Abstract
Long-term primate field research programs contribute to the protection of endangered primate species and their vanishing habitats by informing and fostering local and international conservation programs. The Kibale Chimpanzee Project (KCP) has studied the Kanyawara community of wild chimpanzees continuously since 1987, investigating a wide range of behavioral, ecological, and physiological questions. The study area includes the northwest boundary of Kibale National Park, Uganda, and has experienced habitat change driven by multiple causes, including forest regeneration, an increasingly warmer and wetter climate, and impacts from the neighboring human population. Here, we review the history of research on Kanyawara chimpanzees and examine how their demography, diet, and social behavior have changed over the last 30+ years. While Kanyawara chimpanzees were protected from the major threats of poaching and habitat loss, respiratory diseases of human origin were a major source of mortality. Many individuals were also injured by wire hunting snares. Nevertheless, the study community has grown modestly in size, individuals have become increasingly gregarious, and birth rates have increased. These results are likely attributable to improved habitat productivity that can be traced to decades-long efforts by wildlife authorities and the associated research and conservation programs in Kibale. Overall, research has contributed both to understanding interactions among nutritional ecology, social behavior, physiology, and health of an endangered species, and also to conservation activities in the Kibale community through direct interventions, positive economic impacts, and conservation education programs.
Keywords: primates, ecology, habitat change, human-wildlife conflict, physiology, forests
1. Introduction
1.1. The legacy of long-term primate research
In this paper, we discuss research and conservation contributions from the Kibale Chimpanzee Project, a long-term field study of wild chimpanzees in Uganda. Long-term field research on non-human primates occupies a unique niche at the intersection of tropical ecology, animal behavior, and conservation science. Most diurnal primates are relatively large, live in social groups, can be individually identified, and occupy stable home ranges. Unlike many species, primates require extensive periods of habituation, but can then be readily and safely followed through most or all of their daily activities. They also exhibit complex cognition and social behavior, requiring extended periods of direct observation to adequately characterize. Primates are relatively long-lived, with slow rates of reproduction and long periods of development, meaning that it takes years, or even decades, of study to address key questions about fitness. Thus, out of feasibility and necessity, the model for modern field research in primatology is quite different from most field biology approaches: researchers observe the same groups of animals continuously for many hours a day over many years. In tandem, most studies accumulate meticulous detail on habitat variation and its effects on study groups. While this format is unusual in biology, it has yielded enormously valuable datasets comprising many lifetimes of individual primates.
The legacy of long-term research on great apes began sixty years ago when Jane Goodall initiated her studies of chimpanzees in Tanzania (Wilson et al., this issue). She founded the Gombe Stream Research Center, dedicated to the continued study and protection of chimpanzees, in 1965. This was soon followed by the establishment of the Mahale Mountains Chimpanzee Research Project by Toshisada Nishida (in 1965) and the Karisoke Research Center (in 1967), where Dian Fossey observed mountain gorillas. These projects, which continue today, have inspired the development of many other long-term research sites. Because chimpanzees are our closest living relatives, exhibiting similarities to humans in life history, physiology, and cognition, they are one of the most intensely studied animals in the wild. Chimpanzees have been studied in at least 120 localities in 20 countries, including at least 10 with continuous research lasting a decade or longer (McGrew, 2017). Each study site has unique features that contribute to understanding the ecological, demographic, and behavioral diversity of this species.
1.2. The challenge of chimpanzee conservation
Primates are among the most endangered of mammalian taxa, owing largely to the influence of deforestation and other human activities (Estrada et al., 2017). While chimpanzees face a less critical risk of extinction than some, they are endangered and their numbers are declining in the face of urgent conservation challenges, particularly as the majority of chimpanzees live outside protected areas (Heinicke et al., 2019). The IUCN identifies poaching as the greatest threat to chimpanzees because it can quickly devastate local populations (Humle et al., 2015). Targeting of chimpanzees for meat or body parts varies across their range. However, chimpanzees are also frequently killed incidentally by wildlife traps, in retribution for crop raiding, or as a means to obtain live infants for the pet and entertainment trades. Habitat loss, due to land clearing for subsistence and industrial agriculture or mineral extraction, to extraction of forest products, and to climate change, is a more insidious threat, reducing the figure viability of chimpanzee populations. Habitat loss also exacerbates threats to chimpanzees from poaching and disease, as it increasingly puts chimpanzees in close contact with human populations and provides the infrastructures (e.g., roads, markets) that make chimpanzees more vulnerable to poaching (Morgan et al., 2019).
In Uganda, unlike in most of their range, chimpanzees live primarily in protected areas (national parks and forest reserves) (Plumptre and Cox, 2006). Kibale National Park is reported to have the highest recorded population density of any forest surveyed in Africa, averaging 2.3 per sq km (Plumptre et al., 2003). It also offers nationally significant ecotourism facilities. Long-term research studies are therefore welcomed by the Uganda government as sources of recommendations for conservation. Within Kibale, long-term research on chimpanzees is ongoing at three sites, Kanyawara (since 1987, Wrangham et al., 1996), Ngogo (since 1995, Watts, 2012), and Sebitoli (since 2009, Krief et al., 2014) (Figure 1). Such studies are in a pivotal position to document habitat change and its influences on the health, behavior, and viability of their study groups and to take an active role in catalyzing habitat protection and other conservation actions at a local and international level. In this article, we discuss the successes and challenges of our long-term research program at Kanyawara and its applications to conservation in and around the Kibale ecosystem. In addition, we examine the contributors to habitat change in Kibale and use longitudinal data to assess chimpanzee responses to this change over three decades of study.
Figure 1.
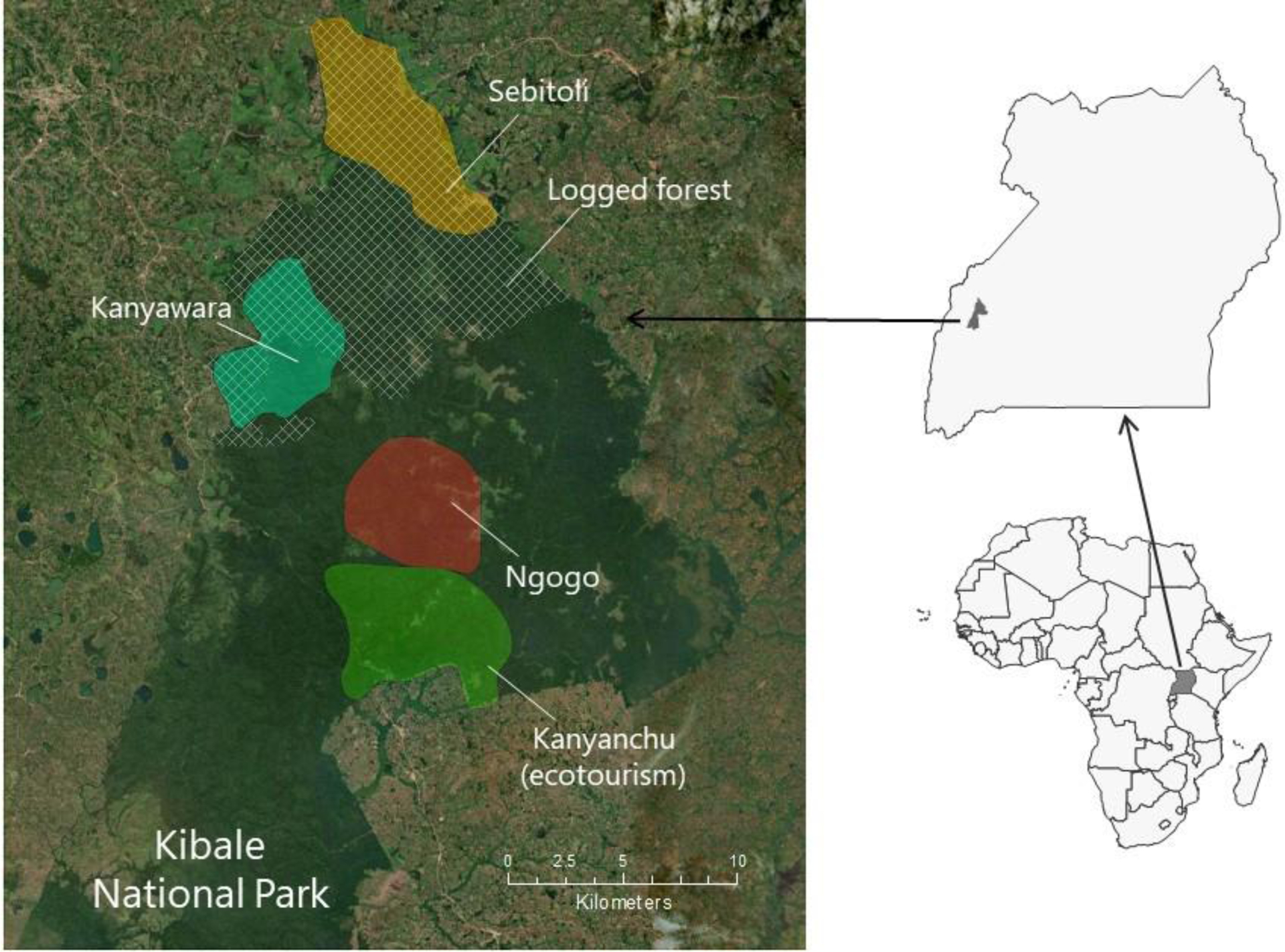
Location of the Kanyawara chimpanzee home range (2009–2017, bright green) within Kibale National Park, Uganda. The margins of Kibale National Park are clearly visible by satellite image, as the heavily forested areas are bounded by villages and agricultural areas. Shown also is the formerly logged portion of the forest (hatched area) and the approximate locations of other major chimpanzee study communities and the ecotourism site. Map courtesy of Jillian Rutherford.
2. The Kibale Chimpanzee Project
2.1. The Kanyawara field site
The Kibale Chimpanzee Project (KCP) is based in Kibale National Park, a 766km2 reserve located just north of the equator and east of the Rwenzori Mountains in southwestern Uganda (Fig. 1). The study area, Kanyawara, sits on the western boundary of the park, at an elevation of ~1500 meters. It is a mosaic of primary forest, logged forest, grassland, and swamp.
Kibale soil quality exceeds that of most tropical forests. Consequently, Kibale trees exhibit low levels of secondary compounds, and support one of the world’s highest reported densities of non-human primates (Struhsaker, 1997). Twelve primate species are known in the park, including Uganda’s largest population of chimpanzees (Pan troglodytes schweinfurthii). Wanyama et al. (2010) estimated a total chimpanzee population of 921, using nest counts, but recent genetic data suggest that this is a considerable underestimate (K. Langergraber, pers. comm.).
The Kanyawara field site was established in 1970 by Tom Struhsaker, who came to study red colobus monkeys in what was then the Kibale Forest Reserve, (gazetted in 1932: Struhsaker, 1997). The study was planned to last two years. However, Struhsaker recognized the potential for comparative behavioral ecology, given the remarkable density and variety of primates, and stayed on for two decades, creating the Kibale Forest Project (KFP).
2.2. Establishment of the long-term chimpanzee study
From 1970–1988 more than two dozen researchers worked in Kibale under the aegis of KFP. The first to systematically study chimpanzees was Michael Ghiglieri. He was based mainly at Ngogo, in the center of the Reserve (Fig. 1), but between 1977 and 1981 he spent around 50 hours observing chimpanzees in Kanyawara (Ghiglieri, 1984). Ghiglieri described the Kanyawara chimpanzees as being particularly tolerant of humans, perhaps because of exposure to forestry workers. He identified 24 individuals, but did not spend enough time at the site to get a good estimate of community size.
The first detailed work on the Kanyawara chimpanzees was Gilbert Isabirye-Basuta’s study of feeding ecology from 1983 to 1985 (Isabirye-Basuta, 1988). Basuta estimated a community size of 50, but only one of his study subjects, a male with a crippled foot, was clearly recognizable from Ghiglieri’s descriptions. Observers were then absent between January 1986 and September 1987, during which time several of the chimpanzees identified by Basuta disappeared.
Richard Wrangham initiated the Kibale Chimpanzee Project in 1987, shortly before Struhsaker’s Kibale Forest Project transitioned into the Makerere University Biological Field Station (MUBFS). Since 1987 the Kanyawara chimpanzees have been under continuous observation by KCP staff. Kibale became a National Park in 1993, and MUBFS continues to maintain the research and training infrastructure that includes an extensive trail system.
The Kanyawara chimpanzees have never been provisioned. In the early days of the project, researchers waited near fruiting trees and revealed themselves to individuals who came to feed. Over time, observers were perceived as less threatening, with adult males the first to become fully habituated. Habituation was achieved through the persistence of daily full-day follows coupled with conservative observation distances. Infants and immigrant females have required considerably less time to habituate, perhaps because they take cues from their groupmates.
In many ways, the Kanyawara chimpanzees are extraordinarily ordinary. Their community size, population density, body size, and food abundance all fall near the mean, across sites. They have avoided the worst anthropogenic influences, enjoying relatively low mortality (Muller and Wrangham, 2014). They have a decidedly limited technological repertoire. Yet Kanyawara is unique among chimpanzee field sites in pairing detailed behavioral observations with ambitious long-term protocols for non-invasive biological sampling and photogrammetric measurement. These approaches have given us an unprecedented ability to examine individual health, physiology, and development, and their links to ecology and social behavior.
2.3. Research Approach
Data on the Kanyawara chimpanzees are collected daily by a team of Ugandan field assistants (typically 2–4 per day), in collaboration with visiting academic researchers. Field assistants are responsible for implementing a consistent, core data collection protocol, and visitors undertake more focused studies that complement and extend the long-term data. Whenever possible, observers follow chimpanzees from the time that they wake in the morning (around 6:00) until they construct their night nests (11–13 hours later). Chimpanzees are usually located at the site where they nested the previous evening, but also by following their tracks, listening for calls, or waiting near fruiting trees. While the chimpanzee community is a clearly defined social unit, members form temporary subgroups - or parties - that fluctuate in size, composition, and duration. Multiple teams sometimes follow different chimpanzee parties simultaneously.
Three types of data are collected daily: (1) assessments of reproductive and health status (presence of clinical signs) for every chimpanzee observed; (2) group-level observations of party composition, location, and behavior; (3) detailed focal data on one or more individuals. Biweekly transect-based phenology surveys are also used to assess the availability of chimpanzee plant foods. Field assistants generally work in pairs, with one person collecting the group-level data, and the other collecting focal observations.
Group-level observations include (a) instantaneous scan samples at 15-minute intervals for party composition, location on a grid map, feeding behavior (species and part of the plant eaten by the majority of chimpanzees), and the presence of monkeys; (b) a continuous ad libitum record of significant behaviors, including grooming, nesting, tool use, and vocalizations; and (c) all-occurrence sampling (on structured datasheets) of specific target behaviors, including hunting, intergroup encounters, aggression, and copulations.
Focal observations record (a) activity (e.g., resting, feeding, traveling), (b) the identity of individuals in close proximity (nearest neighbor and all within a 5m radius), and (c) detailed data on target behaviors such as aggression and copulations. Between 1995 and 2008 these took the form of 10-minute focal follows, with observers switching among available individuals. In 2009 we initiated all-day focal follows, with observers switching targets only if the original focal was lost. Since 2010 we have collected continuous GPS location data on focals. Since 2015 we have collected detailed health data, including ratings of body condition and mobility, breathing rate, clinical signs, and assessment of body parts for wounds or abnormalities. For immature chimpanzees, additional data are collected on distance to the mother and play behavior. While it is not possible to randomize the selection of focal individuals, observers aim to minimize bias by prioritizing chimpanzees that have been followed less frequently or recently. We obtain an average of 14 follows (133 observation hours) per year from each mature chimpanzee and 12 follows (117 hours) per year from each immature chimpanzee.
KCP is unusual among field studies in the extent to which non-invasive fecal and urine collections are integrated into our routine field protocols. When a chimpanzee urinates from a tree, observers trap urine on a disposable plastic bag attached to a two-meter pole. If a bag cannot be placed in time, then urine is pipetted from leaves in the ground layer of vegetation. Fecal sampling was conducted intermittently from the beginning of the project, and has been routine since 2015. We maintain a field laboratory that includes two −20°C solar freezers and employ a variety of other methods for sample preservation (e.g., fixation, drying, buffering of genetic material). Urine and feces have yielded a wealth of information on aspects of chimpanzee biology, including: reproductive condition and drivers of mating effort (Muller and Wrangham, 2004a; Emery Thompson, 2005; Muller and Wrangham, 2005; Emery Thompson et al., 2006; Emery Thompson and Wrangham, 2008a; Emery Thompson and Wrangham, 2008b; Fedurek et al., 2016; Muller, 2017), physiological stress (Muller and Lipson, 2003; Muller and Wrangham, 2004b; Muller et al., 2007; Kahlenberg et al., 2008a; Emery Thompson et al., 2010; Emery Thompson et al., 2020a), growth and development (Emery Thompson et al., 2016; Sabbi et al., 2019), diet and energetic condition (Emery Thompson et al., 2009; Emery Thompson et al., 2012b, 2014; Emery Thompson, 2017; Emery Thompson et al., in press), paternity and relatedness (Surbeck et al., 2019; Muller et al., 2020), sources of infection (Ashford et al., 2000; Rushmore et al., 2015; Negrey et al., 2019), digestive efficiency (Weary et al., 2017), and other biomarkers of health (Kelley et al., 2004; Krief et al., 2005). These sources of data enhance the potential for hypothesis testing but additionally provide a toolkit for monitoring the welfare of the chimpanzees over time.
Since 2012, we have systematically collected photographic data to assess body size, dental emergence, and tooth wear/loss, as well as to document the status of wounds and other clinical indicators (e.g., skin problems) (Smith et al., 2013; Machanda et al., 2015). KCP also curates a skeletal collection of chimpanzees and other mammals, providing a resource for comparative studies of morphology, pathology, and stable isotope ecology (Carter et al., 2008; Nelson, 2013). We maintain a long-term database of nutritional biochemistry values for chimpanzee and monkey foods, and are developing an accompanying virtual herbarium (Conklin-Brittain et al., 1998; Wrangham et al., 1998; Uwimbabazi et al., 2019). In 2019, we implemented a veterinary program, in collaboration with the Uganda Wildlife Authority and the Jane Goodall Institute, to improve health monitoring and permit life-saving interventions in cases of snaring or other human-induced injuries.
KCP data are recorded on paper in the field. Assistants are assigned at least one day per week to review and clean their datasheets. These are subsequently scanned and then transcribed into spreadsheets by a team of Ugandan data assistants. Spreadsheets are reviewed by the field managers before being sent to the project directors. Thus, data undergo three layers of review and quality control before they leave the field. They are inspected for a final time in the US, when they are integrated into KCP’s relational database stored on secure, mirrored servers. To facilitate data sharing, scanned datasheets have been uploaded to the Dataverse Project maintained by the Harvard Institute of Quantitative Social Science. We additionally work with interested collaborators to provide customized data queries.
3. Scientific contributions
Early research at Kanyawara centered on documenting chimpanzee diets and testing the role of feeding ecology in shaping group organization and cooperative behavior. Since then, KCP research has maintained a prominent focus on ecology, and particularly energy availability, as a critical constraint on social behavior and life history strategies. As such, this body of work provides much-needed empirical evidence to evaluate the potential influences of habitat change on chimpanzees. Since 1987, almost 200 publications have incorporated primary data from the Kanyawara chimpanzees. We highlight here five major themes with significant applications to conservation biology: feeding ecology, socioecology, reproductive ecology and behavior, development, and health.
3.1. Feeding ecology
Early studies at Kanyawara confirmed the notion that chimpanzees are ripe fruit specialists, even compared to frugivorous monkeys studied in the same forest (redtail Cercopithecus ascanius, blue monkey C. mitis, gray-cheeked mangabey Lophocebus albigena). Kanyawara chimpanzees ate ripe fruit out of proportion to its abundance and were more sensitive to antifeedants, such as tannins, neutral-detergent fiber, monoterpenes and triterpenes, than were cercopithecines (Isabirye-Basuta, 1988; Conklin-Brittain et al., 1998; Wrangham et al., 1998). Availability of sugary drupes was unpredictable, however, so chimpanzees utilized more reliable, but lower quality figs as fallback foods (Wrangham et al. 1998). Widespread herbaceous piths, consumed more frequently at Kanyawara than at most chimpanzee study sites, were also critical in buffering the chimpanzees from nutritional shortfalls when fruit was scarce (Wrangham et al., 1996). Early observations of feeding behavior at Kanyawara were foundational in the study of chimpanzee self-medication, and particularly the ingestion of whole, rough-surfaced leaves to expel parasites (Huffman and Wrangham, 1994; Wrangham, 1995).
3.2. Socioecology
As predicted by the socioecological model (Wrangham, 1980), heavy reliance on patchily-distributed fruit constrains the sociality of chimpanzees, particularly females. Early work at Kanyawara provided a critical test of this hypothesis, showing that the community fragmented into smaller parties when fruit was scarce (Chapman et al., 1994; Wrangham et al., 1996). Kanyawara males were more gregarious than females and formed stronger bonds (Wrangham, 2000; Machanda et al., 2013). Even when high quality fruit was available, Kanyawara females had reduced energy balance when they associated with more males (Emery Thompson et al., 2014). Mothers were particularly vulnerable, as their juvenile offspring delayed their travel between feeding patches, resulting in lost feeding opportunities (Wrangham, 2000). Mothers may also have avoided groups to reduce the risk of aggression to vulnerable offspring (Otali and Gilchrist, 2005). As in other field sites, Kanyawara males maintained larger home ranges than females, as they defended the community territory, whereas females spent more time in smaller core foraging areas (Chapman and Wrangham, 1993).
Given the energetic constraints on sociality, females rarely exhibited patterns of proximity and grooming with unrelated adults that would indicate strong affiliative bonds (Gilby and Wrangham, 2008). Overt aggression among females was also rare, but females did compete over long-term access to high-quality foraging areas. Female aggression greatly intensified when new immigrants entered the community, threatening residents’ exclusive access to core areas (Kahlenberg et al., 2008b). Consequently, the highest-quality core areas were inhabited by the highest-ranking females. Stress hormone levels were elevated among immigrants, who strategically associated with adult males in an apparent attempt to shield themselves from resident female aggression (Kahlenberg et al., 2008a).
Kanyawara males, by contrast, formed strong and stable bonds with other males, evinced by reciprocated grooming, mutual support in agonistic interactions, and vocal chorusing (Gilby and Wrangham, 2008; Fedurek et al., 2013). The strength of such relationships changed over time, particularly in response to major disruptions in the male dominance hierarchy.
Kanyawara females associated more with males than they did females, but this appears to have resulted primarily from overlapping ranges rather than social preference, as males and females did not exhibit proximity and grooming patterns indicative of strong affiliative bonds (Machanda et al., 2013).
Relations between chimpanzee communities are predictably hostile, sometimes resulting in coalitionary killing (Wilson and Wrangham, 2003). Novel field experiments in Kanyawara established that males were more likely to engage with intruders when they had a numerical advantage that minimized the costs of attacking (Wilson et al., 2001). Consequently, males moved in larger parties and produced fewer vocalizations when they traveled to areas of the range periphery where encounters with strangers were most likely (Wilson et al., 2007). Intergroup interactions occurred more often than expected during periods when one important food species, Uvariopsis congensis, was abundant. This species occurs in groves located along the boundary with a rival chimpanzee community. Thus, the timing of inter-group conflicts appears strongly tied to the distribution and abundance of food resources at Kanyawara (Wilson et al., 2012).
3.3. Reproductive ecology and behavior
Much of the work at Kanyawara has focused on determinants of fertility, including energetic constraints on reproduction, and reproductive strategies of male and female chimpanzees. These studies are unique in the extent to which they incorporate physiological measures, such as assays of steroid hormones or energetic biomarkers, to help quantify reproductive function and the costs and benefits of alternative strategies.
As in humans, the relative levels of ovarian steroids throughout the cycle positively predicted the likelihood of conception in female chimpanzees (Emery Thompson, 2005). Kanyawara females showed increases in estrogen when their preferred foods were abundant, resulting in shorter waiting times to conception (Emery Thompson and Wrangham, 2008a). Females inhabiting resource-rich core areas had shorter birth intervals, higher infant survivorship, and increased ovarian function compared with those in resource-poor areas (Emery Thompson et al., 2007). Assessment of C-peptide of insulin, a biomarker of energy balance, has allowed us to directly examine chimpanzee energetics (Emery Thompson et al., 2009), including the impacts of lactation on mothers (Emery Thompson et al., 2012b). While chimpanzee infants usually nurse for 4–6 years, maternal energy balance recovered substantially during the first two years, indicating that the nutritional demands of older infants on their mothers were relaxed. Resource access was an important mediator of this process, as lactating mothers inhabiting lower-quality foraging areas exhibited lower C-peptide profiles than mothers in food-rich areas (Emery Thompson et al., 2012b). This resulted in considerable variance in interbirth intervals from only 2.2 years to more than 8 years.
Females were frequent victims of aggression from males. Much of this violence functioned as sexual coercion, making females less likely to mate with males other than the aggressor (Muller et al., 2009; Muller, 2017). Specifically, Kanyawara males were most aggressive toward the females who were most likely to conceive, females mated more with their aggressors than with other males, and females who experienced male aggression showed elevated stress levels (Muller et al., 2007; Emery Thompson et al., 2010; Muller et al., 2011). Thus, intersexual conflict was an important contributor to the costs of reproduction.
Male chimpanzees competed intensely over both status and mating opportunities, fighting more over older females, and when females were most likely to conceive (Muller et al., 2006; Emery Thompson and Wrangham, 2008b). Male testosterone levels were not strongly affected by energy availability (Muller and Wrangham, 2005). However, mating competition was physiologically costly in the short-term, as indicated by substantial rises in male testosterone and cortisol, and reductions in feeding time, when females were in estrus (Muller and Wrangham, 2004b, a; Georgiev et al., 2014). Status competition also incurred chronic costs, as high-ranking males exhibited elevated levels of stress hormones and testosterone, and reduced energy balance, compared to lower-ranked males (Muller and Wrangham, 2004b, a; Emery Thompson et al., 2009). As in other sites, dominance rank was a strong predictor of male mating success. However, Kanyawara males who supported the alpha male in aggressive conflicts achieved higher mating success, and copulated with less interference, regardless of their own rank (Duffy et al., 2007). Older males relied on this coalitionary tactic to extend their reproductive careers, despite declining condition (Muller et al., 2020). Shifting behavioral strategies across the life course may be critical to balance the costs and benefits of male competition.
3.4. Development
We are currently investigating chimpanzee development using a wide range of longitudinal data on physiology, morphology, and behavior. Wild chimpanzees exhibited slower growth and delayed maturation compared to captive chimpanzees, indicating that developmental processes are sensitive to differences in energy access (Emery Thompson and Sabbi, in press). Photographic data on dental emergence from Kanyawara suggested that these differences may be magnified post-weaning when juveniles need to forage independently (Machanda et al., 2015). Maternal factors were also important. Immature chimpanzees experienced compromised growth when their mothers reproduced again quickly, transferring their nutritional investment to a new infant (Emery Thompson et al., 2016). These growth deficits persisted even into adolescence. Counterintuitively, these were often the offspring of mothers in the best energetic condition, who funneled their energetic surplus into increasing their reproductive rates.
Physiological development in chimpanzees is also delayed in the wild, so we are now examining the integration among physiological, social, and morphological markers of maturation. Among Kanyawara males, testosterone increased sharply around ages 8–9, when males became socially independent from their mothers (Muller, 2017). Males reached adult levels of testosterone by 15–16, when they began to challenge other adult males for status. Measures of muscle mass followed a similar trajectory, and were highly correlated with testosterone (Emery Thompson et al., 2012a). Males who showed earlier developmental increases in testosterone also showed earlier increases in muscle mass, higher adult levels of testosterone, and greater muscle mass in adulthood. Dominance rank, testosterone, and aggression all showed peaks around the same time in young adulthood. Prior to reaching their physical prime, males were unable to recruit coalition partners to assist them in competition (Enigk et al., 2020). Prior to puberty, adrenal maturation in chimpanzees showed striking similarities to humans during a stage that has been linked to the development of complex social behavior (Sabbi et al., 2019).
3.5. Health
While intraspecific violence and anthropogenic disease (section 4) kill many chimpanzees, there is little understanding of the determinants of individual variation in health and longevity, nor of the natural causes of death in the wild. We are currently investigating these issues by conducting longitudinal health assessments, examinations of microorganisms in feces, and multidimensional analyses of the aging process. Like humans, Kanyawara chimpanzees exhibited glucocorticoid dysregulation during aging (Emery Thompson et al., 2020a), as well as immunosenescence, indicated by increased parasite loads (Phillips et al., 2020) and more frequent signs of respiratory illness (Emery Thompson et al., 2018). On the other hand, they exhibited only moderate changes in physical condition and activity with age (Emery Thompson et al., 2020b), and failed to show an increased burden of oxidative stress until shortly before death (Thompson et al., in press). Males exhibited increased social selectivity as they aged (Machanda and Rosati, 2020; Rosati et al., in press), which may comprise a strategy to counteract the negative effects of male competition on health (Emery Thompson et al., 2020a; Negrey et al., 2020). These kinds of analyses would be impossible with solely cross-sectional data, but are feasible at Kanyawara because some individuals have been observed for decades.
4. Habitat Change at Kanyawara
A key advantage of long-term studies is that they provide scope for examining how habitat changes affect the behavior and welfare of study populations. This has important conservation implications, particularly in species under threat from expanding human populations and their impacts, such as habitat disturbance and hunting. On the one hand, human pressure on wildlife has generally increased worldwide, and is expected to influence behavior and population health. On the other hand, these effects may be counteracted, as in Kibale, by improved protections, community conservation education, and other benefits of long-term research. While quantifying features of habitat quality across populations is difficult, the effects of long-term ecological change within a population can be a useful way to generate and test hypotheses about the sources of between-population variation in behavior and life history features.
Five major sources of ecological change have affected the Kanyawara chimpanzees over 30+ years of study: old-growth maturation, removal of exotics, recovery from logging of hardwoods, climate change, and increasing human population density.
The first two appear to have had positive effects on chimpanzees. First, old-growth forest has continued maturing naturally in areas where it has been protected from major human influences for a century or more. The resulting changes in tree species density affect different primates differently, reducing the food supply for blue monkeys Cercopithecus mitis but likely increasing it for chimpanzees (Isabirye-Basuta and Lwanga, 2008). Second, during the 1990s, several stands of exotic softwoods that had been planted on hilltop grasslands by the Forestry Department from 1963 to 1965 were removed (Chapman et al., 2002). Removal of the exotics led to rapid colonization, first by large herbs, and then within less than a decade by fruiting fig-trees (especially Ficus vallis-choudae) that provided a substantial novel food supply for chimpanzees and other frugivores.
The impacts of logging and climate change are less clear. Twenty-five years prior to the 1993 gazetting of Kibale National Park, parts of the Kanyawara area were subject to 6–9 months of managed selective logging for hardwood timber, mainly Olea welwitschii. In recent years species of trees that colonized these areas subsequent to logging have been increasingly replaced by more mature stands, with resulting changes in food availability for primates (Chapman and Chapman, 2004; Omeja et al., 2016). After managed logging ended in 1969, prior to the onset of our study, densities of monkey species tended to decline (Chapman et al., 2000). However, since the declines occurred both in logged and unlogged areas, the specific effects of logging, and recovery from logging, on food availability for frugivores such as chimpanzees are unknown (Isabirye-Basuta and Lwanga, 2008).
Climate change in the last century has included a ~ 20% increase in rainfall and a larger range of temperatures. In the last quarter of the 20th century, the average maximum monthly temperature grew 3.5°C hotter, and the average minimum monthly temperature became 2°C cooler. Fruiting patterns have responded in complex ways (Chapman et al., 2005). Associations among solar radiation, rainfall, and fruiting productivity differed between Kanyawara and the neighboring Ngogo site during recent years, despite overall increased fruit availability at both sites (Chapman et al., 2018; Potts et al., 2020). This may reflect differences in local climate, forest structure, fauna, and other interacting patterns of forest disturbance and recovery. Nevertheless, ENSO (El Niño Southern Oscillation), a composite measure of climate cycles that is influenced by climate change, positively predicted annual fruit availability at Kanyawara between 1998 and 2013 (Chapman et al., 2018), suggesting that thus far climate change may have had some positive impacts for frugivores in this area.
The final change, increasing human population density, has clear negative effects on chimpanzees. The Kanyawara chimpanzees range along the north-west boundary of Kibale National Park, neighboring a growing human population. Forest outside the Park is contracting, as forest fragments are converted into agricultural plots. Until the early 1990s, Kanyawara chimpanzees sometimes traveled 2–3 km outside the Forest Reserve or Park into village areas to visit fruiting trees, but such journeys are now confined to brief excursions close to the Park boundary. Because Kanyawara includes both a Uganda Wildlife Authority outpost and the permanently-staffed Makerere University Biological Field Station, the area benefits from continuous monitoring against large-scale hunting or habitat disturbance. However, the proximity to villages increases vulnerability to smaller-scale activities, including stem cutting and poaching using snares made of wire, nylon or natural vegetation. It also increases exposure to human diseases. As in other great ape populations (Kaur et al., 2008; Köndgen et al., 2008), the Kanyawara chimpanzees have experienced multiple severe outbreaks of respiratory illness. We estimate that 25% of all deaths of Kanyawara chimpanzees over the past 33 years can be attributed to respiratory illness (Emery Thompson et al., 2018). While the timing of outbreaks is not easily explained by metrics of human contact, such as the number of researchers or the frequency of chimpanzees consuming agricultural crops (Emery Thompson et al., 2018), it is clear that the increasing human contact poses increased disease risks to chimpanzees. The two most recent outbreaks at Kanyawara (in 2013 (5 deaths) and 2017 (1 possible death and widespread morbidity)) have been traced to viruses of human origin (Scully et al., 2018; Negrey et al., 2019).
5. Secular Change in Chimpanzee Demography, Diet, and Social Behavior
In the face of these substantial ecological changes, the Kanyawara community of chimpanzees has fared well. Here we examine secular changes in demography, as well as in feeding and social behavior, over the course of the study. For most analyses we examined data from 1994, when all chimpanzees were identified and data collection standardized. Some interbirth intervals are known from Isabirye-Basuta’s observations as early as 1983.
The Kanyawara chimpanzee community has grown modestly over the course of the long-term study (Pearson correlation 1994–2020, r = 0.49, N = 27 years, p = 0.009, Figure 2). The increase has been most notable over the past 15 years, as the community grew from a low of 41 individuals in 2004 to its current maximum of 57. There has been a net positive rate of female immigration: 16 nulliparous females immigrated into the community, and only 10 emigrated. Immigration cannot explain the increase in community size, however, because the number of adult females has remained relatively stable over time (rP = 0.15, p = 0.46). Instead, the increase in community size reflects an increased number of immatures (rP = 0.60, p = 0.0008). Based on three-year moving averages that minimize interannual stochasticity, the rate of mortality has not changed significantly over time (rP = 0.23, N = 24, p = 0.27) nor has the overall birth rate (births/female: rP = 0.37, N = 24, p = 0.11). However, the rate of producing infants that survive to age 4 years has increased (rP = 0.48, N = 21, p = 0.029).
Figure 2.
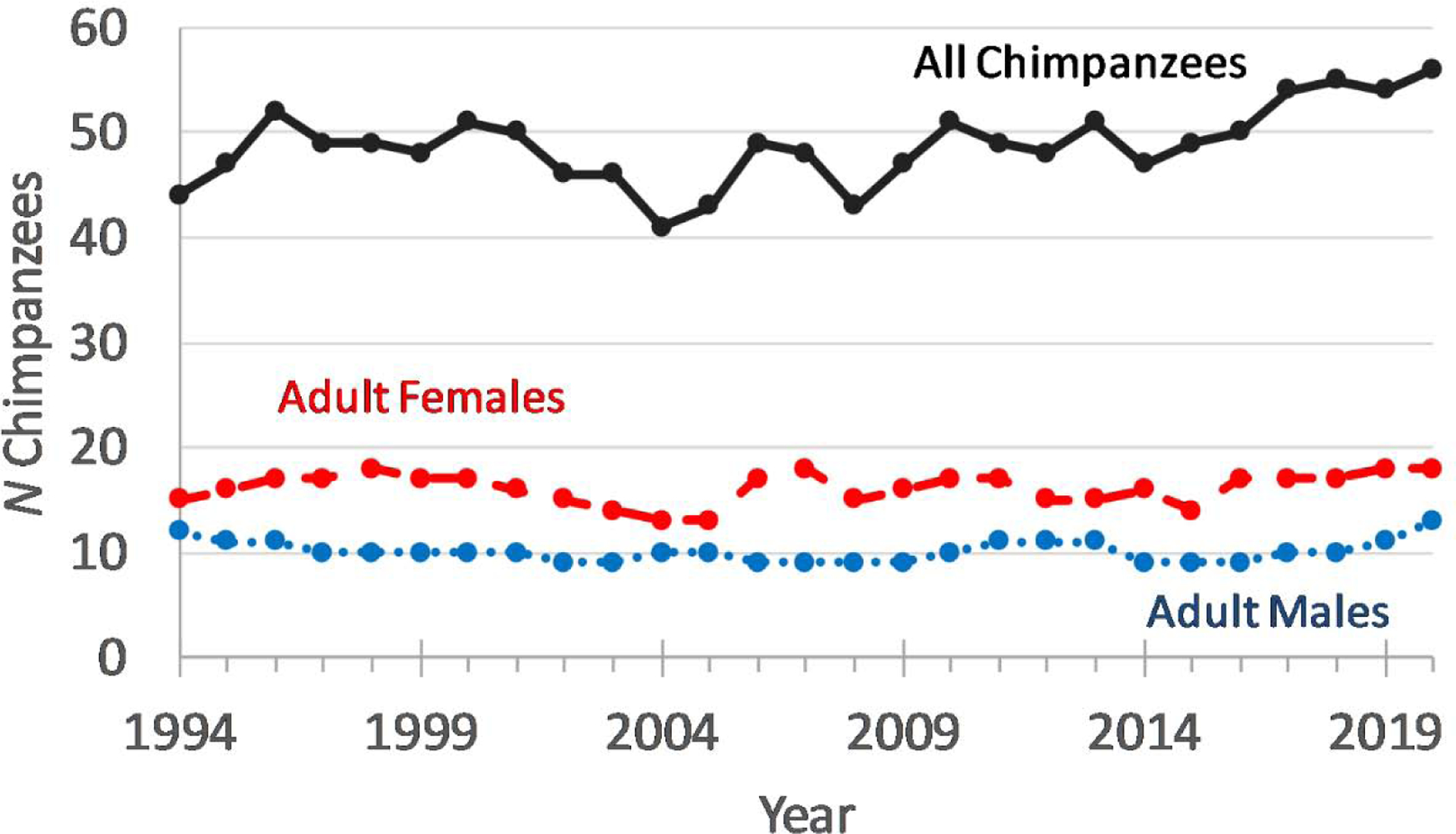
Kanyawara community size from 1994–2020, assessed on January 1 of each year. Adult males (blue, dotted line) are those aged 15 and over. Adult females (red, dashed line) are those who have experienced their first maximal sexual swelling. Total community size (black, solid line) also includes infants, juveniles, and adolescents.
Interbirth intervals across wild chimpanzee study sites average 5–6 years if the first infant has survived to age 4 or to the mother’s next pregnancy (Emery Thompson and Sabbi, in press). Excluding those intervals with uncertain birth dates, we recorded 36 birth intervals between 1983 and 2020 that followed surviving infants and were known to within 3 months’ accuracy. Interbirth intervals have decreased in length significantly (correlation with year at start of interval: rP = −0.50, p = 0.002, Figure 3). We confirmed this within the larger dataset that includes open birth intervals (ending with maternal death or currently open, N = 60). Using a Cox proportional hazards model, controlling for infant sex, we found a nearly 5% increase in the likelihood of birth for each successive year of the study (HR: 1.049, z = 2.248, p = 0.025, model assumptions verified). Intervals that began prior to 2004 had a median length of 5.8 years, while those begun since 2004 have a median length of 4.8 years, lower than reported for any of the 5 wild chimpanzee sites with published fertility data (Emery Thompson and Sabbi, in press).
Figure 3.
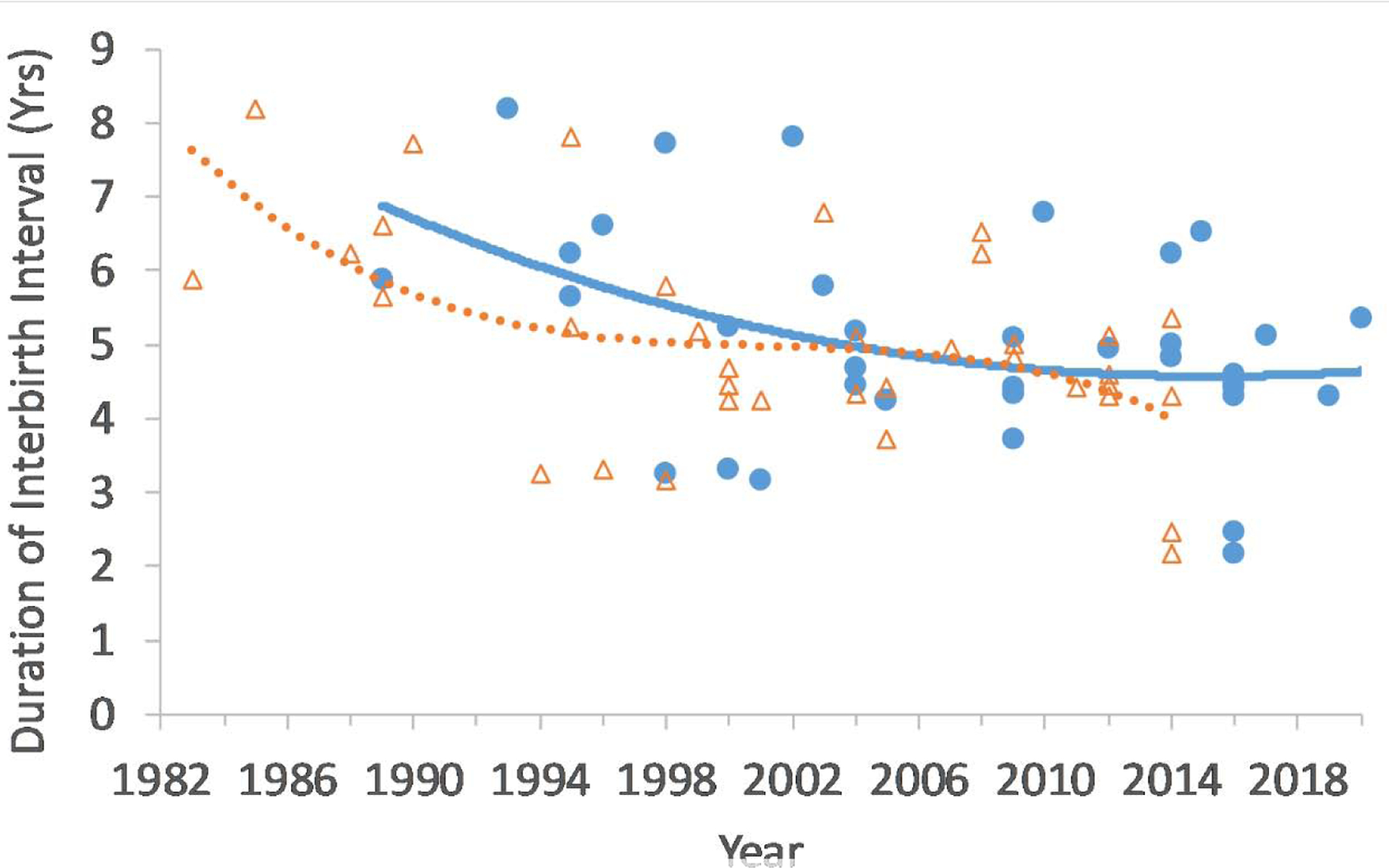
Interbirth intervals for Kanyawara chimpanzee females from 1983–2020. Sample includes only birth intervals following an infant that survived to age 4 or at least to the mother’s next conception and excludes intervals estimated by more than 3 months. The same intervals are plotted twice, once by their start year (orange, dashed line) and once by their end year (blue, solid line), each with third-order polynomial fits.
Because chimpanzees practice fission-fusion grouping, an increase in community size need not mean an increase in party size. However, average party sizes have gradually increased from about 7 to 14 individuals (rS = 0.74, N = 24 years, p < 0.001, Figure 4), despite being defined consistently as all individuals within 50m of others. This was almost exclusively a product of increased gregariousness of adult females (rs = 0.74, p < 0.001) and their dependents, as the average number of adult males in parties has not changed (rs = −0.05, p = 0.83). Greater female gregariousness is particularly notable given that changes to our observation protocols in 2009 (from party follows to all-day focal individual follows) should have reduced any observation bias favoring large parties.
Figure 4.
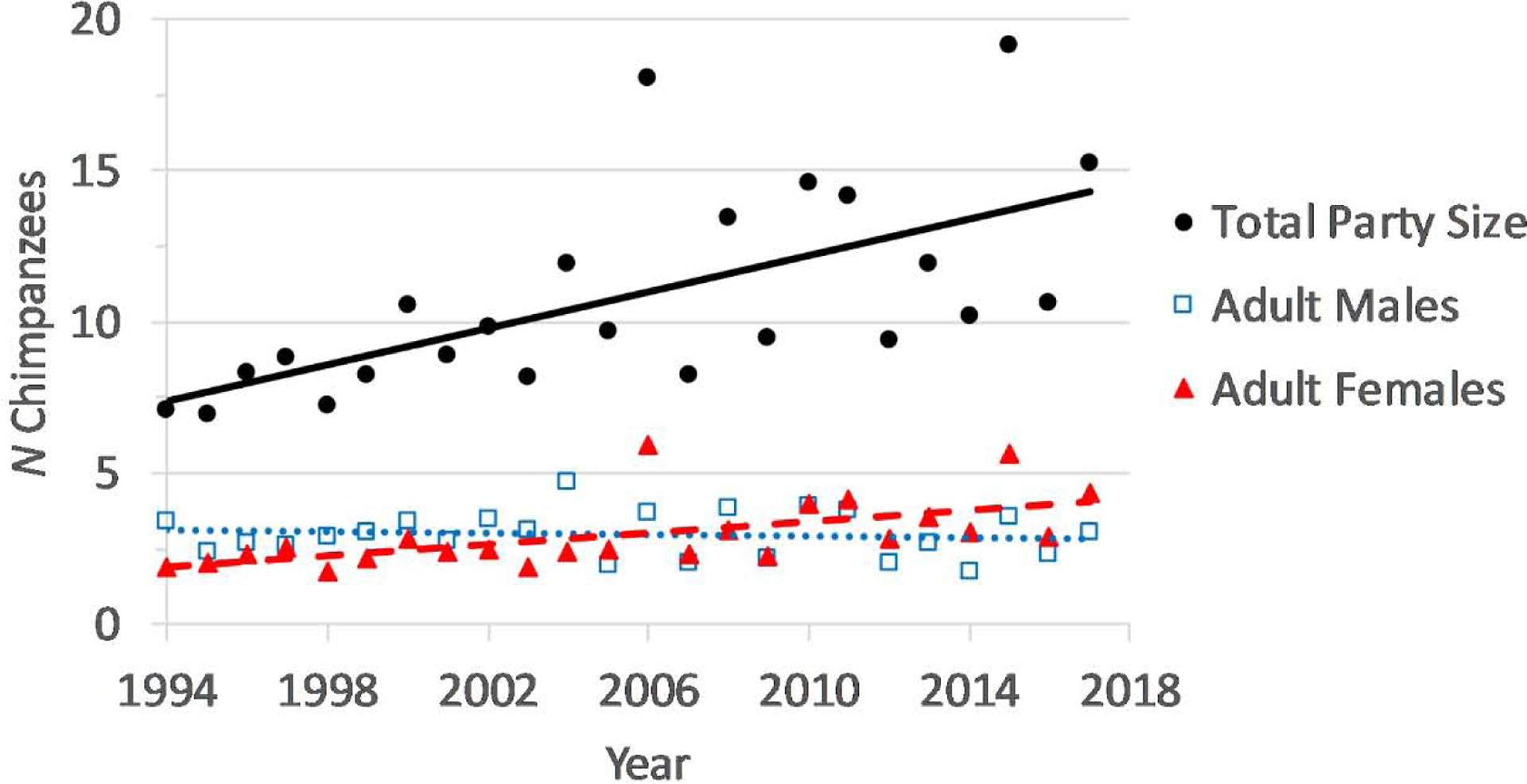
Changes in mean party size from 1994–2017. Shown are the annual means, calculated from daily means of total party size (all individuals, black circle, solid line), adult males (15 years or older, blue squares, dotted line), and adult females (after first maximal swelling, red triangles, dashed line).
In light of prior findings from Kanyawara (section 3), increased fecundity and female gregariousness suggest an improvement in the availability or quality of food resources. Indeed, phenology surveys of both Kanyawara and the nearby Ngogo site have demonstrated increases in fruit availability over the past 20–30 years, perhaps a result of changing climate and/or forest regeneration (Chapman et al., 2018; Potts et al., 2020).
We examined chimpanzee diets from 1994–2018 based on 15-minute scan samples of party activity (N = 240,601 scans with feeding), which are well correlated with results from focal sampling and have been recorded consistently since the onset of the study (Gilby et al., 2010). The broad composition of the diet has changed little (Figure 5a). Leaves, while a minor contributor overall, were the only plant part to change significantly, being consumed slightly more often over time (mean 11.2%, range 6.1–17.1%, rS = 0.54, N = 25 years, p = 0.006). Average consumption of ripe fruits, the major constituent of the diet, showed no consistent secular change (mean 64.0% of feeding time, range 50.0–74.1%, rS = −0.25, p = 0.23). However, the species of fruits that chimpanzees ate did change. First, the overall diversity of fruit species in the diet increased. By 1995, we had recorded 40 fruit species consumed by chimpanzees, and over the next decade, only 9 new species were consumed. Since 2005, 44 new fruit species have been added to the menu, and there is no indication that this trend is reaching a plateau (Figure 5b). This change resulted in an increase in dietary breadth, rather than a mere shift in species composition, as the number of species consumed per year has approximately doubled (rS = 0.93, p < 0.0001). Second, dominant fruit species in the diet changed. Twelve ripe fruit species accounted for a combined 88% of all ripe fruit feeding observations (and 56% of total feeding time) over the study. Several of these are highly seasonal drupes that the chimpanzees gorge on during brief periods. Of these, only Teclea nobilis was consumed more frequently over time (rS = 0.42, p = 0.04). On the other hand, there have been major changes in consumption of fig species. Two of the most commonly consumed species have declined in use (F. natalensis: rS = −0.42, p = 0.04; F. sansibarica: rS = −0.64, p < 0.01), whereas two species that were rarely eaten early in the study have increased to become among the most prominent foods in the diet (F. sur: rS = 0.62, p < 0.01; F. vallis-choudae: rS = 0.95, p < 0.01). While typically eaten ripe, these figs were also among the few species that chimpanzees were willing to eat unripe. We are now investigating whether these dietary shifts have resulted in increased energy intake or reduced energy expenditure. For example, F. sur does not have particularly high energy density per gram (Conklin-Brittain et al., 2006), but its large cauliflorous crops result in efficient foraging, yielding a high rate of energy intake per unit of time (Potts, 2013). Interestingly, no single fruit species appears to be responsible for the increase in party sizes, as larger foraging parties were observed at nearly all of the major fruit species.
Figure 5.
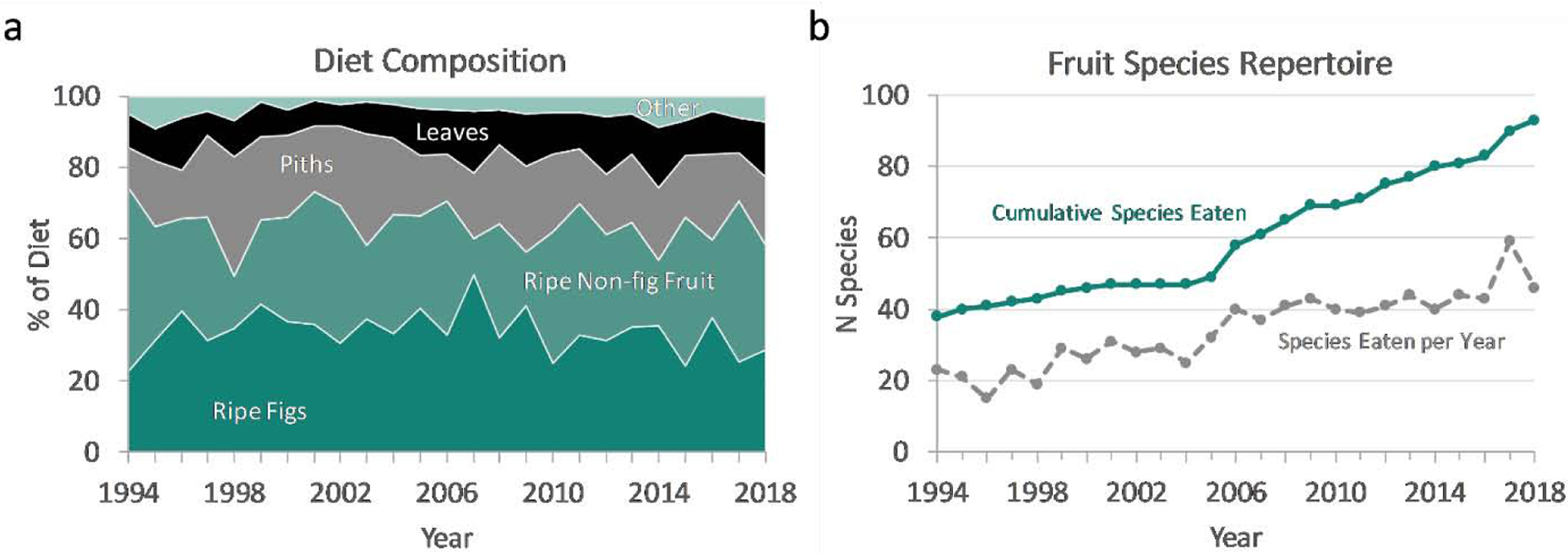
Changes in dietary composition for Kanyawara chimpanzees between 1994–2018, showing (a) major food types in the diet and (b) fruit species repertoire. Percentage of diet calculated from 15-minute scans of feeding at the group level per month and averaged per calendar year. ‘Other’ includes unripe fruit, meat and insects, honey, wood/bark, and flowers. Fruit repertoire indicates counts of cumulative species ever consumed (orange, solid line) and species consumed per calendar year (green, dashed line). Cumulative counts in 1994 include all observations from 1987 to 1994.
The kinds of effects we observed could be consistent with increased habituation, particularly among females, but habituation effects do not appear sufficient to explain many of these patterns. For analyses of community size and behavior, we excluded the first 7 years of observations to constrain our findings to only the time period after which no new adult individuals were identified. As noted above, increases in community size were the product of improved birth rates and infant survival, not the identification of new adults. Our analysis of interbirth intervals excluded those with uncertain dates, a reflection of low observation rates of some females early in the study. These estimated intervals were from 1998 or earlier and were estimated to be relatively long, so excluding them produces a conservative estimate of how much the interbirth intervals have decreased. Further, habituation effects are expected to diminish over time, but most of the change we observed occurred relatively recently or accelerated over time. Such is the case for the chimpanzee diet: some ‘new’ dietary items could be accounted for by chance observations of rare food items, but we should expect the chances of new observations to become increasingly less likely after 30 years of study. Similarly, female party sizes remained low and stable for nearly 20 years of study and only showed appreciable increases after 2006. Notably, increased gregariousness occurred despite female dispersal, meaning that immigrants, most of whom were not previously habituated to observation, played a large role in this increase.
6. Benefits and challenges of long-term research on chimpanzees
Since the Kibale Chimpanzee Project’s inception in 1987, we have endeavored to maintain continuous, daily data collection, often collecting more than 12 hours of observations per day. While the focus on a single community necessarily produces a relatively small sample of individuals, we obtain exceptionally rich longitudinal detail. This approach has several advantages. Longitudinal datasets allow for an increased ability to distinguish cause and effect from correlation. This is crucial for studies of processes like development and aging, to illustrate that phenomena are not driven by particular individuals and to follow-up developmental outcomes. Continuous data collection allows for adequate sampling of rare events, such as hunting or tool use, and precise quantification of demographic events, such as the timing of births, deaths, and dispersal. Perhaps most importantly, complex processes such as the formation and maintenance of social relationships cannot be accurately characterized without thorough sampling of the component behaviors. Given that the lifespan of chimpanzees exceeds 60 years in the wild (Muller and Wrangham, 2014; Wood et al., 2017; Emery Thompson and Sabbi, in press), our research study (30+ years) is still young. Each year of study adds disproportionately to the value of the dataset and increases the capacity to address new questions.
Because we are studying an endangered species in the wild, our study relies on non-invasive methods. We take every precaution to minimize disruptions to the chimpanzees, and due to the possibility of anthropogenic disease transmission, we observe strict post-travel quarantine protocols and maintain an observational distance of at least 5 meters. This is a disadvantage compared with captive studies that can obtain weights and blood samples, and conduct experimental manipulations. However, these limitations are outweighed by the advantages of studying animals in their natural environment. The lives of wild animals are structured around meeting their nutritional needs, so everything from ranging and grouping to cooperation and competition may be affected by the constraints of when and where to feed. Wild studies preserve the influence of important selection pressures, such as energy limitations and infectious disease, that have shaped life history and social behavior over evolutionary time. In the absence of these influences, the data would have little applicability for informing species conservation.
Long-term studies require different methodological considerations than conventional studies. While short-term studies benefit from a narrow set of carefully-tailored methods, longitudinal studies require methods that are more versatile and yet able to be maintained reliably over time. Thus, our daily data collection combines structured and unstructured methods to balance detail and consistency. Alongside standardized checksheets, our team collects extensive, open-ended, ad libitum notes on paper. These require significant field effort and present a challenge for data archiving and extraction. However, as our research agenda evolves, the details from these long-hand notes allow for exploration of new questions that we may not have envisioned previously. They provide a solid foundation for retrospective data analysis, particularly for rare events, and allow for re-coding for harmonization with comparative datasets from other sites. Long-term data often form the basis of pilot studies to launch new research protocols, and in many cases, provide an essential companion to focused studies that collect fine-grained data over shorter periods of time. Similarly, while most field studies collect small quantities of biological samples to address specific questions, we maintain a continuous, open-ended program of urine sample collection. This entails significant supply, storage, and export hurdles, but provides flexibility for us to retrospectively select samples that match new study criteria. As new assay techniques become available, the oversupply also enables us to apply them to previously unused specimens.
An essential ingredient to the success of our project has been a sedulous team of local Ugandan staff and counterparts. Between field assistants, data technicians, support staff, a conservation team, and a veterinarian, we currently employ 25 Ugandans. Since 2005, field operations have been directed by Dr. Emily Otali, whose local knowledge and scientific experience studying chimpanzees combine to make her indispensable for managing routine activities, such as obtaining permits and coordinating payroll, but also for monitoring data quality and dealing with a wide range of emergencies. Rigorous programs for staff training, including periodic assessment and retraining, are essential to ensure that we can make effective use of data collected over many years. Recruiting and maintaining high quality staff is a challenge, as the field work involves long hours and is physically demanding. This requires investment in personnel welfare - competitive salaries, healthcare and benefits. KCP has also made investments in formal education for staff, including master’s and PhDs, to secure the future of our project and promote Ugandan commitment to wildlife conservation. Maintaining such a large permanent field staff is among the biggest challenges for long-term research, as many funding agencies are reluctant to support basic infrastructure costs. In addition to generating the continuous longitudinal dataset, the local field team is essential for training visiting researchers. They further serve as ambassadors to the local community to communicate our conservation message.
7. Contributions of Chimpanzee Research to Conservation
All chimpanzee populations in Uganda are threatened by the same factors that affect chimpanzees across Africa, namely habitat loss, poaching and illegal trafficking, and anthropogenic disease (Humle et al., 2015). Although many long-term studies, including KCP, start with a focus purely on research, over the years they inevitably confer both direct and indirect conservation benefits (Pusey et al., 2007; Wrangham and Ross, 2008; Tagg et al., 2015). Our experience illustrates how research leads to multi-dimensional efforts to engage governments and local people in a common understanding that a particular species and its habitat are worth saving.
First, research can generate political pressure and economic incentives for habitat protection. Chimpanzee researchers have been instrumental in designating the forests in which they work as protected areas, as occurred with Gombe and Mahale National Parks in Tanzania. Similarly, Kibale Forest was designated as a National Park in 1993, 23 years after Tom Struhsaker initiated his research in Kanyawara and began lobbying for this outcome. These protected areas, in turn, attract diverse investigations using the same infrastructure. At Kibale, once MUBFS was established in 1987, other researchers studying monkeys, fish and other communities of chimpanzees began their own long-term projects, each of which organizes their own conservation activities.
Second, research engages the wider public. Long-term research projects have made it possible to highlight complex and fascinating chimpanzee behavior in books, photos, and movies. As a result, chimpanzees have become one of the best known mammals and a leading ‘charismatic’ or flagship species for conservation (Albert et al., 2018). Researchers have also collaborated with local governments to develop, support, and promote chimpanzee ecotourism. In 1988, Wrangham proposed the development of an ecotourism site at Kanyanchu in central Kibale, which was eventually opened in 1991. He served as an advisor to the site in its early years, and KCP field assistants periodically train the Kanyanchu staff in chimpanzee identification and behavior. While ape-based ecotourism can bring the risk of disease transmission, if implemented well, it can promote local economic development, solidify political support for protected areas, and serve as a uniquely effective conduit for spreading conservation messages to an international audience.
Third, research provides data in aid of conservation efforts. For example, at Kanyawara, crop-raiding by primates was reduced when key wild foods were available, indicating that conservation of particular tree species could reduce human-animal conflict (Naughton-Treves et al., 1998). However, chimpanzees consumed maize regardless of fruit abundance in the forest, leading to the practical recommendation that it be planted away from forest boundaries. An understanding of how feeding ecology affects chimpanzee health and reproduction can inform decisions about what kinds of areas should be prioritized for conservation. Data on wild chimpanzee behavior and diet has directly aided the selection of release sites and guided the process of reintroducing wild-born captive chimpanzees (Tutin et al., 2001; Goossens et al., 2005). Chimpanzee researchers at Kanyawara and elsewhere have identified the pathogenesis of major disease epidemics (Leendertz et al., 2004; Kaur et al., 2008; Köndgen et al., 2008; Scully et al., 2018; Negrey et al., 2019), and have additionally provided epidemiological data that are essential to shape long-term risk assessment and management (Lonsdorf et al., 2006; Emery Thompson et al., 2018). Sample collection enables additional health monitoring to uncover the proximate determinants of energetic condition, reproductive function, and stress.
Fourth, long-term researchers directly engage in combating illegal activity. In Uganda, intentional poaching of chimpanzees is rare. However, hunters set large numbers of wire snares, intended to capture small terrestrial mammals, that can also ensnare chimpanzees. At some major study sites, including at Kanyawara, approximately 1 in 3 chimpanzees exhibit injuries, including missing and malformed digits or extremities (Quiatt et al., 2002). In 1997 we established the Kibale Snare Removal Project (KSRP) with the support of the Uganda Wildlife Authority (UWA). Directed by Drs. Jess Hartel and Emily Otali, KSRP works with UWA rangers to search for snares and other illegal activity. Since 1997, snare removers have found and destroyed approximately 8500 snares (averaging between 40–60/month). Parallel snare removal projects are operated elsewhere in Kibale by research teams at Sebitoli and Ngogo. We found that snare density is lower in the home range of the Kanyawara chimpanzees, which is visited daily by research teams, than in neighboring areas that are monitored less often, and the frequency of snare injuries in Kanyawara chimpanzees has fallen (Hartel et al., in press). KSRP patrolling might also contribute to the increase in canopy cover between 2000–2010 in Kanyawara compared to the rest of the park (Hartel et al., in press). KCP and KSRP also provide alerts to UWA about illegal activities and injured wildlife. These have triggered rapid interventions by UWA veterinary teams to remove snares from chimpanzees before they caused loss of limb or life. Recently, with the support of the UWA and with financial support from the Jane Goodall Institute, KCP and KSRP initiated a new veterinary program to allow for a rapid response to any wildlife threats in Kibale National Park. Similar effects of long-term research sites in improving conservation outcomes have been seen across the continent. In Ivory Coast, the presence of a long-term field site positively correlated with measures of biodiversity relative to areas without researchers (Campbell et al., 2011). Specifically, densities of primates, including chimpanzees, are higher closer to research stations (Köndgen et al., 2008; Campbell et al., 2011; N’Goran et al., 2012).
Finally, conservation initiatives can only be successful if local people living around protected areas are treated as partners and can benefit from the presence of the protected areas. In and around Kibale National Park, a number of community development organizations have been established by long-term researchers and their families (Kasenene and Ross, 2008; Goldberg et al., 2012; Chapman et al., 2015). One such organization, the Kasiisi Project, was founded by Dr. Elizabeth Ross in 1997. Kasiisi Project and KCP have been conservation partners for the last 20+ years and while KCP has focused their efforts on conservation initiatives within Kibale National Park, Kasiisi Project works on conservation solutions outside of the Park. Specifically, they work with approximately 10 000 children who attend 16 government primary schools that are within 5km of Kibale. By focusing their efforts in schools and linking educational resources to the well-being of the National Park, Kasiisi Project hopes to create opportunities for children so that they will not view the Park as a source of free food and other materials. Although conservation and wildlife education are the major foci of Kasiisi Project’s efforts, other initiatives concern health, literacy, and scholarship programs as well as construction and sustainable energy initiatives. As a result of these programs, students at Kasiisi Project schools have performed better academically than students at peer schools (MacKenzie et al., 2017; Hartel et al., in press). Over the past ten years, students’ attitudes to both the Park and chimpanzees have therefore significantly improved (Hartel et al., in press). The success of the Kasiisi Project has been credited partly to long-term social relationships between the research community and local people (Kasenene and Ross, 2008).
8. Conclusion
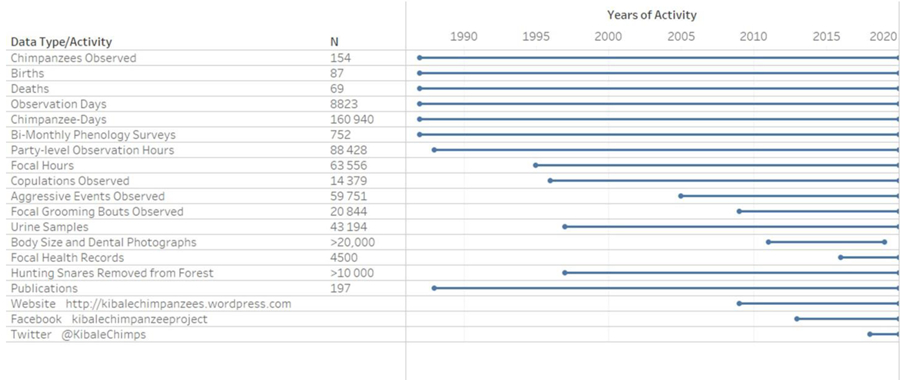
Developing a successful, long-term research project on wildlife means more than simply maintaining access to a study area or population over time. It requires extensive investment in staff development, research infrastructure, and community relations, as well as broad, prospective methods for data collection, and the ability to adapt to new challenges or opportunities while maintaining the highest standards of data quality. Overlaying all these demands, long-term research is intrinsically linked to the welfare of the study system. Over the past 33 years, the Kibale Chimpanzee Project has documented the lives of more than 150 chimpanzees, including three males that have been studied continuously since the project’s beginning. We have compiled over 85 000 hours of observational data and collected more than 40 000 biological specimens (Table 1). Building from foundational work on chimpanzee nutritional and social ecology, KCP has developed one of the richest available longitudinal datasets on wildlife social behavior and physiology, and has become a nexus for collaborative research on a wide range of topics. The growth of our research program has fostered the development of effective conservation initiatives within and outside of Kibale National Park. These efforts may contribute in no small part to what we report here: the Kanyawara chimpanzees have fared well in the face of complex ecological changes. With a healthy community, our success is written not by what we have been able to accomplish thus far but what is in store for the future.
Table 1.
Kibale Chimpanzee Project at a Glance
|
Ns for demography and urine samples are through the end of 2019. Other data types calculated at last count at end of 2017 or 2018.
Highlights.
Kibale Chimpanzee Project has studied wild chimpanzees in Uganda for >30 years
The chimpanzees have thrived despite numerous sources of habitat change
Increased birth rates and female sociality suggest improved feeding conditions
Our research informs on predictors of chimpanzee health, reproduction, and welfare
Long-term primate research sites perform an essential role in species conservation
Acknowledgments
Long-term research at KCP depends on the dedicated work of a large team. We thank present and past field managers Maggy Kobusingye, Adam Arcadi, Colin Chapman, Ken Cochrane, Kim Duffy, Carole Hooven, Alain Houle, Samuel Koojo Mugume, Katharin Pieta, Michael Wilson; conservation partners Elizabeth Ross, Jessica Hartel, Nelson Bukamba, Sonya Kahlenberg, and Amy Pokempner; field assistants Wilberforce Tweheyo, Seezi Atwijuze, James Kyomuhendo, Fred Bugama, Stephen Alio, Bashil Musabe, Michael Mutebi, Francis Mugurusi, the late Donor Muhangyi, John Sunday, Richard Karamagi, Peter Tuhairwe, the late John Barwogeza, Christopher Katongole, the late Christopher Muruuli, Daniel Akaruhanga, Solomon Musana, Japan Musunguzi, and Denis Sebugwawo; data managers Christine Abbe, Joshua Kakwezi, Jovia Mahoro, Edgar Mugenyi, Ashley Menante, Jordan Lucore, Lindsay Hagberg, Kate Burmon, and Jillian Rutherford; research counterparts Gilbert Isabirye-Basuta, the late Jerry Lwanga, John Kasenene, and David Tumusiime; and many collaborators, including Katie Slocombe, Ian Gilby, Nancylou Conklin-Brittain, Tony Goldberg, Drew Enigk, and Kris Sabbi. We thank Reinmar Seidler and Richard Primack for the invitation to contribute to the special issue, and to Cheryl Knott and Michael Wilson for helpful feedback.
Funding: This work was supported by the U.S. National Science Foundation (grant numbers IOS-0416125, BCS-9120960, BCS-1355014, BCS-0849380, BCS-9807448, LTREB-1052693, BCS-1926653, NCSFO-1926352), the National Institute on Aging and National Institutes of Health Office for Research on Women’s Health (grant number R01-AG049395), the Leakey Foundation, the Wenner-Gren Foundation, and the National Geographic Society. The Jane Goodall Institute and Colombus Zoo have provided conservation support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethics permissions: All chimpanzee research has been conducted with approvals from the Institutional Animal Care and Use Committees at Harvard University and the University of New Mexico. Local support and permissions were provided by the Makerere University Biological Field Station, Uganda Wildlife Authority, and Uganda National Council for Science and Technology.
References
- Albert C, Luque GM, Courchamp F, 2018. The twenty most charismatic species. PLOS ONE 13, e0199149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford RW, Reid GDF, Wrangham RW, 2000. Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Annals of Tropical Medicine and Parasitology 94, 173–179. [DOI] [PubMed] [Google Scholar]
- Campbell G, Kuehl H, Diarrassouba A, N’Goran PK, Boesch C, 2011. Long-term research sites as refugia for threatened and over-harvested species. Biology Letters 7, 723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ML, Pontzer H, Wrangham RW, Kerbis-Peterhans J, 2008. Skeletal pathology in Pan troglodytes schweinfurthii in Kibale National Park. Am. J. Phys. Anthropol 135, 389–403. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Balcomb SR, Gillespie TR, Skorupa JP, Struhsaker TT, 2000. Long-term effects of logging on African primate communities: a 28-year comparison from Kibale National Park, Uganda. Conservation Biology 14, 207–217. [Google Scholar]
- Chapman CA, Chapman LJ, 2004. Unfavorable successional pathways and the conservation value of logged tropical forest. Biodiversity & Conservation 13, 2089–2105. [Google Scholar]
- Chapman CA, Chapman LJ, Struhsaker TT, Zanne AE, Clark CJ, Poulsen JR, 2005. A long-term evaluation of fruiting phenology: importance of climate change. Journal of Tropical Ecology 21, 31–45. [Google Scholar]
- Chapman CA, Chapman LJ, Zanne A, Burgess MA, 2002. Does weeding promote regeneration of an indigenous tree community in felled pine plantations in Uganda? Restoration Ecology 10, 408–415. [Google Scholar]
- Chapman CA, Valenta K, Bonnell TR, Brown KA, Chapman LJ, 2018. Solar radiation and ENSO predict fruiting phenology patterns in a 15-year record from Kibale National Park, Uganda. Biotropica 50, 384–395. [Google Scholar]
- Chapman CA, van Bavel B, Boodman C, Ghai RR, Gogarten JF, Hartter J, Mechak LE, Omeja PA, Poonawala S, Tuli D, 2015. Providing health care to improve community perceptions of protected areas. Oryx 49, 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, White FJ, Wrangham RW, 1994. Party size in chimpanzees and bonobos: a reevaluation of theory based on two similarly forested sites, in: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG (Eds.), Chimpanzee Cultures. Harvard University Press, Cambridge, pp. 41–58. [Google Scholar]
- Chapman CA, Wrangham RW, 1993. Range use of the forest chimpanzees of Kibale: implications for the understanding of chimpanzee social organization. Am. J. Primatol 31, 263–273. [DOI] [PubMed] [Google Scholar]
- Conklin-Brittain NL, Knott CD, Wrangham RW, 2006. Energy intake by wild chimpanzees and orangutans: methodological considerations and a preliminary comparison, in: Hohman G, Robbins MM, Boesch C (Eds.), Feeding Ecology in Apes and Other Primates. Cambridge University Press, Cambridge, pp. 445–471. [Google Scholar]
- Conklin-Brittain NL, Wrangham R, Hunt KD, 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. II. Macronutrients. Int. J. Primatol 19, 971–998. [Google Scholar]
- Duffy KG, Wrangham RW, Silk JB, 2007. Male chimpanzees exchange political support for mating opportunities. Current Biology 17, 586–587. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, 2005. Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am. J. Primatol 67, 137–158. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, 2017. Energetics of feeding, social behavior, and life history in non-human primates. Horm. Behav 91, 84–96. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Fox SA, Berghaenel A, Sabbi K, Phillips-Garcia S, Enigk DK, Otali E, Machanda ZP, Wrangham RW, Muller MN, 2020a. Wild chimpanzees exhibit humanlike aging of glucocorticoid regulation. Proc Natl Acad Sci USA [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW, 2007. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim. Behav 73, 501–512. [Google Scholar]
- Emery Thompson M, Machanda ZP, Fox SA, Otali E, Thompson NA, Muller MN, Wrangham RW, in press. Evaluating the impact of physical frailty during aging in wild chimpanzees (Pan troglodytes schweinfurthii). Phil. Trans. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Machanda ZP, Fox SA, Sabbi KH, Otali E, Thompson NA, Muller MN, Wrangham RW, 2020b. Evaluating the impact of physical frailty during aging in wild chimpanzees (Pan troglodytes schweinfurthii). Phil. Trans. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TG, Chapman CA, Wrangham RW, 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Royal Society Open Science 5, 180840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Fitzgerald E, Wrangham RW, 2012a. Variation in muscle mass in wild chimpanzees: application of a modified urinary creatinine method, Am. J. Phys. Anthropol WILEY-BLACKWELL COMMERCE PLACE, 350 MAIN ST, MALDEN 02148, MA USA, pp. 140–140. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW, 2010. Dynamics of social and energetic stress in wild female chimpanzees. Horm. Behav 58, 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Sabbi K, Machanda ZP, Otali E, Wrangham RW, 2016. Faster reproductive rates trade off against offspring growth in wild chimpanzees. Proc Natl Acad Sci USA 113, 7780–7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW, 2012b. The energetics of lactation and the return to fecundity in wild chimpanzees. Behav. Ecol 23, 1234–1241. [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW, 2014. Male chimpanzees compromise the foraging success of their mates in Kibale National Park, Uganda. Behav. Ecol. Sociobiol 68, 1973–1983. [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW, Lwanga JS, Potts KB, 2009. Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm. Behav 55, 299–305. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Sabbi KH, in press. Evolutionary demography of the great apes, in: Sear R, Burger O, Lee R (Eds.), Human Evolutionary Demography. Open Book Publishers, Cambridge, UK, p. https://osf.io/d2thj/. [Google Scholar]
- Emery Thompson M, Wrangham RW, 2008a. Diet and reproductive function in wild female chimpanzees (Pan troglodytes schweinfurthii) at Kibale National Park, Uganda. Am. J. Phys. Anthropol 135, 171–181. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Wrangham RW, 2008b. Male mating interest varies with female fecundity in Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park. Int. J. Primatol 29, 885–905. [Google Scholar]
- Emery Thompson M, Wrangham RW, Reynolds V, 2006. Urinary estrone conjugates and reproductive parameters in Kibale (Kanyawara) and Budongo (Sonso) chimpanzees, in: Newton-Fisher NE, Notman H, Reynolds V, Paterson J (Eds.), Primates of Western Uganda. Springer, New York, pp. 227–246. [Google Scholar]
- Enigk DK, Emery Thompson M, Machanda ZP, Wrangham RW, Muller MN, 2020. Competitive ability determines coalition participation and partner selection during maturation in wild male chimpanzees. Behav. Ecol. Sociobiol 74, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, Nekaris KA-I, Nijman V, Heymann EW, Lambert JE, Rovero F, Barelli C, Setchell JM, Gillespie TR, Mittermeier RA, Arregoitia LV, de Guinea M, Gouveia S, Dobrovolski R, Shanee S, Shanee N, Boyle SA, Fuentes A, MacKinnon KC, Amato KR, Meyer ALS, Wich S, Sussman RW, Pan R, Kone I, Li B, 2017. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv 3, e1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedurek P, Machanda ZP, Schel AM, Slocombe KE, 2013. Pant hoot chorusing and social bonds in male chimpanzees. Anim. Behav 86, 189–196. [Google Scholar]
- Fedurek P, Slocombe KE, Enigk DK, Thompson ME, Wrangham RW, Muller MN, 2016. The relationship between testosterone and long-distance calling in wild male chimpanzees. Behav. Ecol. Sociobiol 70, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev AV, Russell A, Emery Thompson M, Otali E, Muller M, Wrangham R, 2014. The foraging costs of mating effort in male chimpanzees (Pan troglodytes schweinfurthii). Int. J. Primatol 35, 725–745. [Google Scholar]
- Ghiglieri MP, 1984. The chimpanzees of Kibale forest: a field study of ecology and social structure. Columbia University Press, New York. [Google Scholar]
- Gilby IC, Pokempner AA, Wrangham RW, 2010. A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour. Folia Primatol. 81, 254–264. [DOI] [PubMed] [Google Scholar]
- Gilby IC, Wrangham RW, 2008. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav. Ecol. Sociobiol 62, 1831–1842. [Google Scholar]
- Goldberg TL, Paige SB, Chapman CA, 2012. The Kibale Ecohealth Project, in: Aguirre AA, Ostfeld R, Daszak P (Eds.), New Directions in Conservation Medicine: Applied Cases of Ecological Health. Oxford University Press, pp. 452–465. [Google Scholar]
- Goossens B, Setchell JM, Tchidongo E, Dilambaka E, Vidal C, Ancrenaz M, Jamart A, 2005. Survival, interactions with conspecifics and reproduction in 37 chimpanzees released into the wild. Biol. Conserv 123, 461–475. [Google Scholar]
- Hartel JA, Otali E, Machanda Z, Wrangham R, Ross E, in press. Holistic approach for conservation of chimpanzees in Kibale National Park, Uganda, in: Hopper L, Ross S (Eds.), Chimpanzees in Context. University of Chicago Press, Chicago, IL. [Google Scholar]
- Heinicke S, Mundry R, Boesch C, Amarasekaran B, Barrie A, Brncic T, Brugière D, Campbell G, Carvalho J, Danquah E, 2019. Characteristics of positive deviants in western chimpanzee populations. Frontiers in Ecology and Evolution 7, 16. [Google Scholar]
- Huffman MA, Wrangham RW, 1994. Diversity of medicinal plant use by chimpanzees in the wild, in: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG (Eds.), Chimpanzee Cultures. Harvard University Press, Cambridge, pp. 129–148. [Google Scholar]
- Humle T, Maisels F, Oates JF, Plumptre AJ, Williamson EA, 2015. Pan troglodytes. The IUCN Red List of Threatened Species 2016, e.T15933A129038584. [Google Scholar]
- Isabirye-Basuta G, 1988. Food competition among individuals in a free-ranging chimpanzee community in Kibale Forest, Uganda. Behaviour 105, 135–147. [Google Scholar]
- Isabirye-Basuta GM, Lwanga JS, 2008. Primate populations and their interactions with changing habitats. Int. J. Primatol 29, 35–48. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Muller MN, 2008a. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav 76, 1497–1509. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Wrangham RW, 2008b. Female competition over core areas in Pan troglodytes schweinfurthii, Kibale National Park, Uganda. Int. J. Primatol 29, 931–947. [Google Scholar]
- Kasenene JM, Ross E, 2008. Community benefits from long-term research programs: a case-study from Kibale National Park, in: Wrangham RW, Ross E (Eds.), Science and Conservation in African Forests: The Benefits of Longterm Research. Cambridge University Press, Cambridge, UK, pp. 63–74. [Google Scholar]
- Kaur T, Singh J, Tong S, Humphrey C, Clevenger D, Tan W, Szekely B, Wang Y, Li Y, Alex Muse E, Kiyono M, Hanamura S, Inoue E, Nakamura M, Huffman MA, Jiang B, Nishida T, 2008. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. Am. J. Primatol 70, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley T, Sleeman J, Wrangham RW, 2004. Urinalysis in free living chimpanzees (Pan troglodytes schweinfurthii) in Uganda. Veterinary Record 154, 729–730. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Kühl H, N’Goran PK, Walsh PD, Schenk S, Ernst N, Biek R, Formenty P, Mätz-Rensing K, Schweiger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin WI, Pauli G, Boesch C, Leendertz FH, 2008. Pandemic human viruses cause decline of endangered great apes. Current Biology 18, 260–264. [DOI] [PubMed] [Google Scholar]
- Krief S, Cibot M, Bortolamiol S, Seguya A, Krief J-M, Masi S, 2014. Wild chimpanzees on the edge: nocturnal activities in croplands. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Huffman MA, Sevenet T, Guillot J, Bories C, Hladik C-M, Wrangham RW, 2005. Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int. J. Primatol 26, 467–490. [Google Scholar]
- Leendertz FH, Ellerbrok H, Boesch C, Couacy-Hymann E, Matz-Rensing K, Hakenbeck R, Bergmann C, Abaza P, Junglen S, Moebius Y, Vigilant L, Formenty P, Pauli G.n., 2004. Anthrax kills wild chimpanzees in a tropical rainforest. Nature 430, 451–452. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Travis D, Pusey AE, Goodall J, 2006. Using retrospective health data from the Gombe chimpanzee study to inform future monitoring efforts. Am. J. Primatol 68, 897–908. [DOI] [PubMed] [Google Scholar]
- Machanda Z, Brazeau NF, Bernard AB, Donovan RM, Papakyrikos AM, Wrangham R, Smith TM, 2015. Dental eruption in East African wild chimpanzees. J. Hum. Evol 82, 137–144. [DOI] [PubMed] [Google Scholar]
- Machanda ZP, Gilby IC, Wrangham RW, 2013. Male-female association patterns among free-ranging chimpanzees (Pan troglodytes schweinfurthii). Int. J. Primatol 34, 917–938. [Google Scholar]
- Machanda ZP, Rosati AG, 2020. Shifting social bonds during primate aging. Phil. Trans. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie CA, Moffatt SP, Ogwang J, Ahabyona P, Sengupta RR, 2017. Spatial and temporal patterns in primary school enrolment and exam achievement in Rural Uganda. Children’s Geographies 15, 334–348. [Google Scholar]
- McGrew WC, 2017. Field studies of Pan troglodytes reviewed and comprehensively mapped, focussing on Japan’s contribution to cultural primatology. Primates 58, 237–258. [DOI] [PubMed] [Google Scholar]
- Morgan D, Strindberg S, Winston W, Stephens CR, Traub C, Ayina CE, Ndolo Ebika ST, Mayoukou W, Koni D, Iyenguet F, Sanz CM, 2019. Impacts of selective logging and associated anthropogenic disturbance on intact forest landscapes and apes of Northern Congo. Frontiers in Forests and Global Change 2, 28. [Google Scholar]
- Muller MN, 2017. Testosterone and reproductive effort in male primates. Horm. Behav 91, 36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Blurton Jones NG, Colchero F, Emery Thompson M, Enigk DK, Feldblum JT, Hahn BH, Langergraber KE, Scully EJ, Vigilant L, Walker KK, Wrangham RW, Wroblewski EE, Pusey AE, 2020. Sexual dimorphism in chimpanzee (Pan troglodytes schweinfurhii) and human age-specific fertility. J. Hum. Evol 144, 102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Emery Thompson M, Kahlenberg SM, Wrangham RW, 2011. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav. Ecol. Sociobiol 65, 921–933. [Google Scholar]
- Muller MN, Emery Thompson M, Wrangham RW, 2006. Male chimpanzees prefer mating with old females. Current Biology 16, 2234–2238. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW, 2007. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. Roy. Sci. B 274, 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Wrangham RW, 2009. Male aggression against females and sexual coercion in chimpanzees, in: Muller M, Wrangham R (Eds.), Sexual Coercion in Primates: An Evolutionary Perspective on Male Aggression Against Females. Harvard University Press, Cambridge, MA. [Google Scholar]
- Muller MN, Lipson SF, 2003. Diurnal patterns of urinary steroid excretion in wild chimpanzees. Am. J. Primatol 60, 161–166. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW, 2004a. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Anim. Behav 67, 113–123. [Google Scholar]
- Muller MN, Wrangham RW, 2004b. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol 55, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW, 2005. Testosterone and energetics in wild chimpanzees (Pan troglodytes schweinfurthii). Am. J. Primatol 66, 119–130. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW, 2014. Mortality rates among Kanyawara chimpanzees. J. Hum. Evol 66, 107–114. [DOI] [PubMed] [Google Scholar]
- N’Goran PK, Boesch C, Mundry R, N’Goran EK, Herbinger I, Yapi FA, Kuehl HS, 2012. Hunting, law enforcement, and African primate conservation. Conservation Biology 26, 565–571. [DOI] [PubMed] [Google Scholar]
- Naughton-Treves L, Treves A, Chapman C, Wrangham R, 1998. Temporal patterns of crop-raiding by primates: linking food availability in croplands and adjacent forest. Journal of Applied Ecology 35, 596–606. [Google Scholar]
- Negrey JD, Emery Thompson M, Langergraber KE, Machanda ZP, Mitani JC, Muller MN, Otali E, Owens LA, Wrangham RW, Goldberg TL, 2020. Demography, life history trade-offs, and the gastrointestinal virome of wild chimpanzees. Phil. Trans. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrey JD, Reddy RB, Scully EJ, Phillips-Garcia S, Owens L, Langergraber KE, Mitani J, Emery Thompson M, Wrangham RW, Muller MN, Otali E, Machanda ZP, Hyeroba D, Grindle KA, Pappas TS, Palmenberg AC, Gern JE, Goldberg TL, 2019. Simultaneous outbreaks of respiratory disease in wild chimpanzees caused by distinct viruses of human origin. Emerging Microbes and Infections 8, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SV, 2013. Chimpanzee fauna isotopes provide new interpretations of fossil ape and hominin ecologies. Proceedings of the Royal Society B: Biological Sciences 280, 20132324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omeja PA, Lawes MJ, Corriveau A, Valenta K, Sarkar D, Paim FP, Chapman CA, 2016. Recovery of tree and mammal communities during large-scale forest regeneration in Kibale National Park, Uganda. Biotropica 48, 770–779. [Google Scholar]
- Otali E, Gilchrist JS, 2005. Why chimpanzee (Pan troglodytes schweinfurthii) mothers are less gregarious than nonmothers and males: the infant safety hypothesis. Behav. Ecol. Sociobiol [Google Scholar]
- Phillips SR, Goldberg TL, Muller MN, Machanda ZP, Otali E, Friant S, Carag J, Langergraber KE, Mitani JC, Wroblewski EE, Wrangham RW, Emery Thompson M, 2020. Fecal parasites increase with age but not reproductive effort in wild female chimpanzees. Phil. Trans. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumptre AJ, Cox D, 2006. Counting primates for conservation: primate surveys in Uganda. Primates 47, 65–73. [DOI] [PubMed] [Google Scholar]
- Plumptre AJ, Cox D, Mugume S, 2003. Albertine Rift Technical Report Series No. 2 The Status of Chimpanzees in Uganda Wildlife Conservation Society, New York. [Google Scholar]
- Potts KB, 2013. Nutritional ecology and reproductive output in female chimpanzees (Pan troglodytes): variation among and within populations, Building Babies. Springer, pp. 83–100. [Google Scholar]
- Potts KB, Watts DP, Langergraber KE, Mitani JC, 2020. Long-term trends in fruit production in a tropical forest at Ngogo, Kibale National Park, Uganda. Biotropica n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J, 2007. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conservation Biology 21, 623–634. [DOI] [PubMed] [Google Scholar]
- Quiatt D, Reynolds V, Stokes E, 2002. Snare injuries to chimpanzees (Pan troglodytes) at 10 study sites in east and west Africa. African Journal of Ecology 40, 303–305. [Google Scholar]
- Rosati AG, Hagberg L, Enigk D, Otali E, Emery Thompson M, Muller MN, Wrangham RW, Machanda ZP, in press. Social selectivity in aging wild chimpanzees. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore J, Allison AB, Edwards EE, Bagal U, Altizer S, Cranfield MR, Glenn TC, Liu H, Mudakikwa A, Mugisha L, Muller M, Stumpf RM, Emery Thompson M, Wrangham RW, Yabsley MJ, 2015. Screening wild and semi-free ranging great apes for putative sexually transmitted diseases: Evidence of Trichomonadidae infections in wild chimpanzees (Pan troglodytes). Am. J. Primatol 77, 1075–1085. [DOI] [PubMed] [Google Scholar]
- Sabbi KH, Muller MN, Fox SA, Machanda ZP, Otali E, Wrangham RW, Emery Thompson M, 2019. Human-like adrenal development in wild chimpanzees: a longitudinal study of cortisol and DHEAS. Am. J. Primatol, e23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully E, Basnet S, Wrangham RW, Muller MN, Otali E, Hyeroba D, Grindle KA, Pappas TS, Emery Thompson M, Machanda ZP, Watters KE, Palmenberg AC, Gern JE, Goldberg TL, 2018. Lethal respiratory epidemic in wild chimpanzees associated with human rhinovirus C. Emerging Infectious Diseases 24, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Machanda Z, Bernard AB, Donovan RM, Papakyrikos AM, Muller MN, Wrangham R, 2013. First molar eruption, weaning, and life history in living wild chimpanzees. Proc. Natl. Acad. Sci. USA 110, 2787–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhsaker TT, 1997. Ecology of an African Rain Forest: Logging in Kibale and the Conflict between Conservation and Exploitation. University Press of Florida, Gainesville. [Google Scholar]
- Surbeck M, Boesch C, Emery Thompson M, Furuichi T, Fruth B, Hohmann G, Ishizuka S, Machanda Z, Muller M, Pusey A, Sakamaki T, Tokuyama N, Walker K, Wrangham R, Wroblewski E, Zuberbuehler K, Vigilant L, Langergraber K, 2019. Males with a mother living in their community have higher reproductive success in bonobos but not chimpanzees. Current Biology 29, R354–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg N, Willie J, Duarte J, Petre CA, Fa J, 2015. Conservation research presence protects: a case study of great ape abundance in the Dja region, Cameroon. Anim. Conserv 18, 489–498. [Google Scholar]
- Thompson NA, Otali E, Machanda ZP, Muller MN, Wrangham RW, in press. Urinary markers of oxidative stress respond to infection and late life in wild chimpanzees. PLOS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutin CEG, Ancrenaz M, Paredes J, Vacher-Vallas M, Vidal C, Goossens B, Bruford MW, Jamart A, 2001. Conservation biology framework for the release of wild-born orphaned chimpanzees into the Conkouati Reserve, Congo. Conservation Biology 15, 1247–1257. [Google Scholar]
- Uwimbabazi M, Rothman JM, Basuta GI, Machanda ZP, Conklin-Brittain NL, Wrangham RW, 2019. Influence of fruit availability on macronutrient and energy intake by female chimpanzees. African Journal of Ecology 57, 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanyama F, Muhabwe R, Plumptre AJ, Chapman CA, Rothman JM, 2010. Censusing large mammals in Kibale National Park: evaluation of the intensity of sampling required to determine change. African Journal of Ecology 48, 953–961. [Google Scholar]
- Watts DP, 2012. Long-term research on chimpanzee behavioral ecology in Kibale National Park, Uganda, in: Kappeler P, Watts D (Eds.), Long-term Field Studies of Primates. Springer, Berlin, pp. 313–338. [Google Scholar]
- Weary TE, Wrangham RW, Clauss M, 2017. Applying wet sieving fecal particle size measurement to frugivores: A case study of the eastern chimpanzee (Pan troglodytes schweinfurthii). Am. J. Phys. Anthropol 163, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Hauser MD, Wrangham RW, 2001. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav 61, 1203–1216. [Google Scholar]
- Wilson ML, Hauser MD, Wrangham RW, 2007. Chimpanzees (Pan troglodytes) modify grouping and vocal behaviour in response to location-specific risk. Behaviour 144, 1621–1653. [Google Scholar]
- Wilson ML, Kahlenberg SM, Wells M, Wrangham RW, 2012. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim. Behav 83, 277–291. [Google Scholar]
- Wilson ML, Lonsdorf EV, Mjungu DC, Kamenya S, Kimaro EW, Collins DA, Gillespie TR, Travis DA, Lipende I, Mwacha D, Ndimuligo SA, Pintea L, Raphael J, Mtiti ER, Hahn BH, Pusey AE, Goodall J, this issue. Research and Conservation in the Greater Gombe Ecosystem: Challenges and Opportunities. Biol. Conserv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Wrangham RW, 2003. Intergroup relations in chimpanzees. Annu. Rev. Anthropol 32, 363–392. [Google Scholar]
- Wood BM, Watts DP, Mitani JC, Langergraber KE, 2017. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J. Hum. Evol 105, 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham RW, 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300. [Google Scholar]
- Wrangham RW, 1995. Relationship of chimpanzee leaf‐swallowing to a tapeworm infection. Am. J. Primatol 37, 297–303. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, 2000. Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis, in: Kappeler PM (Ed.), Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge University Press, Cambridge, pp. 248–258. [Google Scholar]
- Wrangham RW, Chapman CA, Clark AP, Isabirye-Basuta G, 1996. Social ecology of Kanyawara chimpanzees: implications for understanding the costs of great ape groups, in: McGrew WC, Marchant LF, Nishida T (Eds.), Great Ape Societies. Cambridge University Press, Cambridge, pp. 45–57. [Google Scholar]
- Wrangham RW, Conklin-Brittain NL, Hunt KD, 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. Int. J. Primatol 19, 949–970. [Google Scholar]
- Wrangham RW, Ross E, 2008. Science and Conservation in African Forests: The Benefits of Long-term Research. Cambridge University Press, Cambridge, UK, p. 280. [Google Scholar]


