Abstract
In the paper, “Investigation of histology region in dielectric measurements of heterogeneous tissues,” by Porter and O’Halloran, the authors utilize a flexible phantom in a layered material dielectric property analysis to quantify the effective sensing volume of a coaxial dielectric probe. Ostensibly, this test has been used by others to characterize the region for which percent variation in the material composition in front of the probe corresponds to percent variation in the computed effective dielectric properties. By employing a compressible material, the authors fail to isolate features that are attributable solely to the probe, itself, and inadvertently incorporate confounding characteristics associated with the compressible nature of the material. The net effect is to exaggerate the probe’s sensing volume which undermines conclusions drawn from the subsequent tissue dielectric property studies.
Letter
The open-ended coaxial probe enjoys status as a “go to” tool within the microwave community for measuring dielectric properties of liquids, solid materials and biomedical tissue. Ostensibly, the model used to estimate properties from the microwave reflection measurements makes two primary assumptions – that the medium is homogeneous and that the material extends to infinity [1]. But these assumptions have not stopped practitioners from using probes under other conditions. For the purposes of this Comment, the types of probe measurements that have been reported can be divided into two broad categories – those with homogeneous and those with heterogeneous targets. A common example of a homogeneous target is a well-mixed liquid. Here, open-ended coaxial probes are ideally suited because the homogeneity assumption is well satisfied and extensions to infinity are readily accommodated. Determination of how large the object needs to be to obtain an accurate measure is a practical consideration. The simplest approach, presented by Hagl et al [2] and Perez [3], performs measurements with the probe submerged in a dielectric liquid and then progressively moves a high contrast object closer to the probe until perturbations in the measurements are noted. For the precision probe (2.3 mm diameter) manufactured by Keysight Technologies, Hagl et al and Perez both determined this distance to be roughly 3 mm.
Conversely, understanding the homogeneity assumption and determining the constraints necessary for measuring the effective (homogeneous) properties of heterogeneous objects accurately is more involved. Biological tissue is an excellent example of a heterogeneous target. In some cases, tissue is considered relatively homogeneous appropriately, but for general considerations, it is best treated as heterogeneous. Heterogeneity effects can manifest themselves in dielectric property measurements in any number of ways, most of which are difficult to quantify systematically. One useful framework for studying heterogeneity effects is the layered media case. In this situation, measurement configurations can be constructed in which the probe is immersed in one medium and a second medium of different properties is moved from the point of contact with the probe to some distance away. Similarly to the infinite medium test, composition of the zone immediately in front of the probe can be controlled and correlated with subsequent dielectric property measurements over a series of separation distances. Here, the zone size (or distance away from the probe) for which it produces meaningful dielectric property measurements of the composite (heterogeneous) region is determined by the range over which the relationship between the separation distance and measured properties remains linear. This criterion was used in previous studies by Alanen et al [4], Gregory et al [5], and Meaney et al [6]. In these experiments, no abrupt departure from the linear relationship was observed and one where the measured properties leveled off and asymptotically approached the properties of the liquid was found. In these papers, different but remarkably similar metrics were devised to determine a practical cut off distance which we refer to as the penetration or sensing depth. From these studies, the sensing depth is dependent almost entirely on the probe diameter with minimal variation in terms of frequency or the actual properties of the surrounding liquid [7]. Mayrovitz et al exploited this phenomenon clinically by using a suite of commercial probes (Delfin Technologies, Kuopio, Finland) with varying aperture diameters to assess edema levels at different tissue depths [8].
In the Porter and O’Halloran paper [9], the authors used a deformable (rubber-like) material for penetration depth estimation, presumably because it could be fabricated to have dielectric properties that approximated the tissue samples they encountered and also because the tissue samples they were using were also deformable. Their plots of the dielectric properties as a function of distance begin relatively flat (that of the solid) during the fully compressed state of the deformable material (left portion of the curve – in Figure 5 of Porter and O’Halloran [9] – re-printed here in Fig. 1 along with a plot of the percent liquid in the intervening zone), transition to a straight diagonal line immediately after the probe is fully disengaged from the rubber and eventually level off and asymptotically approach that of the liquid at larger distances. The limitation with this estimate of sensing depth is that for distances when probe is in contact with the solid, no separation occurs between the probe and solid (Fig. 2). No volumetric change in material composition takes place in the space immediately around the probe for which to correlate change in measured dielectric properties. While some change in properties occurs, especially near the disengagement point, it is most likely due to imperfect disconnection between the probe and solid. Accordingly, the distance over which contact is maintained should not be included in the sensing depth estimate since it is outside the span where the measured properties correlate with material composition ratio. Conversely, when a solid dielectric object is used, the dielectric properties begin to change linearly as a function of separation distance immediately after disengagement (Fig. 3).
Fig. 1.
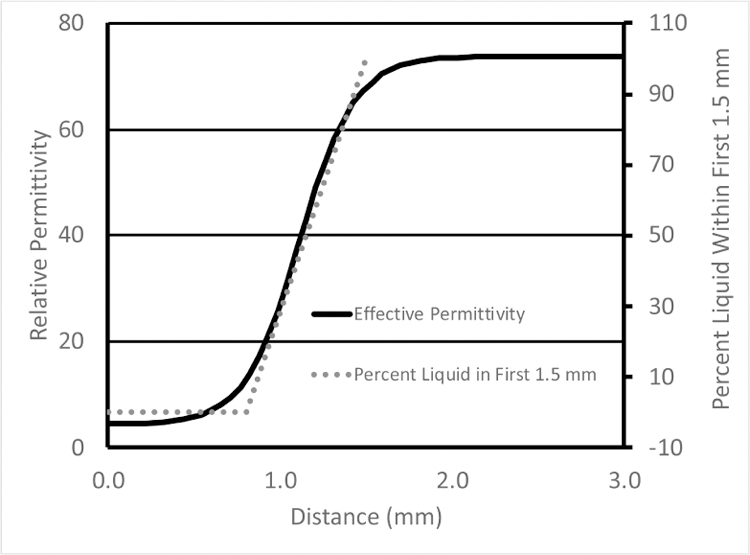
Graph of effective permittivity as a function of distance from the point where the 2.2 mm diameter probe compresses the solid to a state where they are fully separated. Percent liquid is also included for reference.
Fig. 2.
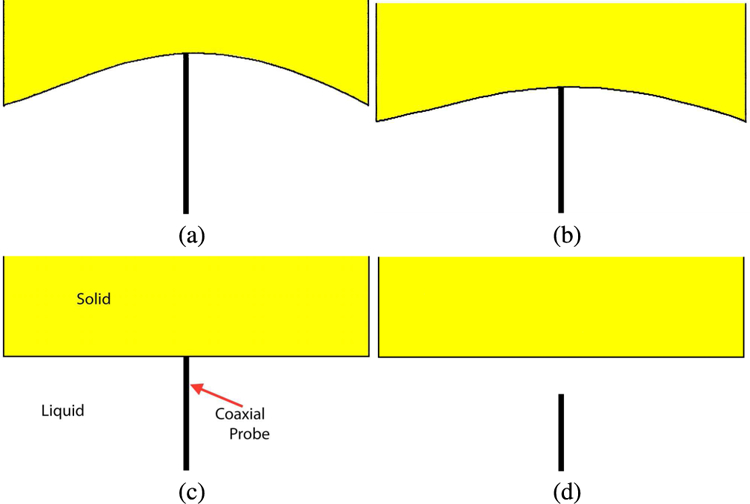
Schematic diagrams of the probe contact (a and b) during different degrees of phantom compression, (c) no compression, and (d) while the probe is not in contact with the phantom.
Fig. 3.
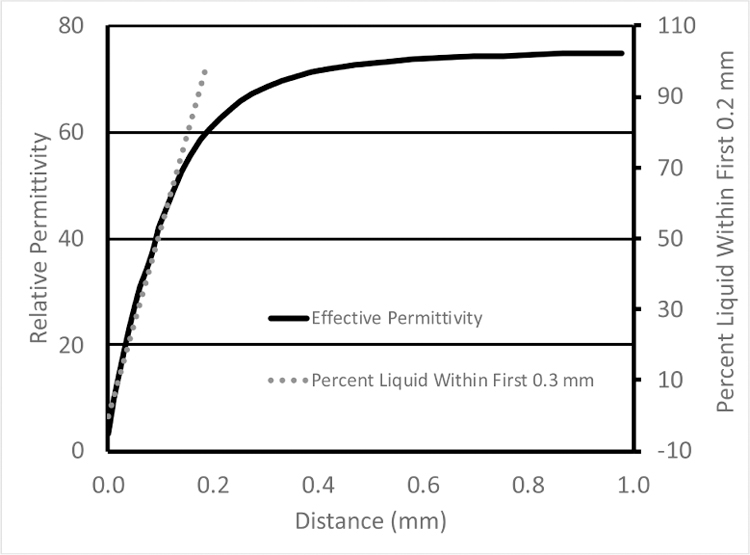
Graph of the effective permittivity as a function of distance from the point where the 2.2mm diameter probe contacts the solid to a state where they are fully separated. Percent liquid is also included for reference.
The implications are important. The authors contend that the penetration depth is considerably deeper when compressible materials – i.e. biological tissue – are used because of the added compression zone. In reality, the experiments do not provide a controlled environment that isolates environmental effects such as the mechanical characteristics of the solid so that the probe’s measurement characteristics can be assessed properly. The analysis is confounded by incorporation of features that are attributable primarily to the compressible nature of the material being tested. While the paper presents several penetration depths based on different interpretations of a single curve, all but one suffer from the same shortcoming by including characteristics belonging to the compressible material and extend the probe’s sensing zone beyond the linear relationship.
Using the Keysight probe, the penetration depth is nominally on the order of 0.3 mm. Based on metrics devised by Porter and O’Halloran, the depth would reach 1.5 to 2.0 mm. Measurement depth has important implications when relating probe property measurements to underlying tissue histology, and how the tissue is processed in pathology relative to its thickness, since differences between 0.2 to 0.3mm and 1.5 to 2.0 mm are substantial. This challenge presents a dilemma to the tissue dielectric property measurement community. While recent tissue property measurement papers published by the O’Halloran group are thorough relative to the methods described, concerns remain about their probe measurement depth estimates and their associated property results.
Acknowledgment
This work was sponsored by NIH/NCI Grant #R01-CA191227.
Contributor Information
Paul M. Meaney, Thayer School of Engineering, Dartmouth College, Hanover, NH 03755 and Chalmers University of Technology, Gothenburg Sweden 412 96.
Andrew P. Gregory, National Physical Laboratory, Teddington, UK TW11 0LW
Tapani Lahtinen, Delfin Technologies, Kuopio, Finland 70210.
Keith D. Paulsen, Thayer School of Engineering, Dartmouth College, Hanover, NH 03755.
References
- [1].Grant JP, Clarke RN, Symm GT, Spyrou NM, “A critical study of the open-ended coaxial line sensor technique for RF and microwave complex permittivity measurements,” J. Phys. E: Sci. Instrum, vol. 22, no. 9, pp. 757–770, September 1989. [Google Scholar]
- [2].Hagl DM, Popovic D, Hagness SC, Booske JH, Okoniewski M, “Sensing volume of open-ended coaxial probes for dielectric characterization of breast tissue at microwave frequencies,” IEEE Trans. Microw. Theory Techn, vol. 51, no. 4, pp. 1194–1206, April 2003. [Google Scholar]
- [3].Perez MD, General effective medium model for the complex permittivity extraction with an open-ended coaxial probe in presence of a multilayer material under test, PhD Thesis, University of Bologna, Bologna, Italy, 2012. [Google Scholar]
- [4].Alanen E, Lahtinen T, Nuutinen J, “Variational formulation of open-ended coaxial line in contact with layered biological medium,” IEEE Trans. Biomed. Eng, vol. 45, no. 10, pp. 1241–1248, October 1998. [DOI] [PubMed] [Google Scholar]
- [5].Gregory AP, Clarke RN, Hodgetts TE, Symm GT, “RF and dielectric measurements upon layered materials using coaxial sensors,” National Physical Laboratory, Report MAT 13, 2008. [Google Scholar]
- [6].Meaney PM, Gregory AP, Epstein NR, Paulsen KD, “Microwave open-ended coaxial dielectric probe: interpretation of the sensing volume revisited,” BMC Med. Phys, vol. 14, no. 3, paper # 1756–6649, June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meaney PM, Gregory AP, Seppälä J, Lahtinen T, “Open-ended coaxial dielectric probe effective penetration depth determination,” IEEE Trans. Micro. Theory Techn, vol. 64, pp. 915–923, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mayrovitz HN, Weingrad DN, Lopez L, “Assessing localized skin-to-fat water in arms of women with breast cancer via tissue dielectric constant measurements in pre-surgery and post-surgery patients,” Ann Surg Oncol, vol. 22, no. 5, pp. 1483–1489, May 2015. [DOI] [PubMed] [Google Scholar]
- [9].Porter E, O’Halloran M, “Investigation of histology region dielectric measurements of heterogeneous tissues,” IEEE Trans. Antennas Propag, vol. 65, no. 10, pp. 5541–5552, October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


