Abstract
Hepatocellular carcinoma (HCC) is a malignant tumor associated with a high recurrence rate after hepatectomy. Recently, preoperative inflammatory and liver function reserve indices were found to predict increased risk of recurrence and decreased survival in HCC patients. This study aims to evaluate the ability of the γ-glutamyl transpeptidase-to-albumin ratio (GAR) and aspartate aminotransferase-to-lymphocyte ratio (ALRI), individually and in combination, to predict the prognosis of HCC patients after hepatectomy.
We retrospectively reviewed 206 HCC patients who underwent radical resection at the General Hospital of Ningxia Medical University from January 2011 to November 2016. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value for GAR and ALRI. The Pearson Chi-Squared test was used to analyze the correlations between GAR, ALRI and clinicopathological characteristics. Univariate and multivariate analyses were used to determine the predictive value of these factors for disease-free survival (DFS) and overall survival (OS). Survival rates were drawn according to the Kaplan-Meier method and differences between subgroups were compared by the log-rank statistics.
GAR and ALRI were significantly correlated with gender, history of smoking, prothrombin time, tumor diameter, T stage and early intrahepatic recurrence by the Pearson Chi-Squared test (all P < .05). Univariate analysis indicated that T stage, GAR and ALRI were significantly correlated with DFS and OS in HCC patients after hepatectomy. Multivariate analysis illustrated that GAR and ALRI were independently related to DFS and OS in HCC patients. Preoperative GAR > 0.946 or ALRI > 18.734 predicted poor prognosis in HCC patients after hepatectomy. Additionally, the predictive scope of GAR combined with ALRI was more sensitive than that of either individual measurement alone.
Our data indicate that there is a close association between the clinicopathological characteristics in HCC patients and increased GAR or ALRI. Higher levels of GAR and ALRI could sensitively and specifically predict a poor prognosis in HCC patients after hepatectomy. Furthermore, combined usage of GAR and ALRI could improve the accuracy of this prediction.
Keywords: hepatocellular carcinoma, γ-glutamyl transpeptidase-to-albumin ratio, aspartate aminotransferase-to-lymphocyte ratio, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common and lethal primary malignant tumors worldwide, with increasing incidence.[1,2] Liver resection remains the curative option for HCC with a 5-year overall survival (OS) rate of 50% to 70% after curative hepatectomy; however, the postoperative recurrence rate remains as high as 40% to 70% within 5 years.[3] Most patients die from recurrence or distant metastases. In China, most HCC cases were reported to be accompanied by chronic liver disease, cirrhosis, or were in an advanced stage at the time of diagnosis. Although the tumor lymph node metastasis (TNM) staging system is an effective independent prognostic factor for HCC, its prognostic value is limited and lagging.
During early stages of tumorigenesis, inflammation can induce tumor cell proliferation, invasion, metastasis, cell survival and angiogenesis. Inflammation can also induce tumoros epithelial-mesenchymal transition (EMT) or produce chemicals represented by reactive oxygen species (ROS), thereby further triggering tumor invasion and metastasis.[4,5] The γ-glutamyl transpeptidase (γ-GGT), which mediates the hydrolysis of extracellular glutathione in a reaction that produces a large amount of hydrogen peroxide and ROS, leads to an oxidative stress response in tissues in vivo.[6] The development of liver disease, diabetes, coronary heart disease, and chronic obstructive pulmonary diseases have been closely related to γ-GGT.[7–10] The γ-GGT is distributed in solid organs such as the kidney, liver and pancreas. Serum γ-GGT, in healthy adults, is mainly secreted by hepatic Kupffer cells and bile duct endothelial cells.[7] In addition, high levels of serum γ-GGT have been reported to indicate poor prognosis in severe hepatitis, cirrhosis and advanced tumors.[11]
Albumin (ALB) is the most significant human protein and is synthesized in liver cells. ALB is involved in maintaining osmotic pressure, possesses anti-oxidative effects, inhibits platelet aggregation, affects arterial permeability and participates in inflammatory responses. These functions reflect liver synthesis, reserve capacity and nutritional status.[12] In particular, some ratios based on the ALB index have been identified as independent prognostic factors for HCC patients. Studies showed that elevated CRP/ALB values were associated with tumor progression and decreased liver function reserve.[13]
Aspartate aminotransferase (AST) is mainly found in hepatocyte mitochondria. The inflammatory response can increase the permeability of the hepatocyte membrane, leading to the release of cytoplasmic (c)-AST into the blood, which causes an increase in serum AST. This increase in AST has been positively correlated with the degree of liver cell damage, which is a reliable and sensitive hallmark of liver damage.[14,15] Lymphocytes were crucial cellular components of the immune response and participate in the anti-tumor immune process. Increased tumor lymphocyte infiltration has been associated with improved patient prognosis.[16,17]
Based on preoperative laboratory examination, we established the serum γ-glutamyl transpeptidase-to-albumin ratio (GAR) and aspartate aminotransferase-to-lymphocyte ratio (ALRI) as markers of HCC. The purpose of this study was to investigate the predictive value of GAR and ALRI on the survival rate of HCC patients who underwent radical resection. We further evaluated whether the combined application of GAR and ALRI could enhance prognostic accuracy.
2. Methods
2.1. Study population
A total of 206 patients diagnosed with HCC at the General Hospital of Ningxia Medical University, Ningxia, China, from January 2011 to November 2016 were included in this study, which was approved by the ethics committee of the General Hospital of Ningxia Medical University. Additionally, this study was in compliance with the ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all patients according to the policies of the ethics committee.
Patients who met the following criteria were included in this study: (a) confirmation of HCC by pathology; (b) no history of transcatheter arterial embolization, chemotherapy or radiotherapy; and (c) no history of other malignancies. Patients who exhibited the following characteristics were excluded from this study: (a) pathologically proven benign liver disease, intrahepatic cholangiocarcinoma (ICC) or secondary (metastatic) hepatocellular carcinoma; (b) gastrointestinal disorder with malnourishment; (c) acute or chronic infection within 2 weeks of admission; (d) occurrence of cerebrovascular accident, surgical history, pulmonary embolism, or rheumatoid disease within 1 month; and (e) incomplete follow-up data.
We used the hospitals electronic medical record management system to collect general information, clinical characteristics and laboratory data.
-
1.
General information included age, gender, and past history.
-
2.
Clinical characteristics included cirrhosis status, ascites status, hepatic capsule invasion, tumor diameter, TNM stage, and intrahepatic recurrence within 1 year after hepatectomy.
-
3.
Laboratory data included lymphocyte count, platelet count, prothrombin time (PT), γ-glutamyl transpeptidase (GGT), alpha-fetoprotein (AFP) and albumin (ALB) levels within 1 week before surgery.
A positive history of drinking was defined as drinking at least once per week for at least 1 year (male ≥40 g/day; female ≥20 g/day).[18] A positive history of smoking was defined as cigarette smoking for ≥4 days per week for at least 5 years.[19] Clinical staging was assigned according to the American Joint Committee on Cancer (AJCC) version 8 TNM staging system and determined through clinical evaluation and postoperative pathological examination. Blood samples for laboratory examination were collected within 7 days before surgery to determine lymphocyte count, platelet count, prothrombin time (PT), γ-glutamyl transpeptidase (γ-GGT), alpha-fetoprotein (AFP) and albumin levels. GAR was calculated as the γ-glutamyl transpeptidase level divided by the albumin level. ALRI was calculated as the aspartate aminotransferase level divided by the lymphocyte level.
2.2. Treatment and follow-up
Hepatectomy was performed when no evidence of distant metastases was found and the tumor was deemed resectable. Patients were underwent regular follow-up for 3 months for the first year after surgery, and every 6 months for the subsequent 2 to 3 years. Enhanced abdominal computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed generally every 3 months. According to imaging data from enhanced abdominal CT or MRI, new lesions on the liver or new metastatic lesions found in other systems were considered as criteria of recurrence in HCC patients. We defined early intrahepatic recurrence as the discovery of new liver lesions within 1 year after hepatectomy. The methods of follow-up included outpatient review and telephone interview. All follow-up visits were completed by November 30, 2019. Overall survival time was calculated from the time of surgery to death, or the last follow-up date.
2.3. Statistical analysis
Receiver operating characteristic (ROC) curve analysis was performed based on the patient survival status to select the most appropriate cut-off values for GAR and ALRI and to group patients at a high risk of death. The Pearson Chi-Squared test was used to analyze the correlations between GAR, ALRI and clinicopathological variables. Disease-free survival (DFS) was calculated from the date of operation to the date of first recurrence or mortality. Overall survival (OS) was calculated as the period from the date of surgery to the date of death, or the last follow-up date. The Student t-test (normal distribution data) and Mann–Whitney U test (non-normal distribution data) were used to compare preoperative GAR and ALRI levels in different HCC subgroups. Univariate and multivariate analyses were performed using Cox proportional hazard regression models. Survival rates in different groups were estimated by the Kaplan–Meier method, and the equivalences of the survival curves were tested by log-rank statistics. All statistical analyses were carried out using SPSS (SPSS for Mac, version 22, IBM Corporation). P values less than .05 were considered statistically significant.
3. Results
3.1. Patient and tumor characteristics
This study included 160 male (77.67%) and 46 female patients (22.33%). The average age was 53 years (range 23-82 years). During the follow-up, 110 patients (53.40%) were diagnosed with early intrahepatic recurrence and 130 patients (63.11%) had died. There were 180 cases (87.38%) of positivity for hepatitis B surface antigen (HBsAg), 178 cases (86.41%) of liver cirrhosis, 117 cases (56.80%) of elevated alpha-fetoprotein (>20 ng/ml), 128 cases (62.14%) in which the tumor diameter was less than 5 cm, and 13 cases (6.31%) of lymph node metastasis. According to the 8th edition of the AJCC for TNM staging, 118 cases (57.28%) were in T stage I-II and 88 cases (42.72%) were in T stage III-IV (Tables 1 and 2).
Table 1.
Relationship between GAR, ALRI, and general clinical data of HCC patients.
| GAR | ALRI | ||||||
| Variables | Cases | GAR ≤ 0.946 | GAR > 0.946 | P value | ALRI ≤ 18.734 | ALRI > 18.734 | P value |
| Gender | |||||||
| Male | 160 | 43 (61.43) | 117 (86.03) | <.001 | 62 (70.45) | 98 (83.05) | .032 |
| Female | 46 | 27 (38.57) | 19 (13.97) | 26 (29.55) | 20 (16.95) | ||
| Age (y) | |||||||
| ≤60 | 163 | 51 (72.86) | 112 (82.35) | .112 | 69 (78.41) | 94 (79.66) | .827 |
| >60 | 43 | 19 (27.14) | 24 (17.65) | 19 (21.59) | 24 (20.34) | ||
| Smoking | |||||||
| No | 128 | 52 (74.29) | 76 (55.88) | .010 | 60 (68.18) | 68 (57.63) | <.001 |
| Yes | 78 | 18 (25.71) | 60 (44.12) | 28 (31.82) | 50 (42.37) | ||
| Drinking | |||||||
| No | 159 | 58 (82.86) | 101 (74.26) | .164 | 70 (79.55) | 89 (75.42) | .486 |
| Yes | 47 | 12 (17.14) | 35 (25.74) | 18 (20.45) | 29 (24.58) | ||
| HBsAg | |||||||
| Negative | 26 | 7 (10.00) | 19 (13.97) | .416 | 13 (14.78) | 13 (11.02) | .422 |
| Positive | 180 | 63 (90.00) | 117 (86.03) | 75 (85.23) | 105 (88.98) | ||
| AFP (ng/ml) | |||||||
| ≤20 | 89 | 24 (34.29) | 65 (47.79) | .064 | 41 (46.59) | 48 (40.68) | .397 |
| >20 | 117 | 46 (65.71) | 71 (52.21) | 47 (53.41) | 70 (59.32) | ||
| PT (s) | |||||||
| ≤14 | 184 | 67 (95.71) | 117 (86.03) | .033 | 83 (94.32) | 101 (85.59) | .045 |
| >14 | 22 | 3 (4.29) | 19 (13.97) | 5 (5.68) | 17 (14.41) | ||
Table 2.
Relationship between GAR, ALRI, and clinicopathological features of HCC patients.
| GAR | ALRI | ||||||
| Variables | Cases | GAR ≤ 0.946 | GAR > 0.946 | P value | ALRI ≤ 18.734 | ALRI > 18.734 | P value |
| Cirrhosis | |||||||
| No | 28 | 9 (12.86) | 19 (13.97) | .825 | 17 (19.32) | 11 (9.32) | .038 |
| Yes | 178 | 61 (87.14) | 117 (86.03) | 71 (80.68) | 107 (90.68) | ||
| Tumor diameter (cm) | |||||||
| ≤5 | 128 | 56 (80.00) | 72 (52.94) | <.001 | 66 (75.00) | 62 (52.54) | .001 |
| >5 | 78 | 14 (20.00) | 64 (47.06) | 22 (25.00) | 56 (47.46) | ||
| Hepatic capsule | |||||||
| No | 103 | 33 (47.14) | 70 (51.47) | .556 | 49 (55.68) | 54 (45.76) | .159 |
| Yes | 103 | 37 (52.86) | 66 (48.53) | 39 (44.32) | 64 (54.24) | ||
| T stage | |||||||
| I–II | 118 | 54 (77.14) | 64 (47.06) | <.001 | 67 (76.14) | 51 (43.22) | <.001 |
| III-IV | 88 | 16 (22.86) | 72 (52.94) | 21 (23.86) | 67 (56.78) | ||
| Lymph node metastasis | |||||||
| N0 | 193 | 66 (94.29) | 127 (93.38) | .801 | 86 (97.73) | 107 (90.68) | .040 |
| N1/NX | 13 | 4 (5.71) | 9 (6.62) | 2 (2.27) | 11 (9.32) | ||
| Intrahepatic recurrence | |||||||
| No | 96 | 42 (60.00) | 54 (39.71) | .006 | 48 (54.55) | 48 (40.68) | .048 |
| Yes | 110 | 28 (40.00) | 82 (60.29) | 40 (45.45) | 70 (59.32) | ||
3.2. Determination of the cut-off value for GAR and ALRI
Using patient survival status as an endpoint, stratification of GAR and ALRI was calculated by ROC curve analyses in accordance with maximum joint sensitivity and specificity values based on the cut-off points. Our results indicated that the optimal cut-off values for GAR and ALRI were 0.946 and 18.734, respectively (Figure 1). The area under the ROC curve of GAR was 0.592 (95% CI: 0.509–0.675, P = .028). The area under the ROC curve of ALRI was 0.624 (95% CI: 0.543–0.704, P = .003).
Figure 1.
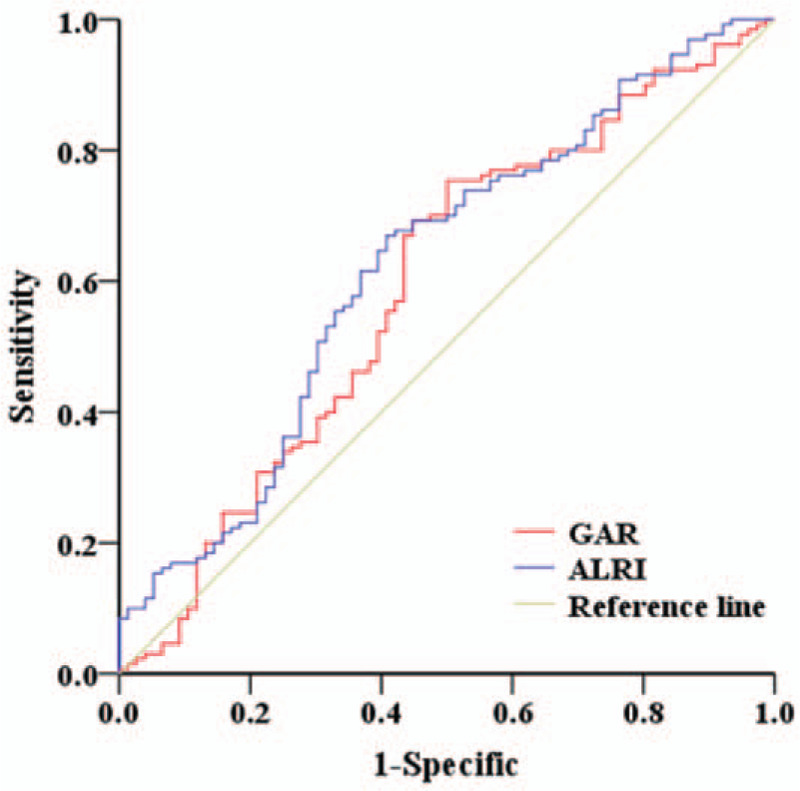
Determination of the GAR and ALRI cut-off values in HCC patients who underwent radical resection.
3.3. The relationship between GAR, ALRI, and clinicopathologic factors of HCC patients.
We divided patients into different groups according to the cut-off values for GAR and ALRI, namely the low GAR group (≤0.946, n = 70), high GAR group (>0.946, n = 136), low ALRI group (≤18.734, n = 88) and high ALRI group (>18.734, n = 118). The relationship between GAR, ALRI, and other clinicopathologic factors of HCC patients are shown in Tables 1 and 2. High GAR was associated with gender (P < .001), smoking (P = .010), prothrombin time (P = .033), tumor diameter (P < .001), T stage (P < .001) and early intrahepatic recurrence (P = .006). A high ALRI ratio was associated with gender (P = .032), smoking (P < .001), cirrhosis (P = .038), prothrombin time (P = .045), tumor diameter (P = .001), T stage (P = .001), lymph node metastasis (P = .040) and early intrahepatic recurrence (P = .048).
3.4. Comparison between preoperative GAR and ALRI levels in different HCC subgroups.
Several factors, such as AFP level, tumor diameter, hepatic capsule invasion and tumor stage, have been established as prognostic indicators for HCC patients. Therefore, we grouped the HCC patients according to the aforementioned clinicopathological features and compared the preoperative level of GAR and ALRI ratio across the subgroups. The preoperative GAR and ALRI levels were higher in HCC patients that exhibited an AFP level greater than 20 ng/ml, a tumor diameter greater than 5 cm, and an advanced T stage (all P < .05, Figure 2). HCC patients with T stage III to IV had significantly higher preoperative GAR and ALRI levels than those with T stage I to II (all P < .05, Figure 2). According to our research, this observed increase in preoperative GAR and ALRI ratio may be related to the aggressiveness and progression of HCC.
Figure 2.
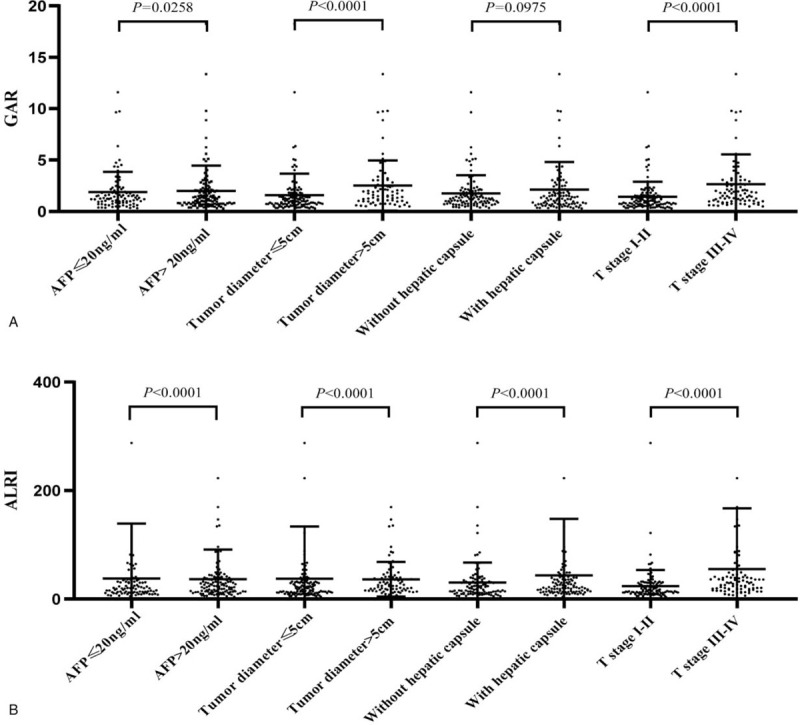
Comparisons of GAR and ALRI ratio between different prognostic indicators of HCC.
3.5. GAR and ALRI are independent prognostic factors for HCC patients.
Univariate analysis and the Cox regression model were used to evaluate the risk factors linked to postoperative DFS and OS. This analysis showed that tumor diameter, T stage, lymph node metastasis, GAR, and ALRI were poor prognostic factors for DFS in HCC patients (all P < .05). Similarly, the significant factors for OS in HCC patients were T stage, GAR, and ALRI (all P < .05) (Table 3).
Table 3.
Univariate analysis reveals prognostic factors for DFS and OS.
| DFS | OS | ||||||
| Variables | Cases | 1-year | 3-years | P value | 1-year | 3-years | P value |
| Gender | |||||||
| Male | 160 | 46.88% | 21.25% | .084 | 76.88% | 49.38% | .161 |
| Female | 46 | 60.87% | 30.43% | 80.43% | 52.17% | ||
| Age (y) | |||||||
| ≤60 | 163 | 52.76% | 25.77% | .361 | 77.30% | 50.92% | .628 |
| >60 | 43 | 39.53% | 13.95% | 65.12% | 46.51% | ||
| Smoking | |||||||
| No | 128 | 50.78% | 25.00% | .265 | 80.78% | 51.22% | .682 |
| Yes | 78 | 48.72% | 20.51% | 75.77% | 59.28% | ||
| Drinking | |||||||
| No | 159 | 52.04% | 24.27% | .917 | 78.62% | 55.43% | .638 |
| Yes | 47 | 51.06% | 23.40% | 74.47% | 48.32% | ||
| HBsAg | |||||||
| Negative | 26 | 69.23% | 30.77% | .232 | 84.62% | 61.54% | .099 |
| Positive | 180 | 50.56% | 24.44% | 78.33% | 53.33% | ||
| AFP (ng/ml) | |||||||
| ≤20 | 89 | 48.31% | 17.98% | .056 | 82.02% | 43.82% | 0.212 |
| >20 | 117 | 51.28% | 27.35% | 74.36% | 54.70% | ||
| PT (s) | |||||||
| ≤14 | 184 | 51.63% | 24.46% | .215 | 79.89% | 52.17% | .220 |
| >14 | 22 | 36.36% | 13.64% | 59.09% | 31.82% | ||
| Cirrhosis | |||||||
| No | 28 | 64.29% | 28.57% | .437 | 82.14% | 53.57% | .847 |
| Yes | 178 | 47.75% | 22.47% | 76.97% | 51.12% | ||
| Tumor diameter (cm) | |||||||
| ≤5 | 128 | 56.25% | 26.56% | .032 | 82.81% | 54.69% | 0.080 |
| >5 | 78 | 39.74% | 17.95% | 69.23% | 42.31% | ||
| Hepatic capsule | |||||||
| No | 103 | 51.46% | 25.24% | .158 | 83.50% | 54.37% | .056 |
| Yes | 103 | 48.54% | 21.36% | 71.84% | 45.63% | ||
| T stage | |||||||
| I-II | 118 | 60.17% | 28.81% | .001 | 87.29% | 58.47% | .007 |
| III-IV | 88 | 36.36% | 15.91% | 64.77% | 38.64% | ||
| Lymph node metastasis | |||||||
| N0 | 193 | 51.81% | 23.83% | .032 | 78.24% | 50.78% | .096 |
| N1/NX | 13 | 23.08% | 15.38% | 69.23% | 38.46% | ||
| GAR | |||||||
| ≤0.946 | 70 | 67.14% | 28.57% | <.001 | 91.43% | 70.00% | <.001 |
| >0.946 | 136 | 41.18% | 20.59% | 70.59% | 39.71% | ||
| ALRI | |||||||
| ≤18.734 | 88 | 59.09% | 29.55% | .001 | 89.77% | 63.64% | <.001 |
| >18.734 | 118 | 43.22% | 18.64% | 68.64% | 39.83% | ||
Multivariate Cox regression analysis was employed to investigate whether GAR and ALRI were independent, significant prognostic factors for HCC patients (Table 2). Model 1, which included gender, age, presence/absence of smoking, presence/absence of drinking, negative/positive for HBsAg, AFP, PT, presence/absence of cirrhosis, tumor diameter, hepatic capsule invasion, T stage, lymph node metastasis, and GAR, showed that GAR was a significant, independent factor associated with the postoperative survival of HCC patients (HR = 2.023, 95% CI: 1.301–3.145, P = .002). Model 2, in which GAR was substituted with ALRI, demonstrated that ALRI was significantly associated with the postoperative survival of HCC patients (HR = 1.845, 95% CI: 1.248–2.729, P = .002). In addition, when GAR and ALRI were simultaneously included as independent variables in Model 3, the addition of ALRI did not affect the significant association of GAR. Multivariate analysis showed that GAR (HR = 1.721, 95% CI: 1.087–2.727, P = .021) and ALRI (HR = 1.571, 95% CI: 1.043–2.368, P = .031) were independent adverse prognostic factors in HCC patients (Table 4).
Table 4.
Independent prognostic factors for OS according to multivariate Cox regression analysis.
| Model 1 | Model 2 | Model 3 | |||||||
| Variables | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI |
| Gender | .806 | 0.937 | 0.559–1.572 | .398 | 0.801 | 0.478–1.340 | .669 | 0.893 | 0.530–1.504 |
| Age (y) | .399 | 1.212 | 0.775–1.896 | .706 | 1.088 | 0.701–1.690 | .476 | 1.176 | 0.754–1.834 |
| Smoking | .789 | 0.944 | 0.617–1.443 | .872 | 1.035 | 0.678–1.581 | .812 | 0.950 | 0.621–1.453 |
| Drinking | .290 | 0.779 | 0.490–1.238 | .342 | 0.796 | 0.499–1.273 | .348 | 0.800 | 0.501–1.276 |
| HBsAg | .342 | 0.769 | 0.448–1.322 | .158 | 0.677 | 0.394–1.164 | .198 | 0.701 | 0.408–1.204 |
| AFP (ng/ml) | .322 | 0.830 | 0.573–1.200 | .187 | 0.779 | 0.537–1.129 | .246 | 0.803 | 0.554–1.163 |
| PT (s) | .825 | 1.067 | 0.600–1.898 | .982 | 0.993 | 0.542–1.820 | .957 | 0.984 | 0.547–1.771 |
| Cirrhosis | .561 | 1.183 | 0.671–2.087 | .981 | 0.993 | 0.567–1.739 | .790 | 1.080 | 0.612–1.908 |
| Tumor diameter (cm) | .371 | 0.745 | 0.391–1.419 | .431 | 0.771 | 0.403–1.473 | .375 | 0.746 | 0.390–1.426 |
| Hepatic capsule | .076 | 1.393 | 0.966–2.009 | .191 | 1.276 | 0.886–1.836 | .094 | 1.370 | 0.947–1.980 |
| T stage | .144 | 1.616 | 0.849–3.076 | .187 | 1.538 | 0.811–2.917 | .237 | 1.477 | 0.774–2.816 |
| Lymph node metastasis | .223 | 1.509 | 0.779–2.925 | .269 | 1.449 | 0.750–2.799 | .237 | 1.487 | 0.770–2.871 |
| GAR | .002 | 2.023 | 1.301–3.145 | .021 | 1.721 | 1.087–2.727 | |||
| ALRI | .002 | 1.845 | 1.248–2.729 | .031 | 1.571 | 1.043–2.368 | |||
3.6. Prognostic values of GAR and ALRI for HCC patients
The overall survival rate (OS) of 206 patients was 1 to 100 months, and the median survival time was 35 months. Our results showed that high GAR and ALRI ratio were significant prognostic indicators of poor OS and disease-free survival (DFS) in HCC patients. The median OS and DFS of patients with high GAR were shorter than patients with low GAR (24 months vs 60 months; 10 months vs 30 months; P < .001). The 1- and 3-year OS rates in the high GAR group were significantly lower than those in the low GAR group (70.59% and 39.71% vs 91.43% and 65.71%, P < .001; Fig. 3A). The 1- and 3-year DFS rates in the high GAR group were significantly lower than those in the low GAR group (39.71% and 19.12% vs 67.14% and 27.14%, P < .001; Fig. 3C).
Figure 3.
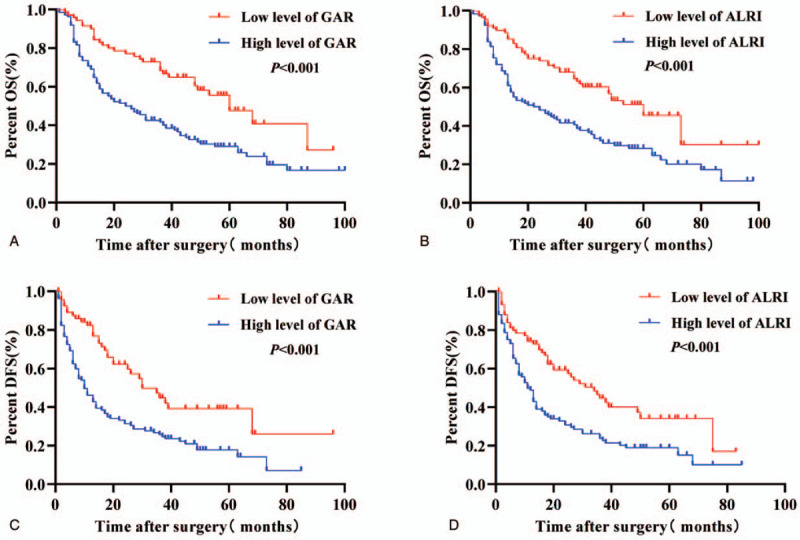
Kaplan-Meier curves for overall survival (OS) and disease-free survival (DFS) according to GAR or ALRI in HCC patients. (A, B) Patients with low GAR or ALRI ratio were associated with significantly better OS compared to patients with high GAR or ALRI ratio (P < .001, log-rank test). (C, D) Patients with low GAR or ALRI were associated with significantly better DFS compared to patients with high GAR or ALRI (P < .001, log-rank test).
Furthermore, the median OS and DFS of patients in the high ALRI group were shorter than patients in the low ALRI group (22 months vs 60 months; 11 months vs 34 months; P < .001). The 1- and 3-year OS rates in the high ALRI group were significantly lower than those in the low ALRI group (68.64% and 39.83% vs 89.77% and 63.64%; P < .001; Fig. 3B). The 1- and 3-year DFS rates in the high ALRI group were significantly lower than those in the low ALRI group (41.53% and 17.80% vs 59.09% and 27.27%; P < .001; Fig. 3D).
3.7. Prognostic values of combining GAR and ALRI for HCC patients
Based on our findings, a high GAR or ALRI level implied poor DFS and OS in HCC patients after surgery. After multivariate analysis, we found that GAR and ALRI were significant independent predictors of OS (all P < .05). GAR > 0.946 or ALRI > 18.734 predicted poor DFS and OS in HCC patients after surgery. GAR was an inflammatory response marker, and ALRI could assess the degree of liver fibrosis and liver damage. Considering the above, when we look at both GAR and ALRI, we can more accurately predict the prognosis in HCC patients. To this end, we established the risk score based on the levels of GAR and ALRI to predict death risk in HCC patients in HCC patients after hepatectomy. Based on the risk score, patients can be divided into 3 categories: low, medium, and high risk. Thus, it is determined that the effect of combining GAR with ALRI for predicting prognosis of HCC patients.
We set GAR ≤ 0.946 or ALRI ≤ 18.734 as the risk score of 0 and GAR > 0.946 or ALRI > 18.734 as the risk score of 1. Then the HCC patients were divided into 3 groups based on the combined GAR with ALRI. Group 1 consisted of HCC patients with GAR ≤ 0.946 and ALRI ≤ 18.734 with a risk score equal to 0. Group 2 was composed of HCC patients with GAR > 0.946 and ALRI ≤ 18.734 or GAR ≤ 0.946 and ALRI > 18.734 with a total risk score of 1. Finally, Group 3 consisted of HCC patients with GAR > 0.946 and ALRI > 18.734 and a total risk score of 2 (Table 5).
Table 5.
Combining GAR and ALRI to predict HCC prognosis.
| Variable | Risk score |
| GAR | |
| ≤0.946 | 0 |
| >0.946 | 1 |
| ALRI | |
| ≤18.734 | 0 |
| >18.734 | 1 |
| Prognostic stratification | |
| 0 | Low risk of death |
| 1 | Medium risk of death |
| 2 | High risk of death |
Our results demonstrate that the OS rates of patients in Group 1 were the highest, followed by those in Group 2 and Group 3, which exhibited the worst prognosis. The 1- and 3-year OS rates in Group 3 were significantly lower than those in Groups 1 (65.66% and 36.36% vs 94.12% and 74.51%; P < .001) and 2 (65.66% and 36.36% vs 83.93% and 51.79%; P = .003) (Fig. 4A). Meanwhile, the DFS rates of patients in Group 1 were highest, followed by those in Group 2 and Group 3, which displayed the worst prognosis. The 1- and 3-year DFS rates in Group 3 were significantly lower than those in Groups 1 (36.36% and 18.18% vs 66.67% and 31.37%; P < .001) and 2 (36.36% and 18.18% vs 55.36% and 19.64%; P = .004) (Fig. 4B).
Figure 4.
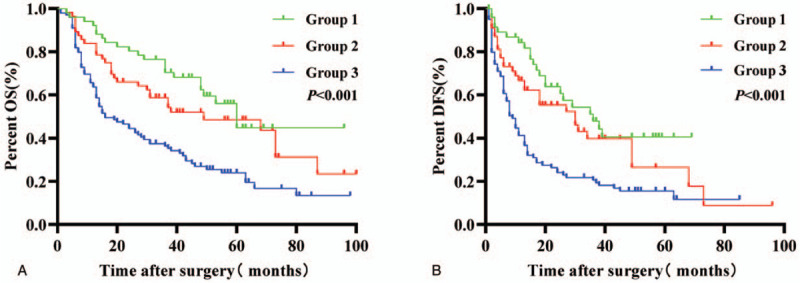
Combination of GAR and ALRI enhances prognostic accuracy for overall survival (OS) and disease-free survival (DFS) in HCC patients. Group 1, GAR ≤ 0.946 and ALRI ≤ 18.734; Group 2, GAR > 0.946 and ALRI ≤ 18.734 or GAR ≤ 0.946 and ALRI > 18.734; Group 3, GAR > 0.946 and ALRI > 18.734.
4. Discussion
Hepatectomy is the most important treatment for HCC patients to attain long-term survival. However, the rate of recurrence after hepatectomy is high, and the overall long-term survival condition in HCC patients is poor. Therefore, a method for estimating prognosis in HCC patients before surgery is critical to provide a basis for treatment, which can improve long-term patient survival.
Theoretically, oxidative stress, inflammatory response, nutrient metabolism abnormalities, and the degree of hepatocellular damage might act as predictors of poor survival in HCC patients after hepatectomy. It has been reported that γ-GGT is a reliable marker of oxidative stress.[20] An increase in γ-GGT expression in tumor cells has been connected to the production of ROS, which promotes the invasion and migration of tumor cells.[21,22] Fu et al[23] retrospectively analyzed 308 HCC patients who had undergone radical liver cancer surgery and identified γ-GGT as a promising, reliable prognostic marker for liver cancer patients after liver resection, especially patients diagnosed with small liver cancer, or those who exhibited AFP ≤ 200 ng/ml. Fu et al[24] examined the levels of preoperative serum γ-GGT of 130 liver cancer patients who had undergone liver transplantation. The results confirmed that γ-GGT was an independently prognostic marker of OS in HCC patients who underwent liver transplantation. Therefore, the γ-GGT level could be used to assess the prognosis of HCC patients.
In contrast, ALB participates in the antioxidant stress response, improved microcirculation, and suppression of the inflammatory response. Researchers have shown that ALB can induce the production of inflammatory mediators such as IL-1 by activating the MAPK and TGF-β signaling pathways. Furthermore, activation of these pathways can stimulate astrocytes and microglia, thereby giving rise to the expression of inflammatory mediators.[25,26] Patients with malignant tumors usually exhibit lower levels of ALB due to malnutrition and tumor consumption. McMillan et al[27] reported that persistent systemic inflammatory response, in patients with advanced lung cancer and gastrointestinal tumors, were associated with decreased ALB concentration. A meta-analysis[28] showed that the administration of ω-3 fish oil fat emulsion supplements to colorectal cancer patients could improve their ALB concentration and systemic immune inflammatory response, which could ultimately improve their prognosis. Therefore, GAR is not only a combination of preoperative laboratory tests, but also a better reflection of the human inflammatory response status, and is therefore useful for estimating the survival prognosis of HCC patients. Li et al.[29] demonstrated that preoperative GAR is an independent prognostic factor for predicting surgical outcomes in patients with pancreatic ductal adenocarcinoma. In our study, we found that preoperative GAR was a significant prognostic factor for DFS and OS in HCC patients. Moreover, we show that a high preoperative GAR is related to poor prognosis in HCC patients. Thus, we hypothesize that anti-inflammatory therapy may improve the prognosis in HCC.
Increasing evidence has indicated that hepatocellular damage is involved in all pathological stages of hepatitis, fibrosis, cirrhosis, and HCC. Aspartate aminotransferase (AST) is a sensitive indicator of hepatocyte injury. Studies showed that higher AST levels were associated with increasing replication of the hepatitis B virus, which was correlated with a lower overall survival rate in HCC patients.[30] Furthermore, serum AST level was found to be significantly higher, suggesting a more severe hepatocyte injury. Conversely, the immune response to tumors was determined by the presence of lymphocytes, which mediate the cytotoxic response and release cytokines that inhibit tumor proliferation and metastasis. Primary tumor infiltrating lymphocytosis is a favorable prognosis for tumor regression, and these patients displayed an improved prognosis.[31,32] Haybaeck et al[33] reported that overexpression of lymphotoxin produced by T lymphocytes could activate the NF-kB signaling pathway in hepatocytes, leading to secretion of inflammatory chemokines. Inflammatory chemokines recruit lymphocytes to perpetuate chronic hepatitis, which might be a prerequisite for tissue remodeling, hepatocyte proliferation and eventual malignant tumor formation. A previous study demonstrated that lymphocyte-mediated anti-tumor immunity during neoadjuvant chemotherapy was extremely beneficial in improving the effectiveness of chemotherapy.[34] For these reasons, ALRI was developed as a non-invasive index for predicting liver fibrosis and cirrhosis.[35] Previous research confirms that ALRI could be used as an independent prognostic factor for OS in HBV-related HCC in clinical practice.[36] Results from our study demonstrate that ALRI is a significant factor for DFS and OS in HCC patients. Overall, our results are consistent with those previously reported.
In our study, the clinical characteristics of HCC patients displaying a high level of GAR were smoking history, prolonged PT, later T stage, stronger tumor proliferation ability and likelihood of intrahepatic recurrence at an early stage. HCC patients with a high level of ALRI were clinically associated with a smoking history, cirrhosis, prolonged PT, larger tumor diameter, advanced TNM stage, and a higher likelihood of intrahepatic recurrence at early stages. Interestingly, our findings suggest that a smoking history is closely associated with preoperative GAR and ALRI levels in HCC patients. Individuals with a history of smoking or drinking had higher GAR and ALRI compared to controls. Previous studies[37,38] demonstrated that drinking, smoking and a viral infection could increase the risk of HCC. In addition, smoking and drinking could induce oxidative stress and elevate the level of free radicals. Oxidative stress is one of the causes of liver injury. However, our results indicate that smoking and drinking are not potential prognostic factors in HCC patients, consistent with Siegel et al.[39] The relationship between smoking and drinking in HCC requires further investigation.
As mentioned above, non-tumor factors such as the inflammatory response, immune status, nutritional status, liver damage and liver fibrosis play a considerable role in determining the survival prognosis of HCC patients after surgery. Our results show that GAR is a better reflection of the inflammatory response in HCC patients, and ALRI could be used to assess the degree of liver fibrosis and damage. Therefore, we concluded that GAR combined with ALRI reflect the range of non-tumor factors, thereby enabling a more accurate prediction of OS and DFS in HCC patients.
Our results show that high GAR and ALRI are significant prognostic indicators of poor OS and DFS in HCC patients. The 1- and 3-year OS and DFS rates of patients with high GAR or ALRI were significantly lower than patients with low GAR or ALRI. Interestingly, our research shows that the combination of GAR and ALRI better reflects the range of non-tumor factors, which could more accurately predict the prognosis of HCC patients with radical resection. Furthermore, our data showed that the 1- and 3-year OS and DFS rates in Group 3 (GAR > 0.946 and ALRI > 18.734) were significantly lower than those in Groups 1 (GAR ≤ 0.946 and ALRI ≤ 18.734) and 2 (GAR > 0.946 and ALRI ≤ 18.734 or GAR ≤ 0.946 and ALRI > 18.734). Significant differences were observed between any 2 groups. The combination of GAR and ALRI could accurately predict the poor prognosis of HCC patients. Following surgery, GAR > 0.946 and ALRI > 18.734 represented a high risk of death, and GAR ≤ 0.946 and ALRI ≤ 18.734 represent a low risk of death. The remainder represent a medium risk of death. Based on these results, we hypothesize that a patient's postoperative survival time can be estimated and that early interventions can be performed, such as preventive trans-arterial chemoembolization (TACE), targeted immunomodulatory therapy and systemic treatment, to develop a better personalized treatment plan.
This study has a few limitations, including its single-center retrospective analysis and small sample size, both of which can create statistical bias. Moreover, the levels of GAR and ALRI were affected by factors such as preoperative laboratory hematology testing techniques and sample collection. In assessing the survival prognosis of HCC patients, we recommend a combination of GAR and ALRI. Furthermore, the degree of tumor differentiation was not mentioned in our report. Therefore, a prospective, well-designed large-sample study should be conducted to examine the relationship between the inflammatory response and liver damage-related indicators and HCC prognosis.
5. Conclusions
In summary, GAR and ALRI are non-invasive, low-cost, easy-to-evaluate and reproducible clinical evaluation indices, and high GAR and ALRI before surgery may indicate a poor prognosis for HCC patients. Preoperative GAR and ALRI levels can also be used as important indicators of tumor TNM stage, early recurrence and distant metastasis. Furthermore, the combination of GAR and ALRI can improve the prediction accuracy of detection, and be used as a clinical evaluation index to further guide patient follow-ups and postoperative treatment.
Author contributions
Conceptualization: Yang Bu, Kejun Liu.
Data curation: Kejun Liu, Yongxue Lv, Yiming Niu.
Formal analysis: Kejun Liu, Yongxue Lv.
Funding acquisition: Yang Bu.
Investigation: Kejun Liu, Yongxue Lv, Yiming Niu.
Methodology: Yang Bu.
Project administration: Yang Bu.
Resources: Yang Bu.
Software: Kejun Liu, Yongxue Lv, Yiming Niu.
Supervision: Yang Bu.
Validation: Kejun Liu.
Visualization: Yang Bu, Kejun Liu.
Writing – original draft: Kejun Liu, Yongxue Lv.
Writing – review & editing: Yang Bu, Kejun Liu, Yongxue Lv.
Footnotes
Abbreviations: ALRI = aspartate aminotransferase-to-lymphocyte ratio, AFP = alpha-fetoprotein, ALB = albumin, AST = aspartate aminotransferase, CI = confidence interval, DFS = disease-free survival, γ-GGT = γ-glutamyl transpeptidase, GAR = γ-glutamyl transpeptidase-to-albumin ratio, HCC = hepatocellular carcinoma, HR = hazard ratio, HBsAg = hepatitis B surface antigen, OS = overall survival, PT = prothrombin time, ROS = reactive oxygen species.
How to cite this article: Liu Kj, LV Yx, Niu Ym, Yang B. Prognostic value of γ-glutamyl transpeptidase to albumin ratio combined with aspartate aminotransferase to lymphocyte ratio in patients with hepatocellular carcinoma after hepatectomy. Medicine. 2020;99:48(e23339).
Ke-jun Liu and Yong-xue Lv contributed equally to this work.
This study was supported by grants from the National Natural Science Foundation of China (81960533) and the Ningxia Natural Science Foundation (NZ15130). The funding agencies had no role in study design, data collection and interpretation, or publication decisions.
All relevant data are within the manuscript.
The authors declare no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
HBsAg = hepatitis B surface antigen; AFP = alpha-fetoprotein; PT = prothrombin time; GAR = γ-glutamyl transpeptidase-to-albumin ratio; ALRI = aspartate aminotransferase-to-lymphocyte ratio.
GAR = γ-glutamyl transpeptidase-to-albumin ratio; ALRI = aspartate aminotransferase-to-lymphocyte ratio.
HBsAg = hepatitis B surface antigen; AFP = alpha-fetoprotein; PT = prothrombin time; GAR = γ-glutamyl transpeptidase-to-albumin ratio; ALRI = aspartate aminotransferase-to-lymphocyte ratio.
HR = hazard ratio; CI = confidence interval; HBsAg = hepatitis B surface antigen; AFP = alpha-fetoprotein; PT = prothrombin time; GAR = γ-glutamyl transpeptidase-to-albumin ratio; ALRI = aspartate aminotransferase-to-lymphocyte ratio.
References
- [1].Christina Fitzmaurice, F Akinyemiju Tomi F, Hasan Al Lami Faris, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol 2018;4:1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Martin WR, Crichton IK, Yang RC, et al. The metabolism of thioinosinic acid by 6-mercaptopurine sensitive and resistant leukemic leukocytes. Proc Soc Exp Biol Med 1972;140:423–8. [DOI] [PubMed] [Google Scholar]
- [4].Coussens Lisa M, Werb Zena. Inflammation and cancer. Nature 2002;420:p.860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Roizen MF. Hallmarks of cancer: the next generation. Yearbook Anesthesiol Pain Management 2012;2012:13. [Google Scholar]
- [6].Tate Suresh S, Meister Alton. γ-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem 1981;39:357–68. [DOI] [PubMed] [Google Scholar]
- [7].Whitfield JB. Gamma Glutamyl Transferase. Crit Rev Clin Lab Sci 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- [8].Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2016;65:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kunutsor Setor K. Gamma-glutamyltransferase-friend or foe within? Liver international: official journal of the International Association for the Study of the Liver 2016;36:1723–34. [DOI] [PubMed] [Google Scholar]
- [10].Strasak Alexander M, Pfeiffer Ruth M, Klenk Jochen, et al. Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer 2008;123:1902–6. [DOI] [PubMed] [Google Scholar]
- [11].Hui Ma, Lan Zhang, Bei Tang, et al. γ-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol 2014;21:3084–9. [DOI] [PubMed] [Google Scholar]
- [12].Xiao J, Chen L, Yang F, et al. Green, yellow and red emitting CdTe QDs decreased the affinities of apigenin and luteolin for human serum albumin in vitro. J Hazardous Materials 2010;182:696–703. [DOI] [PubMed] [Google Scholar]
- [13].Akiyoshi Kinoshita, Hiroshi Onoda, Nami Imai, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803–10. [DOI] [PubMed] [Google Scholar]
- [14].Rosenthal P, Haight M. Aminotransferase as a prognostic index in infants with liver disease. Clin Chem 1990;36:346–8. [PubMed] [Google Scholar]
- [15].Hoshino T, Hosokawa N, Yanai M, et al. A study of serum mitochondrial enzymes (mCK, mAST, mMDH) in rotavirus and adenovirus gastroenteritis in pediatric patients. Rinsho Byori Japanese Journal Clin Pathol 2001;49:1157–61. [PubMed] [Google Scholar]
- [16].Fu J, Zhang Z, Zhou L, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2013;58:139–49. [DOI] [PubMed] [Google Scholar]
- [17].Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860–7. [DOI] [PubMed] [Google Scholar]
- [18].Ofliver EAF. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- [19].Irvin MD, Kevin DLMD, Modlin IM, et al. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59. [DOI] [PubMed] [Google Scholar]
- [20].Tate Suresh S, Meister Alton. Gamma-glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem 1981;39:357–68. [DOI] [PubMed] [Google Scholar]
- [21].Elena Piskounova, Michalis Agathocleous, Murphy Malea M, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015;527:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goo Rhee Sue, signaling Cell. H2O2, a necessary evil for cell signaling. Science 2006;312:1882–3. [DOI] [PubMed] [Google Scholar]
- [23].Fu Shunjun, Guo Zhiyong, Li Shaoqiang, et al. Prognostic value of preoperative serum gamma-glutamyltranspeptidase in patients with hepatocellular carcinoma after hepatectomy. Tumor Biol 2015;37:3433–40. [DOI] [PubMed] [Google Scholar]
- [24].Shun-Jun Fu, Qiang Zhao, Fei Ji, et al. Elevated preoperative serum gamma-glutamyl transpeptidase predicts poor prognosis for hepatocellular carcinoma after liver transplantation. Sci Rep 2016;6:28835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ranaivo Hantamalala Ralay, Patel Fatima, Wainwright Mark S. Albumin activates the canonical TGF receptor-smad signaling pathway but this is not required for activation of astrocytes. Exp Neurol 2010;226:310–9. [DOI] [PubMed] [Google Scholar]
- [26].Hantamalala Ralay Ranaivo, Wainwright Mark S. Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain Res 2010;1313:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McMillan DC, Watson WS, O’Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210–3. [DOI] [PubMed] [Google Scholar]
- [28].Mocellin Michel C, Camargo Carolina Q, Nunes Everson Araujo, et al. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr 2016;35:359–69. [DOI] [PubMed] [Google Scholar]
- [29].Shuo Li, Huaxiang Xu, Chuntao Wu, et al. Prognostic value of γ-glutamyl transferase-to-albumin ratio in patients with pancreatic ductal adenocarcinoma following radical surgery. Cancer Med 2019;8:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Witjes CDM, IJzermans JNM, van der Eijk A, et al. Quantitative HBV DNA and AST are strong predictors for survival after HCC detection in chronic HBV patients. Neth J Med 2011;69:508–13. [PubMed] [Google Scholar]
- [31].Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- [32].Ownby HE, Roi LD, Isenberg R, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983;52:126–30. [DOI] [PubMed] [Google Scholar]
- [33].Johannes Haybaeck, Nicolas Zeller, Julia Wolf Monika, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009;16:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Joji Kitayama, Koji Yasuda, Kazushige Kawai, et al. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol 2010;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jing Ma, Yongfang Jiang, Guozhong Gong. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol 2013;25:428–34. [DOI] [PubMed] [Google Scholar]
- [36].Zongguo Yang, Jianliang Zhang, Yunfei Lu, et al. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget 2015;6:43090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 2005;42:218–24. [DOI] [PubMed] [Google Scholar]
- [38].Hassan M, Spitz MR, Thomas MB, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer 2010;123:1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Siegel Abby B, Conner K, Wang S, et al. Smoking and hepatocellular carcinoma mortality. Exp Therapeutic Med 2012;3:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


