Differences in protein expression of mechanistic Target of Rapamycin (mTOR), vascular endothelial growth factor receptor 2 (VEGFR2), phosphorylated Acetyl-CoA carboxylase, and phosphorylated 5’-prime-AMP-activated protein kinase amongst WT, Cyp1a1−/−, and Cyp1a2−/− mice are also seen [57]. Each of these proteins has a complex pathway that may affect how CYP1A enzymes contribute to the protection against HLI. Moreover, proteome and transcriptome variations in DNA repair, apoptosis, and estrogen response pathways provide insight into the mechanistic roles that these two enzymes play in HLI. However, more in-depth research is needed to isolate and study these complex mechanisms.
5. CONCLUSION
The relationship between CYP1A enzymes and oxidative stress is complicated and is organ-and injury-dependent. In some cases, CYP1A enzymes can contribute to the accumulation of ROS when antioxidant enzymes are inhibited [11]. The pro-oxidant effects of CYP1A enzymes are seen in the liver, lungs, heart, kidneys, and reproductive organs, but the mechanisms and implications of these effects are still being studied. The physiological pathways and endogenous molecules present in these organs seem to have an impact on the relationship between CYP1A enzymes and oxidative stress. The tissue-specific effects of CYP1A1 enzymes, their unique physiological roles, and their polymorphic nature, contribute to or protect against oxidative stress, in relation to various human diseases. Evidence that CYP1A enzymes can affect disease pathways in distant organs via systemic circulation of metabolites broadens the field of research even further [59]. Future research should lead to unravelling the intricate details of the mechanisms by which CYP1A enzymes play roles in oxidative stress. The fact that CYP1A enzymes can be modulated by xenobiotics and drugs renders them to be promising targets for prevention and treatment of a myriad of human diseases.
Acknowledgements:
This work was in part supported by NIH grants R01HL129794, 1R01ES029382, 1P42 ES0327725, and Cancer Prevention Research Institute of Texas (CPRIT) RP190279 to BM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
Special interest (•)
Outstanding interest (••)
- [1].Moorthy B, The CYP1A Subfamily, Issues in Toxicology Cytochromes P450 (2008) 97–135. doi: 10.1039/9781847558428-00097. [DOI] [Google Scholar]
- [2].Walsh AA, Szklarz GD, Scott EE, Human Cytochrome P450 1A1 Structure and Utility in Understanding Drug and Xenobiotic Metabolism, J. Biological Chemistry 288 (2013) 12932–12943. doi: 10.1074/jbc.m113.452953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guengerich FP, Common and uncommon cytochrome p450 reactions related to metabolism and chemical toxicity, Chem Res Toxicol. 14(6) (2001) 611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- [4].Ma Q, Lu AYH, CYP1A Induction and Human Risk Assessment: An Evolving Tale of in Vitro and in Vivo Studies, Drug Metabolism and Disposition 35(7) (2007) 1009–1016. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- ••[5].Ma JK, Eldin WFS, El-Ghareeb WR, Elhelaly AE, Khedr MHE, Li X, Huang XC, Effects of Pyrene on Human Liver HepG2 Cells: Cytotoxicity, Oxidative Stress, and Transcriptomic Changes in Xenobiotic Metabolizing Enzymes and Inflammatory Markers with Protection Trial Using Lycopene, Biomed. Res. Int. 2019 (2019) 7604851. doi: 10.1155/2019/7604851.A recent study that examines the effects of a pollutant on the catalytic cycle of oxidative enzymes and how these changes relate to oxidative stress.
- [6].Sies H, Berndt C, Jones DP, Oxidative stress, Annu Rev Biochem. 8 (2017) 715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- [7].Halliwell BB, Poulsen HE, Oxidative Stress, Cigarette Smoke and Oxidative Stress (2006) 1–4. doi: 10.1007/3-540-32232-9_1. [DOI] [Google Scholar]
- [8].Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N, Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?, Oxidative Medicine and Cellular Longevity (2016) 1–9. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoshikawa T, Naito Y, What Is Oxidative Stress?, J. Japan Medical Association 45(7) (2002) 271–276. doi: 10.1093/ndt/21.suppl_4.iv200. [DOI] [Google Scholar]
- [10].Uttara B, Singh AV, Zamboni P, Mahajan RT, Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options, Curr Neuropharmacol. 7(1) (2009) 65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB, Oxidative stress, inflammation, and cancer: How are they linked?, Free Radic. Biol. Med 49(11) (2010) 1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A, Oxidative Stress: Harms and Benefits for Human Health, Oxid. Med. Cell Longev 2017 (2017) 1–13. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Holme JA, Brinchmann BC, Refsnes M, Låg M, Øvrevik J, Potential role of polycyclic aromatic hydrocarbons as mediators of cardiovascular effects from combustion particles, Environ. Health 18 (2019) 74. doi: 10.1186/s12940-019-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••[14].Albertolle ME, Phan TTN, Pozzi A, Guengerich FP, Sulfenylation of Human Liver and Kidney Microsomal Cytochromes P450 and Other Drug-Metabolizing Enzymes as a Response to Redox Alteration, Mol. Cell. Proteomics 17(5) (2018) 889–900. doi: 10.1074/mcp.RA117.000382.Study that examines the sulfenylation of CYP450 enzymes in the liver and kidney and its effect on the redox balance.
- ••[15].Furue M, Hashimoto-Hachiya A, Tsuji G, Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis, Int. J. Mol. Sci 20(21) (2019) 5424. doi: 10.3390/ijms20215424.Study that examines how the AHR pathway impacts redox balance in the skin and relates to atopic dermatitis and psoriasis.
- ••[16].Nandi A, Yan LJ, Jana CK, Das N, Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases, Oxidative Medicine and Cellular Longevity 2019 (2019) 1–19. doi: 10.1155/2019/9613090.Study that reviews the role of catalase as an antioxidant enzyme in contributing to oxidative stress and how that can affect different diseases and conditions.
- [17].Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A, Oxidative Stress: Harms and Benefits for Human Health, Oxidative Medicine and Cellular Longevity 2017 (2017) 1–13. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jones DP, Sies H, The redox code, Antioxid. Redox Signal 23(9) (2015) 734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zangar RC, Davydov DR, Verma S, Mechanisms that regulate production of reactive oxygen species by cytochrome p450, Toxicol. Appl. Pharmacol 199(3) (2004) 316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- [20].El-Sherbeni AA, El-Kadi AOS, Characterization of Arachidonic Acid Metabolism by Rat Cytochrome P450 Enzymes: The Involvement of CYP1As, Drug Metab. Dispos 42 (2014) 1498–1507. doi: 10.1124/dmd.114.057836. [DOI] [PubMed] [Google Scholar]
- [21].Bae YS, Oh H, Rhee SG, Yoo TD, Regulation of reactive oxygen species generation in cell signaling, Mol. Cells 32(6) (2011) 491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cichoz-Lach H, Michalak A, Oxidative stress as a crucial factor in liver diseases, World J. Gastroenterol 20(25) (2014) 8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ghotbaddini M, Powell JB, The AhR Ligand TCDD, Regulates Androgen Receptor Activity Differently in Androgen-Sensitive versus Castration-Resistant Human Prostate Cancer Cells, Int. J. Environ. Res. Public Health 12(7) (2015) 7506–7518. doi: 10.3390/ijerph120707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Labitzke EM, Diani-Moore S, Rifkind AB, Mitochondrial P450-dependent arachidonic acid metabolism by TCDD-induced hepatic CYP1A5; conversion of EETs to DHETs by mitochondrial soluble epoxide hydrolase, Arch. Biochem. Biophys 468(1) (2007) 70–81. doi: 10.1016/j.abb.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aboutabl ME, Zordoky BNM, El-Kadi AOS, 3-Methylcholanthrene and benzo(a)pyrene modulate cardiac cytochrome P450 gene expression and arachidonic acid metabolism in male Sprague Dawley rats, British J. of Pharm 158 (2009) 1808–1819. doi: 10.1111/j.1476-5381.2009.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zou JG, Ma YT, Xie X, Yang YN, Pan S, Adi D, Liu F, Chen BD, The association between CYP1A1 genetic polymorphisms and coronary artery disease in the Uygur and Han of China, Lipids in Health and Disease 13 (2014) 145. doi: 10.1186/1476-511X-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aliwarga T, Raccor BS, Lemaitre RN, Sotoodehnia N, Gharib SA, Xu L, Totah RA, Enzymatic and Free Radical Formation of Cis- and Trans-Epoxyeicosatrienoic Acids In Vitro and In Vivo, Free Radic. Biol. Med 112 (2017) 131–140. doi: 10.1016/j.freeradbiomed.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •[28].Li Z, Jiang Y, Guengerich FP, Ma L, Li S, Zhang W, Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications, J. of Biological Chem 295 (2019) 833–849. doi: 10.1074/jbc.REV119.008758.Study that analyzes different cytochrome P450 engineering methods and industry applications.
- [29].Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT, Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension, Front. Biosci 13 (2008) 3480–7. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- [30].Neckar J, Kopkan L, Huskova Z, Kolar F, Papousek F, Kramer HJ, Hwang SH, Hammock BD, Imig JD, Maly J, Netuka I, Ostadal B, Cervenka L, Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-1-ylureido)cyclohexyl-oxy]benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension, Clin. Sci. (Lond.) 122(11) (2012) 513–25. doi: 10.1042/CS20110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves A.Ch., Huang Y, Luft FC, Gollasch M, Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice, Arterioscler. Thromb. Vasc. Biol 29(1) (2009) 54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- [32].Honetschlägerová Z, Kitada K, Husková Z, Sporková A, Kopkan L, Bürgelová M, Varcabová S, Nishiyama A, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Kujal P, Vernerová Z, Červenka L, Antihypertensive and renoprotective actions of soluble epoxide hydrolase inhibition in ANG II-dependent malignant hypertension are abolished by pretreatment with L-NAME, J. Hypertens 31(2) (2013) 321–332. doi: 10.1097/HJH.0b013e32835b50aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suryanarayana V, Deenadayal M, Singh L, Association of CYP1A1 gene polymorphism with recurrent pregnancy loss in the South Indian population, Human Reproduction 19(11) (2004) 2648–2652. doi: 10.1093/humrep/deh463. [DOI] [PubMed] [Google Scholar]
- ••[34].Lu J, Shang X, Zhong W, Xu Y, Shi R, Wang X, New insights of CYP1A in endogenous metabolism: a focus on single nucleotide polymorphisms and diseases, Acta Parmaceutica Sinica B 10(1) (2020) 91–104. doi: 10.1016/j.apsb.2019.11.016.Detailed overview of the research being conducted to analyze the relationship between CYP1A SNPs and various diseases.
- [35].Suter MA, Aagaard KM, Coarfa C, Robertson M, Zhou G, Jackson BP, Thompson D, Putluri V, Putluri N, Hagan J, Wang L, Jiang W, Lingappan K, Moorthy B, Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB), Biochem. Biophys. Res. Commun 516(2) (2019) 344–349. doi: 10.1016/j.bbrc.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Esakky P, Hansen DA, Drury AM, Moley KH, Cigarette smoke condensate induces aryl hydrocarbon receptor-dependent changes in gene expression in spermatocytes, Repro. Toxicol 34 (2012) 665–676. doi: 10.1016/j.reprotox.2012.10.005. [DOI] [PubMed] [Google Scholar]
- [37].Cao DH, Ren ZJ, Lu DL, Liu LR, Xu P, Zhang Q, Wei Q, Association between CYP1A1 rs4646903 T > C genetic variations and male infertility risk, Medicine 98 (2019) 31. doi: 10.1097/MD.0000000000016543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang T, Hu T, Zhen J, Zhang L, Zhang Z, Association of MTHFR, NFKB1, NFKBIA, DAZL and CYP1A1 gene polymorphisms risk of idiopathic male infertility in a Han Chinese population, Int. J. Clin. Exp. Pathol 10(7) (2017) 7640–7649. ISSN: 1936-2625/IJCEP0053898. [PMC free article] [PubMed] [Google Scholar]
- ••[39].Veith A, Moorthy B, Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species, Curr. Opin. Toxicol 7 (2018) 44–51. doi: 10.1016/j.cotox.2017.10.003Informative article that examines the effects of oxidative stress from other families and subfamilies of CYP enzymes as well as CYP1A enzymes.
- [40].Frank L, Bucher JR, Roberts RJ, Oxygen toxicity in neonatal and adult animals of various species, J. Appl. Physiol. Respir. Environ. Exerc. Physiol 45(5) (1978) 699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- [41].Freeman BA, Crapo JD, Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria, J. Biol. Chem 256(21) (1981) 10986–10992. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- [42].Turrens JF, Freeman BA, Crapo JD, Hyperoxia increases h2o2 release by lung mitochondria and microsomes, Arch. Biochem. Biophys 217(2) (1982) 411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- [43].Hazinski TA, France M, Kennedy KA, Hansen TN, Cimetidine reduces hyperoxic lung injury in lambs, J. Appl. Physiol 67(6)(1985) 2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- [44].Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE, Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome p-450 inhibitor, 1-aminobenzotriazole, J. Pharmacol. Exp. Ther 292(2) (2000) 553–560. doi: 10.1203/00006450-199704001-01851. [DOI] [PubMed] [Google Scholar]
- [45].Mansour H, Levacher M, Azoulay-Dupuis E, Moreau J, Marquetty C, Gougerot-Pocidalo MA, Genetic differences in response to pulmonary cytochrome p-450 inducers and oxygen toxicity, J. Appl. Physiol 64(4)(1988) 1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- [46].Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y, Induction of cytochrome p450 1a1 and 1a2 by hyperoxia, Biochem. Biophys. Res. Commun 197(2) (1993) 878–885. doi: 10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- [47].Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B, Attenuation of hyperoxic lung injury by the cyp1a inducer beta-naphthoflavone, Toxicol. Sci 87(1) (2005) 204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- [48].Couroucli XI, Liang YH, Jiang W, Wang L, Barrios R, Yang P, Moorthy B, Prenatal administration of the cytochrome p4501a inducer, beta-naphthoflavone (bnf), attenuates hyperoxic lung injury in newborn mice: Implications for bronchopulmonary dysplasia (bpd) in premature infants, Toxicol. Appl. Pharmacol 256(2) (2011) 83–94. doi: 10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Maturu P, Wei-Liang Y, Jiang W, Wang L, Lingappan K, Barrios R, Liang Y, Moorthy B, Couroucli XI, Newborn mice lacking the gene for cyp1a1 are more susceptible to oxygen-mediated lung injury, and are rescued by postnatal beta-naphthoflavone administration: Implications for bronchopulmonary dysplasia in premature infants, Toxicol. Sci 157(1) (2017) 260–271. doi: 10.1093/toxsci/kfx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B, Disruption of the ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes p4501a expression and exacerbates hyperoxic lung injury, J. Pharmacol. Exp. Ther 310(2) (2004) 512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- [51].Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, Moorthy B, Functional Deficiency of Aryl Hydrocarbon Receptor Augments Oxygen Toxicity-Induced Alveolar Simplification in Newborn Mice, Toxicol. Appl. Pharmacol 267(3) (2013) 209–217. doi: 10.1016/j.taap.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shivanna B, Chu C, Welty SE, Jiang W, Wang L, Moorthy B. Omeprazole Attenuates Hyperoxic Injury in H441 Cells via Aryl hydrocarbon Receptor, Free Radic. Biol. Med 51(10) (2011) 1910–1917. doi: 10.1016/j.freeradbiomed.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shivanna B, Jiang W, Wang L, Couroucli XI, Moorthy B, Omeprazole Attenuates Hyperoxic Lung Injury in Mice via Aryl Hydrocarbon Receptor Activation and Is Associated with Increased Expression of Cytochrome P4501A Enzymes, J. Pharm. and Exp. Therap 339 (2011) 106–114. doi: 10.1124/jpet.111.182980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Couroucli XI, Welty SE, Geske RS, Moorthy B, Regulation of Pulmonary and Hepatic Cytochrome P4501A Expression in the Rat by Hyperoxia: Implication of Hyperoxic Lung Injury, Mol. Pharmacol 61(3) (2002) 507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- [55].Lingappan K, Jiang W, Wang L, Couroucli XI, Barrios R, Moorthy B, Sex-specific Differences in Hyperoxic Lung Injury in Mice: Implications for Acute and Chronic Lung Disease in Humans, Toxicol. Appl. Pharmacol 272(2) (2013) 281–290. doi: 10.1016/j.taap.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lingappan K, Jiang W, Wang L, Couroucli XI, Moorthy B, Sex-specific differences in hyperoxic lung injury in mice: role of Cytochrome P450 (CYP)1A, Toxicol. 331 (2015) 14–23. doi: 10.1016/j.tox.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lingappan K, Maity S, Jiang W, Wang L, Couroucli X, Veith A, Zhou G, Coarfa C, Moorthy B, Role of cytochrome p450 (cyp)1a in hyperoxic lung injury: Analysis of the transcriptome and proteome, Sci. Rep 7(1) (2017) 642. doi: 10.1038/s41598-017-00516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Thakur VS, Liang YW, Lingappan K, Jiang W, Wang L, Barrios R, Zhou G, Guntupalli B, Shivanna B, Maturu P, Welty SE, Moorthy B, Couroucli XI, Increased susceptibility to hyperoxic lung injury and alveolar simplification in newborn rats by prenatal administration of benzo[a]pyrene, Toxicol. Lett 230(2) (2014) 322–332. doi: 10.1016/j.toxlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, Barrios R, Wang G, Khan MF, Gonzalez FJ, Roberts LJ, Moorthy B, Disruption of Cytochrome P4501A2 in mice leads to increased susceptibility to hyperoxic lung injury, Free Radic. Biol. Med 82 (2015) 147–159. doi: 10.1016/j.freeradbiomed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


