Abstract
INTRODUCTION:
A recent study in mice points to the gut-derived hormone glucagon-like peptide 2 (GLP-2) as an important regulator of gallbladder motility inducing gallbladder relaxation and refilling. In this study, we evaluated the effect of exogenous GLP-2 on postprandial gallbladder motility in healthy men.
METHODS:
In a randomized, double-blinded, placebo-controlled, crossover study, we evaluated the effect of 4-hour intravenous infusions of high-dose GLP-2 (10 pmol × kg−1 × min−1), low-dose GLP-2 (1 pmol × kg−1 × min−1), and placebo (saline) on postprandial gallbladder motility. A 300-kcal liquid-mixed meal (added 1.5 g of acetaminophen for indirect measurement of gastric emptying) was served 30 minutes after start of intravenous infusions. Gallbladder volume was assessed by ultrasonography.
RESULTS:
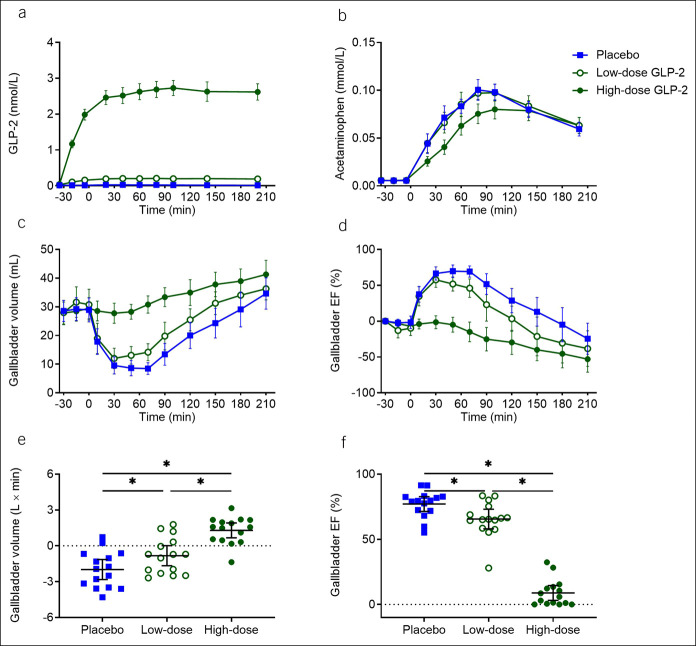
Fifteen healthy men, age 24.3 (22.4–26.1) years (mean [95% confidence interval]) and body mass index 22.5 (21.7–23.4) kg × m−2, were included. Basal plasma GLP-2 concentration was 14 (11–17) pmol/L. During low-dose and high-dose GLP-2 infusions, steady-state postprandial plasma GLP-2 concentrations amounted to 201 (188–214) and 2,658 (2,443–2,873) pmol/L, respectively, compared with maximum postprandial plasma GLP-2 concentration of 34 (25–44) pmol/L during placebo. Gallbladder emptying (assessed as baseline-subtracted area under the curve for gallbladder volume) was reduced by low-dose GLP-2 (−0.8 [0.7–1.9] L × min, P < 0.0001) and nearly abolished by high-dose GLP-2 (1.3 [−1.7 to 0.01] L × min, P = 0.029) compared to placebo (−2.0 [−2.8 to −1.1] L × min). Compared to placebo, gastric emptying was reduced by high-dose GLP-2 (P = 0.0060 and 0.019), whereas low-dose GLP-2 did not affect gastric emptying (P = 0.13 and 0.85).
DISCUSSION:
Exogenous GLP-2 exerts a dose-dependent inhibitory effect on postprandial gallbladder emptying in healthy men.
INTRODUCTION
Glucagon-like peptide (GLP)-2 is a 33-amino acid peptide hormone secreted from enteroendocrine L cells in response to intraluminal nutrients (1). After secretion, GLP-2 is degraded by the enzyme dipeptidyl peptidase 4, resulting in a circulating half-life of approximately 7 minutes (2). GLP-2 is known to exert trophic effects on the intestinal mucosa promoting nutrient absorption (1,3). Due to this intestinotrophic effect, GLP-2 analogs are currently used in the treatment of patients with short bowel syndrome to improve the absorption of orally ingested nutrients and water, thereby reducing the need for parenteral support (4,5). GLP-2 has also been shown to inhibit gastric acid secretion (6), improve (i.e. reduce) gut permeability (7), stimulate pancreatic glucagon secretion (6,8) and attenuate bone resorption leading to increased bone mineral density (9). In line with this broad spectrum of actions, GLP-2 receptor expression has been identified in different human tissues, including (listed according to declining levels of GLP-2 receptor expression) gallbladder, small intestine, duodenum, brain, colon, urinary bladder, fat, stomach, and liver (10). The effect of exogenous GLP-2 on gallbladder motility has not previously been investigated in humans.
The gallbladder is an abdominal organ functioning as a reservoir for bile produced by the liver. Contraction of the gallbladder and relaxation of the sphincter of Oddi ejects bile into the duodenum, where bile acids facilitate the assimilation of ingested lipids (11). Several mechanisms regulate gallbladder smooth muscle activity, and the interaction between these mechanisms leads to postprandial gallbladder contraction and emptying, followed by gallbladder relaxation and refilling. The gut-derived peptide hormone cholecystokinin (CCK), secreted from entero-endocrine I cells after ingestion of nutrients (especially fat), is the most well-acknowledged and potent inducer of postprandial gallbladder contraction (12–14). Other established mechanisms regulating gallbladder motility include parasympathetic signaling through the vagal nerve (contraction), sympathetic activity (relaxation), circulating bile acids (relaxation), and fibroblast growth factor 19 (relaxation) (15–18). Recently, Yusta et al. (19) demonstrated that GLP-2 promotes acute gallbladder refilling in mice through activation of the GLP-2 receptor in gallbladder tissue, and it was proposed that GLP-2 functions as a nutrient-induced feedback to terminate meal-mediated gallbladder contraction.
A better understanding of how gallbladder motility is regulated might disclose pathophysiological processes involved in gallbladder dysmotility and bile stasis and subsequent development of cholelithiasis and/or (calculous and acalculous) cholecystitis (20,21). Of interest, treatment with the GLP-2 analog teduglutide is associated with a small but significantly increased risk of gallbladder-related diseases (cholelithiasis, cholecystitis, and cholangitis) (22), and this association could be speculated to be due to GLP-2 affecting gallbladder motility. Furthermore, understanding how gallbladder motility can be modulated might disclose new targets for the treatment of diseases where a reduction of gallbladder contraction is desired (e.g., biliary colic). In this study, we evaluated the effect of exogenous GLP-2 on postprandial gallbladder motility in healthy men, and we hypothesized that GLP-2 would inhibit postprandial gallbladder emptying in a dose-dependent manner.
METHODS
This study was performed at Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, after approval by the Scientific-Ethical Committee of the Capital Region of Denmark (record no. H-17037571) and the local data protection authorities (record no. 2012-58-0004). The study was registered at ClinicalTrials.gov (NCT03682172) and performed in accordance with the Helsinki Declaration II, including oral and written informed consent from study participants before inclusion.
Participants
Eligible study participants were Caucasian men, aged 18–35 years, with a body mass index of 18.5–24.9 kg × m−2, nonsmokers, with no regular medicine use, and hemoglobin within normal range. Exclusion criteria included intestinal disease, liver disease and/or alanine transaminase >2 times upper normal limit, nephropathy (serum creatinine >130 μmol/L), active or recent malignant disease, medical treatment that could not be paused for 3 days, and cholelithiasis revealed by ultrasonography at the screening.
Study design
The study was designed as a randomized, double-blinded, placebo-controlled, crossover study. All study participants underwent a screening visit followed by 3 study days (separated by at least 48 hours), all involving a liquid-mixed meal test and a concomitant continuous intravenous (IV) infusion of saline (placebo), low-dose GLP-2, or high-dose GLP-2. The primary endpoint was GLP-2-induced change in baseline-subtracted area under the curve (bsAUC) for gallbladder volume (GV).
Peptides
Synthetic human GLP-2 (1–33) was purchased from Bachem Laboratories (Weil am Rhein, Germany) and dissolved in 0.9% NaCl, containing in addition 0.5% human serum albumin and sodium bicarbonate (NaHCO3), the latter added to provide a pH of 8.5 to enhance solubility of GLP-2. The solution was subjected to sterile filtration and dispensed into glass vials and stored at −20 °C until use. At each study day, a vial was thawed, and appropriate amounts (based on the participant's body weight) were injected into an infusion bag containing 250 mL sterile water with 0.9% NaCl and 0.5% human serum albumin. GLP-2 was demonstrated to be >97% pure and identical to the natural human peptide by high-performance liquid chromatography and sequence and mass analysis by Bachem Laboratories. For placebo infusions, an infusion bag containing 250 mL of sterile water with 0.9% NaCl and 0.5% human serum albumin was used.
Experimental procedures
Study participants arrived at the laboratory after an overnight 10-hour fast. They had been instructed to refrain from the use of prescription and nonprescription drugs 3 days before each study day and were instructed not to ingest alcohol or perform strenuous physical exercise 24 hours before each study day. Study participants were placed in a semirecumbent position in a hospital bed, and a cannula was inserted into a cubital vein in each arm: one for collection of blood samples and one for IV infusions. The forearm from which blood samples were collected was wrapped in a heating pad throughout the study day to arterialize venous blood. At time −30 minutes, a 4-hour continuous IV infusion of placebo (saline), low-dose GLP-2 (1 pmol × kg−1 × min−1), or high-dose GLP-2 (10 pmol × kg−1 × min−1) was initiated. At time 0 minutes, the study participants ingested a liquid-mixed meal. The liquid-mixed meal consisted of a premixed protein drink (200 mL, 300 kcal [36.8 g carbohydrate, 11.6 g protein, and 12.0 g lipid]; Nutricia, Danone, Denmark) added 100 mL of water and 1.5 g of acetaminophen (Pinex; Actavis, Dublin, Ireland). Acetaminophen was added for indirect measurement of gastric emptying because acetaminophen is not absorbed in the ventricle. Thus, it is possible to evaluate gastric emptying rate by measuring plasma levels of acetaminophen and determine maximum concentration of acetaminophen and time to maximum concentration of acetaminophen (23). At time 210 minutes, the GLP-2/placebo infusion was stopped.
Data collection and laboratory methods
Gallbladder scans by ultrasonography (LOGIQ E9; GE Healthcare, Waukesha, WI) were performed at baseline (time −60 and −30 minutes) and after infusion start (time −15, 0, 10, 30, 50, 70, 90, 120, 150, 180, and 210 minutes). Three baseline blood samples (time −45, −40, and −35 minutes) were drawn before start of infusion, and blood samples were drawn continuously during the study days (time −20, −5, 20, 40, 60, 80, 100, 140, and 200 minutes). Blood pressure and heart rate were measured at time −60, −30, 0, 30, 50, 70, 90, 120, 150, 180, and 210 minutes. For bedside measurement of plasma glucose, blood was sampled into sodium fluoride tubes and centrifuged immediately at room temperature at 7,400g for 30 seconds. For the analysis of GLP-2, CCK, bile acids, and glucagon, blood was collected in chilled tubes containing EDTA and a specific dipeptidyl peptidase 4 inhibitor (valine pyrrolidide 0.01 mmol/L). For the analysis of insulin and C-peptide, blood was sampled in plain tubes with clot activator for coagulation (10 minutes at room temperature). For the analysis of acetaminophen, total cholesterol, and triglycerides, blood was sampled in tubes with lithium heparin. EDTA, plain, and lithium heparin tubes were centrifuged for 15 minutes at 2,000g and 4°C. Plasma/serum was then distributed to storage tubes kept on ice before being stored at −20 °C or −80 °C until analysis. Intact GLP-2 was measured with antiserum (no. 92160) as previously described (24). Glucagon of pancreatic origin was measured using a C-terminally directed antiserum (no. 4305) (25). CCK (O-sulfated CCK-8, CCK-22, CCK-33, and CCK-58) was measured by radioimmunoassay using antiserum (no. 92128) without cross-reactivity with any gastrin peptide (26). Total bile acids were measured using Sentinel Bile Acid reagent (reference number: 17002B; Sentinel Diagnostics, Milan, Italy) and a KoneLab Analyzer (Thermo Fischer, Vantaa, Finland). Plasma glucose was measured bedside by the glucose oxidase method (Yellow Springs Instrument Model 2900 Series Biochemistry Analyzers; Yellow Springs, OH). Serum insulin and C-peptide concentrations were measured with a 2-side sandwich immunoassay using direct chemiluminescent technology (Siemens Healthcare, Ballerup, Denmark). Plasma concentrations of triglycerides and total cholesterols were measured enzymatically and using absorption photometry.
Calculations and statistical analyses
Based on a SD of the primary endpoint (bsAUC for GV) from a previous study with similar methodology (27), power of 80%, and level of statistical significance of 5%, we calculated the number of study participants to be 13 to detect a minimal relevant difference of 25%. To ensure sufficient power, 15 study participants were included. Results are reported as mean values with 95% confidence intervals unless stated otherwise. Calculations of AUC were based on the trapezoidal rule and presented as bsAUC if nothing else is stated. GV was determined by using gallbladder height (H), width (W), and length (L) and the ellipsoid method: GV = H × W × L × π/6 (28). Gallbladder ejection fraction was calculated according to the following formula: Gallbladder ejection fraction (%) = 100 × (GVbaseline − GVt)/GVbaseline, where GVt is GV at a given time. GV and circulating plasma/serum concentrations of CCK, total bile acids, triglycerides, total cholesterol, glucose, insulin, C-peptide, and glucagon were evaluated by calculation of bsAUC. GLP-2 concentrations were evaluated by steady-state concentrations (on the low-dose and high-dose GLP-2 infusions) and maximal postprandial concentrations (on the placebo day). Maximum gallbladder ejection faction and maximum postprandial glucose concentration was identified. Heart rate and blood pressure were evaluated by mean values. The statistical analysis of the primary endpoint (bsAUC for GV) was performed by 1-way repeated measures ANOVA with Geisser-Greenhouse correction and Tukey multiple comparison correction. The statistical analyses of the secondary endpoints were performed by 1-way repeated measures ANOVA with Geisser-Greenhouse correction, followed by correction for multiple testing by False Discovery Rate correction (Benjamini and Hochberg). Adjusted P values are reported, and a 2-sided P value of <0.05 is used as a significance level. All ANOVAs were calculated by GraphPad Prism 8.4.0 (GraphPad Software, San Diego, CA), and False Discovery Rate correction was performed in RStudio v2.2.5001.
RESULTS
Study participants
Fifteen healthy men with a mean age of 24.3 (22.4–26.1) years and a mean body mass index of 22.5 (21.7–23.4) kg × m−2 were included. Baseline characteristics are summarized in Table 1. One participant experienced 5 minutes of light abdominal discomfort after meal ingestion during high-dose GLP-2 infusion, but otherwise, GLP-2 infusions were well tolerated, and no other adverse events were reported.
Table 1.
Baseline characteristics
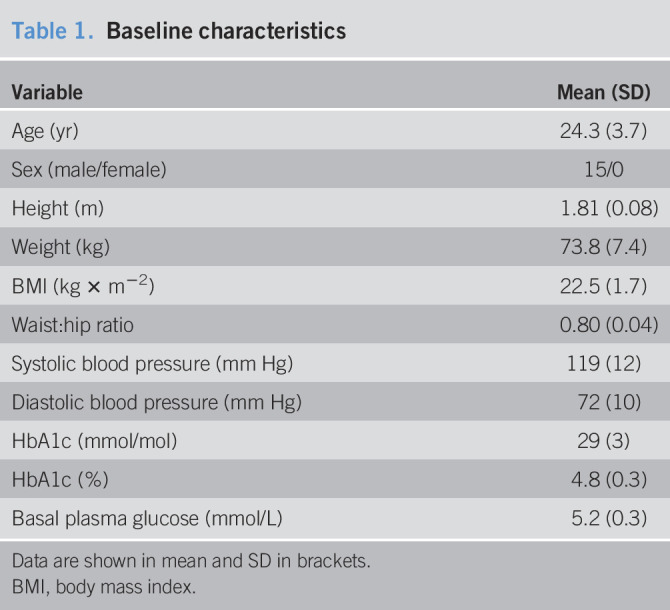
| Variable | Mean (SD) |
| Age (yr) | 24.3 (3.7) |
| Sex (male/female) | 15/0 |
| Height (m) | 1.81 (0.08) |
| Weight (kg) | 73.8 (7.4) |
| BMI (kg × m−2) | 22.5 (1.7) |
| Waist:hip ratio | 0.80 (0.04) |
| Systolic blood pressure (mm Hg) | 119 (12) |
| Diastolic blood pressure (mm Hg) | 72 (10) |
| HbA1c (mmol/mol) | 29 (3) |
| HbA1c (%) | 4.8 (0.3) |
| Basal plasma glucose (mmol/L) | 5.2 (0.3) |
Data are shown in mean and SD in brackets.
BMI, body mass index.
GLP-2 reached high supraphysiological plasma concentrations
Baseline plasma GLP-2 concentrations were similar on all 3 study days. Steady-state GLP-2 concentrations in plasma were obtained 90 minutes after infusion start (at time 60 minutes) and amounted to 201 (188–214) pmol/L during low-dose infusions and 2,658 (2,443–2,873) pmol/L during high-dose GLP-2 infusions (Figure 1a). During placebo infusions, the maximum postprandial GLP-2 concentration in plasma was 34 (25–44) pmol/L. Compared with the maximal postprandial concentration during placebo, GLP-2 levels were significantly higher during low-dose GLP-2 (P < 0.0001) and high-dose GLP-2 (P < 0.0001) infusions.
Figure 1.
GLP-2, gastric emptying, and gallbladder dynamics. Plasma concentration of GLP-2 (a), plasma concentration of acetaminophen (b), gallbladder volume (c), gallbladder ejection fraction (d), baseline-subtracted area under the curve for gallbladder volume (e), and maximum gallbladder ejection fraction (f) during 3 liquid-mixed meal tests with continuous intravenous infusions of placebo (saline), low-dose GLP-2 (1 pmol × kg−1 × min−1), and high-dose GLP-2 (10 pmol × kg−1 × min−1) started at time −30 minutes. The meal was ingested at time 0 minutes. Data were compared by 1-way repeated measurement ANOVA. Data points and wide lines are means; bars are 95% confidence intervals. Asterisk (*) indicates statistical significance (P < 0.05). EF, ejection fraction; GLP-2, glucagon-like peptide 2.
Gastric emptying was delayed by high-dose GLP-2
Gastric emptying, evaluated by maximum plasma concentration of acetaminophen and time to maximum plasma concentration of acetaminophen, was reduced by high-dose GLP-2 infusion (0.088 [0.080–0.097] mmol/L and 127 [103–151] minutes) compared with both low-dose GLP-2 (0.106 [0.098–0.114] mmol/L, P = 0.0081, and 92 [78–106] minutes, P = 0.012) and placebo infusions (0.108 [0.099–0.116] mmol/L, P = 0.019, and 83 [73–92] minutes, P = 0.0060). There was no difference in gastric emptying between low-dose GLP-2 and placebo groups (P = 0.85 and P = 0.13) (Figure 1b).
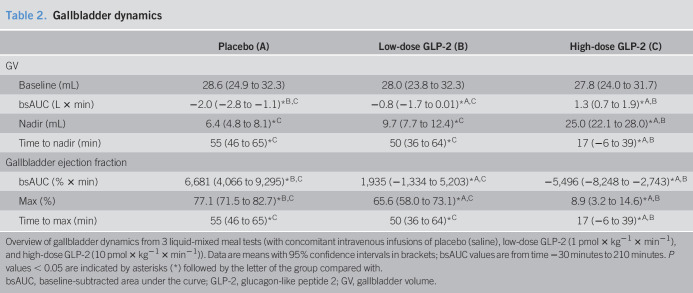
Postprandial gallbladder emptying was reduced by low-dose GLP-2 infusion and nearly abolished by high-dose GLP-2 infusion
Overall gallbladder emptying (assessed as bsAUC for GV) was reduced by low-dose GLP-2 (−0.8 [0.7–1.9] L × minutes, P < 0.0001) and nearly abolished by high-dose GLP-2 (1.3 [−1.7 to 0.01] L × minutes, P = 0.029) compared with placebo (−2.0 [−2.8 to −1.1] L × minutes) (Figure 1c, e and Table 2). Maximum postprandial gallbladder ejection fraction was reduced by low-dose GLP-2 (65.6 [58.0–73.1]%, P = 0.047) and high-dose GLP-2 (8.9 [3.2–14.6]%, P = 0.0009) compared with placebo (77.1 [71.5–82.7]%) (Figure 1d, f).
Table 2.
Gallbladder dynamics
| Placebo (A) | Low-dose GLP-2 (B) | High-dose GLP-2 (C) | |
| GV | |||
| Baseline (mL) | 28.6 (24.9 to 32.3) | 28.0 (23.8 to 32.3) | 27.8 (24.0 to 31.7) |
| bsAUC (L × min) | −2.0 (−2.8 to −1.1)*B,C | −0.8 (−1.7 to 0.01)*A,C | 1.3 (0.7 to 1.9)*A,B |
| Nadir (mL) | 6.4 (4.8 to 8.1)*C | 9.7 (7.7 to 12.4)*C | 25.0 (22.1 to 28.0)*A,B |
| Time to nadir (min) | 55 (46 to 65)*C | 50 (36 to 64)*C | 17 (−6 to 39)*A,B |
| Gallbladder ejection fraction | |||
| bsAUC (% × min) | 6,681 (4,066 to 9,295)*B,C | 1,935 (−1,334 to 5,203)*A,C | −5,496 (−8,248 to −2,743)*A,B |
| Max (%) | 77.1 (71.5 to 82.7)*B,C | 65.6 (58.0 to 73.1)*A,C | 8.9 (3.2 to 14.6)*A,B |
| Time to max (min) | 55 (46 to 65)*C | 50 (36 to 64)*C | 17 (−6 to 39)*A,B |
Overview of gallbladder dynamics from 3 liquid-mixed meal tests (with concomitant intravenous infusions of placebo (saline), low-dose GLP-2 (1 pmol × kg−1 × min−1), and high-dose GLP-2 (10 pmol × kg−1 × min−1)). Data are means with 95% confidence intervals in brackets; bsAUC values are from time −30 minutes to 210 minutes. P values < 0.05 are indicated by asterisks (*) followed by the letter of the group compared with.
bsAUC, baseline-subtracted area under the curve; GLP-2, glucagon-like peptide 2; GV, gallbladder volume.
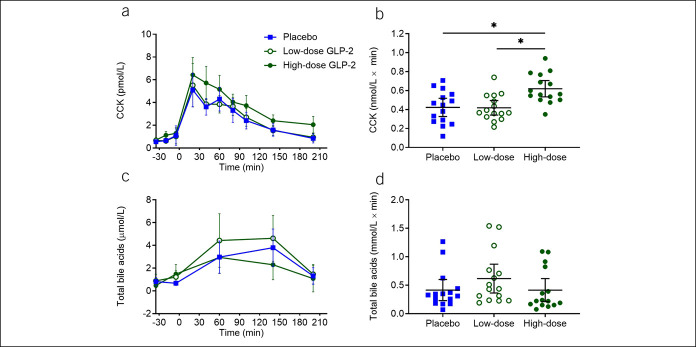
CCK levels were increased by high-dose GLP-2
Basal plasma CCK concentrations were similar on all 3 study days. Postprandial CCK concentrations (assessed as bsAUC) during the study days were higher during high-dose GLP-2 infusion compared with those of both low-dose GLP-2 (P = 0.0026) and placebo infusions (P = 0.0111) (Figure 2b).
Figure 2.
CCK and total bile acids. Plasma concentrations of CCK (a) and total bile acids (c) and corresponding baseline-subtracted area under the curve (b and d) during the 3 liquid-mixed meal tests with continuous intravenous infusions of placebo (saline), low-dose GLP-2 (1 pmol × kg−1 × min−1), and high-dose GLP-2 (10 pmol × kg−1 × min−1). Data were compared by 1-way repeated measurement ANOVA. Data points and wide lines are means; bars are 95% confidence intervals. Asterisk (*) indicates statistical significance (P < 0.05). CCK, cholecystokinin; GLP-2, glucagon-like peptide 2.
Total bile acids were not affected by GLP-2 infusions, high-dose GLP-2 elevated total cholesterols compared with low-dose GLP-2, and high-dose GLP-2 elevated triglycerides compared with placebo
No significant differences in circulating postprandial bile acids (Figure 2d) were observed. Total cholesterols (assessed as bsAUC) were higher on high-dose GLP-2 compared with low-dose GLP-2 (P = 0.020), but no difference between high-dose GLP-2 and placebo was observed (see Supplemental Figure 1b, Supplementary Digital Content 1, http://links.lww.com/CTG/A444). Postprandial triglyceride excursions (as assessed by bsAUC) were higher on the high-dose GLP-2 day compared with placebo (P = 0.012) (see Supplemental Figure 1d, Supplementary Digital Content 1, http://links.lww.com/CTG/A444).
Maximum postprandial plasma glucose was reduced by high-dose GLP-2
Basal plasma glucose concentrations and postprandial plasma glucose excursions (assessed as bsAUC) were similar on all 3 study days, but peak postprandial plasma glucose concentrations were reduced by high-dose GLP-2 (6.4 [6.2–6.7] mmol/L) compared with low-dose GLP-2 (6.9 [6.6–7.3] mmol/L, P = 0.0026) and placebo infusions (7.2 [6.7–7.6] mmol/L, P = 0.020) (Figure 3b). Basal serum insulin concentrations were similar on all 3 study days. During high-dose GLP-2 infusion, postprandial insulin responses (assessed as bsAUC) were reduced compared with low-dose GLP-2 (P = 0.020) but no differences were seen when comparing placebo with high-dose GLP-2 (P = 0.085), and placebo with low-dose GLP-2 (P = 0.62) (Figure 3d). Basal serum C-peptide concentrations and postprandial serum C-peptide responses were similar on all 3 study days (Figure 3e, f).
Figure 3.
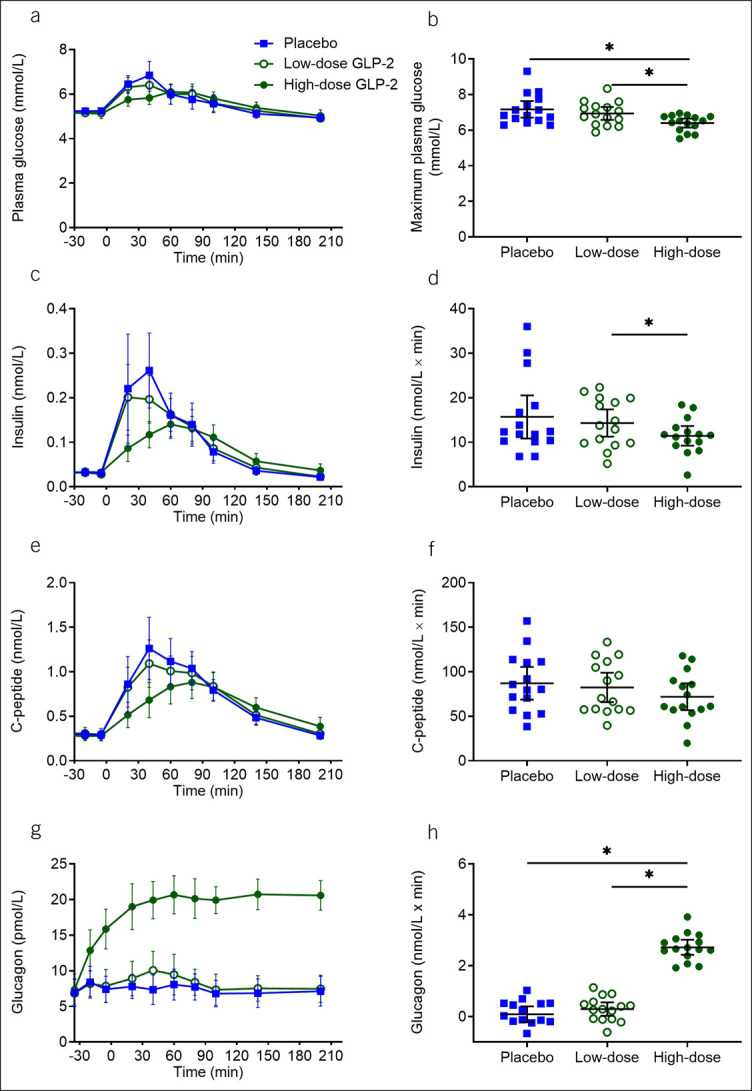
Glucose, insulin, C-peptide, and glucagon. Plasma/serum concentrations of glucose (a), insulin (c), C-peptide (e), and glucagon (g) and corresponding baseline-subtracted area under the curve (d, f, and h) and maximum plasma glucose (b) during the 3 liquid-mixed meal tests with continuous intravenous infusions of placebo (saline), low-dose GLP-2 (1 pmol × kg−1 × min−1), and high-dose GLP-2 (10 pmol × kg−1 × min−1). Data were compared by 1-way repeated measurement ANOVA. Data points and wide lines are means; bars are 95% confidence intervals. Asterisk (*) indicates statistical significance (P < 0.05). GLP-2, glucagon-like peptide 2.
Glucagon levels were elevated by high-dose GLP-2
Basal plasma glucagon concentrations were similar on all 3 study days. High-dose GLP-2 increased plasma concentrations of glucagon (assessed as bsAUC) compared with placebo (P = 0.0009) and low-dose GLP-2 (P = 0.0009) (Figure 3g, h). No differences in bsAUC or steady-state concentrations were observed between placebo and low-dose GLP-2 (P = 0.59).
High-dose GLP-2 infusion elevated heart rate but did not affect blood pressure
The mean heart rate was increased by high-dose GLP-2 (69 [65–72] min−1) compared with low-dose GLP-2 (63 [59–67] min−1, P = 0.0009) and placebo (64 [59–68] min−1, P = 0.0034) infusions (see Supplemental Figure 2b, Supplementary Digital Content 2, http://links.lww.com/CTG/A445). The mean systolic and diastolic blood pressure values were not affected by GLP-2 infusions.
DISCUSSION
Our study demonstrates that IV infusion of synthetic GLP-2 exerts a potent and dose-dependent inhibitory effect on postprandial gallbladder emptying with high supraphysiological concentrations of GLP-2 completely blocking gallbladder emptying. We investigated the acute effect of high supraphysiological concentrations of GLP-2 in healthy young men. Steady-state GLP-2 concentrations during low-dose and high-dose GLP-2 infusions were approximately 6 and 80 folds higher than physiological postprandial GLP-2 levels. Thus, whether endogenous GLP-2 contributes to the physiological regulation of gallbladder motility cannot be inferred from this study. However, the high-dose GLP-2 infusion corresponds to the GLP-2 exposure during treatment with the GLP-2 analog teduglutide. Furthermore, the GLP-2 concentrations reached with the low-dose GLP-2 infusions are similar to those observed after gastric bypass surgery (29). These observations support the clinical relevance of the GLP-2 concentrations in this study. As alluded to in the Introduction, treatment with the GLP-2 analog teduglutide is associated with a small but significantly increased risk of gallbladder-related adverse events (cholelithiasis, cholecystitis, and cholangitis) (22). Our findings suggest that these adverse events might relate to GLP-2 causing gallbladder hypomobility and, thus, bile stasis and stone formation. After gastric bypass surgery the risk of gallbladder disease is increased, which has been attributed to the rapid weight loss (30). However, the increased risk might also be partly driven by the elevated GLP-2 levels (31).
In this study, we found that high-dose GLP-2 reduced gastric emptying measured by the acetaminophen method. Evidence regarding the effect of GLP-2 on gastric emptying is conflicting (6,32,33). This might relate to differences between studies regarding meal type (solid vs liquid) and method for evaluation of gastric emptying (breath test, scintigraphy, and ultrasonography). Previous studies have used considerably lower GLP-2 infusion rates (0.5–2.25 pmol × kg−1 × min−1) than the high-dose GLP-2 (10 pmol × kg−1 × min−1) infusion in this study, and thus, GLP-2 might only inhibit gastric emptying in high supraphysiological concentrations.
In this study, it was necessary to consider a potential effect of GLP-2 on gastric emptying because deceleration of gastric emptying would reduce the entry of nutrients into the duodenum, potentially delaying the release of CCK and consequently slowing gallbladder emptying. Despite a reduced gastric emptying during high-dose GLP-2 infusion, the postprandial CCK concentrations in plasma were higher compared with low-dose GLP-2 and placebo infusions. CCK release is suppressed by intraluminal bile acids, constituting an important part of the feedback regulation of gallbladder motility (34). We believe that the increased postprandial CCK response during high-dose GLP-2 infusion reflects GLP-2-induced inhibition of gallbladder emptying, leading to less intraluminal bile acids and, thus, less suppression of CCK release. The abolished postprandial gallbladder emptying during high-dose GLP-2 infusion in the context of high circulating CCK concentrations suggests that GLP-2-induced inhibition of gallbladder emptying occurs independently of gastric emptying and CCK release. The gallbladder-relaxing effect of GLP-2 might be direct, supported by the expression of the GLP-2 receptor gene in human gallbladder tissue (10,35) and by GLP-2-mediated inhibition of spontaneous and electrically evoked smooth muscle contraction in intestinal and gallbladder tissues from mice (19,36). Furthermore, Yusta et al. (19), whose work inspired this study, found the gallbladder-relaxing effect of GLP-2 to be abolished in GLP-2 receptor knock-out mice. However, further studies are warranted to establish whether exogenous GLP-2 reduces human gallbladder emptying in a direct or indirect fashion.
High-dose GLP-2 infusion reduced postprandial peak plasma glucose. Because glucagon concentrations were increased by the high-dose GLP-2 infusion and insulin secretion was unaffected or even lower during high-dose GLP-2 infusion, the lower postprandial peak in plasma glucose is most likely a consequence of the decelerated gastric emptying rate (37). GLP-2 has previously been shown to stimulate glucagon secretion (6,8,38), and the mechanism is believed to involve activation of GLP-2 receptors on pancreatic alpha cells (39).
In accordance with a previous study, we found that high-dose GLP-2 increased mean heart rate (40). GLP-2 has been shown to increase mesenteric blood flow (41), and the elevated heart rate might be a compensatory mechanism to maintain blood pressure.
We chose to determine GV by two 2-dimensional ultrasonography scans and the ellipsoid method. This might constitute a limitation of our study. Ultrasonography is noninvasive and, therefore, commonly used to evaluate GV, but the quality of ultrasonography depends on the performing investigator(s) and interinvestigator and intrainvestigator variations. In this study, a single investigator performed all ultrasonography scans, leaving just the issue of intrainvestigator variation, which previously has been reported to be approximately 10% (42). The ellipsoid method has been shown to overestimate GV by approximately 10%–15% compared with the sum-of-cylinders (a slightly more accurate but time-consuming method) (43,44). In this study, the GV data from the 3 different study days show clear differences with little variation, and we believe that a systematic overestimation of GV of 10%–15% is unimportant for the conclusions drawn.
In conclusion, we demonstrate that exogenous GLP-2, resulting in supraphysiological plasma concentrations, dose-dependently inhibits postprandial gallbladder emptying in healthy men. We speculate that the increased risk of gallbladder disease after treatment with GLP-2 analogs and after gastric bypass surgery might partly be driven by supraphysiological activation of the GLP-2 receptor, resulting in gallbladder hypomotility. Whether GLP-2-mediated inhibition of gallbladder emptying might be used to temporarily manage conditions in which gallbladder relaxation in desirable, e.g., in biliary colic or gallbladder hyperkinesia, warrants further investigations.
CONFLICTS OF INTEREST
Guarantors of the article: Nina L. Hansen, MD, and Filip K. Knop, MD, PhD.
Specific author contributions: N.L.H., A.B., C.C.N.-L., A.S.C., D.P.S., and F.K.K. designed the study and wrote the study protocol. N.L.H. performed the study. J.F.R., N.J.W.A., B.H., and J.J.H. generated data (J.F.R. measured CCK, N.J.W.A. measured bile acids, and B.H. and J.J.H. measured GLP-2 and glucagon). N.L.H. and A.B. performed the data analysis. N.L.H. and F.K.K. wrote the manuscript. F.K.K. conceptualized the study. All authors critically edited the manuscript and approved the final version.
Financial support: The study was supported by Aase & Ejnar Danielsens Fond and Grosserer L. F. Foghts Fond. These foundations had no commercial interest in the study.
Potential competing interests: N.L.H., A.B., C.C.N.-L., A.S.C., D.P.S., J.F.R., N.J.W.A., and B.H. report no conflict of interest. T.V. has served on scientific advisory panels and/or been a part of speaker's bureaus for, served as a consult to, and/or received research support from Amgan, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, MDS/Merck, Mundipharma, Novo Nordisk, Sanofi, and Sun Pharmaceuticals. J.J.H. is a cofounder and board member of Bainan Biotech. F.K.K. has served on scientific advisory panels and/or been part of speaker's bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, and Zealand Pharma.
Study Highlights.
WHAT IS KNOWN
✓ GLP-2 exerts various effects in the gastrointestinal tract including stimulation of gut mucosal growth, and GLP-2 analogs for the treatment of short bowel syndrome have been developed.
✓ Treatment with GLP-2 analogs is associated with increased risk of gallbladder-related adverse events.
✓ GLP-2 has been shown to induce gallbladder relaxation and refilling in mice.
WHAT IS NEW HERE
✓ Exogenous GLP-2 dose-dependently inhibits postprandial gallbladder emptying in man.
TRANSLATIONAL IMPACT
✓ Our findings suggest that gallbladder-related adverse events observed in patients treated with GLP-2 analogs might arise as a consequence of disturbed gallbladder motility.
✓ In addition, our findings point to a potential link between the elevated postprandial GLP-2 responses and the increased risk of cholelithiasis after gastric bypass surgery.
✓ Our results might position GLP-2 as a potential therapeutic target in the management of biliary colic and gallbladder hyperkinesia (e.g., as bridge-to-surgery).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the study participants for their participation. We thank Lene V. Jespersen, Christine Rasmussen, and Louise S. Larsen (Department of Clinical Biochemistry, Rigshospitalet, Denmark), and Sisse M. Schmidt and Inass Al Nacha (Center for Clinical Metabolic Research, Gentofte Hospital, Denmark) for technical assistance.
Previous presentation: Abstracts with parts of the results have been presented at the American Diabetes Association, Scientific Sessions, 2019 (P-1982) and at the Danish Endocrine Society Annual Meeting, 2020.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A444 and http://links.lww.com/CTG/A445
REFERENCES
- 1.Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol 2014;76(1):561–83. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann B, Harr MB, Jeppesen PB, et al. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab 2000;85(8):2884–8. [DOI] [PubMed] [Google Scholar]
- 3.Jeppesen PB. Gut hormones in the treatment of short-bowel syndrome and intestinal failure. Curr Opin Endocrinol Diabetes Obes 2015;22(1):14–20. [DOI] [PubMed] [Google Scholar]
- 4.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012;143(6):1473–81. [DOI] [PubMed] [Google Scholar]
- 5.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005;54(9):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier JJ, Nauck MA, Pott A, et al. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 2006;130(1):44–54. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Possemiers S, Van De Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58(8):1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen M, Knop FK, Vilsbøll T, et al. Glucagon-like peptide-2, but not glucose-dependent insulinotropic polypeptide, stimulates glucagon release in patients with type 1 diabetes. Regul Pept 2010;163(1-3):96–101. [DOI] [PubMed] [Google Scholar]
- 9.Henriksen DB, Alexandersen P, Hartmann B, et al. Four-month treatment with GLP-2 significantly increases hip BMD: A randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone 2009;45(5):833–42. [DOI] [PubMed] [Google Scholar]
- 10.GLP2R glucagon like peptide 2 receptor [Homo sapiens (human)]. 2018. (https://www.ncbi.nlm.nih.gov/gene/9340?report=expression&bioproject=PRJEB4337). Accessed May 11, 2020.
- 11.Hundt M, Basit H, John S. Physiology, Bile Secretion. StatPearls Publishing: Treasure Island, FL, 2020. [PubMed] [Google Scholar]
- 12.Gether IM, Nexøe-Larsen C, Knop FK. New avenues in the regulation of gallbladder motility—implications for the use of glucagon-like peptide-derived drugs. J Clin Endocrinol Metab 2019;104:2463–72. [DOI] [PubMed] [Google Scholar]
- 13.Rehfeld JF. Cholecystokinin. In: Makhlouf GM. (ed). Handbook of Physiology: The Gastrointestinal System: Neural and Endocrine Biology. American Physiology Society: Bethesda, MD, 1989. [Google Scholar]
- 14.Rehfeld JF. Cholecystokinin-from local gut hormone to ubiquitous messenger. Front Endocrinol (Lausanne) 2017;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol 2011;25(6):1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portincasa P, Di Ciaula A, Van Berge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep 2004;6(2):151–62. [DOI] [PubMed] [Google Scholar]
- 17.Choi M, Moschetta A, Bookout AL, et al. Identification of a hormonal basis for gallbladder filling. Nat Med 2006;12(11):1253–5. [DOI] [PubMed] [Google Scholar]
- 18.Dopico AM, Walsh JV, Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol 2002;119(3):251–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusta B, Matthews D, Flock GB, et al. Glucagon-like peptide-2 promotes gallbladder refilling via a TGR5-independent, GLP-2R-dependent pathway. Mol Metab 2017;6:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poddighe D, Sazonov V. Acute acalculous cholecystitis in children. World J Gastroenterol 2018;24(43):4870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barie PS, Eachempati SR. Acute acalculous cholecystitis. Curr Gastroenterol Rep 2003;5(4):302–9. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LK, O'Keefe SJD, Fujioka K, et al. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol 2016;7:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medhus AW, Lofthus CM, Bredesen J, et al. Gastric emptying: The validity of the paracetamol absorption test adjusted for individual pharmacokinetics. Neurogastroenterol Motil 2001;13(3):179–85. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann B, Johnsen AH, Ørskov C, et al. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides 2000;21(1):73–80. [DOI] [PubMed] [Google Scholar]
- 25.Ørskov C, Jeppesen J, Madsbad S, et al. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 1991;87(2):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem 1998;44(5):991–1001. [PubMed] [Google Scholar]
- 27.Sonne DP, Rehfeld JF, Holst JJ, et al. Postprandial gallbladder emptying in patients with type 2 diabetes: Potential implications for bile-induced secretion of glucagon-like peptide 1. Eur J Endocrinol 2014;171(4):407–19. [DOI] [PubMed] [Google Scholar]
- 28.Dodds WJ, Groh WJ, Darweesh RMA, et al. Sonographic of gallbladder measurement volume. Am Roentgen Ray Sociaty 1985;145:1009–11. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012;22(7):1084–96. [DOI] [PubMed] [Google Scholar]
- 30.Quesada BM, Kohan G, Roff HE, et al. Management of gallstones and gallbladder disease in patients undergoing gastric bypass. World J Gastroenterol 2010;16(17):2075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Kong J, Wu S. Cholesterol gallstone disease: Focusing on the role of gallbladder. Lab Investig 2015;95(2):124–31. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt PT, Näslund E, Grybäck P, et al. Peripheral administration of GLP-2 to humans has no effect on gastric emptying or satiety. Regul Pept 2003;116(1-3):21–5. [DOI] [PubMed] [Google Scholar]
- 33.Nagell CF, Wettergren A, Pedersen JF, et al. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand J Gastroenterol 2004;39(4):353–8. [DOI] [PubMed] [Google Scholar]
- 34.Koop I, Schindler M, Bosshammer A, et al. Physiological control of cholecystokinin release and pancreatic enzyme secretion by intraduodenal bile acids. Gut 1996;39(5):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu X, Li B, Jiang M, et al. RNA sequencing reveals differentially expressed genes as potential diagnostic and prognostic indicators of gallbladder carcinoma. Oncotarget 2015;6(24):20661-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amato A, Rotondo A, Cinci L, et al. Role of cholinergic neurons in the motor effects of glucagon-like peptide-2 in mouse colon. Am J Physiol Gastrointest Liver Physiol 2010;299:1038–44. [DOI] [PubMed] [Google Scholar]
- 37.Marathe CS, Rayner CK, Jones KL, et al. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013;36(5):1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sørensen LB, Flint A, Raben A, et al. No effect of physiological concentrations of glucagon-like peptide-2 on appetite and energy intake in normal weight subjects. Int J Obes 2003;27(4):450–6. [DOI] [PubMed] [Google Scholar]
- 39.De Heer J, Pedersen J, Ørskov C, et al. The alpha cell expresses glucagon-like peptide-2 receptors and glucagon-like peptide-2 stimulates glucagon secretion from the rat pancreas. Diabetologia 2007;50(10):2135–42. [DOI] [PubMed] [Google Scholar]
- 40.Bremholm L, Hornum M, Andersen UB, et al. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept 2010;159(1-3):67–71. [DOI] [PubMed] [Google Scholar]
- 41.Bremholm L, Hornum M, Henriksen BM, et al. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol 2009;44(3):314–9. [DOI] [PubMed] [Google Scholar]
- 42.Everson GT, Braverman DZ, Johnson ML, et al. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology 1980;79(1):40–6. [PubMed] [Google Scholar]
- 43.Stolk MFJ, van Erpecum KJ, van Berge Henegouwen GP, et al. Gallbladder volume and contraction measured by sum-of-cylinders method compared with ellipsoid and area-length methods. Acta Radiol 1990;31:591–6. [PubMed] [Google Scholar]
- 44.Pauletzki J, Sackmann M, Holl J, et al. Evaluation of gallbladder volume and emptying with a novel three-dimensional ultrasound system: Comparison with the sum-of-cylinders and the ellipsoid methods. J Clin Ultrasound 1996;24(6):277–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.