Abstract
Background:
Pulmonary surfactant (PS) is commonly used for the treatment of neonatal respiratory distress syndrome (NRDS), several randomized controlled trials (RCTs) have evaluated the role of nebulized versus invasively delivered PS, yet the results remained inconsistent. Therefore, we aimed to conduct this meta-analysis to evaluate the effects and safety of nebulized versus invasively delivered PS in the treatment of NRDS.
Methods:
We searched PubMed et al databases from inception date to May 15, 2020 for RCTs that compared nebulized vs invasively delivered PS. Two authors independently screened the studies and extracted data from the published articles. Summary odd ratios (OR) or mean differences (MDs) with 95% confidence intervals (CIs) were calculated for each outcome by means of fixed- or random-effects model.
Results:
Two RCTs with a total of 95 preterm neonates were identified, with 48 neonates received PS nebulization and 47 neonates undergone invasive PS administration. There was no significant difference in the SpO2 level (MD = −0.44, 95% CI −6.01 to 5.12) and the A/APaO2 level (MD = 0.01, 95% CI −0.02 to 0.05) 1 hour after treatment among 2 groups. But the duration of mechanical ventilation in the nebulization groups was significantly less than that of invasive group (MD = −30.70, 95% CI −41.45 to 19.95).
Conclusions:
Given the limited evidences, the effects and safety of nebulized versus invasively delivered PS still need further verification.
Keywords: administration, invasive, nebulization, neonatal respiratory distress syndrome, pulmonary surfactant
1. Background
Neonatal respiratory distress syndrome (NRDS) is a common lung disease in the population of neonates, especially for the preterm infants. It is mainly manifested by progressive dyspnea and respiratory failure within a few hours after birth. It has been reported that the incidence of NRDS is 92% for neonates with gestational age of 24 to 25 weeks, 88% for neonates with gestational age of 26 to 27 weeks, 76% for neonates with gestational age of 28 to 29 weeks, and 57% for neonates with gestational age of 30 to 31 weeks.[1,2] The NRDS does increase risk of death or severe morbidity for neonates, early treatments and nursing cares are essential.[3]
Insufficient or lacking pulmonary surfactant (PS) is the most important cause of NRDS.[4] PS has been reported that it can reduce the tension of alveolar air-liquid surface and avoid end-expiratory alveolar collapse.[5] It has been reported that PS therapy plays a vital role in the management of RDS because it reduces pneumothorax and significantly improves the survival of preterm neonates.[6] Clinically, the PS administration requires experienced practitioners with professional intubation skills and mechanical ventilation can be performed whenever needed. The INSURE technique and less invasive surfactant administration (LISA) are the most commonly seen method for PS administration. Currently, the most related research results have favored the use of LISA, it is reasonable with consideration that LISA is less invasive and more stable to the use of continuous positive airway pressure (CPAP).[7,8] However, both of those 2 methods are still invasive. Recently, several studies[9–11] have focused on the potential use of PS delivered by nebulization. With the development of vibrating membrane nebulizers, it is possible to atomize PS, and PS nebulization can be truly non-invasive. Meanwhile, it concerns us that where the nebulized PS is as effective as the PS administered by INSURE or LISA methods. Based on literature review, we have found that several studies have compared the use of PS by nebulization and invasive methods, yet the sample sizes are small and the results remain inconsistent. Therefore, it is necessary to conduct a meta-analysis to identify the role of nebulization of PS in the treatment of NRDS.
2. Methods
Ethical review was not required since our manuscript is meta-analysis. And this meta-analysis was conducted in comply with the guidelines for preferred reporting items for systematic reviews and meta-analyses (PRISMA statement).[12]
2.1. Literature research
Electronic databases, including PubMed, Embase, and the Cochrane Library, China National Knowledge Infrastructure (CNKI) and Wanfang Database, China Biomedical Literature Database (CBM) were searched by 2 independent researchers. Data were last updated on 15 May 2020. The following keywords or corresponding Medical Subject Headings (MeSH) were used: “nebulize” or “nebulization” or “atomize” or “atomization” and “neonates” or “preterm” or “infants” or “newborns” and “surfactant” or “pulmonary surfactant” or “PS”. Reference lists of the relevant articles were also reviewed for any additional relevant studies. The search was not restricted by language.
2.2. Inclusion and exclusion criteria
Studies were identified according to the following inclusion criteria:
-
1.
Participants: neonates with NRDS requiring PS treatment,
-
2.
Intervention: PS treatment,
-
3.
Comparison: nebulized versus invasively delivered surfactant therapy,
-
4.
Outcome: trials that reported important related outcomes, such as the gas analysis results and the length of mechanical ventilation, and
-
5.
Methodological criterion: a prospective randomized controlled trial (RCT).
The following exclusion criteria were applied:
-
1.
insufficient data were available to estimate a risk ratio (RR),
-
2.
animal studies and cadaver studies, and
-
3.
the size of each group in the RCT was less than 10.
2.3. Data extraction
We used a standardized data collection form to extract key information. Any discrepancies in the extraction process were resolved by consensus. We also attempted to contact authors to obtain additional data or to clarify data of missing details. Two reviewers independently extracted the following information: first author, year of publication, study location, patient population, details of PS treatment, main outcomes, and study results.
2.4. Quality assessment
The Cochrane Collaborations risk of bias tool[13] was used by 2 reviewers independently to evaluate the methodological quality and risk of bias of the included RCTs; any disagreements were resolved by discussion and consensus. This tool was also utilized to examine and measure 7 specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other issues. Each domain could be classified as low risk of bias, high risk of bias or unclear risk of bias according to the judgment criteria.
2.5. Data analysis
All statistical analyses were performed with RevMan 5.3 software. Data were used as input and double-checked by 2 reviewers. Data syntheses and interpretations were also performed by 2 authors to ensure the accuracy of the results. Binary outcomes were presented as Mantel–Haenszel-style odds ratios with 95% confidence intervals. Continuous outcomes were reported as mean differences (MDs). A fixed-effect model was adopted in cases of homogeneity (P value of χ2 test >.10 and I2 < 50%), whereas a random-effect model was used in cases of obvious heterogeneity (P value of χ2 test > .10 and I2 ≥ 50%).[14] Publication bias were evaluated by using funnel plots, and asymmetry was assessed by conducting Egger regression test. For funnel plot asymmetry, P < .1 was considered as significantly different.
3. Results
3.1. Study selection
The process for selecting studies is shown in Figure 1. The first search yielded 70 potentially relevant articles. Of these identified articles, 2 studies were excluded as duplicates. After viewing the titles and abstracts of the 68 remaining studies, the full texts of 18 studies were retrieved. Among them, 16 studies were excluded with failure to meet the inclusion criteria. Finally, 2 RCTs[15,16] were included in this present study.
Figure 1.
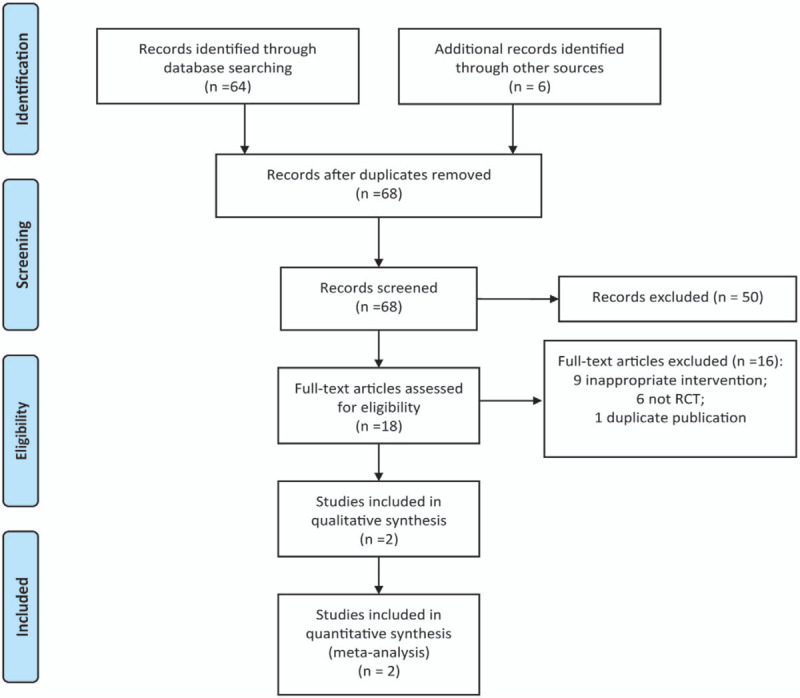
The flow chart of study selection.
3.2. Study characteristics
The characteristics of the 2 included studies are presented in Table 1. All the included 2 RCTs were from China. The dataset consisted of 95 preterm neonates, with 48 in the PS nebulization group and 47 in the invasive PS delivery group. All the PS dose for intervention was 100 mg/kg. For nebulization intervention, the air compressed atomizer[15] and oxygen-driven atomizer[16] were used respectively, but both RCTs used IN-SUR-E technique to perform invasive PS delivery. The demographic baselines of the 2 groups in each included RCT were comparable.
Table 1.
The characteristics of included RCTs.
| Intervention | ||||||
| Study | Country | Populations | Sample (nebulized/invasive) | Nebulized | Invasive | Conclusions |
| Chang, 2012 | China | Preterm infants with NRDS | 63 (32/31) | Curosurf with a dose of 100 mg/kg and 2 ml of normal saline were used, it was mixed and warmed to 37 °C in an air compressed atomizer for continuously inhalation. After the inhalation was completed, oxygen supply pressurized by Balloon were applied. | A dose of 100 mg/kg was given by trachea intubation. After clearing the airway, the drug was instilled through the inserted trachea in different body position, and oxygen supply pressurized by balloon were applied. | Nebulized inhalation of pulmonary surfactant Curosurf might be effective in treatment of NRDS by improving lung function. |
| Guo,2007 | China | Preterm infants with NRDS | 32 (16/16) | The dose of Curosurf was 100 mg/kg. 2 m1 normal saline was used to dissolve the drug and it was warmed to 37°C, then the Curosurf was administered by oxygen-driven atomization. | The dose was 100 mg/kg. After tracheal intubation and clearing of airway secretions, Curosurf was instilled through the inserted trachea in left sided, right sided and supine position respectively, and oxygen supply pressurized by balloon were applied. | Inhalation of pulmonary surfactants can improve the pulmonary ventilation time and diffusion function in a short time and promote oxygenation, decrease the complications and the duration of using ventilator. |
3.3. Quality evaluation
Figures 2 and 3 show the quality of the included studies. Following strict judgments of each included RCT according to the Cochrane handbook, although all of the included RCTs mentioned randomization, no RCT provided a detailed description of the methods used to produce a random sequence. And all included RCTs did not report allocation blinding or the personnel blinding. For the blinding of outcome assessment, all included studies did not report the related information. No selective reporting or other significant biases amongst the 2 included RCTs were found.
Figure 2.
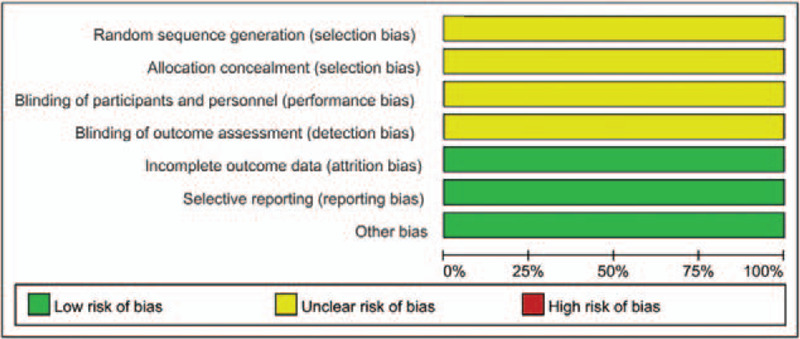
Risk of bias graph.
Figure 3.
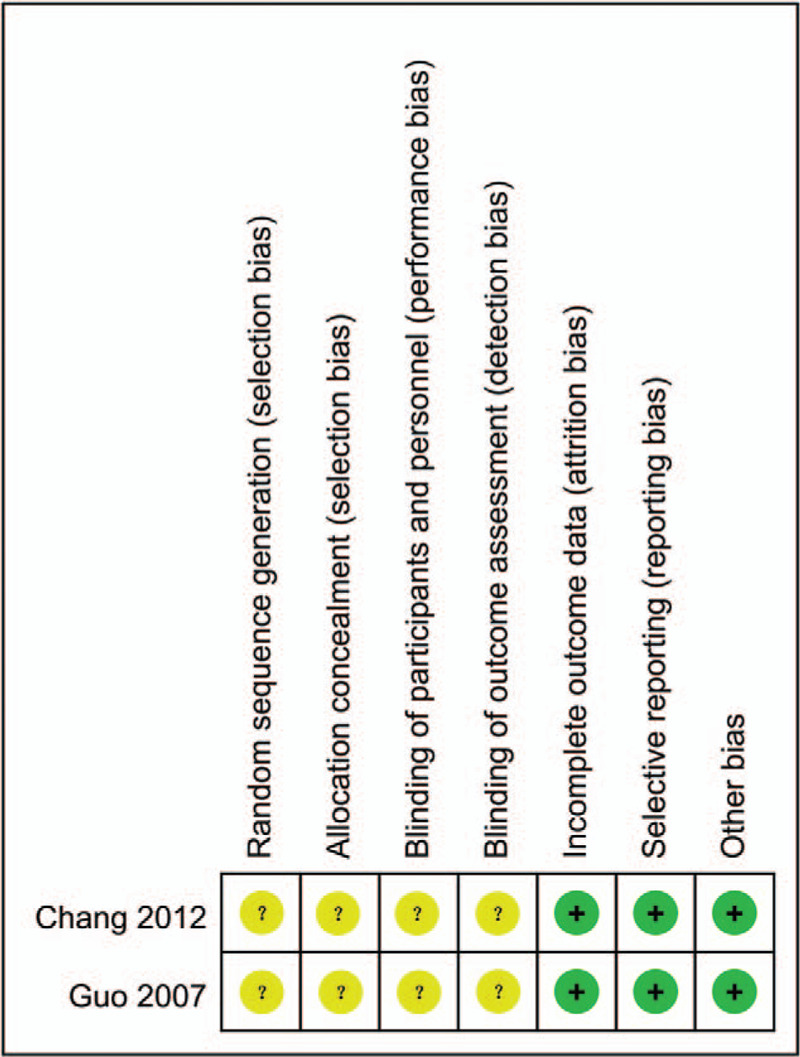
Risk of bias summary.
3.4. Synthesized outcomes
The SpO2 level 1 hour after treatment: Two studies[15,16] reported the SpO2 level 1 hour after treatment among the nebulized and invasive treatment groups, the pooled data from the 2 RCTs revealed that there was no significant difference in the SpO2 level 1 hour after treatment among 2 groups (MD = −0.44, 95% CI −6.01 to 5.12, P = .83, I2 = 0%; Fig. 4).
Figure 4.
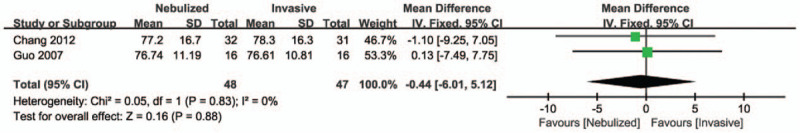
Forest plot for SpO2 level 1 hour after treatment.
The A/APaO2 level 1 hour after treatment: Two studies[15,16] reported the A/APaO2 level 1 hour after treatment among the nebulized and invasive treatment groups, the pooled data from the 2 RCTs revealed that there was no significant difference in the A/APaO2 level 1 hour after treatment among 2 groups (MD = 0.01, 95% CI −0.02 to 0.05, P = .770, I2 = 30%; Fig. 5).
Figure 5.
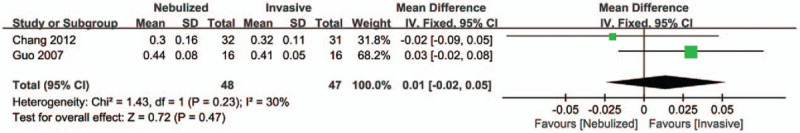
Forest plot for A/APaO2 level 1 hour after treatment.
The duration of mechanical ventilation: One study[16] reported the duration of mechanical ventilation among the nebulized and invasive treatment groups, the data revealed that the duration of mechanical ventilation in the nebulization groups was significantly less than that of invasive group (MD = −30.70, 95% CI −41.45 to 19.95, P < .001; Fig. 6).
Figure 6.

Forest plot for the duration of mechanical ventilation.
3.5. Subgroup and sensitivity analyses
No subgroup analyses were performed in this present study with consideration to the limited data. We attempted to evaluate publication bias by using a funnel plot if 10 or more RCTs were included in an outcome meta-analysis. Limited by the number of included RCTs, we could not perform funnel plot.
Sensitivity analyses, which investigate the influence of 1 study on the overall risk estimate by removing 1 study in each turn, suggested that the overall risk estimates were not substantially changed by any single study.
4. Discussion
Currently, PS therapy has been widely used in the treatment of NRDS. It is well believed that PS therapy is not only beneficial to reduce the mortality of NRDS and the incidence of pneumothorax, but it also can improve lung compliance.[17–19] At present, the INSURE method is the commonly used method for the administration of PS. However, INSURE can lead to barotrauma, volumetric injuries, and many complications related to tracheal intubation.[20,21] Therefore, researchers and clinical health providers have continued to explore more minimally invasive or non-invasive strategies for PS administration. LISA and minimally invasive surfactant therapy (MIST) have been recommended for clinical use in recently years with regards to the advantages that they can significantly reduce the duration of mechanical ventilation and respiratory tract damage.[21–23] However, LISA and MIST are still invasive, and it may require extra sedation during the process.[4,24] PS delivered by nebulization would be truly non-invasive, but its effects and safety merit further verification. This present study has compared the use of PS by nebulization and invasive methods, we have not found significant differences in the SpO2 level, A/APaO2 level 1 hour after treatment for nebulization and invasive methods, the PS nebulization seems to be more superior to invasive methods in reducing the duration of mechanical ventilation. Nevertheless, considering that this present meta-analysis only has included 2 RCTs, those findings should be treated with cautions.
The PS are commonly instilled through the related tubes to lung tissues, but there are several shortcomings such as invasive damage, uneven drug distribution.[25] Besides, it has commonly required tracheal intubation, which is difficult in generalization for community hospitals or clinics without related conditions or techniques. Furthermore, some neonates may have strong cough reflexes when the PS are instilled, the PS can be ejected during the administration process, causing the waste of PS, and affecting curative effects.[26] Technically, the PS can be directly delivery to the respiratory tract through nebulization with consideration that the particles formed during the nebulization are evenly distributed and more suitable for deposition in the respiratory tract, resulting in better therapeutic effects. Besides, PS nebulization is a non-invasive operation, which can avoid the complications such as respiratory tract injury and bronchopulmonary dysplasia caused by invasive operation.[27,28] Previous studies[29–31] have showed that the accumulated exogenous PS in the alveolar of inhaled PS can reach (12.2 ± 1.2)% of the dose, which is 4 times that of the tracheal intubation method. It may be explained that the distribution pattern of PS in alveoli in the nebulization intervention is better. To our knowledge, there are several reports that have favored the nebulized PS in the treatment of NRDS,[32] severe neonatal pneumonia,[33] and meconium aspiration syndrome.[34]
The efficacy of PS nebulization warrants discreet consideration. Linner et al[35] have used piglets animal model to evaluate the deposition rate of e-Flmv nebulizer atomized PS in the lungs. The results have showed that the median lung deposition of inhaled surfactant was 5% via mask, 14% via prongs, and 45% via tracheal tube. It must be aware that nebulized PS is still limited by many issues, such as the diameter of the aerosol, the stability and the potential loss of the PS during the nebulization, the preparation of alveolar surfactants suitable for atomization etc.[25] Previous meta-analysis[36] evaluated the use of inhalation or instillation steroids to prevent bronchopulmonary dysplasia in preterm infants, and it was concluded that early administration of ICS and PS is an effective and safe option for preterm infants with NRDS in preventing BPD and reducing mortality, decreasing the additional PS usage, especially for the ICS intratracheal instillation subgroup. Further researches are needed on preparing a nebulizer with a PS formulation suitable for nebulization and a higher lung deposition rate.
With the development of vibrating membrane atomizers, nebulizing PS for clinical use are possible. One clinical trial has shown that atomizing surfactants on CPAP can reduce the need for MV compared to CPAP alone, but this finding was limited to the subgroup of more mature infants over 32 to 33 weeks.[9] Further testing of PS nebulization is ongoing. PS have also been administered through laryngeal mask, and a clinical trial has shown that this reduces the need for intubation and mechanical ventilation.[22] However, the size of currently available laryngeal masks limits this method to relatively mature premature infants, the routine use for smaller infants may lead to greater risk of BPD,[11] which is currently not recommended in latest guideline.[1]
Several limitations in this present meta-analysis must be considered. Firstly, we only included 2 RCTs for data synthesis, and the sample size was small, it might be not power enough to detect the group differences. Besides, the potential risk of bias in the allocation concealment process, blinding of researchers, blinding of outcome assessments, or selective reporting among the included 2 RCTs must be considered, future RCTs with stricter design are needed. Secondly, the heterogeneity may be existed considering that the nebulization methods among the 2 included RCT, which one is air compressed atomizer and the other one is oxygen-driven nebulizer, the effects of nebulization can be different. Finally, all the reported RCTs were from China, the population and area bias can be existed, more related studies in different countries and populations are highlighted.
In conclusion, the results of this present meta-analysis have revealed that there are no differences in the SpO2 level, A/APaO2 level 1 hour after treatment for nebulization and invasive methods, the PS nebulization seems to be more superior to invasive methods in reducing the duration of mechanical ventilation. However, currently given the limited evidence on the PS nebulization, the superiority of PS nebulization in the treatment of NRDS is not justified, it can be a potential PS administration method, further studies on the effects and safety of PS nebulization are warranted in the future.
Author contributions
HR and ZW designed research; HR, YB, ZW and XC conducted research; ZW, HR and CC analyzed data; ZW wrote the first draft of manuscript; HR and FL had primary responsibility for final content. All authors read and approved the final manuscript.
Conceptualization: Hui Rong, Ying Bao, Zunjia Wen.
Data curation: Zunjia Wen, Xiuli Chen, Cen Chen.
Formal analysis: Hui Rong, Fang Li.
Funding acquisition: Ying Bao, Zunjia Wen.
Investigation: Hui Rong, Ying Bao, Cen Chen, Fang Li.
Methodology: Zunjia Wen, Xiuli Chen.
Project administration: Hui Rong, Cen Chen.
Resources: Hui Rong, Ying Bao, Zunjia Wen, Xiuli Chen, Fang Li.
Software: Zunjia Wen, Cen Chen.
Supervision: Hui Rong, Cen Chen, Fang Li.
Validation: Hui Rong.
Visualization: Fang Li.
Writing – original draft: Hui Rong, Fang Li.
Footnotes
Abbreviations: CIs = confidence intervals, MDs = mean differences, NRDS = neonatal respiratory distress syndrome, OR = odd ratios, PS = pulmonary surfactant, RCTs = randomized controlled trials.
How to cite this article: Rong H, Bao Y, Wen Z, Chen X, Chen C, Li F. Nebulized versus invasively delivered surfactant therapy for neonatal respiratory distress syndrome: a systematic review and meta-analysis. Medicine. 2020;99:48(e23113).
HR, YB, and ZW contributed equally in this study.
All data generated or analyzed during this study are included in this published article.
The authors declare that they have no competing interests.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
N/A = not available, NRDS = neonatal respiratory distress syndrome.
References
- [1].Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology 2019;115:432–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gouyon B, Martin-Mons S, Iacobelli S, et al. Characteristics of prescription in 29 Level 3 Neonatal Wards over a 2-year period (2017-2018). An inventory for future research. PLoS One 2019;14:e0222667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Bisschop B, Derriks F, Cools F. Early predictors for INtubation-SURfactant-Extubation failure in preterm infants with neonatal respiratory distress syndrome: a systematic review. Neonatology 2019;1–3. [DOI] [PubMed] [Google Scholar]
- [4].Kribs A. Minimally invasive surfactant therapy and noninvasive respiratory support. Clin Perinatol 2016;43:755–71. [DOI] [PubMed] [Google Scholar]
- [5].Jasani B, Kabra N, Nanavati R. Surfactant replacement therapy beyond respiratory distress syndrome in neonates. Indian Pediatr 2016;53:229–34. [DOI] [PubMed] [Google Scholar]
- [6].Sardesai S, Biniwale M, Wertheimer F, et al. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr Res 2017;81:240–8. [DOI] [PubMed] [Google Scholar]
- [7].Aldana-Aguirre JC, Pinto M, Featherstone RM, et al. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017;102:F17–23. [DOI] [PubMed] [Google Scholar]
- [8].Kribs A, Roll C, Gopel W, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr 2015;169:723–30. [DOI] [PubMed] [Google Scholar]
- [9].Minocchieri S, Berry CA, Pillow JJ, et al. Nebulised surfactant to reduce severity of respiratory distress: a blinded, parallel, randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2019;104:F313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roberts KD, Brown R, Lampland AL, et al. Laryngeal mask airway for surfactant administration in neonates: a randomized, controlled trial. J Pediatr 2018;193:40–6. e41. [DOI] [PubMed] [Google Scholar]
- [11].Bansal SC, Caoci S, Dempsey E, et al. The Laryngeal mask airway and its use in neonatal resuscitation: a critical review of where we are in 2017/2018. Neonatology 2018;113:152–61. [DOI] [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W264. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dawson DV, Pihlstrom BL, Blanchette DR. Understanding and evaluating meta-analysis. J Am Dent Assoc 2016;147:264–70. [DOI] [PubMed] [Google Scholar]
- [15].Chang S, Pei Z, Cheng X, et al. Nebulized inhalation of pulmonary surfactant in treatment of neonatal respiratory distress syndrome. J Public Med Forum 2012;16:1648–50. [Google Scholar]
- [16].Guo J, Lu Y, Feng X, et al. The clinical study of treatment effects of inhalation of pulmonary surfactant on Neonatal Respiratory Distress Syndrome. Matern Child Health Care China 2007;22:4827–9. [Google Scholar]
- [17].McPherson C, Wambach JA. Prevention and treatment of respiratory distress syndrome in preterm neonates. Neonatal Netw 2018;37:169–77. [DOI] [PubMed] [Google Scholar]
- [18].Foligno S, Luca D. Porcine versus bovine surfactant therapy for RDS in preterm neonates: pragmatic meta-analysis and review of physiopathological plausibility of the effects on extra-pulmonary outcomes. Respir Res 2020;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dani C, Mosca F, Vento G, et al. Effects of surfactant treatment in late preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med 2018;31:1259–66. [DOI] [PubMed] [Google Scholar]
- [20].Dunn MS, Kaempf J, de Klerk A, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128:e1069–76. [DOI] [PubMed] [Google Scholar]
- [21].Niemarkt HJ, Hutten MC, Kramer BW. Surfactant for respiratory distress syndrome: new ideas on a familiar drug with innovative applications. Neonatology 2017;111:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vannozzi I, Ciantelli M, Moscuzza F, et al. Catheter and laryngeal mask endotracheal surfactant therapy: the CALMEST approach as a novel MIST technique. J Matern Fetal Neonatal Med 2017;30:2375–7. [DOI] [PubMed] [Google Scholar]
- [23].Gengaimuthu K. Minimally invasive surfactant therapy using a 2.0 mm uncuffed endotracheal tube as the conduit: an easily adaptable technique. Cureus 2019;11:e5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dekker J, Lopriore E, van Zanten HA, et al. Sedation during minimal invasive surfactant therapy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2019;104:F378–83. [DOI] [PubMed] [Google Scholar]
- [25].Guagliardo R, Perez-Gil J, De Smedt S, et al. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J Control Release 2018;291:116–26. [DOI] [PubMed] [Google Scholar]
- [26].Hidalgo A, Cruz A, Perez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur J Pharm Biopharm 2015;95(Pt A):117–27. [DOI] [PubMed] [Google Scholar]
- [27].Yeh TF, Chen CM, Wu SY, et al. Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 2016;193:86–95. [DOI] [PubMed] [Google Scholar]
- [28].Klotz D, Porcaro U, Fleck T, et al. European perspective on less invasive surfactant administration-a survey. Eur J Pediatr 2017;176:147–54. [DOI] [PubMed] [Google Scholar]
- [29].Hutten MC, Kuypers E, Ophelders DR, et al. Nebulization of Poractant alfa via a vibrating membrane nebulizer in spontaneously breathing preterm lambs with binasal continuous positive pressure ventilation. Pediatr Res 2015;78:664–9. [DOI] [PubMed] [Google Scholar]
- [30].Bianco F, Ricci F, Catozzi C, et al. From bench to bedside: in vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir Res 2019;20:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Milesi I, Tingay DG, Zannin E, et al. Intratracheal atomized surfactant provides similar outcomes as bolus surfactant in preterm lambs with respiratory distress syndrome. Pediatr Res 2016;80:92–100. [DOI] [PubMed] [Google Scholar]
- [32].Sood BG, Cortez J, Kolli M, et al. Aerosolized surfactant in neonatal respiratory distress syndrome: phase I study. Early Hum Dev 2019;134:19–25. [DOI] [PubMed] [Google Scholar]
- [33].Tsybul’kin EK, Rozenberg OA, Seiliev AA, et al. Our experience in the use of a Russian preparation of pulmonary surfactant in the treatment of acute respiratory distress syndrome and severe pneumonia in children. Anesteziol Reanimatol 1999;61–5. [PubMed] [Google Scholar]
- [34].Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 1996;97:48–52. [PubMed] [Google Scholar]
- [35].Linner R, Perez-de-Sa V, Cunha-Goncalves D. Lung deposition of nebulized surfactant in newborn piglets. Neonatology 2015;107:277–82. [DOI] [PubMed] [Google Scholar]
- [36].Zhong YY, Li JC, Liu YL, et al. Early intratracheal administration of corticosteroid and pulmonary surfactant for preventing bronchopulmonary dysplasia in preterm infants with neonatal respiratory distress syndrome: a meta-analysis. Curr Med Sci 2019;39:493–9. [DOI] [PubMed] [Google Scholar]


