SUMMARY
Riboswitches are mRNA domains that make gene-regulatory decisions upon binding their cognate ligands. Bacterial riboswitches that specifically recognize 5-aminoimidazole-4-carboxamide riboside 5'-monophosphate (ZMP) and 5'-triphosphate (ZTP) regulate genes involved in folate and purine metabolism. Now, we have developed synthetic ligands targeting ZTP riboswitches by replacing the sugar-phosphate moiety of ZMP with various functional groups, including simple heterocycles. Despite losing hydrogen bonds from ZMP, these analogs bind ZTP riboswitches with similar affinities as the natural ligand, and activate transcription more strongly than ZMP in vitro. The most active ligand stimulates gene expression ~3 times more than ZMP in a live Escherichia coli reporter. Co-crystal structures of the Fusobacterium ulcerans ZTP riboswitch bound to synthetic ligands suggest stacking of their pyridine moieties on a conserved RNA nucleobase primarily determines their higher activity. Altogether, these findings guide future design of improved riboswitch activators, and yield insights into how RNA-targeted ligand discovery may proceed.
Keywords: RNA, structure, kinetic control, target-based design, transcription, X-ray crystallography, small molecule, microarray
INTRODUCTION
Riboswitches are widespread regulators of central metabolic pathways in bacteria and control gene expression in response to the intracellular concentration of their cognate small molecule (Jones and Ferré-D’Amaré, 2017; Serganov and Nudler, 2013). Purine and folate metabolism are controlled by a class of riboswitches that bind directly to 5-aminoimidazole-4-carboxamide riboside 5'-monophosphate (ZMP, 1, Fig. 1a) and 5'-triphosphate (ZTP), which accumulate when cells are depleted of folate (Bochner and Ames, 1982; Kim et al., 2015). During purine biosynthesis, ZMP is enzymatically converted to inosine through the addition of a single carbon unit provided by 10-formyl-tetrahydrofolate (10f-THF). Consequently, ZTP riboswitches often regulate genes involved in the synthesis of 10f-THF or the synthesis of inosine from ZMP (i.e., the enzyme PurH) (Kim et al., 2015). PurH has been shown to be critical for virulence in several bacterial pathogens, suggesting purine availability is limiting in blood and intracellularly ((Samant et al., 2008; Shaffer et al., 2017) and references therein).
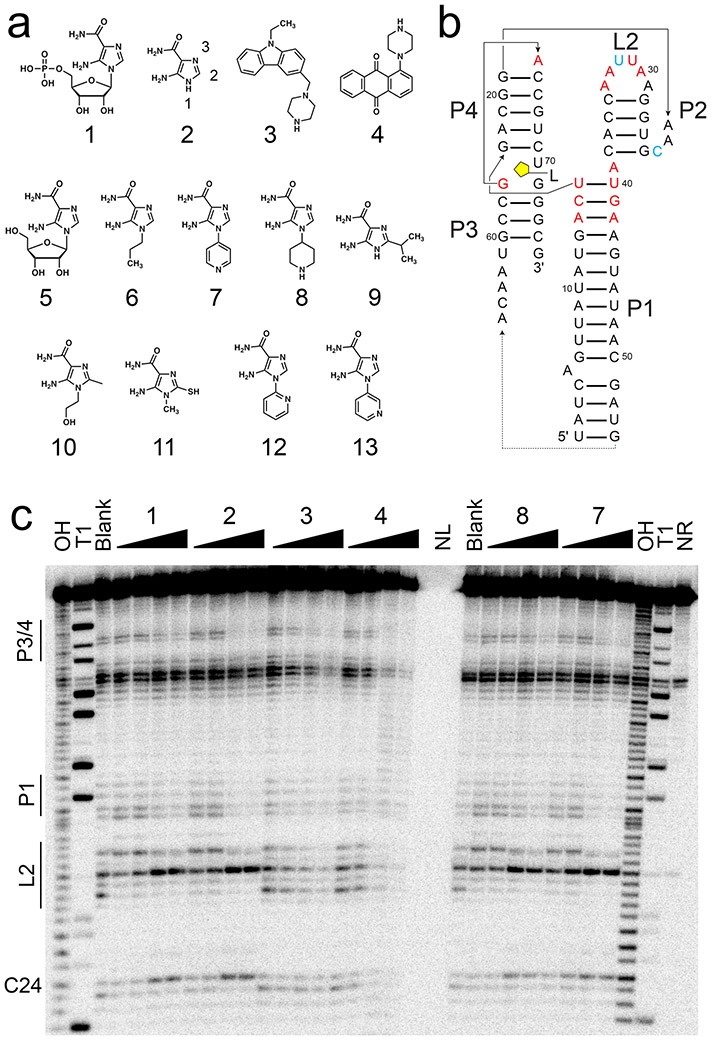
Figure 1.
In-line probing of ZTP riboswitch with AICA derivatives and SMM hits. (a) Chemical structures of compounds used herein—1, 5-aminoimidazole-4-carboxamide-β-1-D-ribofuranoside 5’-monophosphate (ZMP); 2, 5-aminoimidazole-4-carboxamide (AICA), with functional group numbering as indicated; 3, 9-ethyl-3-(1-piperazinylmethyl)-9H-carbazole; 4, 1-(1-piperazinyl)anthra-9,10-quinone; 5, AICA-β-1-D-ribofuranoside (AICAR); 6, 1-propyl-AICA; 7, p-1-pyridinyl-AICA; 8, p-1-piperidinyl-AICA; 9, 2-isopropyl-AICA; 10, 1-hydroxyethyl-2-methyl-AICA; 11, 1-methyl-2-mercapto-AICA; 12, o-1-pyridinyl-AICA; 13, m-1-pyridinyl-AICA. (b) Secondary structure of the F. ulcerans ZTP riboswitch used for in-line probing is shown, with the approximate ligand binding site indicated in yellow. Paired helical elements P1-4 and loop L2 are noted. Nucleotides with decreased cleavage (red) and increased cleavage (cyan) in the presence of 1, 2, 7, and 8 are indicated. (c) On the left side of the gel, regions of the ZTP riboswitch most sensitive to changes in in-line probing reactivity are noted. From left to right, the lanes are: OH, partial alkaline digest; T1, RNase T1 digest; Blank, no-compound control; 1 at 3, 6, 200, 400 nM; 2 at 1.5, 3, 100, 200 μM; 3 at 16, 31, 1000, 2000 μM; 4 at 16, 31, 1000, 2000 μM; NL, no loading; 8 at 0.16, 0.31, 10, 20 μM; 7 at 0.16, 0.31, 10, 20 μM; and NR, no reaction. Example gel is shown (n = 6 independent experiments). See also Figures S1 and S2.
Owing to their natural propensity to bind small molecules in highly conserved pockets, and their role in regulating metabolic pathways essential for growth and virulence, several riboswitches have been pursued as antibiotic targets (reviewed in (Blount and Breaker, 2006; Lunse et al., 2014; Mulhbacher et al., 2010b; Warner et al., 2018). Compounds have been reported that target the thiamine pyrophosphate (Sudarsan et al., 2005), purine (Mulhbacher et al., 2010a; Ster et al., 2013; Yan et al., 2018), flavin mononucleotide (Blount et al., 2015; Howe et al., 2015; Lee et al., 2009; Motika et al., 2020), and lysine (Ataide et al., 2007) riboswitches, as well as the glmS riboswitch-ribozyme (Lunse et al., 2011; Posakony and Ferre-D’Amare, 2013), among others. Lead compounds and antibiotics have been developed through a variety of methods that includes fragment-based screens (Chen et al., 2010; Warner et al., 2014), high-throughput screens (Connelly et al., 2019), and rational design through structure-guided (Gilbert et al., 2009; Shanahan et al., 2011; Vicens et al., 2018) and in silico approaches (Daldrop et al., 2011). These methods can be broadly divided into those that derivatize the natural riboswitch ligand and those that seek to identify structurally distinct synthetic ligands.
More recently, screens utilizing small-molecule microarrays (SMM) (Connelly et al., 2017) have identified structurally distinct synthetic ligands to preQ1 riboswitches capable of binding and transcription activation (Connelly et al., 2019). Importantly, it was possible to determine co-crystal structures of these compounds bound to the preQ1 riboswitch, setting the stage for future efforts to improve ligand binding through structure-guided design. In this manuscript, we have sought to identify ligands to another purine-related riboswitch, the ZTP riboswitch. This riboswitch has been used to report on the potency of antifolates (Perkins et al., 2019), and the best small molecule riboswitch binders discovered thus far include natural ligands such as ZMP, ZTP, and 3',5'-cyclic ZMP (Kim et al., 2015). We now report the discovery of a series of synthetic ZTP riboswitch activators through both SMM screening, as well as systematic modification of 5-aminoimidazole-4-carboxamide (AICA, 2, Fig. 1a), the nucleobase-like moiety of ZMP. These new compounds function in riboswitch-mediated transcription activation in vitro, also activate a genetic reporter in vivo in E. coli, and open the way to the discovery of more potent activators and inhibitors of this bacterial RNA target.
RESULTS
Discovery of ZTP riboswitch binders through SMM screens and targeted design
To identify ZTP-riboswitch-binding ligands, SMM screens were performed using Cy5-labeled F. ulcerans ZTP riboswitch RNAs. In these experiments, small molecules are covalently linked to and spatially organized on a glass surface (Connelly et al., 2017). ZTP riboswitch RNAs were panned over such microarrays consisting of ~22,000 small-molecules in total and resulted in 138 initial hits. Compounds were chosen for validation by their availability, fluorescence brightness above the average using a Z-score cutoff greater than 3, and for hit uniqueness compared to ~35 other RNAs screened previously in the Schneekloth lab (Connelly et al., 2017; Connelly et al., 2019; Felsenstein et al., 2016) (Supplemental Data File 1). In addition, positive hits were inspected for similarities in fluorescence brightness between compound duplicates, as well as spot morphology and fluorescence background.
From these compounds, 88 hits were tested for ZTP riboswitch binding by “in-line probing”, a technique that reports on the ability of small molecule binders to alter rates of spontaneous RNA cleavage through internal transesterification (intramolecular nucleophilic attack of a 2' hydroxyl on an adjacent phosphodiester; (Regulski and Breaker, 2008). In-line probing also reports on RNA structure, as single-stranded regions tend to be more reactive than double-stranded regions; this was also observed here (Fig. 1b,c). 32P-labeled ZTP riboswitch RNA was incubated with 0.2 mM or 2 mM of each SMM hit compound for 40 hours prior to denaturing gel-electrophoretic analysis. For most compounds, no changes in reactivity were apparent, while for other compounds, cleavage across the entire RNA occurred, which was interpreted as nonspecific binding, or the RNA did not enter the gel in the presence of compounds (Fig. S1 and Supplemental Data File 1). Select compounds showing changes were further investigated, such as compounds 3 and 4, which protected and promoted RNA cleavage to differing extents (Fig. 1c, Fig. S2). For example, compound 3 protected highly conserved nucleotides G63 and A64 in the P3/P4 region commonly observed to be protected in the presence of 1 and AICA riboside (AICAR, 5). Unlike 1 and 5 however, 3 did not promote reactivity at nucleotide C24 and destabilized P1 instead, as observed by an increase in reactivity in nucleotides throughout the P1 helix (Fig. S2). Compound 4 also protected the P3/P4 region as well as L2 while also strongly destabilizing the P1 helix (Fig. 1b). Each compound was titrated to estimate apparent dissociation constants (Kd) from in-line probing (Fig. S2a). Both compounds weakly bound the ZTP riboswitch with Kd of ~0.2-0.3 mM at best (Fig. S2b), leading us to attempt an alternative approach for discovering new ZTP riboswitch ligands.
ZTP riboswitches are thought to bind ZMP and ZTP during folate stress in cells. However, in-line probing experiments indicate that, in vitro, ZTP riboswitches are also activated by 2, albeit more weakly than by 1 (Kim et al., 2015). Crystal structures show that the ribose and phosphate moieties of ZMP hydrogen bond with the 2'-OHs of G63 and N4 of C69, respectively. The difference in binding affinity between 1 and 2 is likely explained by the loss of these interactions, as well as the loss of van der Waals interactions between the ribose of 1 and the nucleobase of G63. A co-crystal structure of a ZTP riboswitch bound to 2 suggested it sufficient to organize the ZTP riboswitch binding pocket (data not shown). Likewise, 1 and 2 showed similar patterns of protection by in-line probing (Fig. 1c). Thus, commercially available AICA derivatives (Table 1) were purchased and screened to identify ZTP riboswitch binders, as an alternative to SMM screens. These derivatives initially included a series of analogs substituted at the 1-position of 2 (1-propyl-AICA, 6; p-1-pyridinyl-AICA, 7; p-1-piperidinyl-AICA, 8), 2-position (2-isopropyl-AICA, 9), and both positions (1-hydroxyethyl-2-methyl-AICA, 10; 1-methyl-2-mercapto-AICA, 11) (Fig. 1a). Both 7 and 8 strongly protected the ZTP riboswitch from spontaneous cleavage during in-line probing experiments (Fig. 1c) and were further investigated (below). The in-line probing protection observed included G63 and A64 in the P3/4 region, U40-A43 and G13-U16 in the P1 stem, and A29-A34 in L2, as well as deprotection of C24 (Fig. 1b,c). All of these residues are affected similarly by 1 and 2.
Table 1.
Isothermal titration calorimetry measurements for AICA analogs
| Compound | Kd, μM | ΔH, kcal mol−1 |
|---|---|---|
| 1 | 0.32 ± 0.13 | −9 ± 5 |
| 2 | 41 ± 4 | −17 ± 2 |
| 5 | 2.3 ± 0.6 | −27 ± 3 |
| 7a | 1.0 ± 0.3 | −19 ± 2 |
| 7b | 1.4 ± 0.2 | −22 ± 2 |
| 8a | 11 ± 1 | −17.1 ± 0.5 |
| 8b | 8.7 ± 1.4 | −15 ± 6 |
| 10 | 150 ± 30 | −16 ± 2 |
| 12 | 5.6 ± 3.2 | −20 ± 5 |
| 13 | 0.60 ± 0.06 | −24 ± 8 |
Commercially obtained compound.
Synthesized compound.
Values are the mean ± standard deviation, with n = 3 independent experiments for all compounds except 7, for which n = 6.
AICA derivatives bind and activate ZTP riboswitches in vitro
After observing consistent protection in in-line probing assays with AICA derivatives, we next examined equilibrium binding of 2 and AICA derivatives to the ZTP riboswitch by isothermal titration calorimetry (ITC) (Table 1 and Fig. 2). While 2 (Kd = 41 ± 4 μM) was found to bind the ZTP riboswitch ~120-fold more weakly than 1 (Kd = 0.32 ± 0.13 μM), compound 8 (Kd = 11 ± 1 μM) bound ~3.7-fold better than 2. Notably, compound 7 (Kd = 1.0 ± 0.3 μM) bound the ZTP riboswitch ~40-fold better than 2 and only ~3-fold more weakly than 1. In contrast, compound 10 (Kd = 150 ± 30 μM) bound more poorly than 2, likely due steric clashes between the 2-substituent and the O4 of U70. As even a methyl group is not tolerated at this position, 2-position derivatives were not investigated further.
Figure 2.

Isothermal titration calorimetry of the ZTP riboswitch with AICA derivatives. Example titrations of (a) 2, (b) 8, and (c) 7 binding to the F. ulcerans ZTP riboswitch (n = 3 independent experiments).
To determine if AICA derivatives are capable of activating a ZTP riboswitch in vitro, we performed single-round transcription termination assays (Artsimovitch and Henkin, 2009) with the F. ulcerans ZTP riboswitch (Methods). In these experiments, E. coli RNA polymerase holoenzyme transcribes the ZTP riboswitch from a DNA template, producing either terminated or readthrough products (Fig. 3a). As the F. ulcerans ZTP riboswitch is an “ON” switch, adding increasing ZMP to the transcription reaction stabilizes the aptamer fold of the RNA, prevents termination, and leads to the accumulation of the readthrough product (Fig. 3b), which can be quantified and fit to determine the concentration at which the riboswitch is half activated (T50). In these experiments, T50 values are higher than Kd values determined from other methods, as the riboswitch is kinetically controlled under these conditions (Binas et al., 2020; Strobel et al., 2019; Wickiser et al., 2005b). Thus, the T50 values are non-equilibrium measurements that reflect ligand binding on rates (kon).
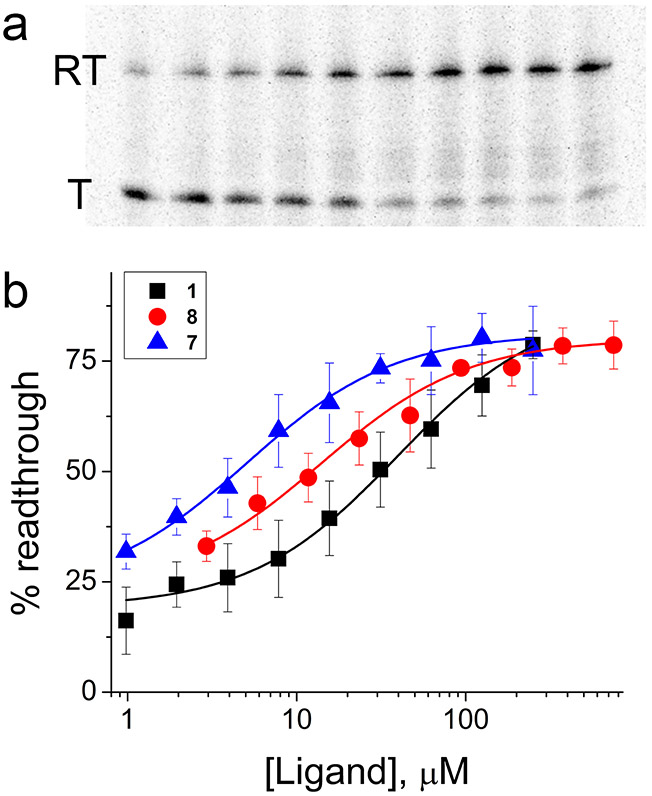
Figure 3.
Activation of transcription readthrough by AICA derivatives in vitro. (a) Example single-round transcription termination assay for 8 (0, 1.0, 2.0, 3.9, 7.8, 15.6, 31.3, 62.5, 125, 250 μM, left to right). Readthrough (RT) and terminated (T) transcription products are labeled. (b) Transcription termination for 7 (blue), 8 (red), and 1 (black) (n = 3 independent experiments).
Compared to ZMP, compounds 7 and 8 potently stimulated transcription readthrough (Table 2 and Fig. 3b), while the more weakly binding SMM hits 3 and 4 failed to show activity at the highest concentration tested (i.e., 2 mM). Surprisingly, compounds 7 (T50 = 5.7 ± 0.9 μM) and 8 (T50 = 16 ± 3 μM) proved to be more potent activators than 1 (T50 = 37 ± 12 μM) under these conditions. As both compounds bind to the ZTP riboswitch more weakly than 1 at equilibrium (as determined by ITC), the most likely explanation is that they bind with larger kon. As a result, both ligands can more potently activate the ZTP riboswitch during the short time window that the RNA is available for binding prior to adopting the default (ligand-free) fold that leads to transcription termination.
Table 2.
Single-round transcription termination efficiencies for ligands to the ZTP riboswitch
| Compound | T50, μM |
|---|---|
| 1 | 37 ± 12 |
| 3 | N.D.a |
| 4 | N.D. |
| 7 | 5.7 ± 0.9 |
| 8 | 16 ± 3 |
| 12 | 4.8 ± 0.9 |
| 13 | 5.8 ± 1.5 |
Not determined (N.D.) as no activity was observed.
Values are the mean ± standard deviation, with n = 3 independent experiments for all compounds.
Based on the robust binding of compound 7 to the ZTP riboswitch, we sought to examine other 1-pyridinyl-AICA regioisomers (Figs. S3-S4 and Methods S1) for binding and transcription activation. Compared to compound 7, the ortho derivative 12 is far less potent in equilibrium binding (Kd = 5.6 ± 3.2 μM) but not in single-round transcription termination assays (T50 = 4.8 ± 0.9 μM). In contrast, the meta derivative 13 binds the ZTP riboswitch ~2-fold better (Kd = 0.60 ± 0.06 μM) than 7 and activates transcription to a similar extent (T50 = 5.8 ± 1.5 μM). We also synthesized compounds 7 and 8 in-house (Figs. S5-S7), and found them to bind with dissociation constants of 1.4 ± 0.2 μM and 8.7 ± 1.4 μM respectively, which are within 2-fold of values for commercially sourced compounds, confirming the chemical identity of the active compounds.
Co-crystal structures of 1-pyridinyl-AICA analogs bound to the ZTP riboswitch
Co-crystallization trials were performed with compounds 3, 4, 7, 8, and 13 and the riboswitch aptamer domain. Although all trials produced crystals, only the co-crystals of the F. ulcerans ZTP riboswitch bound to compounds 7 and 13 (Table S1 and Fig. 4) yielded useful diffraction. This is reminiscent of the observation that affinity and solubility are the best determinants of success of co-crystallization of protein-ligand complexes (Muller, 2017). Their co-crystal structures were each solved at ~3.2 Å resolution by the molecular replacement method, using the ligand-free atomic coordinates of the previously determined ZTP riboswitch structure as a search model (Jones and Ferré-D’Amaré, 2015). After manual rebuilding of poorly resolved residues and simulated annealing, energy, and B-factor refinement, the corresponding ligands were placed into well-defined residual electron density (Fig. 4b,c) in each of the two crystallographically independent RNA molecules in the asymmetric unit of each of the two co-crystal structures.
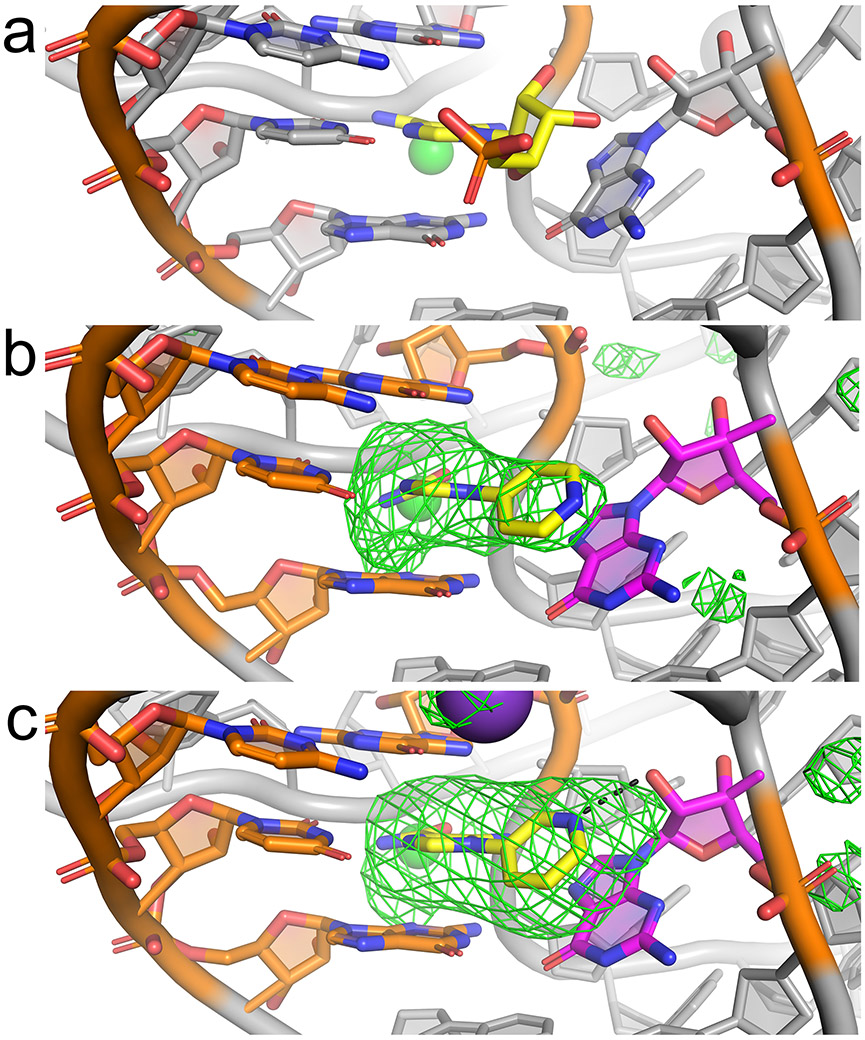
Figure 4.
Co-crystal structures of the ZTP riboswitch bound to 1-pyridinyl-AICA analogs. (a) View of the ligand binding site of the ZTP riboswitch bound to ZMP (yellow) (Jones and Ferré-D’Amaré, 2015). (b) View of the ligand binding site of the ZTP riboswitch bound to 7 (yellow) with nearby base pair G17:C69 and ligand-contacting residues U70 and G71 (orange) and G63 (magenta). The ∣Fo∣-∣Fc∣ Fourier difference map prior to ligand placement is shown, contoured at 3 σ (green mesh). (c) Similar view of the ligand binding site for the ZTP riboswitch bound to 13 (yellow). Black dotted line denotes a putative hydrogen bond (3.0 Å) between the pyridine and G63.
In the cognate complex structure of ZMP bound to the riboswitch, the AICA moiety of the ligand is buried, and its phosphate points into solvent. In this structure, the ribose of 1 makes van der Waals contact and a hydrogen bond with the nucleobase and 2'OH, respectively, of G63 (Fig. 4a). Binding of 7 and 13 appears to be facilitated by π-π stacking between their pyridine moieties and the G63 nucleobase (Fig. 4b,c). This drives the two rings of the pyridinyl-AICA analogs to deviate slightly from coplanarity in each co-crystal structure, leading to a ligand conformation that maximizes stacking of the pyridine ring with the nucleobase of G63. In the para-analog-bound structure (Fig. 4b), the pyrimidine moiety does not appear to participate as a hydrogen bond acceptor with neighboring residues, such as the 2'-OH of G63, which is ~4.5 Å away. In the meta analog-bound structure, the pyrimidine is positioned 3.0 Å away from the 2'-OH of G63 to accept a hydrogen bond. While the precision of the atomic coordinates is limited to 0.50 Å at the current resolution, the most likely explanation for the ~2-fold stronger binding of this compound compared to the para regioisomer is that the pyridine group is oriented toward the 2'-OH of G63 and participates in a hydrogen bond (Fig. 4c).
AICA derivatives activate a riboswitch reporter in cells
As a further test of the ability of AICA derivatives to activate ZTP riboswitch, we next examined their efficacy in a ZTP riboswitch in a previously published reporter assay in E. coli. This assay utilizes the Pectobacterium carotovorum ZTP riboswitch upstream of a β-galactosidase (β-gal) reporter plasmid transformed into E. coli, which naturally lacks ZTP riboswitches but still produces ZMP during purine biosynthesis (Rohlman and Matthews, 1990). As a consequence, the reporter is partially activated in the absence of additional ligands but can be activated in the presence of ligands by more than 10-fold (Fig. 5, Table S2). Growth of cells overnight in rich media supplemented with ligands drives expression of β-gal, which is detected by turnover of a fluorescence substrate analog (Methods). For all compounds, activation of the WT reporter was compared to activation of the M6 mutant riboswitch (Kim et al., 2015), in which an otherwise invariant nucleotide has been mutated, corresponding to C15A in the F. ulcerans ZTP riboswitch sequence. No compounds activated this M6 mutant at the highest compound concentrations tested (5 mM) (Table S2).
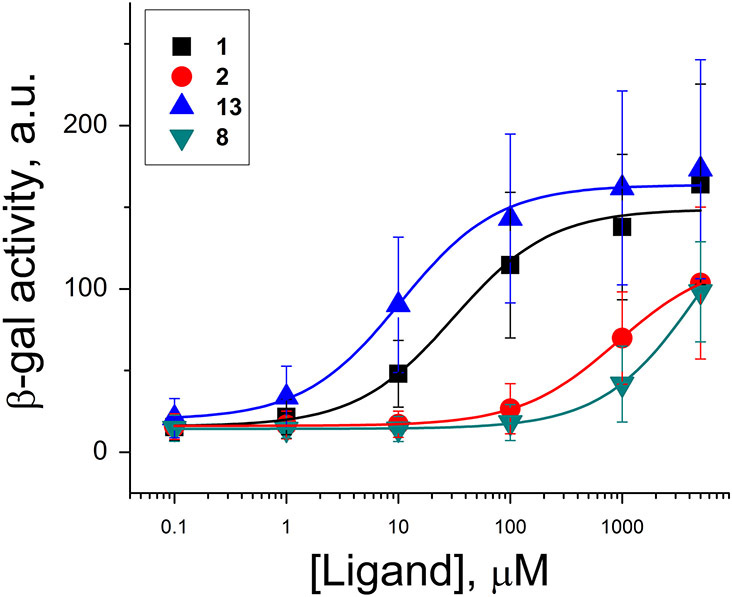
Figure 5.
Activity of various ligands in a ZTP riboswitch reporter in E. coli. Titrations of 1 (black), 2 (red), 13 (blue), and 8 (teal). Values are the means of ten independent experiments (n = 10), and error bars denote standard deviations. See also Tables S2 and S3.
Addition of ligands to cell media resulted in dose-dependent increases in β-gal expression (Fig. 5), characterized by EC50 values of 34 ± 13 μM for ZMP and 27 ± 11 μM for 5 (Table S3), which were not significantly different (p = 0.13, one-tailed Welch’s T test). For these assays, both 1 and 5 were tested as previous reports found that 5 is taken up into cells and phosphorylated ((Kim et al., 2015) and references therein). Compound 2 only weakly activated β-gal expression under these conditions, even at high concentrations of 5 mM. Likewise, 7 only weakly stimulated and did not saturate β-gal expression. In comparison, compounds 8 and 12 appeared to be the weakest activators tested and also failed to saturate β-gal activation at these concentrations. In contrast, 13 was the strongest activator of β-gal expression (EC50 = 12 ± 7 μM), which is ~3-fold more potent than 1 (p = 0.00048) (Fig. 5, Table S3).
DISCUSSION
Here, we have demonstrated that 1-pyridinyl-AICA is a simplified, potent ZMP derivative capable of binding and activating the ZTP riboswitch. The replacement of the ribose and phosphate moieties of ZMP with a pyridine almost entirely compensates for lost hydrogen bonds and allows for riboswitch activation in vitro and in vivo, echoing previous findings regarding the flavin mononucleotide (FMN) riboswitch. The ribose-phosphate moiety of FMN forms vital interactions with that RNA, but replacement with an aryl-alkyl side chain afforded analogues that bound to and activated the riboswitch with the same potency and efficiency, respectively (Blount et al., 2015). Likewise, the 2-methylamino-5-pyrimidine moiety of Ribocil makes π-π stacking interactions with a guanosine in the FMN riboswitch binding pocket (Howe et al., 2015). This suggests that aryl moieties offer a route to strategic formation of favorable interactions during the design of synthetic RNA-targeting ligands. By comparison, synthetic lectin antagonists have used a similar rationale to replace carbohydrates with bi-aryl substituents to facilitate π-π stacking interactions between synthetic ligands and tyrosine residues (Mydock-McGrane et al., 2017). Our para and meta pyridine analogs (7 and 13) could serve as a starting point for structure-guided design of more active compounds in the future. While both synthetic ligands bind to and activate the riboswitch in vitro, 13 is significantly more potent in a cell-based reporter. There are multiple potential reasons that could contribute to these unexpected differences. For example, ligands could have differences in uptake, solubility, permeability, metabolism, retention, or off-target binding between the two compounds. Furthermore, the riboswitch itself may behave slightly differently in vivo than in the biochemical assays above as the P. carotovorum ZTP riboswitch regulates translation by modulating ribosome binding to the Shine-Dalgarno site.
Among the ligands investigated here, the comparison between equilibrium binding and non-equilibrium riboswitch activation during transcription is quite striking, as compounds that are poorer binders than ZMP (Table 1) show stronger activation during transcription (Table 2). This observation is important as ligands with slower koff, given a constant kon, should bind more strongly if measured at equilibrium (Zhang et al., 2010). However, these ligands would not be expected to differ in regulating kinetically controlled riboswitches, which do not reach equilibrium. The difference in activity in equilibrium and non-equilibrium assays has been observed for other riboswitches (Trausch and Batey, 2014; Wickiser et al., 2005a; Wickiser et al., 2005b) and has important implications for screening ligands. That is, ligands with larger kon rate constants may be more potent activators but could be discarded during screening upon determination of weaker Kds. The greatest such disparity is found for 12, which binds to and activates the ZTP riboswitch with similar affinity and efficiency in vitro, respectively. We speculate that the pyridine moiety of the 1-pyridinyl-AICA series enhances the ligand’s kon while the relative position of the pyridine affects the ligand’s koff, potentially in part by the ability of 13 achieving a hydrogen bond with the 2'-OH of G63. These findings suggest future screening efforts should initiate with activity assays that depend on kon rather than relying only on affinity measurements. This argument contrasts with enzyme-binding ligands, where residence time (i.e., 1/koff) can be a key factor in determining efficacy and is now often utilized as the primary feature for drug optimization (Copeland et al., 2006). In those cases, over the course of development, a series of compounds often have similar kon while residence time is maximized. Notably, an activation mechanism reliant on kon would be affected by any of the parameters mentioned above that would alter the effective ligand concentration in vivo.
Each of the above compounds activates the riboswitch presumably by stabilizing the folded, ligand-bound state. Although potent, long-lasting riboswitch activators could dysregulate purine and folate metabolism, riboswitch inhibition could prevent the expression of genes critical for virulence, such as PurH. Potent activators may also be useful in activating synthetic riboswitches; however, basal activation by naturally occurring ZMP and ZTP would likely occur. Development of chemical inhibitors would necessitate an alternative strategy that prevents readthrough and gene expression. From SMM screens, 3 and 4 were identified, albeit destabilizing the P1 helix with weak activities. Although weak in effect, these compounds could be thought of as allosteric modulators that alter RNA conformation without causing the same effect achieved by the cognate ligand. An alternative strategy could be to target upstream transcription intermediates predicted to fold into a stemloop and compete with proper folding for this class of riboswitches (Strobel et al., 2019). A disadvantage of that approach is that the upstream sequence is less conserved and less information-rich than the natural ligand binding site, consisting of G17:C69, G63, U70, and G71 (Fig. 4). Taken together, the observations in this work indicate that in-depth structural and mechanistic understanding of the target RNA is key to developing efficacious small molecule modulators.
SIGNIFICANCE
While many RNAs are recognized as important therapeutic targets, the development of small molecule methods for RNAs has lagged behind those for proteins. Found primarily in bacteria, and many important pathogens, riboswitches are non-coding RNA motifs that bind directly to metabolites to modulate gene expression via controlling transcription or translation. As natural small-molecule binders, riboswitches are model systems for understanding how synthetic ligand/RNA interactions can be achieved. ZTP riboswitches specifically recognize the purine biosynthetic metabolite 5-aminoimidazole-4-carboxamide riboside 5'-monophosphate (ZMP) and its triphosphorylated form (ZTP), which accumulate in cells stressed for folates. In this work, we sought to develop synthetic ligands against ZTP riboswitches using screens for novel ligands, as well as derivatives of the natural ligand. The latter approach yielded a series of derivatives in which the ribose and phosphate moieties of ZMP were replaced with pyridine. While the best of these synthetic ligands, 13, binds to the ZTP riboswitch two fold more poorly than ZMP, it more potently activates the riboswitch during transcription in vitro, suggestive of improved binding kinetics. This synthetic ligand is ~3-fold more potent than ZMP in vivo in an E. coli riboswitch reporter. Co-crystal structures of these ligands bound to the ZTP riboswitch suggest that their activity is achieved largely through π-π stacking interactions with a highly conserved residue in the ligand binding pocket. In addition to providing a foundation for structure-guided design of more potent ligands in the future, this work also gives insight into how modification of a natural ligand can lead to improved binding kinetics and activation.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Christopher P. Jones (christopher.jones2@nih.gov), Biochemistry and Biophysics Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
Materials availability
Requests for reagents may be sent to John S. Schneekloth, Jr., (jay.schneekloth@nih.gov) and Christopher P. Jones (christopher.jones2@nih.gov).
Data and code availability
Structure coordinates for the Fusobacterium ulcerans ZTP riboswitch bound to p-1-pyridinyl-AICA (PDB ID: 6WZR) and m-1-pyridinyl-AICA (PDB ID: 6WZS) have been deposited to the Protein Data Bank (Berman et al., 2000).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
For β-galactosidase reporter assays, Escherichia coli, strain BW25113, was grown at 37 °C on agar plates or in liquid media.
METHOD DETAILS
Materials and reagents
RNAs were in vitro transcribed as previously described (Jones and Ferré-D’Amaré, 2015) using PCR-amplified DNA templates prepared from plasmids encoding the ZTP riboswitch preceded by a hammerhead ribozyme (Ferré-D’Amaré and Doudna, 1996). The Tris-Borate-EDTA (TBE) buffer used throughout consists of 90 mM Tris base (Fisher), 90 mM boric acid (Fisher), and 2 mM sodium ethylenediamine tetraacetate (EDTA, Fisher), pH 8.0. RNAs were purified using a 1X TBE 8% denaturing PAGE (containing 8 M urea) gel and electroeluted from the gel overnight (Elutrap, Fisher). RNA was washed once with 1M KCl, desalted by washing with DEPC-treated water several times, concentrated in a 10K molecular-weight cutoff Amicon centrifugal concentrator, and filtered through a 0.22 μM centrifugal filter. RNA concentrations were determined using an extinction coefficient at 260 nm (ε260) of 655,000 M−1 cm−1 for the 75-nt F. ulcerans ZTP riboswitch. RNAs were stored at −20 °C until further use.
For SMM screens, a 79-nt construct of the ZTP riboswitch containing a 4-nt extension on the 3' end was used. The 3' end of this RNA was oxidized with sodium periodate and labeled with Cy5-hydrazide (Fisher), and the mixture was purified using an Illustra MicroSpin G-25 column (GE Healthcare) to remove free dye. The concentration was calculated using the absorbances at 260 nm and 649 nm, and the labeling efficiency was estimated to be ~80%. Labeled RNA was stored at −20 °C until use in SMM assays.
Purchased AICA derivatives—2 (Sigma-Aldrich), 7 (Enamine), 8 (Enamine), 10 (ChemBridge)—were resuspended in DEPC-treated water, diluted as needed, and stored at −20 °C. Synthesized AICA derivatives—7 and 12—were resuspended in DMSO, except 13, which was resuspended in DEPC-treated water, diluted as needed, and stored at −20 °C.
Small-molecule microarray screening
SMM screens were performed as previously described (Connelly et al., 2017). Cy5-labeled ZTP riboswitch RNA was panned against slides, and fluorescence was recorded on InnoScan 1100 AL Fluorescence Scanner (Innopsys) prior to analysis with Mapix (Innopsys). Positive hits were ranked and compared to hits found for all other nucleic acids screened by the Schneekloth lab to identify positive hits relatively unique to the ZTP riboswitch (Supplemental Data File 1).
In-line probing
RNA was end-labeled by incubating 45 pmol RNA with 65 μCi [γ32P]-ATP (Perkin Elmer) and 6.4 U T4 polynucleotide kinase (NEB) for 1 hour at 37 °C. Labeled RNA was purified via denaturing gel electrophoresis as above and eluted overnight by rotisserie in a buffer containing 0.2 M NaCl, 10 mM Tris-HCl pH 7.5, and 1 mM EDTA at room temperature. Labeled RNA was concentrated, filtered, purified using a G-25 column, and stored at −20 °C until further use.
In-line probing reactions contained 0.1 M Tris pH 8.5, 150 mM KCl, 10 mM MgCl2, and varying concentrations of ligand as described previously (Regulski and Breaker, 2008). Compounds identified in SMM screens were purchased from ChemDiv and ChemBridge, resuspended in DMSO, and compared against controls containing equal amounts of DMSO. SMM hits were initially screened at 0.2 and 2 mM in duplicate, and compounds demonstrating protection under these conditions were further examined. In-line probing reactions were incubated at room temperature for 40 hours before mixing with equal volumes of loading dye containing 8 M urea, 20% (w/v) sucrose, 1X TBE, 0.1% (w/v) sodium dodecyl sulfate, 0.001% (w/v) xylene cyanol, and 0.001% (w/v) bromophenol blue. An RNase digest ladder was prepared by incubating radiolabeled RNA in a solution containing 7 M urea, 1.05 mM EDTA, and 0.1 U of RNase T1 enzyme or RNase A enzyme (Ambion) at 55 °C for 10 minutes before mixing with loading dye. A partial alkaline digest ladder was prepared by incubating radiolabeled RNA in 50 mM Na2CO3 at 90°C for 10 minutes before mixing with equal volume of loading dye. In-line probing reaction samples were separated by a 1X TBE 12% denaturing PAGE sequencing gel (0.3 mm width spacers) at 45 W for 4.5 hours. The gel was transferred to Whatman filter paper, exposed to a storage phosphor screen overnight, and imaged using an Amersham Typhoon PhosphorImager (GE Healthcare). To estimate Kd for titrations of 3 and 4, in-line probing gel band intensities were measured with ImageQuant software (GE Healthcare). To account for loading differences among lanes, normalized band intensities were calculated by dividing intensities for ligand-sensitive bands (nts 41-49) by those of position 23. Normalized intensities were fit to a simple binding isotherm, assuming 1:1 binding.
Isothermal titration calorimetry
ITC measurements were performed using a MicroCal iTC200 microcalorimeter (Malvern) and data were fit using NITPIC and SEDPHAT as previously described (Jones et al., 2019). All experiments were performed at 37 °C in 50 mM Hepes-KOH, pH 7.4, 150 mM KCl, 10 mM MgCl2, except for 7 and 13, which were supplemented with 3% DMSO. Typically, 30 μM F. ulcerans pfl RNA in the cell was titrated with 300 μM ligand, but RNA and ligand concentrations were increased up 300 μM and 6 mM, respectively, for weaker binding ligands (e.g., compound 10). Titrations contained 18 or 40 points, and the first point was always removed.
Single-round transcription termination assays
DNA templates containing a lambda phage promoter and 29-nucleotide C-less sequence followed by the F. ulcerans ZTP riboswitch, termination hairpin, and downstream sequence (50 nts) were PCR amplified as previously described (Jones and Ferré-D’Amaré, 2015). Halted transcription complexes were formed using a reaction mix containing 80 nM DNA template, transcription buffer (20 mM Tris-HCl, pH 8.0, 2 mM MgCl2, 0.1 mM DTT, 0.1 mM EDTA, 4% (v/v) glycerol, 2 mM NaCl), 1.5 μM GTP, 2.5 μM ATP, 2.5 μM UTP, 130 μM ApU, 100 μM anti-26 nt oligonucleotide, ~5-10 μCi 32P-[α]-GTP, and 10 U/mL of E. coli RNA polymerase holoenzyme (NEB). This mixture was incubated at 37 °C for 10 minutes to allow for halted complexes to form. Transcription was restarted by adding 9 μL of halted complex to tubes containing 3 μL of ligand in transcription buffer, except for 7 and 12, which were supplemented with 3% DMSO, and 3 μL of an NTP mix containing 250 μM NTPs, 0.5 mg/mL heparin, 20 mM MgCl2, and transcription buffer. Transcription reactions proceeded for 15 minutes by incubating tubes at 37 °C. To quench the reaction, an equal volume of loading dye (15 μL) containing 8 M urea, 20% (v/v) sucrose, 0.1% (w/v) SDS, 0.001% (w/v) xylene cyanol, and 0.001% (w/v) bromophenol blue was added. Samples were separated using 8% denaturing PAGE and imaged by exposing the gel to a storage phosphor screen overnight. Band intensities were measured using ImageQuant software. After background correcting all bands, the percentage of readthrough was calculated by dividing the intensity of full-length readthrough band by the total intensity of terminated plus readthrough bands. These values were fitted to a simple binding isotherm assuming a 1:1 binding stoichiometry to determine the T50 value.
Crystallization
Crystals of F. ulcerans ZTP riboswitch aptamer domain bound to 7 were grown by hanging drop vapor diffusion at 21 °C by adding 1.05-1.4 μL of 96-172 μM RNA solution containing 50 mM Hepes-KOH, pH 7.4, 150 mM KCl, 10 mM MgCl2, and 3-fold excess 7, to 0.7-1.05 μL of reservoir solution containing 26% (w/v) polyethylene glycol (PEG) 4000, 4% (v/v) PEG 400, 180 mM Li2SO4, and 100 mM Tris-HCl, pH 8.5, and equilibrated against 500 μL of reservoir solution. Crystals grew out of precipitate typically within 3-5 days, and were ~200 μm in length. Crystals were cryoprotected in a solution consisting of the reservoir solution supplemented with 15% (v/v) PEG 400.
Crystals of the same RNA bound to 13 were grown by hanging drop vapor diffusion at 21 °C by adding 1.4 μL of 115 μM RNA solution containing 50 mM Hepes-KOH, pH 7.4, 150 mM KCl, 20 mM MgCl2, and 3-fold excess 13, to 0.7 μL of reservoir solution containing 32.5% (w/v) polyethylene glycol (PEG-MME) 2000 monomethyl ether, 200 mM Li2SO4, and 100 mM Tris-HCl, pH 8.5, and equilibrated against 500 μL of reservoir solution. Crystals grew out of precipitate typically within 3-5 days, and were ~100 μm in length. Crystals were cryoprotected in a solution consisting of the reservoir solution supplemented with 9% (v/v) PEG 400.
All crystals were flash-frozen in liquid nitrogen and shipped to beamline 5.0.1 or 5.0.2 of the Advanced Light Source (ALS), Lawrence Berkeley Laboratory, or beamline 24-ID of the Advanced Photon Source, Argonne National Laboratory, where data were collected at 100 K.
Structure determination and refinement
The previously determined model for the F. ulcerans ZTP riboswitch bound to ZMP (5BTP) was used for as a molecular replacement search model for the compound 7 dataset after removing ZMP, ions, and seven base pairs of the P1 helix, which is the most disordered part of the structure. The same molecular replacement procedure was used for the compound 13 dataset except that the entire 5BTP RNA was used as a search model. Molecular replacement was performed using PHASER (McCoy et al., 2007), and all subsequent refinement was performed using Phenix (Adams et al., 2010). After an initial round of rigid-body refinement, refinement was performed using simulated annealing, secondary structure restraints, and multiple rounds of individual B-factor refinement interspersed with model building in Coot (Emsley and Cowtan, 2004). A single bond restraint between the O6 of G63 and N1 of G71 was used to prevent G63 from migrating into the binding pocket during refinement. Residues in the P1 helix were rebuilt prior to placement of the ligand and ligand-contacting magnesium ions into a large positive feature in the ∣Fo∣-∣Fc∣ Fourier difference map (Fig. 4b,c). Waters were placed adjacent to the ligand-bound Mg2+ ions to account for positive features and restrained in subsequent refinements. Other ions were assigned as Mg2+, refined, and changed to K+ ions if positive features remained, which was the case for only a single ion. Additional residues in the P1 helix were subsequently rebuilt, and TLS parameters were refined during the final rounds of refinement (Table S1).
Liquid β-galactosidase reporter assay
Assays were performed as previous described (Perkins et al., 2019), with slight modifications. WT and M6 riboswitch reporters in pRS414 plasmids were transformed into E. coli strain BW25113 (Fisher) and plated on agar plates supplemented with ampicillin. Ten individual colonies were selected for separated overnight cultures in Luria broth at 37 °C. After 16 hours, 1 μL cells were diluted into 0.2 mL fresh media supplemented with antibiotic and small molecule (e.g., compound 7) at a maximum concentration of 5 mM, and dilutions thereof. After 20 hours of growth at 37 °C in Clear 96-well U-bottom Tissue Culture Plates with Low Evaporation Lid (Falcon), 40 μL of cells were transferred in triplicate to Black 96-well Half Area μClear Cellstar Culture Microplates (Greiner Bio-One), and absorbance at 595 nm were recorded using a Multimode Multiplate Reader Infinite M200 Pro NanoQuant (Tecan). Cells were supplemented with 40 μL Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, pH 7.0) and 40 μL 1 mg/mL 4-methylumbelliferyl-β-galactopyranoside. After 15 min, reactions were quenched by adding 20 μL of 1 M Na2CO3, and fluorescence was recorded via excitation at 380 nm and emission at 485 nm using a Multimode Multiplate Reader Infinite F200 Pro NanoQuant (Tecan). Absorbance was used to normalize fluorescence to cell density. The error-weighted data for each clone (i.e., the averages and deviations for N = 3 technical replicates) were individually fit to a single-site binding model to estimate EC50 values using Origin 8.0 (OriginLab Corporation, Northampton, MA). Averages and standard deviations of EC50 values were calculated from all clones (N = 10 before removing outliers). Outliers were identified as being two standard deviations away from the mean, and included 1 outlier for 1, 1 outlier for 2, 1 outlier for 12, and 1 outlier for 5. A one-tailed Welch’s T-test, assuming unequal variances, was used to test for significance.
QUANTIFICATION AND STATISTICAL ANALYSIS
Using Microsoft Excel, averages and standard deviations were calculated from independent experiments (e.g., independent titrations), as reported in each Figure legend and Table. For statistical tests comparing treatments in the β-galactosidase reporter, p-values from one-tailed Welch’s T tests were calculated using Excel, and outliers were excluded if they fell two standard deviations outside the mean. The criterion for significance was a p-value less than 0.05.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| BW25113 | ThermoFisher | Cat. #OEC5042 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| List of chemicals described in Fig. 1a. | Refer to Methods and Supplemental Information | Refer to Fig. 1a. |
| Deposited Data | ||
| Structure of F. ulcerans ZTP riboswitch bound to p-1-pyridinyl-AICA | Protein Data Bank (Berman et al, 2000) | PDB:6WZR |
| Structure of F. ulcerans ZTP riboswitch bound to m-1-pyridinyl-AICA | Protein Data Bank (Berman et al, 2000) | PDB: 6WZS |
| Oligonucleotides | ||
| F. ulcerans ZTP riboswitch crystal construct RNA, UAUCAGUUAUAUGACUGACGGAACGUGGAAUUAACCACAUGAAGUAUAACGAUGACAAUGCCGACCGUCUGGGCG | In vitro transcription, as described in Jones and Ferré-D’Amaré, Nat Struct Mol Biol 22(9):679-85 (2015). | N/A |
| F. ulcerans ZTP riboswitch crystal construct forward primer, TAATACGACTCACTATAGG | Ibid. | Integrated DNA Technologies |
| F. ulcerans ZTP riboswitch crystal construct reverse primer, mCmGCCCAGACGGTCGGCAT | Ibid. | Integrated DNA Technologies |
| F. ulcerans ZTP riboswitch single-round transcription termination template forward primer, CTTGATTCTAGCTGATCGTGGACC | N/A | Integrated DNA Technologies |
| F. ulcerans ZTP riboswitch single-round transcription termination template reverse primer, TCAACAATTATATTTGATATTCCC | N/A | Integrated DNA Technologies |
| Recombinant DNA | ||
| WT Pectobacterium carotovorum ZTP riboswitch in pRS414 | Perkins et al, ACS Chem Biol 14(12):2841-2850 (2019). | WT |
| M6 Pectobacterium carotovorum ZTP riboswitch in pRS414 | Ibid. | M6 |
| F. ulcerans ZTP riboswitch crystal construct plasmid | Jones and Ferré-D’Amaré, Nat Struct Mol Biol 22(9):679-85 (2015). | Refer to Methods. |
| F. ulcerans ZTP riboswitch single-round transcription termination template in PCR2.1 plasmid, CTTGATTCTAGCTGATCGTGGACCGGAAGGTGAGCCAGTGAGTTGATTGCAGTCCAGTTACGCTGGAGTCTGAGGCTCGTCCTGAATGATATGCGGCCTCTTGACTATTTTACCTCTGGCGGTGATAATGGTTGCAATGTAGTAAGGAGGTTGTATGGAAGATTATCAGTTATATGACTGACGGAACGTGGAATTAACCACATGAAGTATAACGATGACAATGCCGACCGTCTGGGCGAACAAGCCTAGATTGTCGGTTTTTTTTATACATTTTTTTAGGAGGAGATAATAAGGGAATATCAAATATAATTGTTGA | N/A | Integrated DNA Technologies |
ACKNOWLEDGMENTS
We thank R. Breaker and K. Corbino for providing WT and M6 plasmids for the reporter assay; M. Banco, N. Demeshkina, T. Numata, L. Truong, and R. Trachman, III, for discussions; G. Piszczek and D. Wu of the Biophysics Core of the National Heart, Lung and Blood Institute (NHLBI) for ITC support; K. Neumann and R. Hogg, Jr., for use of Tecan fluorimeters; and the staff at beamlines 5.0.1 and 5.0.2 of the Advanced Light Source (ALS). ALS is a U.S. DOE Office of Science User Facility under Contract No. DE-AC02-05CH11231, which is supported in part by the ALS-ENABLE program funded by the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) (grant P30 GM124169-0). This work is partly based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the NIH, NIGMS (grant P30 GM124165). The Pilatus 6M detector on 24-ID-C beam line is funded by a NIH-ORIP HEI grant (S10 RR029205). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. C.P.J. is the recipient of NIH transition award K22HL139920-01. B.T. and P.P. were part of the Post-baccalaureate program of NHLBI, and L.T. of the Post-baccalaureate program of the National Cancer Institute (NCI). This work was supported in part by the Intramural AIDS Targeted Antiviral Program and by the intramural programs of NCI and NHLBI of the NIH.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica Section D, Biological crystallography 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsimovitch I, and Henkin TM (2009). In vitro approaches to analysis of transcription termination. Methods 47, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataide SF, Wilson SN, Dang S, Rogers TE, Roy B, Banerjee R, Henkin TM, and Ibba M (2007). Mechanisms of resistance to an amino acid antibiotic that targets translation. ACS chemical biology 2, 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, and Bourne PE (2000). The Protein Data Bank. Nucleic acids research 28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binas O, Schamber T, and Schwalbe H (2020). The conformational landscape of transcription intermediates involved in the regulation of the ZMP-sensing riboswitch from Thermosinus carboxydivorans. Nucleic acids research 48, 6970–6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount KF, and Breaker RR (2006). Riboswitches as antibacterial drug targets. Nature biotechnology 24, 1558–1564. [DOI] [PubMed] [Google Scholar]
- Blount KF, Megyola C, Plummer M, Osterman D, O'Connell T, Aristoff P, Quinn C, Chrusciel RA, Poel TJ, Schostarez HJ, et al. (2015). Novel riboswitch-binding flavin analog that protects mice against Clostridium difficile infection without inhibiting cecal flora. Antimicrobial agents and chemotherapy 59, 5736–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR, and Ames BN (1982). ZTP (5-amino 4-imidazole carboxamide riboside 5'-triphosphate): a proposed alarmone for 10-formyl-tetrahydrofolate deficiency. Cell 29, 929–937. [DOI] [PubMed] [Google Scholar]
- Chen L, Cressina E, Leeper FJ, Smith AG, and Abell C (2010). A fragment-based approach to identifying ligands for riboswitches. ACS chemical biology 5, 355–358. [DOI] [PubMed] [Google Scholar]
- Connelly CM, Abulwerdi FA, and Schneekloth JS Jr. (2017). Discovery of RNA Binding Small Molecules Using Small Molecule Microarrays. Methods in molecular biology 1518, 157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CM, Numata T, Boer RE, Moon MH, Sinniah RS, Barchi JJ, Ferre-D'Amare AR, and Schneekloth JS Jr. (2019). Synthetic ligands for PreQ1 riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure. Nature communications 10, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland RA, Pompliano DL, and Meek TD (2006). Drug-target residence time and its implications for lead optimization. Nature reviews Drug discovery 5, 730–739. [DOI] [PubMed] [Google Scholar]
- Daldrop P, Reyes FE, Robinson DA, Hammond CM, Lilley DM, Batey RT, and Brenk R (2011). Novel ligands for a purine riboswitch discovered by RNA-ligand docking. Chemistry & biology 18, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta crystallographica Section D, Biological crystallography 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Felsenstein KM, Saunders LB, Simmons JK, Leon E, Calabrese DR, Zhang S, Michalowski A, Gareiss P, Mock BA, and Schneekloth JS Jr. (2016). Small Molecule Microarrays Enable the Identification of a Selective, Quadruplex-Binding Inhibitor of MYC Expression. ACS chemical biology 11, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré-D'Amaré AR, and Doudna JA (1996). Use of cis- and trans-ribozymes to remove 5' and 3' heterogeneities from milligrams of in vitro transcribed RNA. Nucleic acids research 24, 977–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SD, Reyes FE, Edwards AL, and Batey RT (2009). Adaptive ligand binding by the purine riboswitch in the recognition of guanine and adenine analogs. Structure 17, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JA, Wang H, Fischmann TO, Balibar CJ, Xiao L, Galgoci AM, Malinverni JC, Mayhood T, Villafania A, Nahvi A, et al. (2015). Selective small-molecule inhibition of an RNA structural element. Nature 526, 672–677. [DOI] [PubMed] [Google Scholar]
- Jones CP, and Ferré-D’Amaré AR (2015). Recognition of the bacterial alarmone ZMP through long-distance association of two RNA subdomains. Nature structural & molecular biology 22, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CP, and Ferré-D’Amaré AR (2017). Long-Range Interactions in Riboswitch Control of Gene Expression. Annual review of biophysics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CP, Piszczek G, and Ferre-D’Amare AR (2019). Isothermal Titration Calorimetry Measurements of Riboswitch-Ligand Interactions. Methods in molecular biology 1964, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PB, Nelson JW, and Breaker RR (2015). An ancient riboswitch class in bacteria regulates purine biosynthesis and one-carbon metabolism. Molecular cell 57, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ER, Blount KF, and Breaker RR (2009). Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA biology 6, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunse CE, Schmidt MS, Wittmann V, and Mayer G (2011). Carba-sugars activate the glmS-riboswitch of Staphylococcus aureus. ACS chemical biology 6, 675–678. [DOI] [PubMed] [Google Scholar]
- Lunse CE, Schuller A, and Mayer G (2014). The promise of riboswitches as potential antibacterial drug targets. International journal of medical microbiology : IJMM 304, 79–92. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007). Phaser crystallographic software. Journal of applied crystallography 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motika SE, Ulrich RJ, Geddes EJ, Lee HY, Lau GW, and Hergenrother PJ (2020). Gram-Negative Antibiotic Active Through Inhibition of an Essential Riboswitch. Journal of the American Chemical Society 142, 10856–10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhbacher J, Brouillette E, Allard M, Fortier LC, Malouin F, and Lafontaine DA (2010a). Novel riboswitch ligand analogs as selective inhibitors of guanine-related metabolic pathways. PLoS pathogens 6, e1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhbacher J, St-Pierre P, and Lafontaine DA (2010b). Therapeutic applications of ribozymes and riboswitches. Current opinion in pharmacology 10, 551–556. [DOI] [PubMed] [Google Scholar]
- Muller I (2017). Guidelines for the successful generation of protein-ligand complex crystals. Acta crystallographica Section D, Structural biology 73, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydock-McGrane LK, Hannan TJ, and Janetka JW (2017). Rational design strategies for FimH antagonists: new drugs on the horizon for urinary tract infection and Crohn’s disease. Expert opinion on drug discovery 12, 711–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KR, Atilho RM, Moon MH, and Breaker RR (2019). Employing a ZTP Riboswitch to Detect Bacterial Folate Biosynthesis Inhibitors in a Small Molecule High-Throughput Screen. ACS chemical biology 14, 2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony JJ, and Ferre-D’Amare AR (2013). Glucosamine and glucosamine-6-phosphate derivatives: catalytic cofactor analogues for the glmS ribozyme. The Journal of organic chemistry 78, 4730–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulski EE, and Breaker RR (2008). In-line probing analysis of riboswitches. Methods in molecular biology 419, 53–67. [DOI] [PubMed] [Google Scholar]
- Rohlman CE, and Matthews RG (1990). Role of purine biosynthetic intermediates in response to folate stress in Escherichia coli. Journal of bacteriology 172, 7200–7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samant S, Lee H, Ghassemi M, Chen J, Cook JL, Mankin AS, and Neyfakh AA (2008). Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS pathogens 4, e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A, and Nudler E (2013). A decade of riboswitches. Cell 152, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer CL, Zhang EW, Dudley AG, Dixon B, Guckes KR, Breland EJ, Floyd KA, Casella DP, Algood HMS, Clayton DB, et al. (2017). Purine Biosynthesis Metabolically Constrains Intracellular Survival of Uropathogenic Escherichia coli. Infection and immunity 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CA, Gaffney BL, Jones RA, and Strobel SA (2011). Differential analogue binding by two classes of c-di-GMP riboswitches. Journal of the American Chemical Society 133, 15578–15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster C, Allard M, Boulanger S, Lamontagne Boulet M, Mulhbacher J, Lafontaine DA, Marsault E, Lacasse P, and Malouin F (2013). Experimental treatment of Staphylococcus aureus bovine intramammary infection using a guanine riboswitch ligand analog. Journal of dairy science 96, 1000–1008. [DOI] [PubMed] [Google Scholar]
- Strobel EJ, Cheng L, Berman KE, Carlson PD, and Lucks JB (2019). A ligand-gated strand displacement mechanism for ZTP riboswitch transcription control. Nature chemical biology 15, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan N, Cohen-Chalamish S, Nakamura S, Emilsson GM, and Breaker RR (2005). Thiamine pyrophosphate riboswitches are targets for the antimicrobial compound pyrithiamine. Chemistry & biology 12, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Trausch JJ, and Batey RT (2014). A disconnect between high-affinity binding and efficient regulation by antifolates and purines in the tetrahydrofolate riboswitch. Chemistry & biology 21, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens Q, Mondragon E, Reyes FE, Coish P, Aristoff P, Berman J, Kaur H, Kells KW, Wickens P, Wilson J, et al. (2018). Structure-Activity Relationship of Flavin Analogues That Target the Flavin Mononucleotide Riboswitch. ACS chemical biology 13, 2908–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KD, Hajdin CE, and Weeks KM (2018). Principles for targeting RNA with drug-like small molecules. Nature reviews Drug discovery 17, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KD, Homan P, Weeks KM, Smith AG, Abell C, and Ferre-D’Amare AR (2014). Validating fragment-based drug discovery for biological RNAs: lead fragments bind and remodel the TPP riboswitch specifically. Chemistry & biology 21, 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickiser JK, Cheah MT, Breaker RR, and Crothers DM (2005a). The kinetics of ligand binding by an adenine-sensing riboswitch. Biochemistry 44, 13404–13414. [DOI] [PubMed] [Google Scholar]
- Wickiser JK, Winkler WC, Breaker RR, and Crothers DM (2005b). The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Molecular cell 18, 49–60. [DOI] [PubMed] [Google Scholar]
- Yan LH, Le Roux A, Boyapelly K, Lamontagne AM, Archambault MA, Picard-Jean F, Lalonde-Seguin D, St-Pierre E, Najmanovich RJ, Fortier LC, et al. (2018). Purine analogs targeting the guanine riboswitch as potential antibiotics against Clostridioides difficile. European journal of medicinal chemistry 143, 755–768. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lau MW, and Ferré-D’Amaré AR (2010). Ribozymes and riboswitches: modulation of RNA function by small molecules. Biochemistry 49, 9123–9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structure coordinates for the Fusobacterium ulcerans ZTP riboswitch bound to p-1-pyridinyl-AICA (PDB ID: 6WZR) and m-1-pyridinyl-AICA (PDB ID: 6WZS) have been deposited to the Protein Data Bank (Berman et al., 2000).