Abstract
During development of the Caenorhabditis elegans gonad, the gonadal leader cells, called distal tip cells (DTCs), migrate in a U-shaped pattern to form the U-shaped gonad arms. The ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family metalloproteases MIG-17 and GON-1 are required for correct DTC migration. Mutations in mig-17 result in misshapen gonads due to the misdirected DTC migration, and mutations in gon-1 result in shortened and swollen gonads due to the premature termination of DTC migration. Although the phenotypes shown by mig-17 and gon-1 mutants are very different from one another, mutations that result in amino acid substitutions in the same basement membrane protein genes, emb-9/collagen IV α1, let-2/collagen IV α2 and fbl-1/fibulin-1, were identified as genetic suppressors of mig-17 and gon-1 mutants. To understand the roles shared by these two proteases, we examined the effects of the mig-17 suppressors on gon-1 and the effects of the gon-1 suppressors and enhancers on mig-17 gonadal defects. Some of the emb-9, let-2 and fbl-1 mutations suppressed both mig-17 and gon-1, whereas others acted only on mig-17 or gon-1. These results suggest that mig-17 and gon-1 have their specific functions as well as functions commonly shared between them for gonad formation. The levels of collagen IV accumulation in the DTC basement membrane were significantly higher in the gon-1 mutants as compared with wild type and were reduced to the wild-type levels when combined with suppressor mutations, but not with enhancer mutations, suggesting that the ability to reduce collagen IV levels is important for gon-1 suppression.
Introduction
Members of the ADAMTS family of secreted zinc metalloproteases have important roles in animal development. Most of these proteases degrade extracellular matrix components such as proteoglycans or collagens [1]. Nineteen ADAMTS genes have been identified in the human genome, and mutations in many of them result in hereditary diseases that are related to disorders of the extracellular matrix [2, 3]. Multiple ADAMTS proteases often function in a common developmental process. For example, the functions of ADAMTS-5, -9 and -20 are required for interdigital web regression [4]. ADAMTS-9 and -20 are needed for closure of the palate and for craniofacial morphogenesis and neural tube closure through their function in ciliogenesis [4, 5]. ADAMTS-5 and -15 act in myoblast fusion [6]. These enzymes appear to function in a partially overlapping manner. However, the roles shared by these ADAMTS proteases in development still remain elusive.
Among five ADAMTS genes in C. elegans, gon-1 and mig-17 play essential roles in the development of the somatic gonad [7, 8]. Both GON-1 and MIG-17 localize to the gonadal basement membrane (BM) [9, 10]. In the mig-17 mutants, DTCs meander and stray, resulting in an abnormal gonadal shape. In contrast, in the gon-1 mutants, DTCs rarely migrate and the gonads remain small. We have isolated and analyzed the genetic suppressors for DTC migration defects in mig-17 mutants. Dominant gain-of-function mutations in the fbl-1/fibulin-1 and let-2/collagen IV α2 chain, which encode BM molecules, have been frequently isolated as suppressors [11, 12]. The fbl-1(gf) mutations are amino acid substitutions in the second epidermal growth factor−like motif of FBL-1C, one of the two splicing isoforms, and FBL-1C protein is secreted by the intestine to be released into the gonadal BM in a MIG-17 activity-dependent manner [11]. Suppression of mig-17 by fbl-1(gf) mutations is dependent on the BM molecule NID-1/nidogen [12]. let-2(gf) mutations are associated with amino acid changes within the triple helix domain or the C-terminal non-collagenous domain (NC)1 of collagen IV. LET-2 protein is secreted from body wall muscle cells and DTCs and localized to the gonadal BM in a MIG-17 activity-independent manner. Unlike the case of fbl-1(gf), suppression of mig-17 by let-2(gf) mutations does not require NID-1 [12].
Genetic suppressor analysis of gon-1 mutants revealed that loss-of-function (deletion) mutations in fbl-1 can suppress the shortened-gonad phenotype of gon-1 mutants. Because fbl-1 deletion mutants also exhibit the shortened-gonad phenotype, GON-1 and FBL-1 act antagonistically to regulate gonad formation [13]. This genetic interaction is mediated by the control of collagen IV accumulation in the gonadal BM: GON-1 acts to reduce collagen IV levels, whereas FBL-1 acts to maintain collagen IV levels [14].
Although mig-17 and gon-1 mutants are phenotypically very different, they are both suppressed or enhanced by mutations in let-2 and fbl-1. In this study, we isolated novel suppressor mutations in emb-9 for mig-17 and in let-2 for gon-1 gonadal defects. Together with the previously isolated suppressors and enhancers, we investigated the consequences when suppressors for mig-17 were combined with gon-1 mutations, and when gon-1 suppressors and enhancers were combined with mig-17 mutations. We found that some of the emb-9, let-2 and fbl-1 mutations suppressed both mig-17 and gon-1, whereas others suppressed only mig-17 or gon-1. These results suggest that mig-17 and gon-1 have their specific functions as well as the functions commonly shared between them for gonad formation.
Materials and methods
Strains and genetic analysis
Culture, handling and ethyl methanesulfonate (EMS) mutagenesis of C. elegans were conducted as described [15]. The following mutations and transgenes were used in this work: mig-17(k174), gon-1(e1254, g518), fbl-1(k201, tk45), let-2(g25, b246, k193, k196), emb-9(tk75, g34, g23cg46) and tkTi1[emb-9::mCherry] [7, 8, 11, 12, 14, 16–18]. The suppressor mutations were genetically mapped with single-nucleotide polymorphism mapping using mig-17(k174) and gon-1(e1254) mutant strains, which are in the CB4856 background [19]. Among the 11 mig-17(k174) suppressors, k204 and k207 were mapped to the center of linkage group (LG) III. Next-generation sequence analysis identified missense mutations in emb-9 in these suppressors. Of the two gon-1(e1254) suppressors, one was mapped to the right end of LG X and was identified by next-generation sequence analysis as corresponding to a missense mutation in let-2. All experiments were conducted at 20°C. The temperature-sensitive mutants emb-9(g34) and let-2(g25, b246), which arrest during embryogenesis or early larval stages at 25°C, do proliferate at 20°C. Because gon-1(e1254, g518) and fbl-1(tk45) mutants were sterile, we used the genetic balancer nT1[qIs51] (IV;V) to maintain these mutants and to generate double mutants containing these mutations.
Microscopy
Gonad migration phenotypes were scored using a Nomarski microscope (Axioplan 2; Zeiss). Analysis of gonadal phenotypes was performed at the young-adult stage as described [20]. Although the gonadal phenotypes of some strains used are published, we reevaluated them in this study. The levels of EMB-9-mCherry were quantified as follows. For each sample, confocal images of a sagittal section of the DTCs were obtained with a Zeiss Imager M2 microscope equipped with a spinning-disk confocal scan head (CSU-X1; Yokogawa) and an ImageEM CCD camera (ImageEM; Hamamatsu Photonics). Using ImageJ software, we measured the fluorescence intensities along three drawn lines, each of which crossed the DTC BM; the average background intensities inside the gonad were subtracted from the peak values of the line scan, and the resulting corrected values were averaged.
Transgenic analysis of suppressors
Germline transformation was carried out as described [21]. Plasmids containing emb-9(k204), emb-9(k207) and let-2(tk101) were constructed by introducing these mutations individually into their respective wild-type constructs [12, 14]. These plasmids were injected into the unc-119(e2498) gonad at 1−2 ng/μl with 10 ng/μl unc-119+ plasmid [22], 70 ng/μl sur-5::gfp plasmid [23] and 70 ng/μl pBSIIKS(−), and the resulting extrachromosomal arrays were transferred to either mig-17(k174); unc-119(e2498) or gon-1(e1254); unc-119(e2498) animals by mating.
Homology modeling of NC1 domains
Homology modeling was conducted based on the crystal structures of bovine collagen IV NC1 domains [24] using the SWISS-MODEL server. Ribbon diagrams were created and edited with the Swiss-Pdb Viewer software.
Results
Isolation of novel suppressors of mig-17 and gon-1 mutants
The C. elegans hermaphrodite gonad arms extend to the anterior-right and posterior-left areas of the body cavity. The U shape of the gonad arms are generated by migration of the gonadal leader cells, the DTCs, over the body wall during larval development (Fig 1). gon-1 mutants exhibit shortened gonads due to the premature termination of DTC migration and are sterile. In contrast, mig-17 mutants show misshapen gonad arms due to the misdirected migration of DTCs, but they are still fertile. Although both of these genes encode ADAMTS family metalloproteases, the phenotypes shown by these mutants are very different.
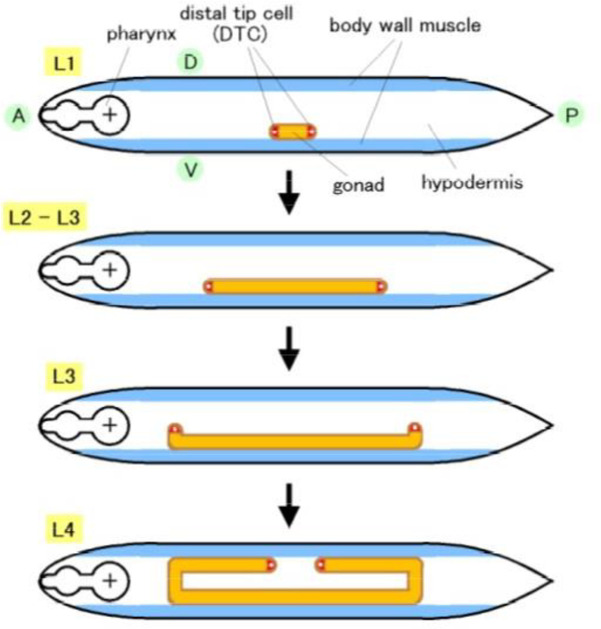
Fig 1. Schematic presentation of gonad formation in the C. elegans hermaphrodite.
The hermaphrodite U-shaped gonad arms are generated by migration of two DTCs. DTCs are generated at the anterior (A) and posterior (P) ends of the gonad primordium at the first larval stage (L1) and migrate along the anteroposterior axis on the ventral (V) body wall muscle (L2−L3). The DTCs turn dorsally (D) and migrate along the lateral hypodermis (L3). Finally, the DTCs undergo a second turn and migrate along the dorsal body wall muscle to form the symmetrical U-shaped arms (L4).
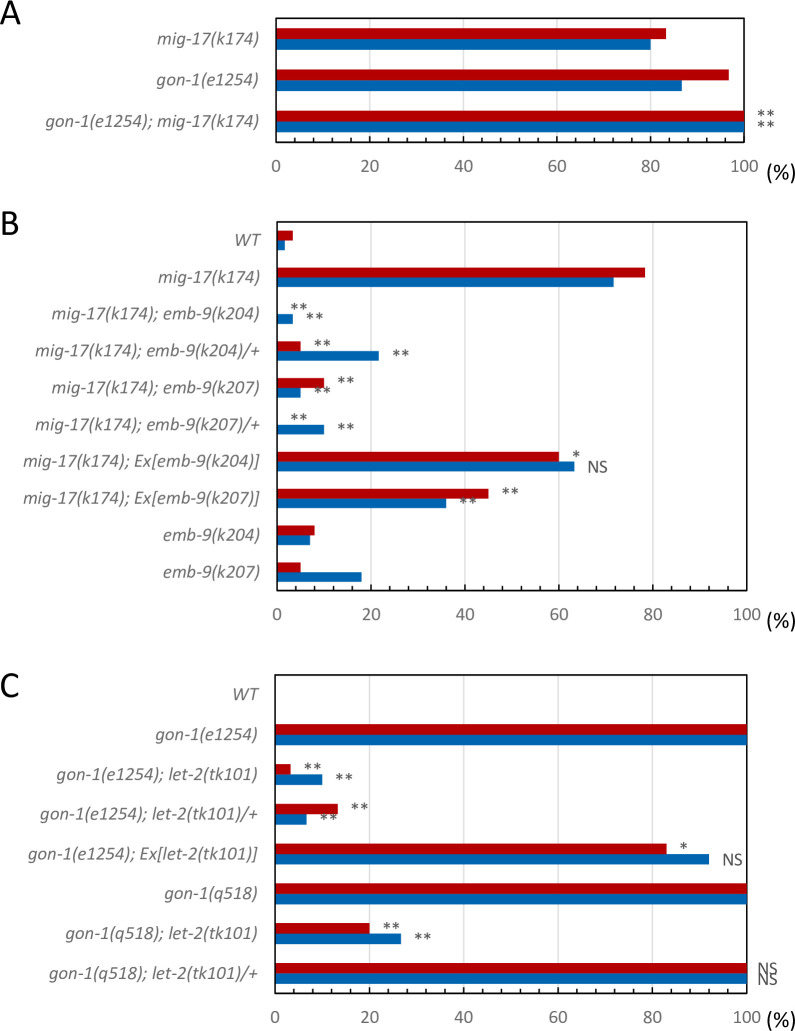
The null allele gon-1(q518) exhibits a fully penetrant short gonad phenotype that cannot be distinguished from that of the mig-17(k174); gon-1(q518) double null mutants [8]. The phenotypic penetrance of gon-1(e1254), a partial loss-of-function allele that results in a milder gonad phenotype as compared with gon-1(q518), was enhanced in combination with the mig-17(k174) null allele (Fig 2A; S1 Fig), indicating that gon-1 and mig-17 function in partially overlapping pathways.
Fig 2. DTC migration phenotypes of mig-17, gon-1 and the suppressors.
A. Percentage of DTC migration defects in mig-17(k174) and gon-1(e1254) single mutants and their double mutants. P-values from Fisher’s exact test comparing the double mutants with mig-17(k174) animals: **P < 0.01. B. Percentage of DTC migration defects in mig-17(k174) animals with the emb-9(k204) or emb-9(k207) mutation or with extrachromosomal arrays carrying these mutant genes. P-values from Fisher’s exact test comparing the double mutants with mig-17(k174) animals: **P < 0.01; *P < 0.05; NS, not significant. C. Percentage of DTC migration defects in gon-1(e1254) or gon-1(q518) animals with a let-2(tk101) mutation or with an extrachromosomal array carrying the let-2(tk101) mutant gene. P-values from Fisher’s exact test comparing the double mutants with gon-1(e1254) animals: **P < 0.01; *P < 0.05; NS, not significant. Red and blue bars represent defects in anterior and posterior gonad arms, respectively.
To identify genes interacting with mig-17 and gon-1, we isolated novel suppressor mutations for DTC migration defects of mig-17 and gon-1 mutants using EMS mutagenesis. We isolated 11 suppressor mutants of mig-17(k174), a null allele that has a nonsense mutation in the pro-domain (S1 Fig). Two of these mutants, k204 and k207, were genetically mapped near the center of LG III and acted as dominant suppressors for mig-17 (Fig 2B). Next-generation sequence analysis of k204 and k207 identified mutations in emb-9, which encodes the α1 subunit of collagen IV. Both mutations were amino acid substitutions in the C-terminal NC1 domain. We generated plasmids carrying these mutant alleles of emb-9 and introduced them into mig-17 mutants. The extrachromosomal arrays containing these plasmids partially rescued the gonadal phenotype of mig-17 mutants (Fig 2B), indicating that emb-9(k204) and emb-9(k207) mutations are causative for mig-17 suppression.
We used EMS mutagenesis to isolate suppressors of gon-1(e1254), a strong loss-of-function allele with a nonsense mutation in the C-terminal domain, which contains thrombospondin type 1 motifs (S1 Fig). Although gon-1(e1254) homozygotes are sterile, the transgenic strain gon-1(e1254); tkEx370[gon-1 fosmid, rol-6(su1006), sur-5::GFP], which carries an extrachromosomal array that consists of multiple copies of the wild-type gon-1 fosmid (WRM0622dB04), mutant rol-6(1006) plasmid and sur-5::GFP plasmid, does proliferate as a homozygote. rol-6(su1006) and sur-5::GFP are marker plasmids that result in the roller movement phenotype and GFP expression in all somatic nuclei, respectively. We isolated non-roller and GFP− fertile animals from the F2 or F3 generation of transgenic animals treated with EMS (S2 Fig). One of the two gon-1 suppressors, tk101, acted as a dominant suppressor of gon-1(e1254) and was genetically mapped to the right end of LG X. tk101was a recessive suppressor for the gon-1(q518) null allele (Fig 2C). Next-generation sequence analysis revealed an amino acid substitution in the N-terminal region of the triple helical domain of let-2, which encodes the α2 subunit of collagen IV. The extrachromosomal array containing this let-2 mutant plasmid partially rescued the gonadal phenotype of gon-1(e1254) (Fig 2C), indicating that let-2(tk101) is the causative mutation for gon-1 suppression.
Swapping experiments for mig-17 and gon-1 suppressors or enhancers
Thus far, our genetic screening had identified various mutant alleles in let-2 and fbl-1 that act as suppressors of mig-17 [11, 12] and in fbl-1 that act as a suppressor of gon-1 [14], among which the suppressor mutant alleles differed between mig-17 and gon-1. We also previously showed that loss-of-function mutations emb-9(g34 and g23cg46) and let-2(g25 and b246) act as suppressors of mig-17 [12] and that emb-9(tk75), which was originally isolated as a suppressor of fbl-1(tk45), acts as an enhancer of gon-1 [14]. emb-9(g34 and g23cg46) also enhance gon-1 [14]. In this study, we identified novel suppressors let-2(tk101) for gon-1 and emb-9(k204 and k207) for mig-17 (Fig 3A and 3B). These results imply that MIG-17 and GON-1 ADAMTS proteases functionally interact with the same BM proteins collagen IV α1 and α2 subunits and fibulin-1.
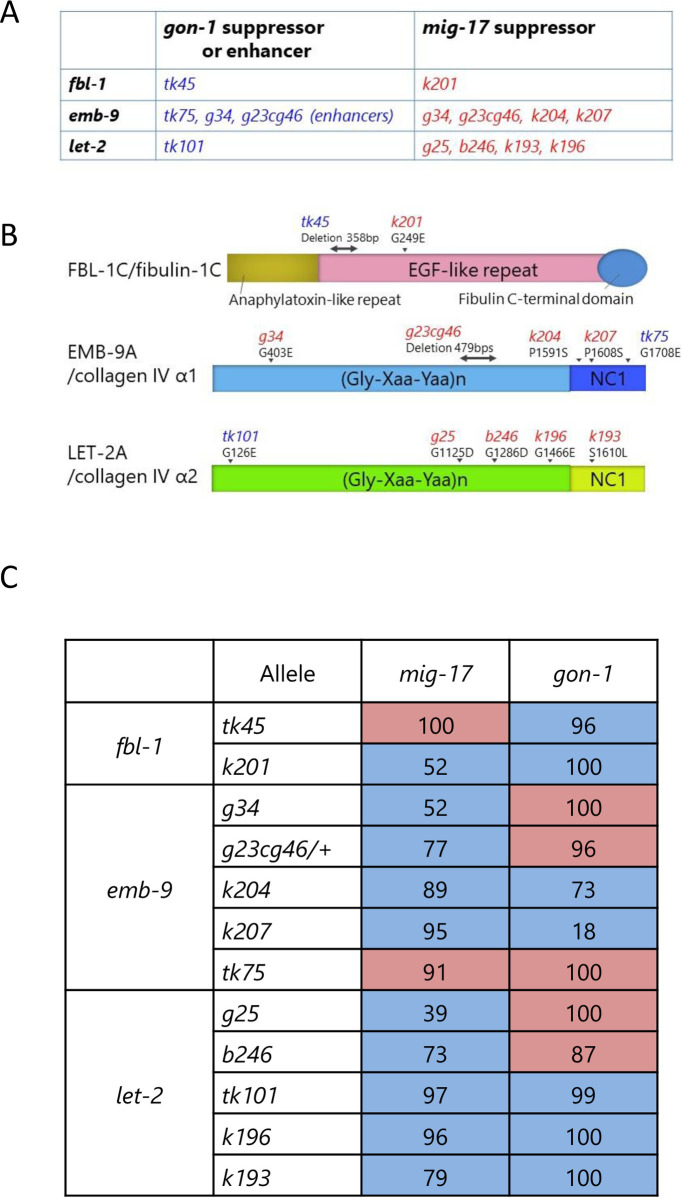
Fig 3. Suppressor and enhancer mutations for gon-1 and mig-17, and summary for swapping experiments of suppressors and enhancers.
A. fbl-1, emb-9 and let-2 alleles that suppress or enhance the gonadal defects of gon-1(e1254) or mig-17(k174). B. Locations of mutations in FBL-1C, EMB-9A and LET-2A proteins. The mutation sites are shown by arrowheads (amino acid substitutions) or bidirectional arrows (deletions). Both fbl-1(tk45) and emb-9(g23cg46) deletions are potential null alleles, as they are expected to introduce termination codons shortly after the deleted region [11, 17]. C. Summary of effects of fbl-1, emb-9 and let-2 alleles on mig-17(k174) and gon-1(e1254) mutants. Blue and red boxes represent suppression and enhancement, respectively. Numeric values representing strength of suppression or enhancement were calculated as follows. Percentages of anterior and posterior gonadal defects were averaged for each strain. Strength of suppression indicates percent recovery of the gonadal defect in double mutants relative to that of the mig-17 or gon-1 single mutants. Strength of enhancement indicates percent decrease of the ratio of non-defective gonads in double mutants relative to that of the mig-17 or gon-1 single mutants.
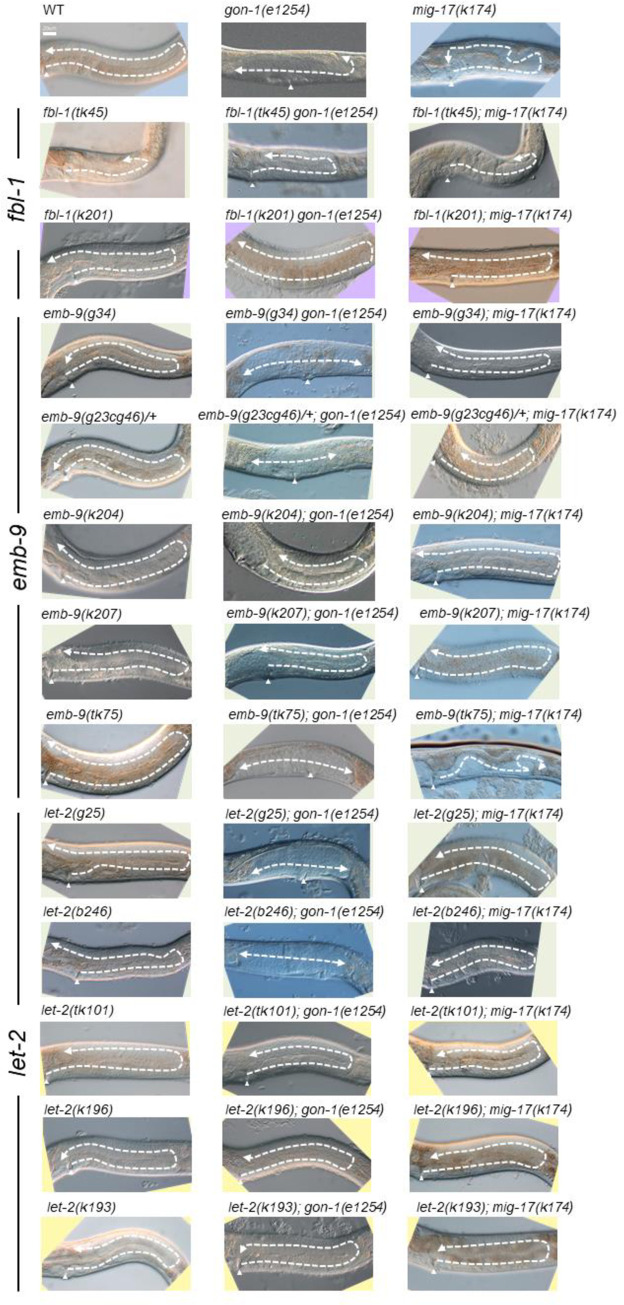
To understand the roles shared by these two proteases, we examined how the suppressors of mig-17 affect gon-1 and how the suppressors and enhancers of gon-1 affect mig-17 gonadal defects. We examined all the combinations of double mutants. The representative phenotypes exhibited by these double mutants are shown in Fig 4, and their phenotypic penetrance scored at the young adult stage is shown in Figs 5 and 6. In mig-17(k174) animals and in double mutants containing mig-17(k174), suppression was assessed by whether the U-shaped gonad was formed as in the wild type. In gon-1(e1254) animals and in the double mutants containing gon-1(e1254), suppression was assessed by whether the gonad arms reached the dorsal muscle.
Fig 4. Representative Nomarski images of young adult hermaphrodite gonads of wild-type, gon-1(e1254), mig-17(k174) and double-mutant animals analyzed in this study.
The gonad morphology is shown by dashed arrows. Anterior is to the left, dorsal up. Arrowheads point to the vulva. Bar: 20 μm.
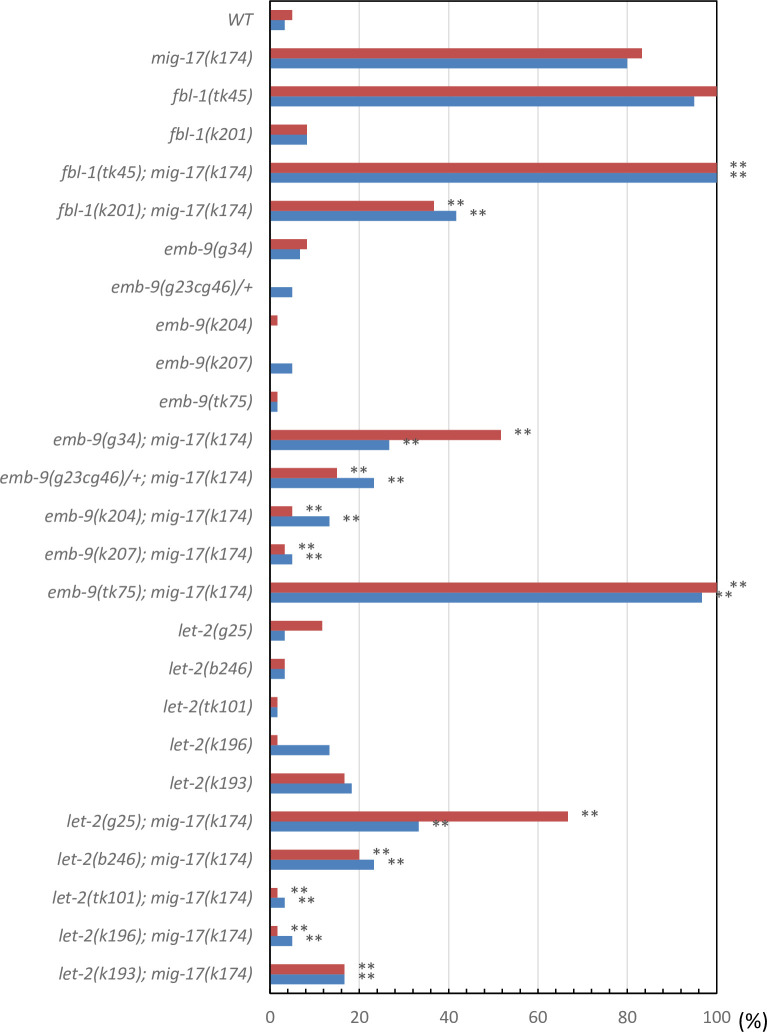
Fig 5. Percentage of DTC migration defects in mig-17(k174) mutants in the presence of fbl-1, emb-9 and let-2 alleles.
Red and blue bars represent defects in anterior and posterior gonad arms, respectively. n = 60 for each experiment. P-values from Fisher’s exact test comparing the double mutants with mig-17(k174) animals: **P < 0.01; *P < 0.05; NS, not significant.
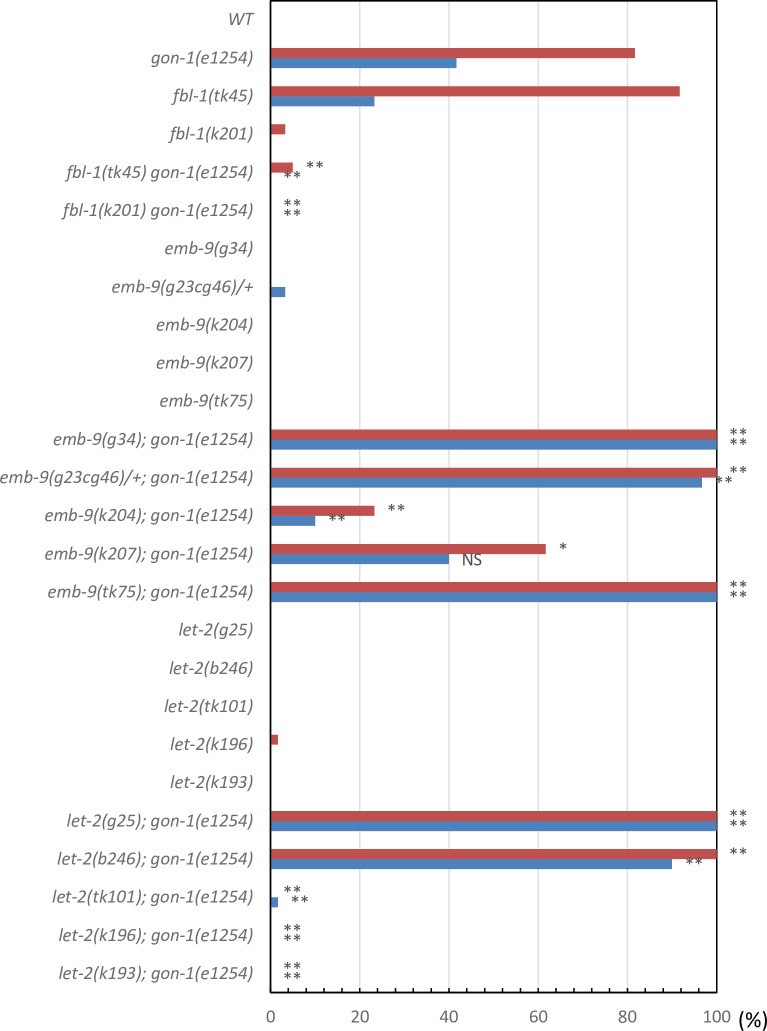
Fig 6. Percentage of gonad arms that failed to reach the dorsal muscle of gon-1(e1254) mutants in the presence of fbl-1, emb-9 and let-2 alleles.
Red and blue bars represent defects in anterior and posterior gonad arms, respectively. n = 60 for each experiment. P-values from Fisher’s exact test comparing the double mutants with gon-1(e1254) animals: **P < 0.01; *P < 0.05; NS, not significant.
For the fbl-1 alleles, the fbl-1(k201) mutation suppressed both mig-17 and gon-1. Although fbl-1(tk45) suppressed gon-1, it rather enhanced mig-17. For the emb-9 alleles, although emb-9 (g34 and g23cg46/+) suppressed mig-17, they both enhanced gon-1. emb-9(k204 and k207) suppressed mig-17 strongly, whereas they suppressed gon-1 somewhat weakly. emb-9(tk75) acted as a strong enhancer for both mig-17 and gon-1. For the let-2 alleles, although let-2(g25 and b246) suppressed mig-17, they both enhanced gon-1. let-2(tk101, k196 and k193) suppressed both mig-17 and gon-1. These data are summarized in Fig 3C. Because gon-1 mutants are 100% sterile, we also analyzed fertility in the double mutants (S3 Fig). We found that among fbl-1, emb-9 and let-2, some alleles suppressed or enhanced both mig-17 and gon-1, whereas others affected mig-17 and gon-1 differentially. In the latter case, the alleles that suppressed mig-17 or gon-1 rather enhanced gon-1 or mig-17, respectively. These results suggested that the former suppressor alleles suppress the common functional defects in mig-17 and gon-1, whereas the latter alleles suppress gene-specific defects in either mig-17 or gon-1.
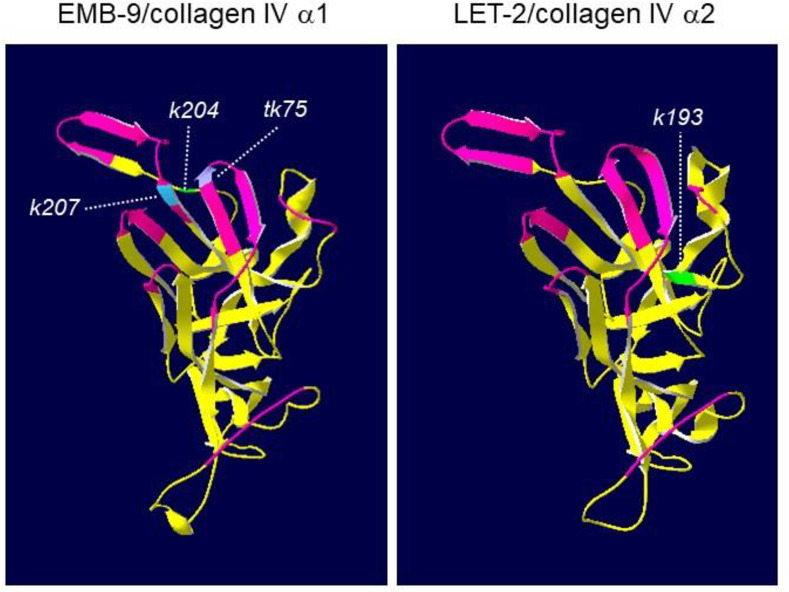
Three mutations found in EMB-9 (k204, k207 and tk75) and one in LET-2 (k193) were localized to the C-terminal NC1 domain of these collagen IV molecules. Using SWISS-MODEL, we deduced the three-dimensional structures of the NC1 domains of EMB-9 and LET-2 based on the crystal structures of bovine collagen IV NC1 domains [24] (Fig 7). We found that three amino acid substitutions in EMB-9, which are separated from one another in the primary sequence, were closely apposed in the three-dimensional structure. In the type IV collagen meshwork, triple-helical collagen molecules connect to one another through NC1-NC1 domain interactions. EMB-9(k207 and tk75) mutations were localized to the NC1-NC1 interface regions, and EMB-9(k204) was close to these interface regions, suggesting that these amino acid substitutions may affect physical interactions between two NC1 trimers. However, because these mutants were able to proliferate as homozygotes, their mutant collagen molecules were most likely successfully assembled into the functional network in the BM. It is interesting that the amino acid substitutions EMB-9(k204 and k207), which strongly suppressed gon-1 and mig-17, and EMB-9(tk75), which strongly enhanced gon-1 and mig-17, were closely localized in the three-dimensional structure.
Fig 7. Predicted three-dimensional structures of NC1 domains of C. elegans type IV collagen subunits EMB-9 and LET-2.
The segments corresponding to the interface region of two NC1 trimers are shown in magenta. The mutated amino acids in the k204, k207, tk75 and k193 mutations are highlighted each one in a different color.
Collagen IV accumulation in the DTC BM
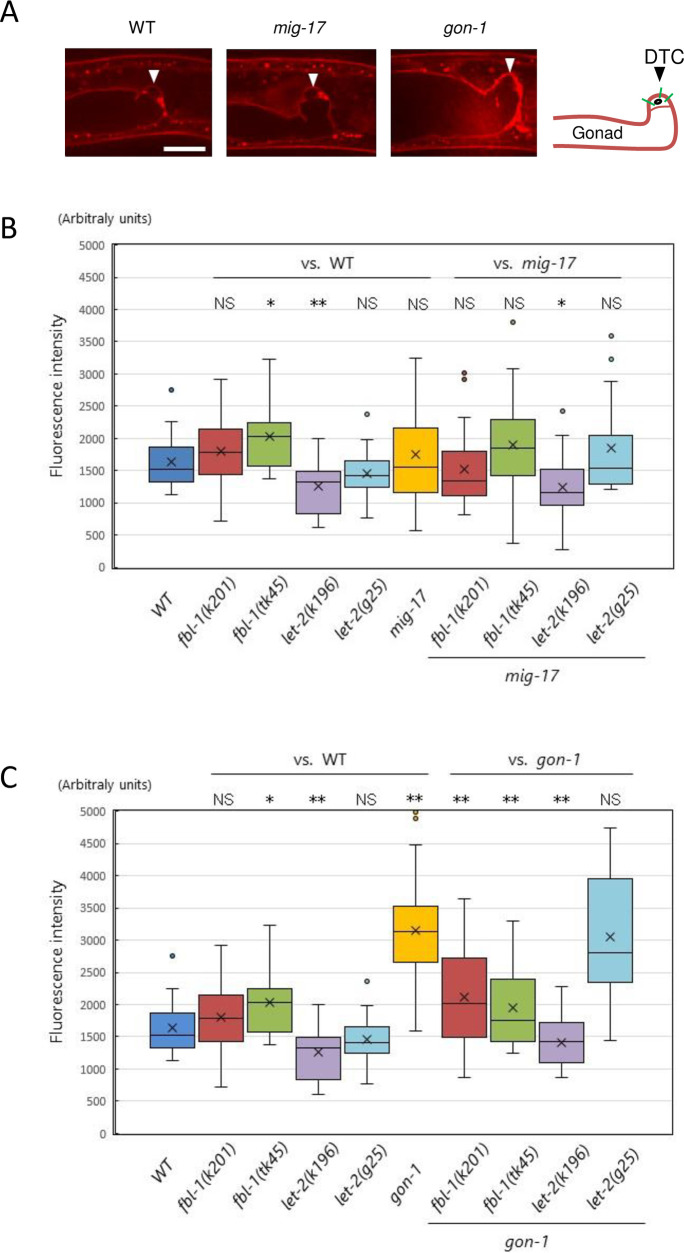
Based on an immunohistochemical analysis, we previously reported that the reduced accumulation of collagen IV in the gonadal BM in animals with fbl-1(tk45), a null mutation, can be compensated by gon-1(e1254) [14]. To examine the amount of collagen IV accumulation in the BM quantitatively, we used a functional emb-9::mCherry fusion reporter [18]. Third larval stage animals in which their DTCs were at or shortly beyond the first turn were selected, and the fluorescence intensity of the BM surrounding the DTCs was measured (Fig 8A–8C). Among the six single mutants examined, we observed that the intensity was slightly higher in fbl-1(tk45), significantly lower in let-2(k196) and significantly higher in gon-1(e1254) as compared with wild type. When combined with fbl-1(k201 and tk45) or let-2(k196 and g25) mutations, the level of EMB-9-mCherry accumulation in mig-17 mutants was not affected except for the case of let-2(k196), in which a slightly lower accumulation was observed (Fig 8B). Because the three mutations fbl-1(k201), let-2(k196) and let-2(g25) suppressed mig-17 but fbl-1(tk45) did not, the levels of EMB-9-mCherry accumulation were not correlated with mig-17 suppression. We then combined fbl-1(k201 and tk45) or let-2(k196 and g25) mutations with gon-1. We observed that the levels of EMB-9-mCherry accumulation in fbl-1(k201), fbl-1(tk45) and let-2(k196), all of which suppress gon-1, were significantly lower than that of the gon-1 single mutants, whereas the level was not affected in let-2(g25), which enhances gon-1 (Fig 8C). Thus, it is possible that the reduction in EMB-9-mCherry accumulation is indicative of gon-1 suppression.
Fig 8. Accumulation of EMB-9-mCherry in the BM.
A. Representative images of optical sections of the gonadal tip shortly after the first turn of the DTCs in wild-type, mig-17(k174) and gon-1(e1254) animals expressing EMB-9-mCherry. Arrowheads point to DTCs. Bar: 20 μm. The right panel illustrates the gonadal BM (brown). The fluorescence intensity of the DTC BM was quantified by averaging the peak values along three lines (green) that cross the DTC BM (see Materials and methods). B, C. Box-and-whisker plot of the fluorescence intensity of EMB-9-mCherry in the DTC BM in animals with wild-type and mutant fbl-1 and let-2 alleles and with those mutant alleles in combination with mig-17(k174) (B) or gon-1(e1254) (C); n = 20. Boxplots indicate the median and the interquartile range. Whiskers extend to the minimum and maximum values within 1.5 times the interquartile range. Points indicate outliers. P-values from Student’s t-test are indicated: **P < 0.01; *P < 0.05; NS, not significant.
Discussion
In the present study, we isolated novel suppressor mutations of gonadal defects related to mig-17 and gon-1 mutants. We identified alleles of emb-9 as mig-17 suppressors and an allele of let-2 as a gon-1 suppressor for the first time. We found that some of the emb-9 (collagen IV α1), let-2 (collagen IV α2) and fbl-1 (fibulin-1) mutations suppressed both gon-1 and mig-17, whereas others suppressed only gon-1 or mig-17. These results suggest that gon-1 and mig-17 have their specific functions as well as functions in common that relate to gonad formation. Probably, the loss of the gene-specific functions is the cause of the very different phenotypes of the gon-1 and mig-17 mutants.
We presented a diagram of expected mechanisms of the action for MIG-17 and GON-1 which will be discussed here (Fig 9). The fbl-1(tk45) null mutation suppressed gon-1 but enhanced mig-17. The gon-1 suppression is likely to be due to the reduction of collagen levels in the late larval stages that results from loss of fbl-1 activity [14], although the collagen levels in fbl-1(tk45) were not reduced in the mid-L3 stage when the DTCs make their first turn (Fig 8C). FBL-1 may inhibit the GON-1 activity to reduce collagen (Fig 9E). Alternatively, it may act to maintain collagen levels independently of GON-1. FBL-1C acts downstream of MIG-17 to recruit NID-1/nidogen-1 to regulate directed DTC migration [12]. Thus, mig-17 is enhanced probably because of the reduction of NID-1 in the DTC BM (Fig 9A–9D). In mig-17 mutants, the DTCs do not migrate along their normal U-shaped route because they often detach from the body wall (their normal migratory substratum) and mis-attach to the intestine [8]. Therefore, NID-1 is likely to be required for appropriate adhesiveness between the DTC and body wall BMs. The fbl-1(k201) gain-of-function mutation suppressed both mig-17 and gon-1. fbl-1(k201) suppressed the collagen accumulation in gon-1, as did fbl-1(tk45), but fbl-1(k201) is fully fertile on its own, unlike fbl-1(tk45) (S3 Fig). Therefore, it is possible that the fbl-1(k201) mutation may partially compromise the ability of FBL-1C to maintain collagen IV without affecting its ability to recruit NID-1.
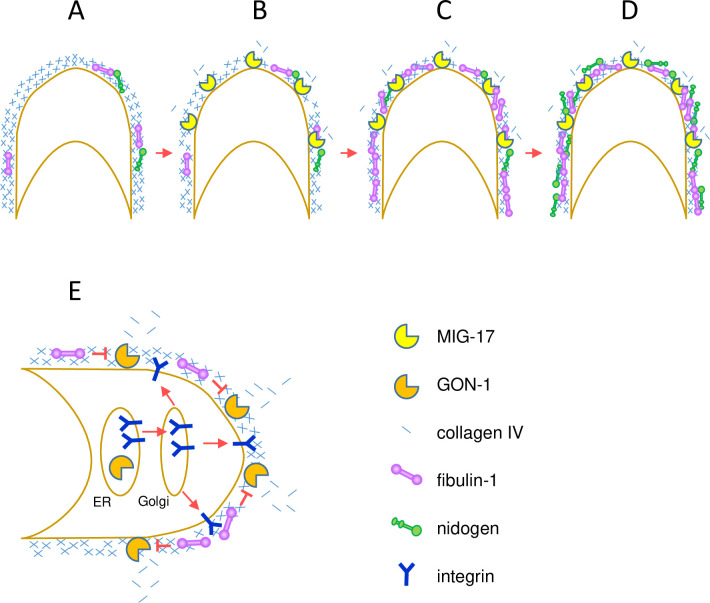
Fig 9. Models for the action of MIG-17 and GON-1 in DTC migration.
MIG-17 and GON-1 are postulated as collagenases in these models. Plasma membrane of DTCs is shown in brown. A-D. Model for MIG-17 action. MIG-17 localizes to and is activated in the gonadal BM shortly after the first turn of DTCs at the L3 stage [8, 25]. Although the levels of BM localized FBL-1 and NID-1 are low before the activation of MIG-17 (A), collagen degradation by MIG-17 promotes more FBL-1 accumulation (B, C). The BM localized FBL-1 then recruits NID-1, which facilitates directional DTC migration [12] (D). E. Model for GON-1 action. GON-1 likely localizes to the gonadal BM from the L1 stage because the null allele gon-1(q518) completely blocks DTC migration [26]. The GON-1 collagenase activity is inhibited by FBL-1 localized to the BM and controlled in appropriate levels. GON-1 also acts in the endoplasmic reticulum to transport integrin receptors from the endoplasmic reticulum to the Golgi, and therefore to the plasma membrane.
The gain-of-function mutations of collagen IV emb-9(k204, k207) and let-2(tk101, k196 and k193) were potent suppressors of mig-17 and gon-1. We found that the BM collagen levels in let-2(k196) were significantly decreased and that let-2(k196) suppressed the dramatic increase in collagen accumulation in gon-1 animals. Although we did not examine the other gain-of-function collagen mutations, it is possible that the levels of BM collagen are similarly affected. In contrast, the gain-of-function mutation emb-9(tk75) enhanced both mig-17 and gon-1. We previously showed that the levels of BM collagen in animals expressing the mutant EMB-9(tk75) α1 subunit can be maintained in the absence of FBL-1, which is otherwise required for the maintenance of BM collagen levels [14]. Therefore, it is likely that too much accumulation of collagen in the BM could be causative for both the mig-17 and gon-1 mutant phenotypes (Fig 9). Although we could not detect over-accumulation of collagen in the mig-17 mutant, this could be because of the insufficient sensitivity of our assay condition.
This model is not, however, consistent with the fact that the loss-of-function mutations of collagen IV, emb-9(g34 and g23cg46/+) and let-2(g25 and b246), which conceivably reduce the levels of BM collagen, all acted as enhancers of gon-1 even though they acted as suppressors of mig-17. The loss-of-function emb-9(xd51) mutation also enhances gon-1 in the presynaptic bouton overgrowth phenotype [27]. In contrast, emb-9(g34) and emb-9(b189) can suppress the synaptic defect of gon-1 when emb-9; gon-1 double mutants are shifted up from 16 to 25°C after completion of embryogenesis [28]. In this case, it is possible that collagen IV containing the temperature-sensitive mutant proteins EMB-9(g34) and EMB-9(b189) form intracellular aggregates and thus are not secreted [16], and therefore the BM accumulation of collagen could be considerably decreased. Our analysis using the EMB-9-mCherry reporter revealed that the collagen levels of let-2(g25); gon-1 or let-2(g25); mig-17 double mutants were not lowered relative to the respective gon-1 or mig-17 single mutant (Fig 8B and 8C). It might be possible that a subtle reduction in collagen accumulation is sufficient for amelioration of BM physiology in mig-17 mutants but instead worsens that in gon-1 mutants. The slight collagen reduction may promote NID-1 accumulation in the BM and suppress the mig-17 gonadal defect.
Why is gon-1 enhanced by collagen IV loss-of-function mutations? This seemingly contradictory phenomenon may be related to the dual function of GON-1. In addition to its predicted extracellular protease activity, GON-1 functions in the endoplasmic reticulum to transport secreted or membrane proteins from the endoplasmic reticulum to the Golgi. This transport activity is dependent on the C-terminal GON domain but is independent of protease activity [29]. For example, cell surface expression of the integrin receptors that are required for cell migration may be reduced and, therefore, the DTC migration activity in gon-1 mutants may be weakened (Fig 9E). The fact that RNAi knockdown of the integrin α subunit ina-1results in a short-arm gonad phenotype similar to that of gon-1 supports this possibility [30]. Because the remodeling of the BM is coupled with epithelial cell migration [31, 32], reduced migration of DTCs could also lead to downregulation of BM remodeling, resulting in thick accumulation of collagen, which can further block DTC migration. Remodeling of the BM is likely to be mediated by the GON-1 protease activity.
We previously showed that a reduction in collagen IV lowers PAT-3/β-integrin expression in DTCs [14]. A similar correlation between collagen IV and the integrin expression level has been observed in pancreatic β cells migration on Matrigel [33]. At the early second larval stage, the fluorescence levels of EMB-9-mCherry in gon-1 mutants were closer to those in the wild type (K.N., unpublished). Thus, it might be possible that when combined with loss-of-function collagen IV mutants, the reduced collagen levels may further impair integrin expression in DTCs of gon-1 animals in the second larval or younger stages. This might be the reason for the enhancement of the gon-1 phenotype in the presence of collagen IV loss-of-function mutations.
It is unclear why the gain-of-function collagen IV mutation let-2(k196) did not enhance gon-1, as did let-2(g25), even though let-2(k196) also reduced collagen accumulation. We speculate that GON-1 is the major enzyme responsible for turnover of BM collagen IV and that the mutant LET(k196) protein may confer the property by which the collagen IV meshwork is turned over quickly as compared with the wild-type meshwork even with the weakened activity of GON-1(e1254) mutant enzyme. If this is the case, the collagen meshwork containing EMB-9(tk75) may have gained a slower turnover rate.
Our observations suggest that both MIG-17 and GON-1 function to reduce collagen IV in the BM. Although this is consistent with the idea that collagen IV is the direct substrate of these enzymes, we still do not have evidence of this interaction. Future biochemical analysis is thus needed to determine the substrates. In addition, molecular structural analysis of interactions among these ADAMTS proteases and the BM molecules should shed light on the detailed mechanism of BM remodeling during organogenesis.
Supporting information
Domain organization is shown by colored boxes. Mutation sites for gon-1(q518 and e1254) and mig-17(k174) are indicated.
(TIF)
Gonad arms (arrows) of young adult hermaphrodites are shown. A, B. Nomarski images of wild type (A) and gon-1(e1254) (B). C, D. Nomarski (C) and fluorescence (D) images of a gon-1(e1254); tkEx370[gon-1 fosmid, rol-6(su1006), sur-5::GFP] young adult hermaphrodite. E, F. Nomarski (E) and fluorescence (F) images of a let-2(k101); gon-1(e1254) young adult hermaphrodite, which lost the tkEx370[gon-1 fosmid, rol-6(su1006), sur-5::GFP] extrachromosomal array. Bar: 20 μm.
(TIF)
For each strain, 20 first larval stage hermaphrodites were grown at 20°C, and the number of animals that produced offspring were counted. Green and red bars represent % fertile and % sterile animals, respectively.
(TIF)
Acknowledgments
We thank Noriko Nakagawa and Nami Okahashi for technical assistance. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Grant-in-Aid for Scientific Research on Innovative Areas by Ministry of Education, Culture, Sports, Science and Technology to KN (22111005), https://www.mext.go.jp/en/index.htm. The Naito Grant for the advancement of natural science to KN, https://www.naito-f.or.jp/jp/joseikn/jo_index.php?data=about. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284(46):31493–7. 10.1074/jbc.R109.052340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol. 2018;71–72:225–39. 10.1016/j.matbio.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, et al. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17(5):687–98. 10.1016/j.devcel.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandadasa S, Kraft CM, Wang LW, O'Donnell A, Patel R, Gee HY, et al. Secreted metalloproteases ADAMTS9 and ADAMTS20 have a non-canonical role in ciliary vesicle growth during ciliogenesis. Nat Commun. 2019;10(1):953 10.1038/s41467-019-08520-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupka N, Kintakas C, White JD, Fraser FW, Hanciu M, Aramaki-Hattori N, et al. Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and -15 facilitates myoblast fusion. J Biol Chem. 2013;288(3):1907–17. 10.1074/jbc.M112.429647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399(6736):586–90. 10.1038/21196 . [DOI] [PubMed] [Google Scholar]
- 8.Nishiwaki K, Hisamoto N, Matsumoto K. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science. 2000;288(5474):2205–8. 10.1126/science.288.5474.2205 . [DOI] [PubMed] [Google Scholar]
- 9.Ihara S, Nishiwaki K. Prodomain-dependent tissue targeting of an ADAMTS protease controls cell migration in Caenorhabditis elegans. EMBO J. 2007;26(11):2607–20. 10.1038/sj.emboj.7601718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keeley DP, Hastie E, Jayadev R, Kelley LC, Chi Q, Payne SG, et al. Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement within the Matrix Scaffolding. Dev Cell. 2020;54(1):60–74 e7. 10.1016/j.devcel.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota Y, Kuroki R, Nishiwaki K. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr Biol. 2004;14(22):2011–8. 10.1016/j.cub.2004.10.047 . [DOI] [PubMed] [Google Scholar]
- 12.Kubota Y, Ohkura K, Tamai KK, Nagata K, Nishiwaki K. MIG-17/ADAMTS controls cell migration by recruiting nidogen to the basement membrane in C. elegans. Proc Natl Acad Sci U S A. 2008;105(52):20804–9. 10.1073/pnas.0804055106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesselson D, Newman C, Kim KW, Kimble J. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr Biol. 2004;14(22):2005–10. 10.1016/j.cub.2004.11.006 . [DOI] [PubMed] [Google Scholar]
- 14.Kubota Y, Nagata K, Sugimoto A, Nishiwaki K. Tissue architecture in the Caenorhabditis elegans gonad depends on interactions among fibulin-1, type IV collagen and the ADAMTS extracellular protease. Genetics. 2012;190(4):1379–88. 10.1534/genetics.111.133173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta MC, Graham PL, Kramer JM. Characterization of α1(IV) collagen mutations in Caenorhabditis elegans and the effects of α1 and α2(IV) mutations on type IV collagen distribution. J Cell Biol. 1997;137(5):1185–96. 10.1083/jcb.137.5.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sibley MH, Graham PL, von Mende N, Kramer JM. Mutations in the α2(IV) basement membrane collagen gene of Caenorhabditis elegans produce phenotypes of differing severities. EMBO J. 1994;13(14):3278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Kitano Y, Mori M, Takano T, Harbaugh TE, Mizutani K, et al. The novel secreted factor MIG-18 acts with MIG-17/ADAMTS to control cell migration in Caenorhabditis elegans. Genetics. 2014;196(2):471–9. 10.1534/genetics.113.157685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet. 2001;28(2):160–4. 10.1038/88878 . [DOI] [PubMed] [Google Scholar]
- 20.Nishiwaki K. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics. 1999;152(3):985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10(12):3959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maduro M, Pilgrim D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 1995;141(3):977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yochem J, Gu T, Han M. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics. 1998;149(3):1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundaramoorthy M, Meiyappan M, Todd P, Hudson BG. Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J Biol Chem. 2002;277(34):31142–53. 10.1074/jbc.M201740200 . [DOI] [PubMed] [Google Scholar]
- 25.Ihara S, Nishiwaki K. Stage-specific activation of MIG-17/ADAMTS controls cell migration in Caenorhabditis elegans. FEBS J. 2008;275(17):4296–305. 10.1111/j.1742-4658.2008.06573.x . [DOI] [PubMed] [Google Scholar]
- 26.Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev Biol. 1999;216(1):382–93. 10.1006/dbio.1999.9491 . [DOI] [PubMed] [Google Scholar]
- 27.Qin J, Liang J, Ding M. Perlecan antagonizes collagen IV and ADAMTS9/GON-1 in restricting the growth of presynaptic boutons. J Neurosci. 2014;34(31):10311–24. 10.1523/JNEUROSCI.5128-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurshan PT, Phan AQ, Wang GJ, Crane MM, Lu H, Shen K. Regulation of synaptic extracellular matrix composition is critical for proper synapse morphology. J Neurosci. 2014;34(38):12678–89. 10.1523/JNEUROSCI.1183-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshina S, Sakaki K, Yonezumi-Hayashi A, Gengyo-Ando K, Inoue H, Iino Y, et al. Identification of a novel ADAMTS9/GON-1 function for protein transport from the ER to the Golgi. Mol Biol Cell. 2012;23(9):1728–41. 10.1091/mbc.E11-10-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meighan CM, Schwarzbauer JE. Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev. 2007;21(13):1615–20. 10.1101/gad.1534807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada KM, Collins JW, Cruz Walma DA, Doyle AD, Morales SG, Lu J, et al. Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int J Exp Pathol. 2019;100(3):144–52. 10.1111/iep.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrissey MA, Sherwood DR. An active role for basement membrane assembly and modification in tissue sculpting. J Cell Sci. 2015;128(9):1661–8. 10.1242/jcs.168021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor α1β1. J Biol Chem. 2004;279(51):53762–9. 10.1074/jbc.M411202200 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Domain organization is shown by colored boxes. Mutation sites for gon-1(q518 and e1254) and mig-17(k174) are indicated.
(TIF)
Gonad arms (arrows) of young adult hermaphrodites are shown. A, B. Nomarski images of wild type (A) and gon-1(e1254) (B). C, D. Nomarski (C) and fluorescence (D) images of a gon-1(e1254); tkEx370[gon-1 fosmid, rol-6(su1006), sur-5::GFP] young adult hermaphrodite. E, F. Nomarski (E) and fluorescence (F) images of a let-2(k101); gon-1(e1254) young adult hermaphrodite, which lost the tkEx370[gon-1 fosmid, rol-6(su1006), sur-5::GFP] extrachromosomal array. Bar: 20 μm.
(TIF)
For each strain, 20 first larval stage hermaphrodites were grown at 20°C, and the number of animals that produced offspring were counted. Green and red bars represent % fertile and % sterile animals, respectively.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.