Abstract
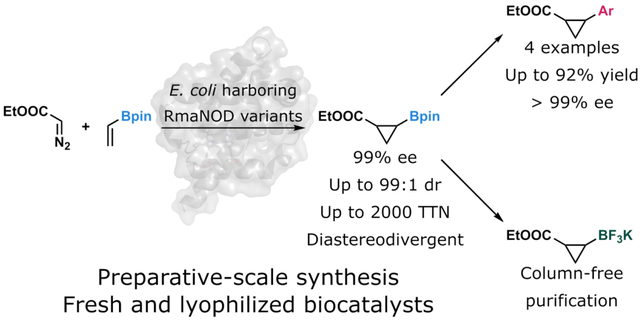
While biocatalysis is increasingly incorporated into drug development pipelines, it is less commonly used in the early stages of drug discovery. By engineering a protein to produce a chiral motif with a derivatizable functional handle, biocatalysts can be used to help generate diverse building blocks for drug discovery. Here we show the engineering of two variants of Rhodothermus marinus nitric oxide dioxygenase (RmaNOD) to catalyze the formation of cis- and tran- diastereomers of a pinacolboronate-substituted cyclopropane which can be readily derivatized to generate diverse stereopure cyclopropane building blocks.
Keywords: biocatalysis, cyclopropanation, carbene transfer, diastereodivergence, diversity-oriented synthesis
Graphical Abstract
Enzymes are increasingly used in industrial processes to produce value-added compounds and drugs.1 As a drug moves further down the development pipeline, highly selective biocatalytic processes may become desirable alternatives to chemical ones for synthesis. Prominent examples include the production of sitagliptin2 and islatravir3. Usually, the enzymes used in these processes are highly engineered and show exceptional activity and selectivity for a given task, features which often result in a narrow substrate scope. While this specialization is useful for biocatalysts in the production stage, it limits the utility of enzymes in the initial phases of drug discovery, where broad substrate scopes are key to rapidly generating diverse molecules.4
An emerging strategy for the incorporation of biocatalysis into earlier stages of drug development is the enzymatic synthesis of a core motif that can be further derivatized.5–8 An enantiopure core motif can be generated with high selectivity, followed by the use of reactions commonly applied in diversity-oriented synthesis to derivatize the molecule. One chiral motif found in several pharmaceutical and agrochemical compounds is the substituted cyclopropane.9–11 The Arnold lab and others have shown that heme proteins can catalyze cyclopropanation via carbene transfer12, and engineered heme proteins have been used to generate the cyclopropane-containing pharmaceuticals levomilnacipran,13 ticagrelor,14,15 and grazoprevir16.
To bring the benefits of biocatalytic cyclopropanation to early drug discovery, we envisioned a chemoenzymatic approach in which a tandem enzymatic cyclopropanation-chemical derivatization sequence would enable preparation of diverse enantiopure cyclopropanes. To this end, we engineered heme proteins which catalyze carbene transfer to vinyl boronic acid pinacol ester (1) from ethyl diazoacetate (EDA, 2) to produce cis- and trans-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)cyclopropanecarboxylic acid ethyl ester (3) with high diastereo- and enantioselectivity (Figure 1). Unlike small-molecule approaches for the stereoselective construction of cyclopropylboronates,17–21 this biocatalytic approach avoids the need for chiral ligands, expensive transition-metal catalysts, or highly reactive substrates. It also reduces waste in the form of organic solvents and undesired regio- and stereoisomers. The boronate functional group in 3 allows for the rapid generation of enantiopure cyclopropanes using Suzuki-Miyaura coupling.22–24 This method provides a new avenue for using biocatalysts in medicinal chemistry and other processes where the rapid generation of molecular diversity is desired.
Figure 1.

Directed evolution of RmaNOD for production of cyclopropylboronate 3. All reactions were performed with whole E. coli resuspended in 20 mM MOPS buffer (pH = 7) to OD600=30, 20 mM 1, 40 mM 2, anaerobic conditions, 5% EtOH cosolvent. Experimental details are provided in the Supporting Information, Materials and Methods section. (a) Diastereomeric ratio (%cis) and total turnover number (TTN) of the evolutionary lineage toward production of cis-3. (b) Diastereomeric ratio (%trans) and TTN of the evolutionary lineage of trans-3 production.
To find a starting enzyme having some level of the desired cyclopropanation activity, we screened a panel of heme proteins (including variants of cytochromes P411 (serine-ligated P450s), cytochromes c, and globins) for the ability to produce 3 using EDA as a carbene precursor for the cyclopropanation of 1 (Supporting Information Table S1). We found that the Rhodothermus marinus nitric oxide dioxygenase (RmaNOD) variant RmaNOD Q52A catalyzed the desired reaction with low activity (17 total turnovers (TTN)) to preferentially produce trans-3 (20:80 cis:trans diastereomeric ratio (dr)). The Q52A mutation was found during engineering for unactivated alkene cyclopropanation25 and is analogous to mutations found to enhance myoglobin-catalyzed cyclopropanation reactions.26
With RmaNOD Q52A as a starting point and using a crystal structure we obtained of RmaNOD Q52V (PDB ID: 6WK3) to guide selection of amino-acid residues in the distal heme pocket for mutagenesis, we began iterative rounds of single-site-saturation mutagenesis and screening and recombined the beneficial mutations (Supporting Information). In an initial round of site-saturation mutagenesis, we discovered that mutation Y32T caused an inversion of diastereoselectivity to favor production of cis-3 and boosted overall enzymatic activity 24-fold (420 TTN, 90:10 dr, >99% enantiomeric excess (ee)). One further round of single-site-saturation mutagenesis and recombination yielded the variant RmaNOD Y32T Y39H L48R Q52A R79W (RmaNOD THRAW), which exhibited both higher activity and diastereoselectivity than RmaNOD Y32T Q52A while maintaining high enantioselectivity (Figure 1a). At analytical scale, RmaNOD THRAW produces cis-3 with a dr of 94:6, ee of >99%, and TTN of 1300.
In parallel to the evolution of the cis-lineage, we also focused on engineering an enzyme that produces trans-3. Starting from RmaNOD Q52A, we performed three sequential rounds of single-site-saturation mutagenesis and recombination to generate RmaNOD L20W Q52A L56I L60H L101N I105M (RmaNOD WAIHNM), which produces trans-3 with a dr of <1:99, ee of >99%, and TTN of 2000 (Figure 1b). As we would expect, the most impactful mutations identified in both the cis- and trans-lineages are found at first-shell residue positions (Supporting Information Figures S1 and S3). Mutations to first-shell residues can be expected to affect substrate and reactive intermediate binding, leading to changes in diastereoselectivity and activity. Future mechanistic studies might elucidate why these mutations exert the observed effects.
We performed a gram-scale reaction using RmaNOD THRAW. Whole E. coli cells (OD600=30 in 1x M9-N, 60 mM 1, 120 mM 2, anaerobic conditions, 5% EtOH cosolvent, details in Supporting Information, Compound Synthesis and Characterization) expressing RmaNOD THRAW were used to produce 3.7 g of cis-3 (>99% ee, 95:5 dr, 36% isolated yield, 41% isolated yield based on recovered starting material). Some of the chiral product was converted to the potassium trifluoroborate salt (4, Figure 2a), which was crystallized to determine that the absolute stereochemistry of the enzymatic product was (1R,2S) (Supporting Information). During preparative-scale reactions, we found that 3 in aqueous buffer partially converted to the pinacol-deprotected form, cyclopropylboronic acid. We found that the deprotected boronic acid could either be re-protected with additional pinacol or converted into the trifluoroborate salt (Supporting Information, Compound Synthesis and Characterization).
Figure 2.
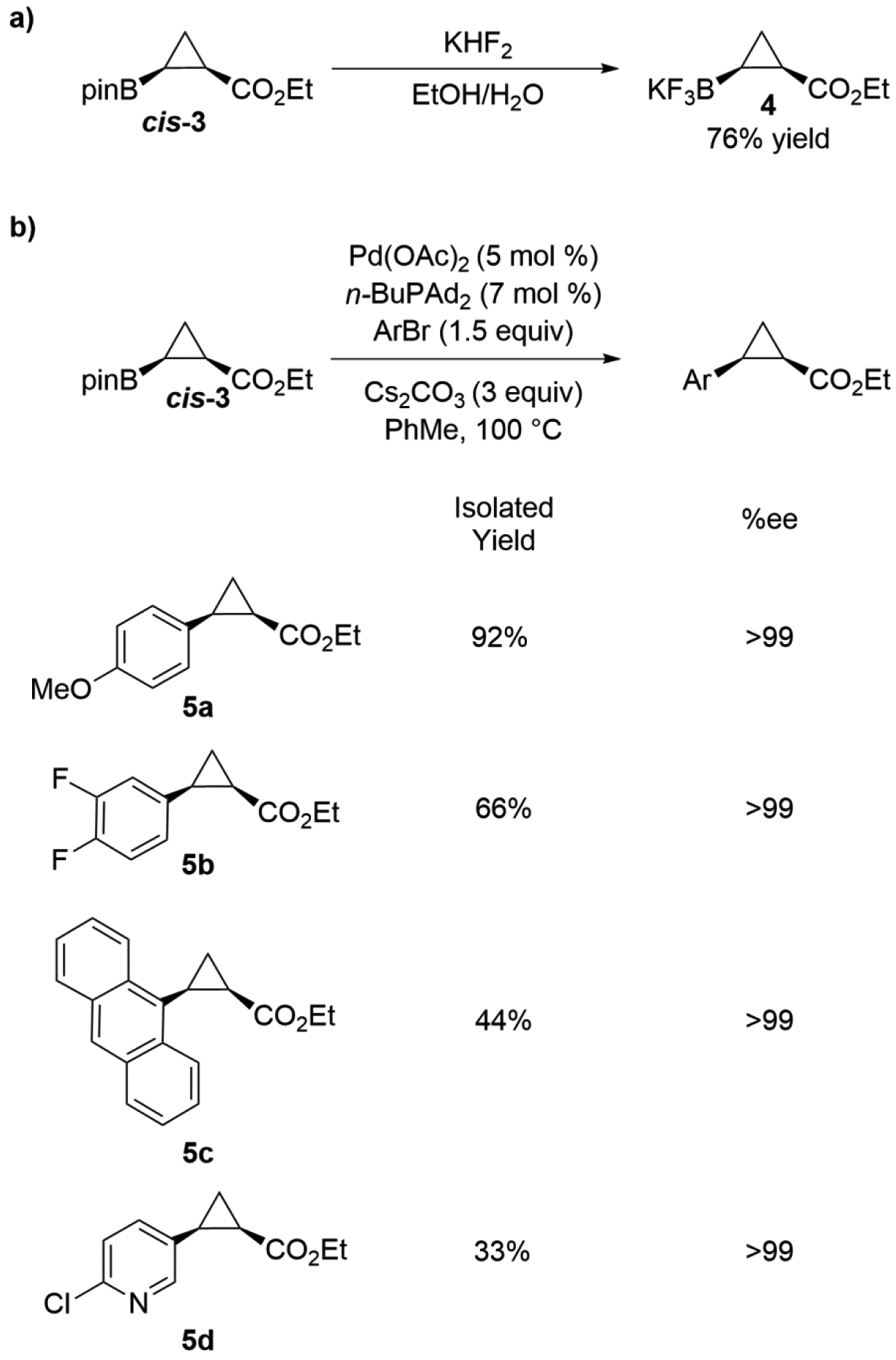
Derivatization of cis-3. (a) Conversion of cis-3 to the trifluoroborate salt (4). Products are drawn with absolute stereochemistry, which was determined as (1R,2S) from X-ray diffraction experiments on 4. (b) Conversion of cis-3 to a variety of aryl cyclopropanes using stereoretentive Suzuki-Miyaura coupling. Isolated yields are reported for the derivatization step.
We also sought to improve access to the final product by simplifying both the catalyst formulation and the product isolation procedure. Currently most biocatalytic carbene-transfer reactions are catalyzed using freshly prepared whole E. coli cells harboring engineered heme proteins.12 While reactions run using whole cells are straightforward to perform, the whole cells have a multi-day preparation prior to use, have a short shelf life, and require biological infrastructure not available to many chemists. In contrast, using lyophilized biocatalysts enables preparation of a large batch of catalyst (e.g. via fermentation) which can be shelf-stable29 and used by researchers without cell culture experience. We found that both RmaNOD THRAW and RmaNOD WAIHNM function as lyophilized enzyme in whole E. coli cells resuspended in aqueous buffer, and that they could be used in preparative-scale reactions (Supporting Information section, Materials and Methods).
A challenging step in the isolation of 3 was efficient separation of 3 from the two EDA dimers, diethyl maleate and diethyl fumarate, via silica column chromatography. We therefore modified the procedure to isolate the product from starting material and EDA dimer byproducts without the use of chromatography. After aqueous work-up and extraction of the crude reaction mix into organic solvents, unreacted 2 can be removed under reduced pressure. Then, to efficiently separate the cyclopropane product from the maleate and fumarate byproducts, 3 can be derivatized in crude extract to form 4.30 The trifluoroborate salt is then easily separated from the remaining impurities (Supporting Information section, Materials and Methods). Through the combination of using lyophilized whole cells harboring RmaNOD THRAW and the improved product isolation procedure, we prepared (1R,2S)-4 on 1 mmol scale at 52% isolated yield.
Using diverse coupling partners, many compounds could be produced from cis- and trans-3 or their respective trifluoroborate derivatives. In addition to cross-coupling reactions, conversions of cyclopropylboronates to cyclopropylamines and cyclopropanols are known.20,22,23,27,28 As a proof of concept, chiral product from the gram-scale reaction was used in stereoretentive Suzuki-Miyaura coupling reactions to produce select aromatic cyclopropanes 5a–5d (Figure 2b). These examples demonstrate that Suzuki-Miyaura cross-coupling with cis-3 tolerates different functional groups (5a, 5b, 5d), bulky aromatic groups (5c), and heterocycles (5d), with complete retention of stereochemistry. The full synthetic pathway to compounds 5a–5d demonstrates how combining small-molecule and biocatalytic systems takes advantage of the stereoselectivity of biocatalysts and the general activity of small-molecule catalysts. sWhile we have focused on cross-coupling reactions on purified cyclopropylboronates, one could consider performing these derivatizations immediately following the enzymatic reaction. Work by Lipshutz and coworkers has shown that cross-coupling reactions can be carried out in aqueous buffer using specialty surfactants.31 Further reaction optimization could provide a one-pot, two-step system utilizing these surfactants, in which the enzymatic cyclopropanation is followed by cross-coupling to provide the desired derivatized cyclopropane product.
By coupling the specificity of biocatalysts with the broad substrate scope of small-molecule catalysts, this study presents a chemoenzymatic approach that rapidly generates diverse enantiopure cyclopropane-containing compounds, a strategy that could be useful for preparing chiral small-molecule libraries at the early stages of drug discovery.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NSF MCB (grant 1513007 to F. H. A.), NSF STTR (grant 1738308 to F. H. A.), and the NIH (grant R35GM118191-01 to S. E. R.). Graduate student support from NIH T32 training grants GM112592 (A. M. K.) and GM07616 (B. J. W.), and the NSF Graduate Research Fellowship Program (A. M. K. and J. L. H, DGE-1144469), is acknowledged. The authors thank Mr. Lawrence M. Henling for assistance with small-molecule X-ray crystallography, as well as Dr. Mona Shahgholi and Naseem Torian for assistance with mass spectrometry measurements. Crystallography experiments were supported by Jens Kaiser and the Caltech Molecular Observatory. The authors thank Jingzhou Wang for technical assistance, David Rozzell, Nathaniel Goldberg, Ferdinand Huber, Nicholas Porter, and Kari Hernandez for valuable discussions, and Sabine Brinkmann-Chen and Zhen Liu for critical reading of the manuscript.
Funding Sources
NSF MCB (Grant 1513007), NSF DGE (Grant 1144469), NIH T32 (Grant GM07616, Grant GM112592), NSF STTR (Grant 1738308), NIH (grant R35GM118191-01).
ABBREVIATIONS
- Bpin
pinacolborane
- EDA
ethyl diazoacetate
- E. coli
Escherichia coli
- RmaNOD
Rhodothermus marinus nitric oxide dioxygenase
- TTN
Total turnover number
- dr
diastereomeric ratio
- ee
enantiomeric excess
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Materials and experimental methods, compound characterization data (PDF)
Full nucleotide and amino-acid sequences for all reported enzyme variants (XLSX)
X-ray crystallographic information file for (1R,2S)-4 (CIF).
CONFLICT OF INTEREST
A provisional patent, on which A. M. K., B. J. W., and S. B. J. K. are inventors, has been filed through the California Institute of Technology based on the results presented here.
REFERENCES
- (1).Fryszkowska A; Devine PN Biocatalysis in Drug Discovery and Development. Curr. Opin. Chem. Biol 2020, 55, 151–160. 10.1016/j.cbpa.2020.01.012. [DOI] [PubMed] [Google Scholar]
- (2).Savile CK; Janey JM; Mundorff EC; Moore JC; Tam S; Jarvis WR; Colbeck JC; Krebber A; Fleitz FJ; Brands J; Devine PN; Huisman GW; Hughes GJ Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- (3).Huffman MA; Fryszkowska A; Alvizo O; Borra-Garske M; Campos KR; Canada KA; Devine PN; Duan D; Forstater JH; Grosser ST; Halsey HM; Hughes GJ; Jo J; Joyce LA; Kolev JN; Liang J; Maloney KM; Mann BF; Marshall NM; McLaughlin M; Moore JC; Murphy GS; Nawrat CC; Nazor J; Novick S; Patel NR; Rodriguez-Granillo A; Robaire SA; Sherer EC; Truppo MD; Whittaker AM; Verma D; Xiao L; Xu Y; Yang H Design of an in Vitro Biocatalytic Cascade for the Manufacture of Islatravir. Science 2019, 366, 1255–1259. 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- (4).Brown DG; Bostrom J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?. J. Med. Chem 2016, 59, 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- (5).Baraibar AG; Reichert D; Mugge C; Seger S; Groger H; Kourist R A One-Pot Cascade Reaction Combining an Encapsulated Decarboxylase with a Metathesis Catalyst for the Synthesis of Bio-Based Antioxidants. Angew. Chem., Int. Ed 2016, 55, 14823–14827. 10.1002/anie.201607777. [DOI] [PubMed] [Google Scholar]
- (6).Latham J; Henry JM; Sharif HH; Menon BRK; Shepherd SA; Greaney MF; Micklefield J Integrated Catalysis Opens New Arylation Pathways via Regiodivergent Enzymatic C-H Activation. Nat. Commun 2016, 7, 1–8. 10.1038/ncomms11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Frese M; Schnepel C; Minges H; VoB H; Feiner R; Sewald N Modular Combination of Enzymatic Halogenation of Tryptophan with Suzuki-Miyaura Cross-Coupling Reactions. ChemCatChem 2016, 8, 1799–1803. 10.1002/cctc.201600317. [DOI] [Google Scholar]
- (8).Chandgude AL; Fasan R Highly Diastereoand Enantioselective Synthesis of Nitrile-Substituted Cyclopropanes by Myoglobin-Mediated Carbene Transfer Catalysis. Angew. Chem., Int. Ed 2018, 57, 15852–15856. 10.1002/anie.201810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Talele TT The “Cyclopropyl Fragment” Is a Versatile Player That Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]
- (10).Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med, Chem 2014, 57, 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- (11).Katsuda Y Development of and Future Prospects for Pyrethroid Chemistry. Pest Manag. Sci 1999, 55, 775–782. 10.1002/ps.2780550803. [DOI] [Google Scholar]
- (12).Brandenberg OF; Fasan R; Arnold FH Exploiting and Engineering Hemoproteins for Abiological Carbene and Nitrene Transfer Reactions. Curr. Opin. Biotechnol 2017, 47, 102–111. 10.1016/jxopbio.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang ZJ; Renata H; Peck NE; Farwell CC; Coelho PS; Arnold FH Improved Cyclopropanation Activity of Histidine-Ligated Cytochrome P450 Enables the Enantioselective Formal Synthesis of Levomilnacipran. Angew. Chem., Int. Ed 2014, 53, 6810–6813. 10.1002/anie.201402809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bajaj P; Sreenilayam G; Tyagi V; Fasan R Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity. Angew. Chem., Int. Ed 2016, 55, 16110–16114. 10.1002/anie.201608680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hernandez KE; Renata H; Lewis RD; Kan SBJ; Zhang C; Forte J; Rozzell D; McIntosh JA; Arnold FH Highly Stereoselective Biocatalytic Synthesis of Key Cyclopropane Intermediate to Ticagrelor. ACS Catal. 2016, 6, 7810–7813. 10.1021/acscatal.6b02550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kim T; Kassim AM; Botejue A; Zhang C; Forte J; Rozzell D; Huffman MA; Devine PN; McIntosh JA Hemoprotein-Catalyzed Cyclopropanation En Route to the Chiral Cyclopropanol Fragment of Grazoprevir. ChemBioChem 2019, 20, 1129–1132. 10.1002/cbic.201800652. [DOI] [PubMed] [Google Scholar]
- (17).Fontani P; Carboni B; Vaultier M; Carrie R A Convenient Highly Stereoselective Synthesis of Cyclopropylboronates. Tetrahedron Lett. 1989, 30, 4815–4818. 10.1016/S0040-4039(01)80516-5 [DOI] [Google Scholar]
- (18).Benoit G; Charette AB Diastereoselective Borocyclopropanation of Allylic Ethers Using a Boromethylzinc Carbenoid. J. Am. Chem. Soc 2017, 139, 1364–1367. 10.1021/jacs.6b09090. [DOI] [PubMed] [Google Scholar]
- (19).Parra A; Amenos L; Guisan-Ceinos M; Lopez A; Ruano JLG; Tortosa M Copper Catalyzed Diastereo and Enantioselective Desymmetrization of Cyclopropenes: Synthesis of Cyclopropylboronates. J. Am. Chem. Soc 2014, 136, 15833–15836. 10.1021/ja510419z. [DOI] [PubMed] [Google Scholar]
- (20).Liskey CW; Hartwig JF Iridium-Catalyzed C–H Borylation of Cyclopropanes. J. Am. Chem. Soc 2013, 135, 3375–3378. 10.1021/ja400103p. [DOI] [PubMed] [Google Scholar]
- (21).Carreras J; Caballero A; Perez PJ Enantioand Diastereoselective Cyclopropanation of 1-Alkenylboronates: Synthesis of 1-Boryl-2,3-Disubstituted Cyclopropanes. Angew. Chem., Int. Ed 2018, 57, 2334–2338. 10.1002/anie.201710415. [DOI] [PubMed] [Google Scholar]
- (22).Blaser H; Busacca CA; Chung CK; Crawley ML; Fandrick DR; Senanayake CH; Shen HC; Song JJ; Szabo WA; Terrell L; Thiel OR; Yeh V; Yin J Applications of Transition Metal Catalysis in Drug Discovery and Development [Online]; Crawley ML, Trost BM, Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. 10.1002/9781118309872. [DOI] [Google Scholar]
- (23).Lee JCH; Hall DG State-of-the-Art in Metal-Catalyzed Cross-Coupling Reactions of Organoboron Compounds with Organic Electrophiles. In Metal Catalyzed Cross-Coupling Reactions and More [Online]; Meijere A, Brase S, Oestreich M; 2014 Wiley-VCH Verlag GmbH & Co. KGaA: Boschstr; 12, 69469 Weinheim, Germany: 2014; pp 65–132. 10.1002/9783527655588.ch2. [DOI] [Google Scholar]
- (24).Zhao S; Gensch T; Murray B; Niemeyer ZL; Sigman MS; Biscoe MR Enantiodivergent Pd-Catalyzed C–C Bond Formation Enabled through Ligand Parameterization. Science 2018, 362, 670–674. 10.1126/science.aat2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Knight AM; Kan SBJ; Lewis RD; Brandenberg OF; Chen K; Arnold FH Diverse Engineered Heme Proteins Enable Stereodivergent Cyclopropanation of Unactivated Alkenes. ACS Cent. Sci 2018, 4, 372–377. 10.1021/acscentsci.7b00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bordeaux M; Tyagi V; Fasan R Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts. Angew. Chem., Int. Ed 2015, 54, 1744–1748. 10.1002/anie.201409928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Leonori D; Aggarwal VK Stereospecific Couplings of Secondary and Tertiary Boronic Esters. Angew. Chem., Int. Ed 2015, 54, 1082–1096. 10.1002/anie.201407701. [DOI] [PubMed] [Google Scholar]
- (28).Sandford C; Aggarwal VK Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem. Commun 2017, 53, 5481–5494. 10.1039/c7cc01254c. [DOI] [PubMed] [Google Scholar]
- (29).Jakoblinnert A; Rother D A Two-Step Biocatalytic Cascade in Micro-Aqueous Medium: Using Whole Cells to Obtain High Concentrations of a Vicinal Diol. Green Chem. 2014, 16, 3472–3482. 10.1039/c4gc00010b. [DOI] [Google Scholar]
- (30).Bagutski V; Ros A; Aggarwal VK Improved Method for the Conversion of Pinacolboronic Esters into Trifluoroborate Salts: Facile Synthesis of Chiral Secondary and Tertiary Trifluoroborates. Tetrahedron 2009, 65, 9956–9960. https://doi.org/10.1016Zj.tet.2009.10.002. [Google Scholar]
- (31).Lipshutz BH; Ghorai S “Designer”-Surfactant-Enabled Cross-Couplings in Water at Room Temperature. Aldrichim. Acta 2012, 45, 3–16. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


