Abstract
Cystic fibrosis (CF) is due to mutations in the CF-transmembrane conductance regulator (CFTR) and CF-related diabetes (CFRD) is its most common co-morbidity, affecting ~50% of all CF patients, significantly influencing pulmonary function and longevity. Yet, the complex pathogenesis of CFRD remains unclear. Two non-mutually exclusive underlying mechanisms have been proposed in CFRD: i) damage of the endocrine cells secondary to the severe exocrine pancreatic pathology and ii) intrinsic β-cell impairment of the secretory response in combination with other factors. The later has proven difficult to determine due to low expression of CFTR in β-cells, which results in the general perception that this Cl−channel does not participate in the modulation of insulin secretion or the development of CFRD. The objective of the present work is to demonstrate CFTR expression at the molecular and functional levels in insulin-secreting β-cells in normal human islets, where it seems to play a role. Towards this end, we have used immunofluorescence confocal and immunofluorescence microscopy, immunohistochemistry, RT-qPCR, Western blotting, pharmacology, electrophysiology and insulin secretory studies in normal human, rat and mouse islets. Our results demonstrate heterogeneous CFTR expression in human, mouse and rat β-cells and provide evidence that pharmacological inhibition of CFTR influences basal and stimulated insulin secretion in normal mouse islets but not in islets lacking this channel, despite being detected by electrophysiological means in ~30% of β-cells. Therefore, our results demonstrate a potential role for CFTR in the pancreatic β-cell secretory response suggesting that intrinsic β-cell dysfunction may also participate in the pathogenesis of CFRD.
Introduction
As the treatment for patients with cystic fibrosis (CF) improved, they in turn became the population with the highest risk for developing age-dependent diabetes, the most common co-morbidity (50% in CF [1]), referred to as CF-related diabetes (CFRD). This is a highly relevant clinical issue because it worsens lung function and increases mortality rate [2]. CFRD is a complex hyperglycemic metabolic syndrome with features of type 1 and 2 diabetes mellitus that has been classified as pancreatogenic in origin (Type 3c) [3]. However, at least in infancy, built on our recent findings CFRD cannot be attributed to exocrine pancreatic pathology, but rather to a reduction in β-cell density and replication [4].
The direct role that CFTR plays in the insulin secretory response remain challenging due, at least in part, to two experimental limitations including: i) lack of animal models fully recapitulating the clinical picture present in human CF/CFRD [5], and ii) few reliable immunological [6] and pharmacological [7] tools to adequately identify and characterize channel expression and activity in vitro. The lack of tools is further exacerbated by the low and heterogeneous expression pattern of CFTR compared to the usual target tissues, i.e., lung, gut and exocrine pancreas [8–10], in the pancreatic islet; clearly adding important methodological obstacles for its detection and subsequent characterization. As a result, immunoreactive CFTR and/or its function have not been detected in the pancreatic islet by some [11,12] yet it has been by others [13–18]. Islet or β-cell CFTR mRNA expression has been recently interrogated [11], or not found [19] based on RNA-sequencing data. New results by White et al. suggest that CFTR is indeed expressed in a few β-cells in the adult human islet, but considered it too low and unlikely to play a role on β-cell function [12]. The inconsistency in the results from several laboratories could be explained, at least in part, by the fact that islet β-cells are of diverse morphology and functionally heterogeneous, which would make their detection difficult [20–22]. Hence, it remains possible that CFTR may be expressed in a subpopulation of β-cells and therefore, play a role in their secretory response.
CFTR, like any other Cl−channel in the islet, has the potential to regulate the insulin secretory response. Indeed, as we have recently reviewed in detail [23], intracellular Cl−ions tend to electrogenically exit from insulin-secreting β-cells through Cl−channels thus contributing to plasma membrane depolarization [24] and therefore insulin secretion [25]. The objective of the present study was to detect CFTR expression at the molecular and functional levels in the human and rodent pancreatic islet whilst testing the hypothesis that CFTR expression occurs in a heterogeneous pattern in a subpopulation of β-cells. Our results confirm and extend previous reports on the expression, localization and function of CFTR in human and rodent β-cells and for the first time, we present electrophysiological data suggesting that ~30% of all β-cells tested express functional CFTR.
Materials and methods
Materials
Platinum Pfx thermostable DNA polymerase, RNaseOUT, SuperScript-III reverse transcriptase, random hexamers, and tissue culture media were from Invitrogen (Carlsbad, CA); dNTPs and molecular biology grade chemicals were from Affymetrix (Cleveland, OH); custom PCR primers were from Integrated DNA Technologies (Coralville, IA). Tissue culture supplements and general chemicals were from Sigma-Aldrich Co. (St. Louis, MO). Culture media low in glucose (5.5mM) was from Hyclone-GE (Logan, UT). Microscopy materials were from EMS (Hatfield, PA) and ThermoFisher Sci. (Waltham, MA). Collagenase type IV from Clostridium histolyticum (≥160U/mg) was from Worthington (Lakewood, NJ). CFTR inh-172 and the adenylyl cyclase activator forskolin where from Tocris and R&D (Minneapolis, MN).
Human tissue and islet donors
Normal adult and juvenile human pancreatic tissue for IHC was obtained from Dr. Cecilia Ridaura (Departamento de Patología, Instituto Nacional de Pediatría, Mexico City) and from the Network for Pancreatic Organ Donors (nPOD, JDRF). At the beginning of this project, we obtained 36 tissue samples; 20 CF cases, out of which 16 were less than one year of age and 4 between 20 and 40 years and 11 control samples from patients younger than 24 months of age and 5 adults, who died of non-pancreatic pathology [4]. Tissue sections from a large subset of this group was used to test 21 antibodies against CFTR (see Table 1 and S1–S4 Figs), of those, sections from 6/12 and 12/12 CF patients (homozygous for the CFTR mutations F508del and G542X, of Mestizo origin), as well as an 11/12 control case are presented in Figs 1 and 2. All the experiments with these de-identified archival autopsy tissues obtained from deceased patients were carried out with the approval of the Institutional Review Board of the Instituto Nacional de Pediatría. The excellent quality of these tissues was incontestable [4]. Primary human islets from normal donors were obtained from Prodo Laboratories (Aliso Viejo, CA). Detail demographics are presented in Table 2. Upon arrival, primary islets were allowed to recover 24-48hs in PIM(R) media supplemented with PIM (ABS, G & 3X) as per provider instructions and subsequently used for functional, molecular and secretory studies.
Table 1. List of primary antibodies raised against the indicated antigens are identified by their clone/catalogue number from vendors/suppliers.
| Antibody | Supplier | Dilution | Tested application | Comments |
|---|---|---|---|---|
| Cftr (CFF-432) | Cystic Fibrosis Foundation (CFF) | 1:250 | IHC/WB | Specific, tested against KO tissues, not suitable for WB |
| Cftr (CFF-217) | CFF | 1:250 | IHC/WB/IF | Specific, tested against KO tissues, not suitable for WB |
| Cftr (CFF-412) | CFF | 1:250 | IHC/WB | Specific, tested against KO tissues, not suitable for WB |
| Cftr (CFF-596) | CFF | 1:100 | WB | Not specific, not suitable for WB |
| Cftr (10B6.2) | CFF | 1:100 | WB | Specific, not suitable for WB |
| Cftr (CFF-570) | CFF | 1:100 | IHC/WB | Specific, not suitable for WB |
| Cftr (CFF-770) | CFF | 1:100 | IHC/WB | Not suitable for IHC/WB |
| Cftr (CFF-154) | CFF | 1:50 | IHC | No signal |
| Cftr (CFF-660) | CFF | 1:100 | IHC | Not specific, high background |
| Cftr (CFF-450) | CFF | 1:50 | IHC | Strong staining in islets and weak in ducts |
| Cftr (MA1-935) | Thermo Scientific | 1:400 | IHC | Stains islets and ducts |
| Cftr (Ab4067) | Abcam | 1:100 | IHC | No signal in human pancreas, weak in mouse pancreas |
| Cftr (Ab-3 L1B4) | Neomarkers | 1:50 | IHC | No signal |
| Cftr (acl-006) | Alomone | 1:250 | IF | Specific |
| Cftr (24–1) | R&D | 1:250 | WB | Specific, not suitable for IF |
| Cftr (MrPink) | CFTR Folding Consortium | 1:250 | IF | Specific |
| Cftr (3G11) | CFTR Folding Consortium | 1:250 | IF | Specific |
| Cftr (LS-B5115) | Ls-Bio | 1:200 | IF | No signal |
| Cftr (NB300-511) | Novus Biologicals | 1:100 | IF | Very weak signal |
| Cftr (20738-1-ap) | Proteintech | 1:100 | IF | No signal |
| Cftr (Mab3484) | Millipore | 1:250 | IHC/IF | Not suitable for IF |
| Insulin (273A-16) | Cell Marque | 1:500 | IF/IHC | Widely used, considered specific |
| Glucagon (K79bB10) | Abcam | 1:500 | IF/IHC | Widely used, considered specific |
| Glucagon (259A-15) | Cell Marque | 1:500 | IF/IHC | Widely used, considered specific |
| Somatostatin (ab30788) | Abcam | 1:250 | IF/IHC | Widely used, considered specific |
| Synaptophysin (ab8049) | Abcam | 1:200 | IF/IHC | Widely used, considered specific |
| PP (ab77192) | Abcam | 1:250 | IF/IHC | Widely used, considered specific |
| E-cadherin (ab6528) | Abcam | 1:100 | IF | Widely used |
| Actin (JLA20) | DHSB | 1:5000 | WB | Specific |
Dilutions and applications used are indicated together with comments regarding their specificity/reliability/suitability. Note: suitability refers to the ability of the antibody to detect low expression of the antigen in the pancreatic islet. CFTR antibodies were tested in pancreatic tissue slides and/or protein extracts, as indicated.
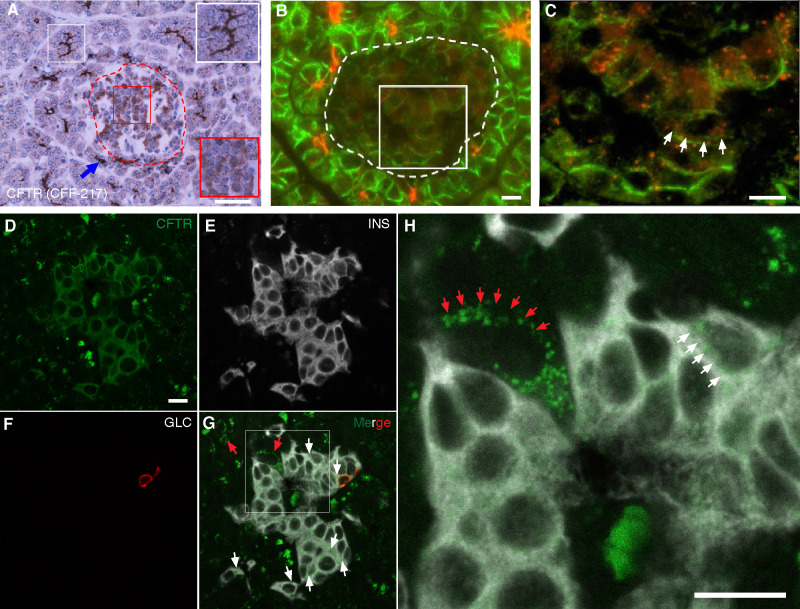
Fig 1. CFTR is expressed in human islets.
A-C. Immunohistochemistry (A) and immunofluorescence (B-C) images of human pancreas (normal tissue from an 11-month-old control) immunostained by using CFF-217 antibody (Table 1). The islet shown in A, encircled by a dashed red trace, contains endocrine cells stained by the antibody, which is shown in the magnified red square in the right bottom corner of the figure. CFTR-positive pancreatic duct cells are shown within the white squares. Shown in B is CFTR immunoreactivity in human exocrine and endocrine cells. The edges of all cells were immunolabeled by using E-cadherin antibodies (Table 1). The islet is encircled by a dashed trace and the square represents the area magnified and shown in C. D-H. In situ hybridization of normal human pancreas tissue by using fluorophore-labeled RNA probes directed against CFTR (green, D), insulin (INS, white, E) and glucagon (GLC, red, F) transcripts. Red and white arrows in H, a magnification of G, indicate specific CFTR labeling on acinar and endocrine cells, respectively. Bars in A and B, C, D, and in H correspond to 50μm and 10μm, respectively.
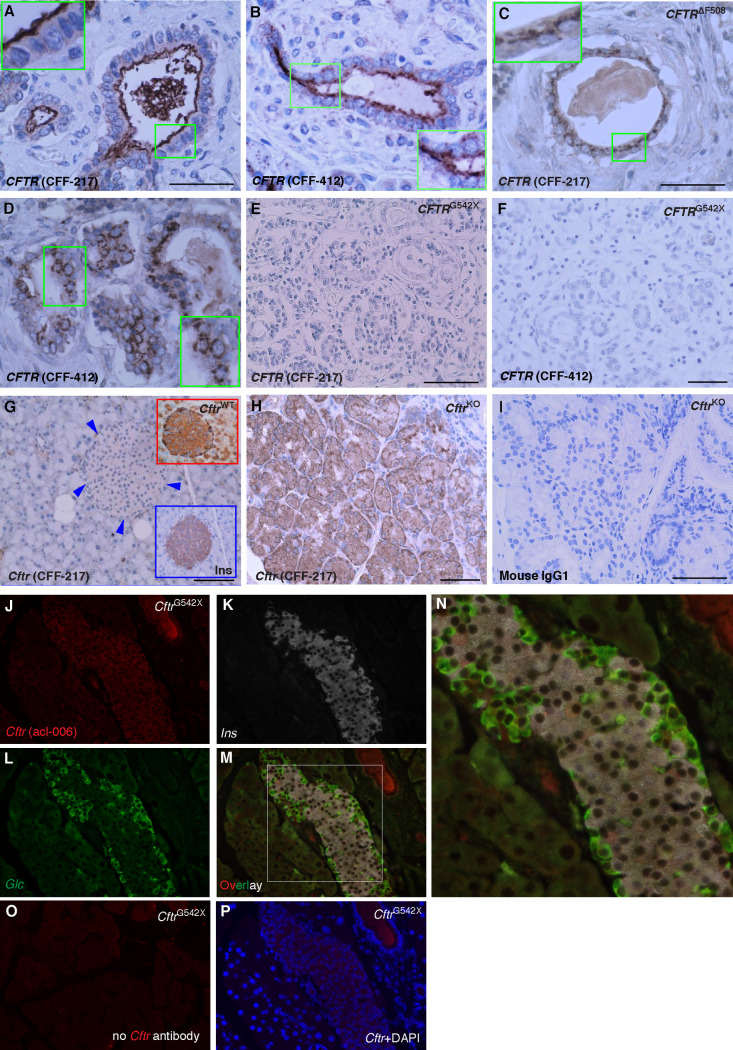
Fig 2. CFTR antibody validation.
A-D. Shown are microscopy images of human pancreas from a normal donor (A, B, 11-month-old control) or homozygous for the CFTR mutation F508del (C, D, 6-month-old CF patient) immunostained using CFF-217 (A, C) or CFF 412 (B, D). Note the expected apical and intracellular immunolabeling of CFTR in normal and mutant pancreas, respectively. The insets indicate higher magnification. E, F. Microscopy images of human pancreas from a 1 yr. old CF patient homozygous for CFTRG542X using the antibodies CFF-217 (E) and CFF-412 (F). Shown is absence of immunostaining with both antibodies. G-I. Microscopy images of pancreas (G) and intestine (H) of CftrKO mice immunolabeled using CFF-217. Shown are the expected patterns of immunostaining for specific antibodies. For comparison, shown are magnified images of CftrWT pancreas islets using CFTR and insulin antibodies (top and bottom right corners in G, respectively). I. Omission of the CFTR antibody did not generate immunostaining in the intestine. Bars in A-I represents 50μm. J-L. Pancreatic tissue from CftrG542X mice co-immunolabeled by using the indicated mouse CFTR-specific antibody acl-006 (J), insulin (K, Ins) and glucagon (L, Glc). M-N. Overlay image of J-L and magnified squared area shown in N. O-P. Control images obtained from CftrG542X mice in the absence of primary (O) or secondary antibodies (P). DAPI was used to counterstain nuclei in P.
Table 2. Human islet donor demographics.
| Age (years) | Sex | Ethnicity | Height (cm) | Weight (kg) | BMI | HbA1c (%) | Cause of Death | Purity (%) | Viability (%) | Secretion | Gene expression |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 | M | White | 172.0 | 81.7 | 26.3 | 5.1 | Stroke | 90 | 95 | ✓ | |
| 61 | F | White | 162.5 | 66.3 | 24.5 | 5.1 | Stroke | 95 | 95 | ✓ | |
| 52 | F | White | 170.1 | 73.5 | 25.6 | 4.5 | Stroke | 95 | 95 | ✓ | |
| 38 | M | Latino | 175.3 | 86.5 | 28.0 | 5.9 | Head trauma | 90 | 95 | ✓ | |
| 46 | F | White | 170.0 | 55.8 | 19.0 | 5.8 | Stroke | 90 | 95 | ✓ | |
| 31 | M | White | 175.2 | 72.2 | 22.0 | 5.5 | Anoxic event | 95 | 95 | ✓ | |
| 33 | F | White | 172.7 | 90.8 | 30.3 | 4.9 | Head trauma | 85 | 95 | ✓ | |
| 49 | M | White | 170.2 | 86.3 | 30.0 | 5.5 | Anoxic event | 90 | 95 | ✓ | |
| 35 | M | African-American | 170.0 | 85.0 | 30.2 | 5.4 | Anoxic event | 90 | 95 | ✓ | |
| 33 | F | White | 172.7 | 90.8 | 30.3 | 4.9 | Head trauma | 85 | 95 | ✓ | |
| 61 | F | Latino | 149.9 | 64.9 | 28.9 | 5.6 | Head trauma | 90 | 95 | ✓ | |
| 54 | M | White | 180.3 | 72.6 | 22.3 | 5.8 | Stroke | 90 | 95 | ✓ | |
| 35 | F | White | 160.0 | 62.7 | 24.0 | 4.8 | Anoxic event | 90 | 95 | ✓ | |
| 47 | M | White | 160.0 | 71.0 | 28.1 | 5.8 | Head trauma | 85 | 95 | ✓ | |
| 43 | F | Latino | 152.4 | 68.1 | 30.0 | 5.5 | Head trauma | 95 | 95 | ✓ | |
| 46 | M | Latino | 167.6 | 93.0 | 33.0 | 5.4 | Anoxic event | 90 | 95 | ✓ | |
| 30 | F | White | 170.2 | 54.5 | 18.0 | 4.4 | Anoxic event | 90 | 95 | ✓ | |
| 40 | M | Latino | 175.3 | 78.1 | 25.0 | 5.3 | Head trauma | 95 | 95 | ✓ | |
| 49 | F | White | 155.0 | 81.3 | 33.0 | 5.9 | Stroke | 90 | 95 | ✓ | |
| 52 | F | African-American | 170.2 | 69.5 | 23.0 | 4.8 | Stroke | 90 | 95 | ✓ | |
| 57 | F | White | 167.6 | 59.9 | 21.4 | 5.8 | Stroke | 95 | 95 | ✓ | |
| 63 | F | White | 152.0 | 52.7 | 27.0 | 5.8 | Stroke | 85 | 95 | ✓ | |
| 66 | M | White | 170.2 | 79.0 | 27.0 | 4.7 | Stroke | 95 | 95 | ✓ | |
| 50 | M | Latino | 160.0 | 84.0 | 32.6 | 6.0 | Anoxic event | 90 | 95 | ✓ |
Mouse pancreas collection and processing for immunomicroscopy
Fixed pancreas tissues from wild-type (WT) mice of the C57BL/6J genetic background and homozygous for the CftrG542X allele were kindly provided by Dr. Mitch Drumm (Case Western Reserve University). Tissues from mice homozygous for the Cftr mutation S489X and expressing the human CFTR transgene in the intestine [Cftrtm1Unc Tg(FABPCFTR)1Jaw/J, Stock No: 002364, Jackson Labs], referred to as CftrKO mice throughout the text were obtained and processed as described [26] with some modifications. Briefly, deeply anesthetized mice (Euthasol®, ip 150mg/kg) were transcardially perfused with ~20ml ice-cold 1xPBS (0.1mM, pH7.4) containing 1000U/ml of heparin (Meitheal Pharmaceuticals, IL) at a rate of 1.6ml/min with the help of a peristaltic minipump (Model EP-1 Econo pump, Bio-Rad) and then perfused with freshly made ice-cold 4% paraformaldehyde (PFA) fixative. After mice were euthanized by perfusion with PFA, pancreatic tissue was collected, placed at 4°C overnight in 4% PFA, washed in PBS and post-fixed for additional 24hs in 4%PFA containing 30% sucrose. Post-fixed tissues were transferred to 30% sucrose in PBS and collected after tissues sunk at the bottom of the tube. Tissues were paraffin-embedded and sectioned at 5 μm (AML Laboratories, Inc., Baltimore, MD). For immunolabeling protocols, tissue slides were deparaffinized in xylene and re-hydrated in ethanol solutions of decreasing concentrations (100%-0%). Antigens were retrieved in sodium citrate buffer (10 mM) at 100°C for 30 min, tissue slides permeabilized in 4% PFA containing 0.3% Triton X100, blocked in 3% host serum solution and incubated with primary antibodies as indicated in the next section.
Immunofluorescence/confocal microscopy, immunohistochemistry and in situ hybridization
The twenty-one primary antibodies used in these experiments and their sources are listed in Table 1. Tissue sections from mouse or human pancreas were immunolabeled with different dilutions of the primary antibodies at 4°C overnight with gentle rocking followed by 2hs incubation with appropriate fluorescently-labeled secondary antibodies [Cy3-, AlexaFluor (AF488)- and DyLight405-conjugated (Jackson Immunoresearch, West Grove, PA). Slides were allowed to dry at room temperature before adding mounting media containing DAPI (Vector Laboratories, Burlingame, CA) and cover the sections with acetone-washed coverslips. Labeled slides were immediately viewed using an Olympus Epi Fluorescence Spot microscope equipped with RT color camera. Digital images were obtained using a Diagnostics Instrument Spot 6 digital camera (Spot Imaging Solutions, Sterling Heights, MI). For immunohistochemistry (IHC) experiments, tissue sections were incubated overnight with primary antibodies. Sections were washed in 1xPBS and then incubated with biotinylated- or fluorescent-labeled secondary antibodies for 1h at room temperature. The peroxidase (HRP) reaction was developed by incubating sections in 0.3% H2O2 and 0.15% diaminobenzidine tetrachloride (Sigma, St. Louis, MO). For CFTR, detection the antibodies we used were all validation against CFTR null tissues following identical conditions (see Figs 2C, 2E, 2F, 2H–2P and 4A). Classic negative controls were also performed by omitting primary antibodies (Fig 2O) or by staining with isotype-matched antibodies (Fig 2I). Some of the antibodies not suitable for immunohistochemistry experiments are shown in S3 Fig. Neither of these two approaches generated staining. In situ RNA hybridization was performed using RNAscope technology (ACD, Advanced Cell Diagnostics, CA). Normal human pancreas sections provided by ACD were permeabilized and hybridized with combinations of mRNA probes specific for human CFTR, insulin (INS) and glucagon (GLC) according to the manufacturer’s instructions. A multiplex fluorescent kit was used to detect mRNA signals, which were analyzed by epi-fluorescence and confocal microscopy. High-resolution confocal images were taken by using the FV1000 Confocal Microscope (Olympus, PA, USA). When DyLight405-conjugated antibodies were used to visualize insulin-positive β-cells, images were taken in gray-scale instead of blue color to increase contrast against red- and green-stained antigens.
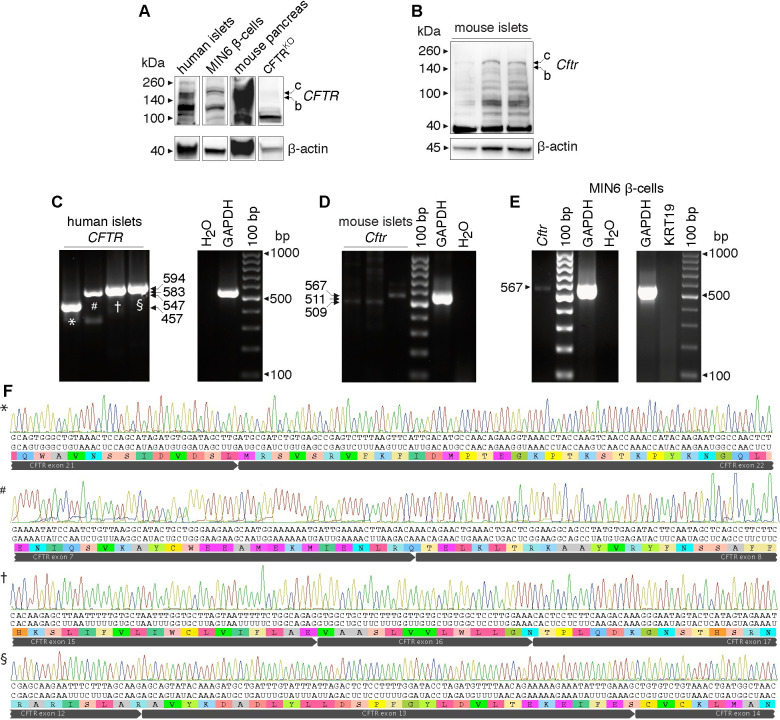
Fig 4. CFTR expression in human and mouse islets and in MIN6 β-cells.
A, B. Immunoblots of protein extracts obtained from purified human islets, the β-cell line MIN6 and mouse pancreas as positive control (A) and primary mouse islets (B). Bands of expected size are shown for human/mouse Cftr (C band: ~170kDa, B band: ~150kDa) detected using Cftr antibody 24–1. Actin was used as loading control. C-E. Representative original RT-PCR experiments showing Cftr transcripts of expected sizes (Table 3) amplified from total RNA obtained from primary human (n = 5 donors), mouse islets (n = 3 preparations) (C and D, respectively) and MIN6 β-cells (n = 4 preparations) (E). F. Partial sequence chromatograms of purified human CFTR amplicons, as indicated in C.
Mice and rats
CftrWT, CftrKO [i.e., Cftrtm1UncTg(FABPCFTR)1Jaw/J] and C57BL/6J mice (Jackson Laboratories) were housed under controlled conditions at the Cell Function Analysis Core of the Diabetes Research Center (DRC, University of Washington, Seattle, WA). Male and female mice (8–10 weeks old) were used. The Animal Care Committee of the University of Washington approved all methods involving mice. Male Wistar rats (12 young adults, 250-280g) were obtained from the local animal facility and housed at the Instituto de Fisiología Celular, Universidad Nacional Autónoma de México. Rats were kept in a 12:12h light-dark cycle under standard laboratory conditions including and fed with standard rat chow (Laboratory rodent diet 5001, LabDiet St Louis, MO, USA) and tap water. The Animal Care Committee of the Instituto de Fisiología Celular, Universidad Nacional Autónoma de México approved all methods involving rats. Animal care (mice and rats) was performed according to the International Guiding Principles for Biomedical Research Involving Animals, Council for International Organizations of Medical Sciences, 2010.
Primary islet and tissue isolation
Mouse islets for secretory studies were isolated from by the DRC Cell Function Analysis Core as previously described [27]. Mice and rat pancreas were obtained from deeply anesthetized animals (Euthasol® ip 150mg/kg or 40mg/Kg sodium pentobarbital for mice and rats, respectively) by the terminal collagenase digestion method as described [28]. After surgical removal of the collagenase-digested pancreas, mice and rats were immediately euthanized. Tissues for gene expression analysis were surgically removed from deeply anesthetized mice (Euthasol® ip 150mg/kg) and immediately processed.
Insulin secretion
Primary islets were handpicked into individual wells of 12-well plates with mesh inserts [15 islet equivalents (iEq)/well] containing KRBH (in mM: 118.5 NaCl, 2.5 CaCl2, 1.2 KH2PO4, 4.7 KCl, 25 NaHCO3, 1.2 MgSO4, 10 HEPES and 0.1% BSA pH 7.4) plus 5.5mM glucose. The mesh inserts containing islets were transferred to new wells containing KRBH+5.5mM glucose and incubated at 37°C (5% CO2) for 30 minutes. This wash step was repeated once more. The islets were then transferred into their respective experimental wells containing KRBH + 5.5mM glucose plus vehicle (DMSO) or drugs for 2h at 37°C (5% CO2). Islets were transferred into new wells containing KRBH + 12.5mM glucose plus vehicle or drugs, incubated 2h at 37°C (5% CO2) and transferred to new wells containing acidified ethanol. The KRBH from experimental wells was frozen at –20°C until analysis. Insulin secreted into the media or contained in cells was estimated by using ELISA (#10-1247-01, Mercodia, Salem, NC). Results are expressed as the % ratio between secreted insulin and the sum of secreted and islet/cell insulin content. When assayed at DRC, the insulin secretory response (ISR) was determined statically with multiple conditions, as described previously [29]. Briefly, primary islets were handpicked into a petri dish containing KRBH+5.5mM glucose and incubated at 37°C (5% CO2) for 90min. Subsequently, islets were picked into 96-well plates containing desired amounts of glucose and drugs, as indicated, and incubated for additional 60min. At the end of this period, supernatant was assayed for insulin by radioimmunoassay (Linco Research Inc., Billerica, MA). Results are expressed as ISR per min, per 100iEq. Single rat islet cells were obtained by incubating handpicked islets in a shaker bath at 37°C in Ca2+-free spinner solution containing 10mM glucose, 0.5% BSA and 0.01% trypsin, followed by mechanical disruption. Single cells were cultured overnight on poly-L-lysine pre-coated glass coverslips and incubated in RPMI-1640 (11.6mM glucose) supplemented with 10% FCS, 200U/ml penicillin G, 200 μg/ml streptomycin and 0.5 μg/ml amphotericin-B for recover from dissociation. Mouse MIN6 β-cells for secretory studies were kept, cultured and assayed as previously described [30]. Basal or glucose-stimulated insulin secretion (GSIS) was determined in these cells two hours after incubation in the presence of basal (5.5mM) or stimulatory glucose concentrations (12.5mM) by ELISA (EZRMI-13K, EMD Millipore, Billerica, MA) in accordance with the manufacturer’s directions.
Electrophysiology
Whole-cell configuration of patch-clamp technique was used on isolated primary rat β-cells. All the experiments were done at 22°C. Ionic currents were recorded with a data acquisition system (DigiData 1322A) and Axopatch 200A amplifier (Axon Instruments, Foster City, CA USA). Patch pipettes from capillary tubes KIMAX-51 (Kimble Glass, Vineland NJ) were coated with dental wax and pulled with micropipette puller (P-97, Sutter Instruments Co., USA) to a resistance of 2–4 MΩ after being filled with intracellular solution. The cells were bathed with an external solution (in mM): 118 NaCl, 20 tetra-ethyl-ammonium-chloride, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2 and 5 HEPES with 5.5 or 12.5 D-Glucose pH 7.4, supplemented with 10μM forskolin and 5μM Inh172. The pipettes were filled with solution (in mM): 125 CsOH, 125 glutamate, 10 CsCl, 10 NaCl, 1 MgCl2, 3 MgATP, 4 EGTA, 5 HEPES pH 7.2. When the Giga seal was performed cells with capacitances between 5-12pF were considered as β-cells [28,31,32]. The whole-cell current was obtained by depolarizing test pulses from –100 to +100mV, at 10mV increments, from a holding potential of –80mV. The command voltages and analysis of currents were done with pClamp v9 software.
Extraction of total RNA and RT-PCR
Total RNA was obtained from freshly isolated mouse as indicated above and human islets or β-cell lines using the RNeasy minikit (Qiagen, Valencia, CA). These RNA samples or purified total RNA from human islets (kindly provided by Dr. Patrick MacDonald, University of Alberta, Canada) were quantified by Nanodrop and used in RT-PCR/qPCR experiments as described [30]. The sets of primers used to amplify transcripts of interest are indicated in Table 3. RT-PCR amplicons were sequenced (Beckman Genomics, Beverly, MA) and aligned in silico using Geneious Suite R10 (Biomatters Ltd., New Zealand) against relevant sequences of reference (RefSeq). Thermal conditions used for PCR were previously described [30]. RT-PCR products were separated on 2% agarose gels stained with ethidium bromide and directly photographed by using the ChemiDoc™ MP Imaging System with Image Lab™ Software (Bio-Rad, Berkeley, CA). The digital images were inverted for clarity and cropped to exclude gel edges or empty/non-relevant lanes.
Table 3. List of primer-sets used in RT-PCR experiments named after the target transcript followed by numbers indicating amplicon sizes in base pairs (bp).
| Primer set | Sense | Antisense | RefSeq | Species |
|---|---|---|---|---|
| Cftr-509 | GTCATTCGACGAGTTCTAAAACAAG | CAAGAGTATATCCACAGGTATTGTCC | NM_021050 | mouse |
| Cftr-511 | CTAGTAGTCTTTATTTTACTGAGGGCC | CTAAAACGTCAGATGATCCTTCTCTAG | ||
| Cftr-567 | ATGTCGAGTCCAACCTGAATTTAG | CGGCTTGACAACTTTAAAGTCTTC | ||
| CFTR-457 | CATTCTGTTCTCAGTTTTCCTGGATTATGC | CTAAAGTCTGGCTGTAGATTTTGGAGTTCT | NM_000492 | human |
| CFTR-547 | CTCAAGAAACTGGCTTGGAAATAAGTGAAG | ATGAAGTCAAATATGGTAAGAGGCAGAAG | ||
| CFTR-583 | AGGGAGAATGATGATGAAGTACAGAGATCA | GAGAAATTACTGAAGAAGAGGCTGTCATC | ||
| CFTR-594 | CTGAATTTACATACTGCCAACTGGTTCTTG | CAAAGTTATTGAATCCCAAGACACACCATC | ||
| Krt19-544 | CCTGAAGAAGAACCATGAGGAGGA | CAGATTGTTGTAGTGGGCTTCCTG | NM_008471 | mouse |
| GAPDH-555 | GTGAAGGTCGGAGTCAACGGATTT | CACAGTCTTCTGGGTGGCAGTGAT | NM_002046 | human |
Primer sequences were designed according to mouse, rat or human cDNAs and/or RefSeq nucleotide sequences of reference, as indicated.
Western blotting
Proteins from tissues and cell lines were obtained as formerly described [30]. Protein extracts from human islets were freshly obtained either from Dr. Patrick MacDonald or after sonication of freshly isolated primary human (Prodo Labs, Table 2)/mouse islets on ice by using RIPA buffer (Teknova, Hollister, CA) supplemented with protease/phosphatase inhibitor cocktails (ThermoFisher, Sci.). Up to 75μg of total proteins were loaded onto pre-casted 4–20% Tris-HEPES protein gels (Thermo Scientific-Pierce) or Bolt 4–12% Bis-Tris Plus gels (Invitrogen/ThermoFisher Sci.), run under denaturing conditions and electro-transferred onto PDVF membranes at 4°C by using the Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad) or the iBlot-2 Gel Transfer Device (Invitrogen). After blocking overnight with 4% BSA, membranes were incubated with suitable primary antibodies (overnight at 4°C) and developed with HRP-conjugated secondary ones (2h at room temperature). We used ChemiDoc™ MP Imaging System with Image Lab™ Software (Bio-Rad, Berkeley, CA) to detect antigen-antibody reactions. High-resolution gray-scale digital images taken by using this imaging system were cropped to exclude irrelevant lanes and used without further modification. Original blots used to construct Figures are shown in S4 Fig.
Statistics
Data has been analyzed by using Prism v5 (GraphPad Software, San Diego, CA) and pClamp v9 Electrophysiology Data Acquisition and Analysis Software (Molecular Devices, LLC. San José, CA). Results are represented as mean values ± SEM, with the number of individual replicates (n). A p value ≤0.05 was considered significant and was obtained by using one-way or two-way analyses of variance (ANOVA), as appropriate, followed by the Tukey-Kramer post-hoc test.
Results
CFTR is expressed in endocrine cells in the human islet
CFTR protein and mRNA expression were determined in the human pancreatic islet by using immunomicroscopy and in situ hybridization, respectively. We have evaluated twenty-one different antibodies against CFTR, of which only a handful were specific or suitable for immunomicroscopy experiments (Table 1 and S1–S3 Figs). As shown in Fig 1A–1C, CFTR antibodies CFF-217 and CFF-412 detect CFTR in a fraction of the normal human islet endocrine cells with the expected intense staining and apical localization of the pancreatic duct cells (inset Fig 1A). Similar results are shown in S2 Fig using CFTR antibodies CFF-432, -412 and -570. Corroborating the concept that CFTR is expressed in the human islet, the channel was sought at the mRNA level by using in situ hybridization. As shown in Fig 1D–1H, very low expression of CFTR transcripts is detected as a small punctate in some but not all β-cells in the human islet. Importantly, the CFTR antibodies used in our experiments gave the predicted predominantly apical as well as cytoplasmic immunostaining pattern in pancreatic sections from control patients (Figs 2A, S1 and S2), or only cytoplasmic in the case of patients homozygous for the mutation F508del (ΔF508, Fig 2B) but did not generate an immunological signal in pancreas sections from a CF patient homozygous for the truncating CFTR mutation G542X (Fig 2C and 2D) leading to protein absence, or mice lacking Cftr expression (Fig 2E and 2F).
To further ratify and extend the previous observations, glucagon, insulin, somatostatin, pancreatic polypeptide (PP) and Cftr immunoreactivity were also sought in normal rodent islets. CFTR was detected by using the Cftr antibody acl-006 (Table 1), also validated against pancreatic sections from mouse islets homozygous for CftrG542X allele (Figs 2H and 3). As shown in Fig 3, immunoreactive Cftr is detected in some but not all CftrWT mouse α-, β- (Fig 3A–3E), δ- and PP-cells (Fig 3F–3J). In a similar fashion, Fig 3K and 3L show the expected immunolabeling of the endocrine and exocrine components of the human pancreas. To cross-validate these results, Cftr expression was demonstrated at the protein (Fig 4A and 4B) and mRNA (Fig 4C and, 4D) levels in human islets ~95% free of exocrine cells (Table 2), mice primary islets as well as in the mouse β-cell line MIN6 (Fig 4E), which is clearly exempt of exocrine contamination. Of note, the molecular identity of the CFTR mRNAs amplified from human islets was verified by direct DNA sequencing (Fig 4F). When taken together, these results confirm and broaden previous observations that CFTR is in fact expressed in the human and mouse islet, albeit at a low level and in a small percentage of endocrine cells.
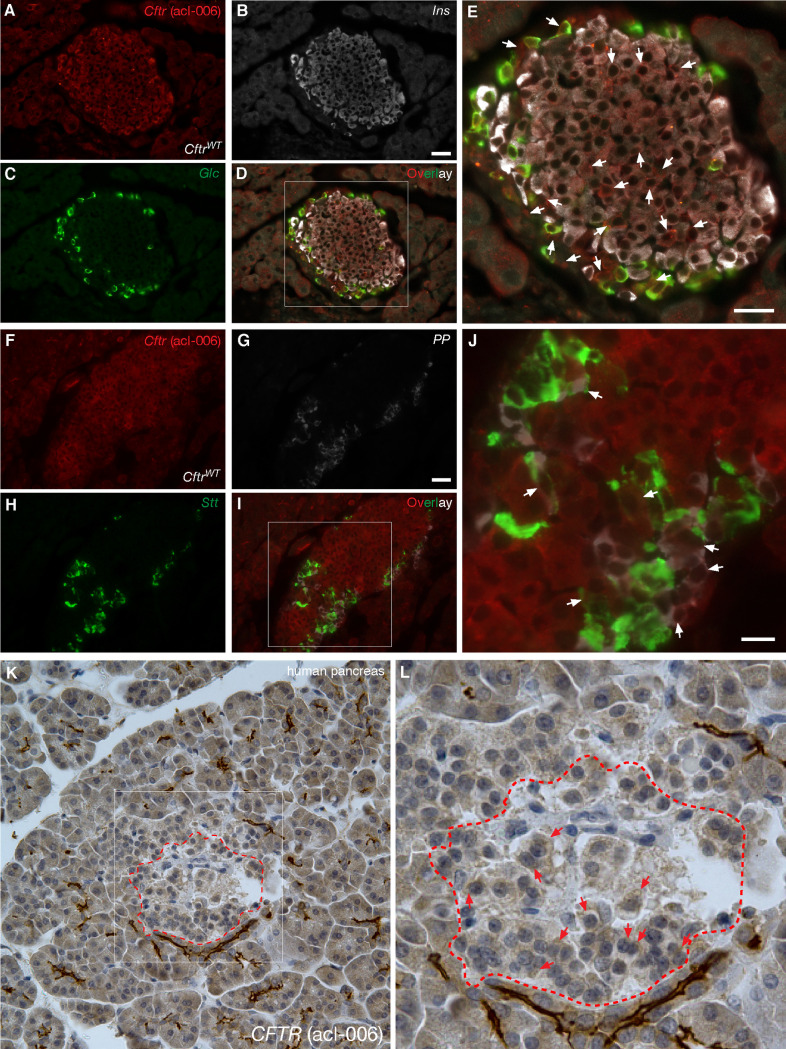
Fig 3. Cftr is expressed in endocrine cells of the mouse islet.
A-J. Immunofluorescence images of WT mouse (CftrWT) pancreas immunostained with the Cftr antibody acl-006 [(Table 1), A and F] in combination with antibodies against insulin (Ins, B), glucagon (Glc, C), somatostatin (Stt, H) and pancreatic polypeptide (PP, G). Magnified overlay images of D and I are shown in E and J, respectively, wherein Cftr immunoreactivity in α-, β-, δ- and PP-cells of the mouse islet is indicated by arrows. K-L. Immunohistochemistry images of normal human pancreas immunostained with acl-006. The islet encircled by a dashed red trace in K is magnified in L to show CFTR immunoreactivity. Bars in A-G represent 25μm.
CFTR modulates insulin secretion
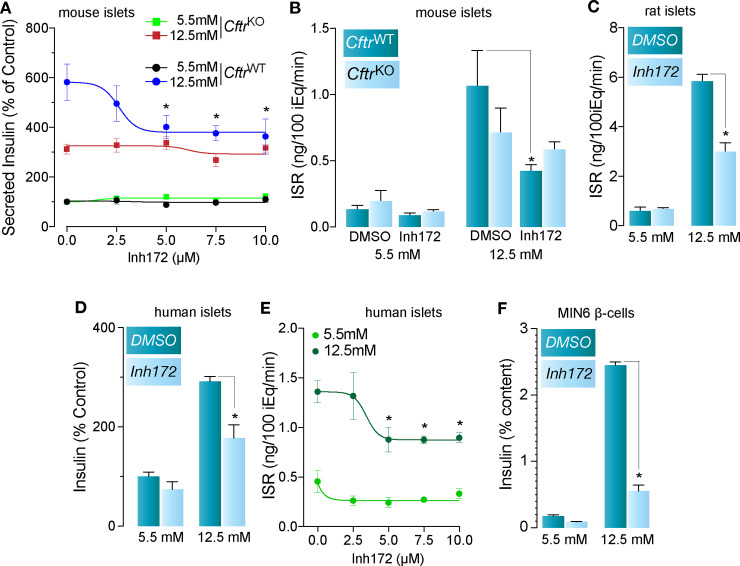
To continue, using primary islets in vitro, we sought to validate previous physiological results. First, we corroborated the specificity of CFTRinh-172 on the secretory response using primary islets obtained from CftrWT and CftrKO [i.e., Cftrtm1Unc Tg(FABPCFTR)1Jaw/J] mice. As shown in Fig 5A, CFTRinh-172 (2.5–10μM) does not affect insulin secretion in CftrKO islets in response to basal or stimulating glucose, as expected for the concentration range already demonstrated to be specific for CFTR [7]. Worthy of note, 5μM of the CFTR inhibitor was the lowest concentration to reduce the secretory response to glucose in CftrWT islets. Therefore, we used that concentration of the inhibitor to interrogate CFTR-specific secretory responses in the islet. Accordingly, as shown in Fig 5B–5E, 5μM CFTRinh-172 reduced, rather than completely inhibiting, the overall secretory response in mouse, rat and human islets as well as MIN6 β-cells (Fig 5F). Taken together, these results suggest that CFTR participates, at least in part, in the secretory response in primary islets.
Fig 5. Cftr contributes to the insulin secretory response in mouse, rat and human islets and the MIN6 β-cell line.
A. Proof of specificity for CFTRinh-172 (0–10μM) on the secretory response using islets obtained from transgenic mice lacking Cftr (CftrKO) and CftrWT mice, in response to 5.5mM and 12.5mM glucose (n = 8, *p<0.05). B, C. Effect of 5μM CFTRinh-172 on insulin secretion of mouse CftrKO and CftrWT islets (B, n = 5, *p<0.05) and rat islets (C, n = 3, *p<0.05) in response to 5.5mM and 12.5mM glucose. D. Basal (5.5mM glucose) and stimulated (12.5mM glucose) insulin secretory response of freshly isolated primary human islets in the presence of vehicle (DMSO) or 5μM CFTRinh-172 (n = 5 donors, *p<0.05). E. Dose-response curve of basal (5.5mM glucose) and stimulated (12.5mM glucose) insulin secretion from human islets (n = 4 donors, *p<0.05) treated with the indicated concentrations of CFTRinh-172. F. Basal (5.5mM glucose) and stimulated (12.5mM glucose) insulin secretory response of MIN6 β-cells incubated with vehicle (DMSO) or 5μM Inh172 (n = 3, *p<0.05).
Cftr is functionally detected in a small proportion of freshly dissociated rat β-cells
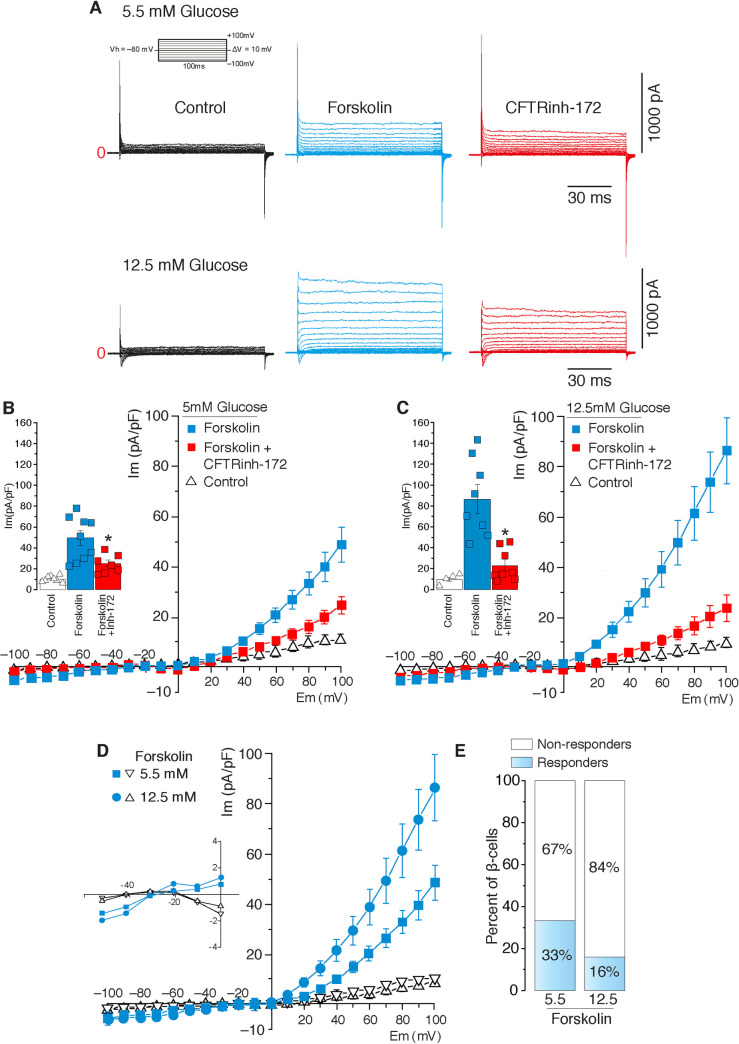
Previous functional data supports the likelihood that a fraction of the total insulin secretory capacity of human and rodent islets as well as MIN6 β-cells is modulated by CFTR (12, 15). To provide further evidence of functional Cftr expression, even in a small number of β-cells, channel activity was determined at the single cell level by using the whole-cell patch-clamp configuration on isolated primary rat β-cells. Chloride currents were measured upon imposing a 6-fold asymmetrical Cl−concentration difference across the membrane patch, given by [Cl–]o = 151.2mM and [Cl–]i = 22mM. As shown in Fig 6A, under basal non-insulinotropic or stimulated insulinotropic conditions (5.5mM and 12.5mM glucose, respectively), rat β-cells exhibit a small Cl−current that increases in response to forskolin (10μM [33]) and is partially inhibited by CFTRinh-172 (5μM), (Figs 6A–6C and 5A). The corresponding I/V relationship plots are shown in Fig 6B and 6C. Note that this conductance became curvilinear and positive as a function of voltage. Indeed, as the voltage increased from negative values to positive ones (becoming >> ECl), Cl−increases its probability to move through CFTRinh-172-sensitive channel(s) due to the higher electrochemical potential and therefore, the conductance is predicted to increase asymptotically. An important observation is that CFTRinh-172 blocks CFTR with an IC50 of 0.3μM and that at 5μM, the inhibitor does not alter Ca2+-activated (Ano1/Ano2) or volume-regulated Cl−currents (VRAC) [7] whereas it has no effect on the secretory response in CftrKO mouse islets (Fig 5A). Remarkably, as shown in Fig 6D and 6E, a relatively low proportion of β-cells revealed Cl−currents under basal and stimulated glucose conditions i.e., ~33% and ~16%, respectively (Fig 6E). The fact that CFTR is expressed in a minor and heterogenous subpopulation of β-cells may help explain, at least in part, why others have not been able to detect channel activity by electrophysiological means.
Fig 6. Cftr is functionally detected in rat β-cells.
A. Representative Cl−currents recorded using rat primary pancreatic β-cells pre-incubated for 1h in 5.5 or 12.5mM glucose before (control, black trace), after forskolin addition (10μM, blue trace) alone or 5 min after adding CFTRinh-172 (5μM, red trace). B, C. Current density-voltage relationships of β-cell Cl−currents recorded in 5.5mM (n = 10) glucose (B) or 12.5mM (n = 8) glucose (C) in the presence of forskolin (10μM, blue squares) alone or plus CFTR-inh-172 (5μM, red squares). Insets in B and C denote peak currents at +100mV (n = 8, for all conditions, *p<0.05). D. Current density-voltage relationship of β-cell Cl−currents in 5.5mM or 12.5 mM glucose after addition of forskolin (10μM). Traces show β-cell responders (blue circles/squares, n = 10 and n = 8 for 5.5mM and 12.5 mM respectively) and non-responders to forskolin in 5.5mM or 12.5mM glucose (open triangles, n = 19 and n = 41, respectively). E. Numerical proportion of the latter observation.
Discussion
We have provided evidence supporting the thought that CFTR is expressed at low levels in a heterogeneous manner in β-cells and that this Cl−channel may play a role in the modulation of insulin secretion. To start afresh and to minimize misinterpretations based on previous results, we systematically screened 21 antibodies raised against human and mouse CFTR and validated them against pancreatic tissues expressing and lacking this protein (Figs 2 and 3). Six of these antibodies were indeed specific when tested against human and mouse pancreatic tissues (Table 1) but the remainder, were either not sensitive enough to detect low levels of CFTR in the islet or produced unexpected staining patterns or high background. For instance, CFF-596, a specific antibody gave the expected staining in the exocrine pancreas, but failed to detect CFTR in the islet (S3A Fig), a finding recently corroborated [12], thus suggesting a reduced affinity/sensitivity of the antibody for its antigen. Moreover, in our hands, antibodies CFF-660, -450 and Ab4067 (S3 Fig) did not produce strong staining in pancreatic sections whereas those from the CFTR Folding Consortium [34] did, but mainly on the islets (S3E–S3J Fig). Antibodies CFF-432, -570 (S2 Fig), 10B6.2 and MA1-935 were all only suitable for IHC, as shown for CFF-217 and -412 (Figs 1–3 and S1 Fig). Although these results may provide the experimental basis of the general tenet that CFTR is absent in the islet, they highlight the need for the use of validated tools to minimize false negative results. In fact, few of the CFTR antibodies we tested and validated do support the conclusion that CFTR is expressed at low levels and in a heterogeneous way in the pancreatic islet in human (Fig 1A–1C) and mouse (Fig 3A–3E) sections. We consistently detected CFTR close to the boundaries of the endocrine cells in human (Fig 1C) and mouse (Fig 3E) islets, confirming the possibility that a small fraction of CFTR localizes to the plasma membrane where channel function can be measured. These data agree with epithelial CFTR sub-membranous localization, where less than 20% of channels reach the plasma membrane [35], giving the distinctive "apical" immunolabeling pattern observed in epithelial tissues.
In situ hybridization experiments also demonstrate very low and diverse levels of CFTR transcripts in some β-cells, but not in all of them (Fig 1D–1H), complementing previous results suggesting that CFTR transcripts are indeed expressed at very low levels in sorted β-cells [11,36,37]. However, it remains to be determined whether these RNA-sequencing studies, that show low expression of CFTR reflects β-cell heterogeneity, as recently suggested [12]. In fact, RNA-sequencing data analysis usually fails to provide reliable and accurate gene expression measurements [38,39], in particular related to the expression of genes in heterogeneous populations expressing low levels [40]. This limitation becomes important in pancreatic islets where they comprise subpopulations of β-cells with different genetic programs and functional properties, including the secretory response [22,41]. As previously described, human islets, for instance, contain at least four antigenically distinguishable β-cells with different transcriptomes and secretory responses [42].
CFTR’s involvement in the modulation of β-cell electrical activity and insulin secretion has been suggested by several investigators [14,17] but disapproved by others [19]. In fact, CFTR, which was originally detected at the protein level in rat α-cells as well as β-cell lines [13], has been functionally implicated in the insulin [14,17] and glucagon [15] secretory responses of human and mouse islets. Our results corroborate specificity for CFTRinh-172 in the secretory response in human, mouse and rat islets (Fig 5A–5E) as well as in MIN6 β-cells (Fig 5F). Importantly, CFTRinh-172 did modulate insulin secretion in WT, but not in islets lacking Cftr in a concentration range between 2.5μM and 10μM (Fig 5A), contrasting with the recent suggestion that CFTRinh-172 is not specific [11,18]. Actually, the inhibitor’s lack of specificity appears to reflect its use at or above 10μM [18], as already previously reported [7]. Our results show that specific inhibition of CFTR was insufficient to abolish islet insulin secretion in response to glucose (Fig 5A–5E). Similarly, elimination of VRAC in mouse β-cells did not eliminate, but rather decreased insulin secretion [43], whereas mouse islets lacking Cftr exhibited an apparently normal secretory response [11]. In addition, the reduced plasma membrane depolarization, Ca2+ oscillations and insulin secretion [17] or reduced Cl−currents and granule exocytosis [14] demonstrated in response to >10μM CFTRinh-172 may suggest that at this concentrations, CFTR is not the only CFTRinh-172-sensitive Cl−channel involved in the insulin secretory response. In fact, >10μM CFTRinh-172 blocks Ca+2- and volume-activated Cl−channels [7], regardless of CFTR expression [44] whereas ≥20μM also impairs mitochondrial function [45]. Therefore, the inhibition of the insulin secretory response documented in CftrKO ferret islets treated with 20μM CFTRinh-172 [18] could be attributed to off-target effects, including the inhibition of other Cl−channels (Ano1/2/VRAC) and/or decreased mitochondrial function, both known to alter the insulin secretory response [46,47]. Therefore, the effect of high concentrations of CFTRinh-172 on normal β-cells could be attributed to a combined participation of CFTR, VRAC, Ano1 and/or any other potential functional target of the inhibitor, which await identification. Altogether these data suggest that inhibition/elimination of CFTR or VRAC alone are not sufficient to fully abolish the insulin secretory response and that additional Cl−channels are at play, supporting the concept that a multicomponent Cl−channel system is present in β-cells, that have the ability to modulate the secretory response [23].
The electrophysiological investigation measuring CFTR channel activity in β-cells has been a challenging and daunting task due, at least in part, to the very low and heterogeneous expression of CFTR in pancreatic β-cells. Therefore, analyses of an unusual number of β-cells was required to detect CFTRinh-172-sensitive anionic (Cl–) currents. We also used a validated whole-cell recording protocol similar to that of Edlund et al. [14] instead of the perforated patch strategy used by Hart et al. [11] to detect CFTR in rat primary β-cells (Fig 6A–6C). These conceptual and methodological considerations may have contributed to the lack of detection of CFTR currents in β-cells by other investigators [11]. Recordings show the reversal potential for Cl−currents under control conditions or in cells which did not respond to forskolin to be consistent with that predicted by the Nernst equation. In addition, the forskolin-activated Cl−current was shifted to the right suggesting additional cAMP-activated currents. Nevertheless, this forskolin-dependent current was partially inhibited by 5uM CFTRinh-172, a concentration that specifically affects CFTR currents. The presence of a forskolin-dependent current is also consistent with functional expression of Cftr in these cells. However, it is important to emphasize that less than ~30% of β-cells exhibited these Cl−currents under basal or stimulated conditions (Fig 6D and 6E), suggesting that the expression pattern of Cftr in the islet is heterogeneous and not functionally measurable in all β-cells by electrophysiological means. As briefly mentioned earlier, there is ample evidence supporting β-cell functional variability within the islets [20], where it has been evident that β-cell functional heterogeneity shapes overall islet function under normal and pathological states [21,22]. Therefore, our results demonstrating functional Cftr in ~30% of β-cells under basal conditions advocate for a role for this channel in the secretory response in at least 1/3 of β-cells, a finding that helps mitigate at least partially, some of the disagreements around CFTR expression and function in the mammalian islet [11,12,19].
In summary, it remains clinically and mechanistically relevant to validate the hypothesis that CFTR, and/or any other Cl−channel expressed in β-cells modulate the β-cell secretory response. Demonstrating that CFTR is expressed in a subset of β-cells and takes part in their function adds an extra layer of complexity to the pathogenesis of CFRD. Clearly, this, as any other interpretation driven from CFTR expression in β-cells, cannot simply discard other mechanisms already proposed as players in the pathophysiology of CFRD. At this point, it is important to note that CFTR, irrespective of its low expression and/or independently of its Cl−channel activity, may have long-term effects on β-cells physiology or pathology. Further, CFTR does not need to be abundant or a Cl−channel to play a role in any cell type (reviewed in [23]). Therefore, disregarding the possibility that CFTR may have a role in β-cell physiology because its Cl−channel function is impaired or because its expression is low still remain premature at this point. Although the involvement of β-cell CFTR in the appearance of the insidious intermittent hyperglycemia in CFRD remains to be determined, the concept is relevant when considering that the existing relationship between CFRD and exocrine pancreatic insufficiency [48] does not explain the fact that CFRD also occurs in patients without pancreatic insufficiency and that the latter is not a necessary condition to develop diabetes [49]. Together, our observations place CFTR as a component of a complex Cl−channel machinery in the modulation of insulin secretion and provide an additional piece of information to help explain the impaired insulin secretion that results in CFRD.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
The authors thank the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative Type 1 Diabetes Research Project sponsored by JDRF, for providing pancreatic tissues from control and CF subjects. Dr. Ian Sweet and Austin Rountree at the University of Washington DRC, Cell Function Analysis Core (P30 DK017047). The Human Organ Procurement and Exchange (HOPE) program and the Trillium Gift of Life Network (TGLN) for their work in obtaining human pancreas for research. Dr. Tatsuya Kin and Mr. James Lyon for their efforts in human pancreatic islet isolation. Dr. Mourad Ferdaoussi for assistance with qPCR of CFTR expression. Dr. Jun-Ichi Miyazaki for the MIN6 β-cell line. Antibodies from the Cystic Fibrosis Foundation, the CFTR Folding Consortium and the University of North Carolina. We are most grateful to Drs. Peter Brown and Len Best (University of Manchester, UK) for their careful and critical analysis of the manuscript and their constructive suggestions and to Luis Galietta (Cell Biology and Disease Mechanisms, Telethon Institute of Genetics and Medicine, Italy) and Alan Verkman (University of California San Francisco, US) for their initial guidance on the pharmacology of Ano1 and Cftr.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
LA-B: NIDDK R01 097829 National Institutes of Health https://www.nih.gov/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MDiF: 1-17-IBS-258 American Diabetes Association https://www.diabetes.org/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. R21DK113446-01 National Institutes of Health https://www.nih.gov/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, et al. (2009) Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 32: 1626–1631. 10.2337/dc09-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ (2005) Cystic fibrosis. N Engl J Med 352: 1992–2001. 10.1056/NEJMra043184 [DOI] [PubMed] [Google Scholar]
- 3.Kelly A, Moran A (2013) Update on cystic fibrosis-related diabetes. J Cyst Fibros 12: 318–331. 10.1016/j.jcf.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Bogdani M, Blackman SM, Ridaura C, Bellocq JP, Powers AC, et al. (2017) Structural abnormalities in islets from very young children with cystic fibrosis may contribute to cystic fibrosis-related diabetes. Sci Rep 7: 17231 10.1038/s41598-017-17404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, et al. (2018) Animal and model systems for studying cystic fibrosis. J Cyst Fibros 17: S28–S34. 10.1016/j.jcf.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendes F, Farinha CM, Roxo-Rosa M, Fanen P, Edelman A, et al. (2004) Antibodies for CFTR studies. J Cyst Fibros 3 Suppl 2: 69–72. 10.1016/j.jcf.2004.05.016 [DOI] [PubMed] [Google Scholar]
- 7.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, et al. (2002) Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658. 10.1172/JCI16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farinha CM, Mendes F, Roxo-Rosa M, Penque D, Amaral MD (2004) A comparison of 14 antibodies for the biochemical detection of the cystic fibrosis transmembrane conductance regulator protein. Mol Cell Probes 18: 235–242. 10.1016/j.mcp.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 9.Farinha CM, Penque D, Roxo-Rosa M, Lukacs G, Dormer R, et al. (2004) Biochemical methods to assess CFTR expression and membrane localization. J Cyst Fibros 3 Suppl 2: 73–77. 10.1016/j.jcf.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 10.Saint-Criq V, Gray MA (2017) Role of CFTR in epithelial physiology. Cell Mol Life Sci 74: 93–115. 10.1007/s00018-016-2391-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart NJ, Aramandla R, Poffenberger G, Fayolle C, Thames AH, et al. (2018) Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 3 10.1172/jci.insight.98240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White MG, Maheshwari RR, Anderson SJ, Berlinguer-Palmini R, Jones C, et al. (2020) In Situ Analysis Reveals That CFTR Is Expressed in Only a Small Minority of beta-Cells in Normal Adult Human Pancreas. J Clin Endocrinol Metab 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boom A, Lybaert P, Pollet JF, Jacobs P, Jijakli H, et al. (2007) Expression and localization of cystic fibrosis transmembrane conductance regulator in the rat endocrine pancreas. Endocrine 32: 197–205. 10.1007/s12020-007-9026-x [DOI] [PubMed] [Google Scholar]
- 14.Edlund A, Esguerra JL, Wendt A, Flodstrom-Tullberg M, Eliasson L (2014) CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med 12: 87 10.1186/1741-7015-12-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edlund A, Pedersen MG, Lindqvist A, Wierup N, Flodstrom-Tullberg M, et al. (2017) CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep 7: 90 10.1038/s41598-017-00098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang WQ, Guo JH, Yuan C, Cui YG, Diao FY, et al. (2017) Abnormal CFTR Affects Glucagon Production by Islet alpha Cells in Cystic Fibrosis and Polycystic Ovarian Syndrome. Front Physiol 8: 835 10.3389/fphys.2017.00835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JH, Chen H, Ruan YC, Zhang XL, Zhang XH, et al. (2014) Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nat Commun 5: 4420 10.1038/ncomms5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Yi Y, Xie W, Liang B, Winter MC, et al. (2017) CFTR Influences Beta Cell Function and Insulin Secretion Through Non-Cell Autonomous Exocrine-Derived Factors. Endocrinology 158: 3325–3338. 10.1210/en.2017-00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rorsman P, Ashcroft FM (2018) Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev 98: 117–214. 10.1152/physrev.00008.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasteska D, Hodson DJ (2018) The role of beta cell heterogeneity in islet function and insulin release. J Mol Endocrinol 61: R43–R60. 10.1530/JME-18-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez GD, Gromada J, Sussel L (2017) Heterogeneity of the Pancreatic Beta Cell. Front Genet 8: 22 10.3389/fgene.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benninger RKP, Hodson DJ (2018) New Understanding of beta-Cell Heterogeneity and In Situ Islet Function. Diabetes 67: 537–547. 10.2337/dbi17-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Fulvio M, Aguilar-Bryan L (2019) Chloride transporters and channels in beta-cell physiology: revisiting a 40-year-old model. Biochem Soc Trans 47: 1843–1855. 10.1042/BST20190513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best L (2005) Glucose-induced electrical activity in rat pancreatic β-cells: dependence on intracellular chloride concentration. J Physiol 568: 137–144. 10.1113/jphysiol.2005.093740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best L, Brown PD, Tomlinson S (1997) Anion fluxes, volume regulation and electrical activity in the mammalian pancreatic β-cell. Exp Physiol 82: 957–966. 10.1113/expphysiol.1997.sp004081 [DOI] [PubMed] [Google Scholar]
- 26.Alshahrani S, Almutairi MM, Kursan S, Dias-Junior E, Almiahuob MM, et al. (2015) Increased Slc12a1 expression in β-cells and improved glucose disposal in Slc12a2 heterozygous mice. J Endocrinol 227: 153–165. 10.1530/JOE-15-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rountree AM, Neal AS, Lisowski M, Rizzo N, Radtke J, et al. (2014) Control of insulin secretion by cytochrome C and calcium signaling in islets with impaired metabolism. J Biol Chem 289: 19110–19119. 10.1074/jbc.M114.556050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velasco M, Larque C, Gutierrez-Reyes G, Arredondo R, Sanchez-Soto C, et al. (2012) Metabolic syndrome induces changes in KATP-channels and calcium currents in pancreatic beta-cells. Islets 4: 302–311. 10.4161/isl.21374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung SR, Reed BJ, Sweet IR (2009) A highly energetic process couples calcium influx through L-type calcium channels to insulin secretion in pancreatic beta-cells. Am J Physiol Endocrinol Metab 297: E717–727. 10.1152/ajpendo.00282.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kursan S, McMillen TS, Beesetty P, Dias-Junior E, Almutairi MM, et al. (2017) The neuronal K+Cl- co-transporter 2 (Slc12a5) modulates insulin secretion. Sci Rep 7: 1732 10.1038/s41598-017-01814-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Delgado N, Velasco M, Sanchez-Soto C, Diaz-Garcia CM, Hiriart M (2018) Calcium Channels in Postnatal Development of Rat Pancreatic Beta Cells and Their Role in Insulin Secretion. Front Endocrinol (Lausanne) 9: 40 10.3389/fendo.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastoy B, Godazgar M, Clark A, Nylander V, Spiliotis I, et al. (2018) Electrophysiological properties of human beta-cell lines EndoC-betaH1 and -betaH2 conform with human beta-cells. Sci Rep 8: 16994 10.1038/s41598-018-34743-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran O, Zegarra-Moran O (2008) On the measurement of the functional properties of the CFTR. J Cyst Fibros 7: 483–494. 10.1016/j.jcf.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 34.Peters KW, Okiyoneda T, Balch WE, Braakman I, Brodsky JL, et al. (2011) CFTR Folding Consortium: methods available for studies of CFTR folding and correction. Methods Mol Biol 742: 335–353. 10.1007/978-1-61779-120-8_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho-Oliveira I, Efthymiadou A, Malho R, Nogueira P, Tzetis M, et al. (2004) CFTR localization in native airway cells and cell lines expressing wild-type or F508del-CFTR by a panel of different antibodies. J Histochem Cytochem 52: 193–203. 10.1177/002215540405200207 [DOI] [PubMed] [Google Scholar]
- 36.Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, et al. (2016) Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab 24: 593–607. 10.1016/j.cmet.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, et al. (2015) Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes 64: 3172–3181. 10.2337/db15-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Consortium SM-I (2014) A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat Biotechnol 32: 903–914. 10.1038/nbt.2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora S, Pattwell SS, Holland EC, Bolouri H (2020) Variability in estimated gene expression among commonly used RNA-seq pipelines. Scientific Reports 10: 2734 10.1038/s41598-020-59516-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang B, Lee JH, Bang D (2018) Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 50: 96 10.1038/s12276-018-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, et al. (2016) Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab 24: 389–401. 10.1016/j.cmet.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, et al. (2016) Human islets contain four distinct subtypes of beta cells. Nat Commun 7: 11756 10.1038/ncomms11756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuhlmann T, Planells-Cases R, Jentsch TJ (2018) LRRC8/VRAC anion channels enhance beta-cell glucose sensing and insulin secretion. Nat Commun 9: 1974 10.1038/s41467-018-04353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melis N, Tauc M, Cougnon M, Bendahhou S, Giuliano S, et al. (2014) Revisiting CFTR inhibition: a comparative study of CFTRinh -172 and GlyH-101 inhibitors. Br J Pharmacol 171: 3716–3727. 10.1111/bph.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, et al. (2010) Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther 333: 60–69. 10.1124/jpet.109.162032 [DOI] [PubMed] [Google Scholar]
- 46.Best L, Brown PD, Sener A, Malaisse WJ (2010) Electrical activity in pancreatic islet cells: The VRAC hypothesis. Islets 2: 59–64. 10.4161/isl.2.2.11171 [DOI] [PubMed] [Google Scholar]
- 47.Maechler P (2013) Mitochondrial function and insulin secretion. Mol Cell Endocrinol 379: 12–18. 10.1016/j.mce.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 48.Soave D, Miller MR, Keenan K, Li W, Gong J, et al. (2014) Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study. Diabetes 63: 2114–2119. 10.2337/db13-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wooldridge JL, Szczesniak RD, Fenchel MC, Elder DA (2015) Insulin secretion abnormalities in exocrine pancreatic sufficient cystic fibrosis patients. J Cyst Fibros 14: 792–797. 10.1016/j.jcf.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.