Abstract
To evaluate arterial fluorodeoxyglucose (FDG) uptake as a marker of arterial inflammation in multiple vascular beds in patients treated with anthracycline-based chemotherapy for Hodgkin lymphoma (HL).
We used maximum standardized uptake value (SUVmax) and target-to-background ratio (TBR) to quantify arterial FDG uptake in the carotid artery, ascending aorta, abdominal aorta, and femoral artery obtained on positron emission tomography/computed tomography (PET/CT) imaging performed at baseline before chemotherapy and after completion of chemotherapy in patients with HL treated with an anthracycline-containing regimen. We compared the SUVmax and TBR obtained at baseline with that obtained post-chemotherapy for each arterial bed to evaluate the effect of anthracycline-based chemotherapy. We evaluated the effect of cardiovascular risk factors such as human immunodeficiency virus (HIV) infection, smoking, hypertension, and diabetes on the changes in SUVmax and TBR seen in the different arterial beds after anthracycline-based chemotherapy.
Fifty-two patients were included with a mean age of 34.56 ± 10.19 years. There were 33 males, and 18 patients were HIV-infected. The mean interval between completion of chemotherapy and follow-up flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) scan was 65 weeks. We found no significant difference in arterial FDG uptake measured by SUVmax and TBR in all arterial beds between the pre- and post-chemotherapy FDG PET/CT. There was no significant impact of HIV infection, smoking, and hypertension on the changes in arterial FDG uptake following treatment with anthracycline-based chemotherapy.
In patients with HL who were treated with anthracycline-based chemotherapy, we found no significant increase in arterial inflammation measured by FDG PET/CT after an average follow-up period of about 65 weeks since completion of chemotherapy.
Keywords: arterial inflammation, anthracyclines, FDG PET/CT, Hodgkin lymphoma
1. Introduction
Hodgkin lymphoma (HL) is a malignant disease with an excellent response to therapy. Ten-year overall survival is reported in over 80% of patients treated for HL.[1,2] Over 90% of patients treated for HL in their childhood or adolescence survive into adulthood.[3] Anthracyclines are a group of anti-cancer chemotherapeutic agents that form the backbone of HL treatment. Avoiding them in HL treatment leads to less favorable treatment outcome.[4] Despite their efficacy, anthracyclines increase cardiovascular mortality and morbidity of patients in the long run. One of the pathogenic mechanisms by which anthracyclines predispose to atherosclerotic cardiovascular disease (ASCVD) is by causing impairment in the repair of endothelial injury. Anthracycline agents cause an increase in the circulating level of functionally incapacitated endothelial progenitor cells,[5] which are responsible for the repair of endothelial injury through the process of re-endothelization after injury.[6] Unrepaired endothelial injury is the bedrock lesion for the formation of vascular atheroma formation.
Long-term survival of patients treated for HL and the negative impact of anthracycline on vascular endothelial function, therefore, expose these patients to significant cardiovascular morbidity and mortality. A recent study with an extended follow-up of 40 years post HL treatment reported a 4- to 7-fold increased risk of coronary artery disease among HL survivors compared with the general population, which translates to 857 excess cardiovascular events per 10,000 person-years.[7]
Inflammation is the hallmark process that drives vascular atheroma formation, progression, and its catastrophic completion.[8] The prominent role played by inflammation in ASCVD has formed the basis for the keen interest in the use of anti-inflammatory agents as a primary and secondary preventive therapeutic intervention in many recent randomized trials.[9,10] Florine-18 fluorodeoxyglucose (FDG) is a radioactive analog of glucose which is trapped by cancer cells and activated inflammatory cells. Photons emitted when FDG undergoes radioactive decay can be imaged using hybrid positron emission tomography and computed tomography (PET/CT) scanner. Flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) has, therefore, emerged in the last 2 decades as a sensitive tool for non-invasive imaging of biological processes, including inflammation, infection, and neoplasm. The intensity of FDG uptake in the vessel has been shown to correlate with the level of vascular macrophage invasion.[11,12] Abdelbaky et al showed, in a longitudinal FDG PET/CT study, that arterial inflammation precedes subsequent calcification in the same location as a marker of plaque progression.[13] The level of inflammation in the vessel in general or in an atherosclerotic lesion in specific correlates with the risk of cardiovascular events in a patient.[14–16] FDG PET/CT is the most accurate imaging modality for the staging and re-staging of HL and is the most commonly used imaging modality for these indications in routine clinical practice.[17,18] FDG PET/CT can, therefore, serve the dual role for evaluating HL and vascular inflammation as a risk for ASCVD in patients treated with anthracycline-based chemotherapy. To our knowledge, no published study has evaluated the clinical utility of FDG PET/CT in characterizing arterial inflammation in the patients treated with anthracycline-based chemotherapy for HL. In this study, we aimed to evaluate arterial FDG uptake as a marker of arterial inflammation in multiple vascular beds of patients treated with anthracycline-based chemotherapy for HL. We hypothesized that arterial FDG uptake would be higher on post-chemotherapy FDG PET/CT compared with pre-therapy FDG PET/CT.
2. Materials and methods
2.1. Patients
We performed a retrospective review of FDG PET/CT scans of patients referred to the Department of Nuclear Medicine at Steve Biko Academic Hospital, Pretoria, between August 2015 and September 2019 for baseline staging and post-chemotherapy assessment of HL. We included patients who were aged 18 years and older and were treated with a standard course of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD). We excluded patients in whom arterial FDG uptake could not be quantified without interference from adjacent lymphoma. Other exclusion criteria were renal failure (estimated glomerular filtration rate of <60 mL/min/m2), treatment with chemotherapy regime other than ABVD, additional radiotherapy, technically inadequate scans, ongoing use of steroid or other anti-inflammatory medications, and suspected or confirmed vasculitis. We reviewed the patients’ hospital records to obtain their demographic information, the associated co-morbidities, and social history. Patients were defined as smokers if they smoke at least a stick of cigarette per day. Patients were classified as hypertensive or diabetic if they self-identify as such based on previous hospital diagnosis or they are on treatment for these chronic medical conditions. Our institutional review board approved the study and waived the need for patients’ consent due to the retrospective design of this study.
2.2. FDG PET/CT scan imaging
FDG PET/CT scan imaging was performed as previously described and according to published guidelines.[19,20] Briefly, all patients had a minimum of 4 hours of fasting. The blood sugar level was less than 11.1 mmol/L in all patients at the time of FDG injection. The activity of FDG administered was adjusted for patient weight. Imaging commenced 60 minutes after the intravenous administration of FDG. Imaging was acquired on a Biograph 40 Truepoint hybrid PET/CT scanner (Siemens Medical Solution, Illinois). Imaging was acquired from vertex to mid-thigh. PET imaging was acquired in 3D mode at 3 minutes per bed position with a 50% overlap. We performed image reconstruction using the ordered subset expectation maximization algorithm (4 iterations and 8 subsets) with a Gaussian filter applied at full-width half-maximum of 5.0 mm.
2.3. Image analysis
We performed image analysis on a dedicated workstation equipped with a Syngo.via software. We used the maximum standardized uptake value (SUVmax) and the target-to-background ratio (TBR) as the parameters for arterial FDG uptake quantification following the recommendation of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM).[21] To evaluate systemic arterial inflammation using vascular FDG uptake, we selected the distal common carotid artery, the ascending aorta, the infra-renal abdominal aorta, and the proximal femoral artery as the representative arterial beds on both the baseline and post-chemotherapy FDG PET/CT scans. We determined SUVmax and TBR at these arterial beds. For SUVmax, we drew multiple regions of interest (ROI) around the arterial bed of interest and obtained the mean of the SUVmax measurements obtained from each ROI. For TBR (target-to-background ratio), the target represents the mean SUVmax obtained in the arterial bed of interest, while background represents the mean of SUVmean obtained from multiple ROI drawn in the lumen of the vein adjacent to the arterial bed of interest. For the common carotid artery, venous SUVmean was obtained from the adjacent internal jugular vein, superior vena cava for the ascending aorta, the inferior vena cava for the abdominal aorta, and the femoral vein for the femoral artery. TBR was obtained by dividing the mean arterial SUVmax by the mean venous SUVmean. In patients who had multiple FDG PET/CT scans after completion of chemotherapy, the analysis was done on the latest scan to obtain the maximum interval between completion of chemotherapy and follow-up FDG PET/CT.
2.4. Statistical analysis
We expressed continuous variables as mean ± standard deviation (SD). We expressed categorical data as proportions (percentages). We used the paired samples T test to compare SUVmax and TBR obtained at the 4 arterial beds of interest between the pre-chemotherapy and post-chemotherapy FDG PET/CT scans. We also compared the patients’ parameters such as fasting blood sugar, the activity of FDG administered for PET/CT scan, patients’ weight, and patients’ body mass index between the pre- and post-chemotherapy FDG PET/CT scans. We performed sub-group analysis in patients with human immunodeficiency virus (HIV) infection by comparing pre- and post-chemotherapy variables using independent samples T test. We evaluated the impact of specific cardiovascular risk factors, including smoking, hypertension, and diabetes mellitus, on the changes in arterial FDG uptake in response to chemotherapy. All statistical analyses were two-tailed, and P-value <.05 was considered statistically significant. We performed statistical analyses using the IBM SPSS Statistics 21.0 (IBM Corp, Armonk, New York).
3. Results
A total of 52 patients were included with a mean age of 34.56 ± 10.19 years. There were 33 males, and 18 patients were HIV-infected. The mean duration between the pre- and post-chemotherapy FDG PET/CT scan was 24.06 months, while the average interval between completion of chemotherapy and follow-up FDG PET/CT was 65 weeks. Table 1 shows the baseline clinical and demographic characteristics of the patients included.
Table 1.
Baseline clinical and demographic characteristics of the patients.
| Variable | Frequency | Percent (%) |
| Age (yr) | ||
| Mean ± SD | 34.56 ± 10.19 | |
| Range | 18–60 | |
| Gender | ||
| Male | 33 | 63.5 |
| Female | 19 | 36.5 |
| HIV | ||
| Yes | 18 | 34.6 |
| No | 34 | 65.4 |
| Smoking | ||
| Yes | 15 | 28.8 |
| No | 37 | 71.2 |
| HTN | ||
| Yes | 3 | 5.8 |
| No | 49 | 94.2 |
| DM | ||
| Yes | 2 | 3.8 |
| No | 50 | 96.2 |
| Weight (kg) | ||
| Mean ± SD | 70.62 ± 17.90 | |
| BMI (kg/m2) | ||
| Mean ± SD | 25.23 ± 5.98 | |
| CD4 (cells/μL) | ||
| Mean ± SD | 282.44 ± 131.94 | |
| Interval between the 2 PET scans (mo) | ||
| Mean ± SD | 24.06 ± 21.53 | |
| Interval between completion of chemotherapy and post-chemo-PET scan (wk) | ||
| Mean ± SD | 64.67 ± 88.41 | |
Patients’ weight and, consequently, their body mass index (BMI) were significantly higher at the time of post-chemotherapy FDG PET/CT compared with pre-chemotherapy FDG PET/CT. Fasting blood sugar at the time of FDG administration was similar between pre- and post-chemotherapy FDG PET/CT scans. Similarly, the activity of FDG administered for both pre- and post-chemotherapy scans was comparable. We found no significant difference in the SUVmax and TBR measured in the carotid artery, ascending aorta, abdominal aorta, and femoral artery between the pre- and post-chemotherapy FDG PET/CT scans. Table 2 shows a detailed comparison between pre- and post-chemotherapy FDG PET/CT scans.
Table 2.
Comparison of parameters obtained at pre-chemotherapy and post-chemotherapy FDG PET/CT scans.
| Variable | Pre-chemo | Post-chemo | t | P |
| Mean ± SD | Mean ± SD | |||
| Weight (kg) | 70.62 ± 17.90 | 74.51 ± 14.38 | −3.092 | .003∗ |
| BMI (kg/m2) | 25.23 ± 5.98 | 26.73 ± 5.25 | −3.230 | .002∗ |
| FBS (mmol/L) | 5.94 ± 1.66 | 5.54 ± 1.20 | 1.461 | .150 |
| Administered FDG (mCi) | 8.78 ± 2.32 | 9.05 ± 2.15 | −1.281 | .206 |
| Ascending aorta | ||||
| SUVmax | 2.67 ± 0.75 | 2.66 ± 0.65 | 0.144 | .886 |
| TBR | 1.77 ± 0.38 | 1.83 ± 0.48 | −0.765 | .448 |
| Carotid artery | ||||
| SUVmax | 2.02 ± 0.59 | 2.01 ± 0.53 | 0.039 | .969 |
| TBR | 1.44 ± 0.35 | 1.45 ± 0.41 | −0.076 | .940 |
| Abdominal aorta | ||||
| SUVmax | 2.63 ± 0.81 | 2.47 ± 0.61 | 1.363 | .179 |
| TBR | 1.81 ± 0.46 | 1.69 ± 0.35 | 1.410 | .164 |
| Femoral artery | ||||
| SUVmax | 1.48 ± 0.49 | 1.50 ± 0.47 | −0.314 | .755 |
| TBR | 1.28 ± 0.31 | 1.38 ± 0.36 | −1.390 | .171 |
We compared HIV-infected patients, and those without regarding changes in baseline characteristics and PET-derived variables. There was no significant difference in the weight and BMI of HIV-infected patients and those who were not. Similarly, there was no significant difference in the activity of FDG administered for PET/CT scan and the blood sugar levels at the time of FDG administration between HIV-infected patients versus HIV-uninfected patients. Table 3 shows comparative data between HIV-infected and HIV-uninfected patients regarding changes in patients’ and PET-derived variables between pre- and post-chemotherapy variables.
Table 3.
Comparison of the change in parameters between the 2 FDG PET/CT scans according to patients’ HIV serostatus.
| HIV | ||||
| Mean ± SD | Mean ± SD | |||
| Change in parameters | Yes (n = 18) | No (n = 34) | t | P |
| Weight (kg) | 2.77 ± 5.92 | 4.49 ± 10.41 | −0.648 | .520 |
| BMI (kg/m2) | 1.03 ± 2.10 | 1.75 ± 3.86 | −0.738 | .464 |
| FBS (mmol/L) | 0.05 ± 1.2 | −0.63 ± 2.21 | 1.203 | .235 |
| Administered FDG (mCi) | −0.26 ± 1.37 | 0.55 ± 1.55 | −1.875 | .067 |
| Ascending aorta | ||||
| SUVmax | 0.02 ± 0.81 | −0.04 ± 0.94 | 0.208 | .836 |
| TBR | 0.07 ± 0.42 | 0.05 ± 0.61 | 0.085 | .933 |
| Carotid artery | ||||
| SUVmax | −0.13 ± 0.59 | 0.06 ± 0.92 | −0.803 | .426 |
| TBR | 0.01 ± 0.46 | 0.00 ± 0.57 | 0.087 | .931 |
| Abdominal aorta | ||||
| SUVmax | −0.18 ± 1.08 | −0.15 ± 0.72 | −0.122 | .904 |
| TBR | −0.06 ± 0.66 | −0.16 ± 0.62 | 0.519 | .606 |
| Femoral artery | ||||
| SUVmax | −0.10 ± 0.36 | 0.09 ± 0.63 | −1.166 | .249 |
| TBR | −0.05 ± 0.45 | 0.18 ± 0.53 | −1.548 | .128 |
We evaluated the effects of the presence of certain ASCVD risk factors on the differences in FDG PET-derived variables between the 2 scans. We found no significant difference between patients who were smokers versus those who were not (Table 4). Regarding systemic hypertension, we did not find any significant difference between hypertensive and normotensive patients (Table 5). Among patients who were diabetic, there was a mean decline in patients’ weight of 10.10 kg between pre- and post-chemotherapy FDG PET/CT scan while weight increased by a mean of 4.46 kg among non-diabetic patients (P = .025). Consequently, there was a significant difference in weight and BMI between patients grouped based on a history of diabetes mellitus between the 2 FDG PET/CT scans. We found no significant difference in the activity of FDG administered and the blood sugar level between patients who were diabetic and those who were not (Table 6). Among the changes in PET-derived variables between the pre- and post-therapy FDG PET/CT, patients who were diabetic, had a decline in SUVmax measured at the ascending aorta while non-diabetic patients had an increase in the same parameter (P = .035). In the femoral artery, diabetic patients had a significant rise in TBR compared with non-diabetic patients, P = .002 (Table 6). The rest of the quantitative parameters were not significantly different between diabetics and non-diabetics.
Table 4.
Comparison between patients grouped according to smoking history.
| Smoking | ||||
| Yes | No | |||
| Change in parameters | Mean ± SD | Mean ± SD | t | P |
| Weight (kg) | 2.97 ± 12.93 | 4.27 ± 7.17 | −0.462 | .646 |
| BMI (kg/m2) | 1.21 ± 4.65 | 1.62 ± 2.73 | −0.398 | .692 |
| FBS (mmol/L) | −0.51 ± 2.28 | −0.35 ± 1.83 | −0.263 | .794 |
| Administered FDG (mCi) | 0.33 ± 1.93 | 0.25 ± 1.36 | 0.185 | .854 |
| Ascending aorta | ||||
| SUVmax | 0.08 ± 0.79 | −0.06 ± 0.94 | 0.482 | .632 |
| TBR | 0.08 ± 0.79 | −0.06 ± 0.94 | 0.180 | .858 |
| Carotid artery | ||||
| SUVmax | −0.19 ± 0.81 | 0.07 ± 0.82 | −1.065 | .292 |
| TBR | −0.21 ± 0.57 | 0.09 ± 0.49 | −1.951 | .057 |
| Abdominal aorta | ||||
| SUVmax | −0.10 ± 0.88 | −0.18 ± 0.85 | 0.302 | .764 |
| TBR | −0.22 ± O.62 | −0.08 ± 0.63 | −0.684 | .497 |
| Femoral artery | ||||
| SUVmax | 0.13 ± 0.49 | −0.02 ± 0.58 | 0.850 | .399 |
| TBR | 0.16 ± 0.72 | 0.07 ± 0.40 | 0.534 | .596 |
Table 5.
Comparison between patients grouped according to the history of systemic hypertension.
| Hypertension | ||||
| Yes | No | |||
| Change in parameters | Mean ± SD | Mean ± SD | t | P |
| Weight (kg) | 4.33 ± 9.95 | 3.87 ± 9.14 | 0.085 | .932 |
| BMI (kg/m2) | 1.71 ± 3.74 | 1.49 ± 3.37 | 0.108 | .915 |
| FBS (mmol/L) | 1.30 ± 2.08 | −0.50 ± 1.91 | 1.576 | .121 |
| Administered FDG (mCi) | 1.27 ± 1.27 | 0.21 ± 1.53 | 1.168 | .248 |
| Ascending aorta | ||||
| SUVmax | 0.51 ± 1.15 | −0.05 ± 0.88 | 1.049 | .299 |
| TBR | −0.45 ± 0.31 | 0.09 ± 0.55 | −1.680 | .099 |
| Carotid artery | ||||
| SUVmax | 0.55 ± 1.41 | −0.04 ± 0.78 | 1.210 | .232 |
| TBR | 0.09 ± 0.23 | 0.00 ± 0.54 | 0.281 | .780 |
| Abdominal aorta | ||||
| SUVmax | 0.51 ± 1.13 | −0.20 ± 0.83 | 1.411 | .164 |
| TBR | −0.01 ± 0.15 | −0.13 ± 0.64 | 0.327 | .745 |
| Femoral artery | ||||
| SUVmax | 0.40 ± 0.85 | 0.00 ± 0.53 | 1.219 | .288 |
| TBR | 0.13 ± 0.07 | 0/.10 ± 0.52 | 0.112 | .911 |
Table 6.
Comparison between patients grouped according to the history of diabetes mellitus.
| Diabetes mellitus | ||||
| Yes | No | |||
| Change in parameters | Mean ± SD | Mean ± SD | t | P |
| Weight (kg) | −10.10 ± 2.69 | 4.46 ± 8.79 | −2.315 | .025∗ |
| BMI (kg/m2) | −3.39 ± 0.93 | 1.69 ± 3.27 | −2.183 | .034∗ |
| FBS (mmol/L) | −0.10 ± 3.68 | −0.41 ± 1.91 | 0.216 | .830 |
| Administered FDG (mCi) | −0.95 ± 0.21 | 0.32 ± 1.54 | −1.158 | .252 |
| Ascending aorta | ||||
| SUVmax | −1.32 ± 0.99 | 0.03 ± 0.86 | −2.170 | .035∗ |
| TBR | 0.68 ± 1.02 | 0.03 ± 0.53 | 1.660 | .103 |
| Carotid artery | ||||
| SUVmax | −0.21 ± 0.04 | 0.00 ± 0.84 | −0.349 | .728 |
| TBR | −0.11 ± 1.72 | 0.01 ± 0.48 | −0.298 | .767 |
| Abdominal aorta | ||||
| SUVmax | −0.97 ± 0.79 | −0.13 ± 0.85 | −1.372 | .176 |
| TBR | 0.09 ± 0.47 | −0.13 ± 0.63 | 0.474 | .637 |
| Femoral artery | ||||
| SUVmax | 0.48 ± 0.86 | 0.01 ± 0.54 | 1.181 | .243 |
| TBR | 1.16 ± 1.07 | 0.06 ± 0.44 | 3.294 | .002∗ |
In order to determine the trends in arterial FDG uptake after anthracycline-based chemotherapy, we grouped patients according to the interval between completion of chemotherapy and the time of follow-up FDG PET/CT (at increments of 4 weeks). We plotted SUVmax and TBR obtained on pre- and post-chemotherapy for each arterial bed. At most time points, SUVmax and TBR were lower on the post-chemotherapy FDG PET/CT compared with the pre-chemotherapy scan. In patients who had their post-chemotherapy FDG PET/CT at 24 weeks or more after completion of chemotherapy, SUVmax, and TBR obtained at this follow-up scan were similar or higher than baseline pre-chemotherapy SUVmax and TBR. Figures 1 and 2 show the trends over time of SUVmax and TBR in patients grouped according to the interval between completion of chemotherapy and the follow-up FDG PET/CT scan. Figure 3 shows how TBR and SUVmax were computed.
Figure 1.
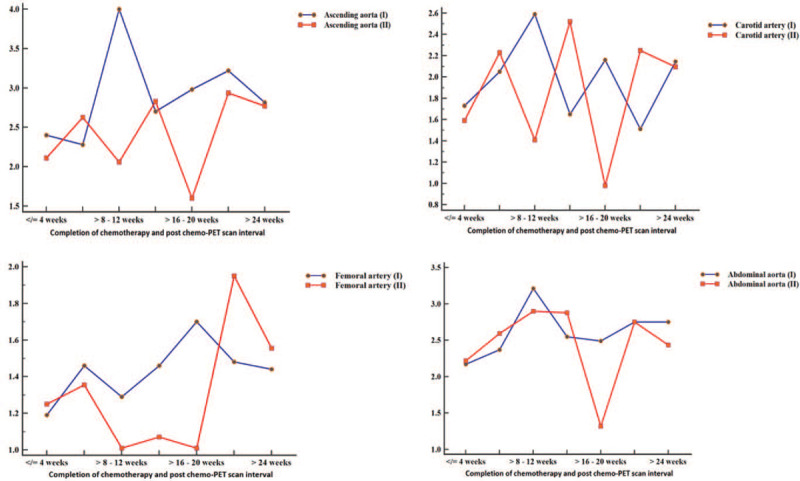
Time trend in SUVmax in patients grouped according to the interval between completion of chemotherapy and the time of follow-up FDG PET/CT. The blue curve represents the SUVmax obtained on the pre-chemotherapy FDG PET/CT, while the red curve represents SUVmax obtained in different groups at post-chemotherapy FDG PET/CT. At more than half of the time-points in the 4 arterial beds, SUVmax was lower for the post-therapy FDG PET/CT compared with pre-chemotherapy FDG PET/CT scan. In the group in whom post-chemotherapy FDG PET/CT was obtained more than 24 wk after completion of chemotherapy, SUVmax in the arterials from the post-chemotherapy FDG PET/CT equaled (ascending aorta and carotid artery) or surpassed (femoral artery) that of the pre-chemotherapy FDG PET/CT.
Figure 2.
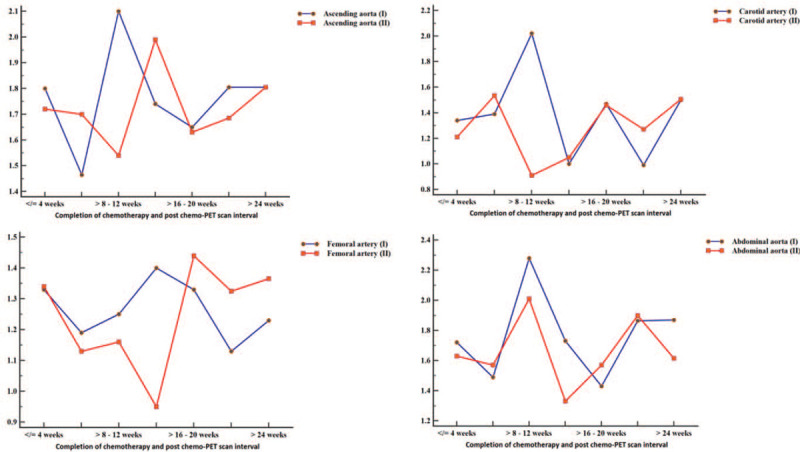
Time trend in TBR in patients grouped according to the interval between completion of chemotherapy and the time of follow-up FDG PET/CT scan. The blue curve represents the TBR obtained on the pre-chemotherapy FDG PET/CT, while the red curve represents TBR obtained in different groups at post-chemotherapy FDG PET/CT. Like for SUVmax shown in Fig. 1, TBR was lower for the post-therapy FDG PET/CT compared with pre-chemotherapy FDG PET/CT in multiple time-points in the trend. In the group in whom post-chemotherapy FDG PET/CT was obtained more than 24 wk after completion of chemotherapy, TBR in the arterials from the post-chemotherapy FDG PET/CT equaled (ascending aorta, carotid artery) or surpassed (femoral artery) that of the pre-chemotherapy FDG PET/CT. TBR of post-chemotherapy FDG PET/CT remained below that of the pre-chemotherapy FDG PET/CT scan in the abdominal aorta.
Figure 3.
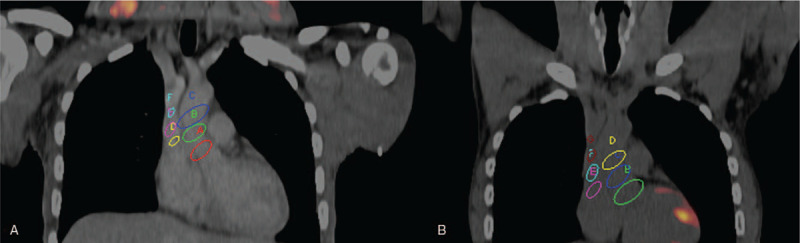
Fused FDG PET/CT images through the chest of a 40-year-old male who had baseline (A) and follow-up imaging post-chemotherapy (B) for Hodgkin lymphoma. This figure depicts how SUVmax and TBR were calculated for each arterial bed using the ascending aorta and the superior vena cava for demonstration. Multiple regions of interests (ROI) are shown encircling the ascending aorta. The mean SUVmax obtained from these ROIs represents the aortic SUVmax. Multiple ROIs are shown within the lumen of the superior vena cava (SVC). The mean of the SUVmean from these ROIs represents the SVC SUVmean. TBR for the ascending aorta was computed by dividing the aortic SUVmax by the SVC SUVmean. Aortic SUVmax and aortic TBR were computed for the baseline and post-chemotherapy FDG PET/CT scans. These parameters were also obtained for the coronary vessels, the abdominal aorta (using the inferior vena cava for background correction), and the femoral vessels.
4. Discussion
We evaluated systemic arterial inflammation in patients treated with anthracycline-based chemotherapy for HL and found no significant difference in the pre- and post-chemotherapy arterial FDG uptake. We selected 4 arterial beds; carotid artery in the neck, ascending aorta in the chest, infra-renal abdominal aorta in the abdomen, and the proximal femoral artery in the proximal lower limbs; for a holistic evaluation of the effect of anthracycline-based chemotherapy on the systemic arterial vascular wall. Several observational studies have reported a higher incidence of cardiac morbidity and mortality contributed by ASCVD in patients treated with anthracycline-based chemotherapy for HL.[7,22,23] Invasion of the arterial wall by inflammatory cells is one of the earliest events in the formation of vascular atheroma, which makes arterial inflammation an early process in ASCVD. Arterial FDG accumulation is a commonly used technique to measure arterial inflammation in cardiovascular risk assessment.[15,24] An increase in arterial FDG accumulation over time is associated with the progression of ASCVD.[25]
In our study, we could not demonstrate an increase in arterial FDG uptake as a marker of arterial inflammation. Many factors could account for this negative result. ASCVD complicating anthracycline treatment occurs after a long latent period. The mean interval between the completion of chemotherapy and follow-up FDG PET/CT in our study was 65 weeks. It may be possible that a more extended follow-up period is necessary for the development of a significant arterial inflammation that will be detectable by FDG PET imaging. Another putative reason for our results may be due to the cytokine-induced by HL itself, causing an accentuated arterial FDG uptake on the baseline pre-chemotherapy FDG PET/CT scan. This may explain why arterial FDG uptake was lower post-chemotherapy compared with pre-chemotherapy in some patients who were imaged earlier than 24 weeks after completion of chemotherapy (Figs. 1 and 2). A recent study found similar levels of circulating dysfunctional endothelial progenitor cells in treatment-naïve HL patients and at 2 years during remission induced by anthracycline-based chemotherapy.[5] Endothelial progenitor cells are responsible for the repair of endothelial damage induced by factors such as diabetes and smoking that predispose to vascular atheroma formation. This suggests that both HL and its treatment with an anthracycline-containing chemotherapy regime increase the risk ASCVD. The primary pathogenic mechanism by which HL and its treatment predispose to ASCVD has been reported to be through impairment in the re-endothelization of damaged vascular endothelium due to dysfunctional endothelial progenitor cells. This pathogenic mechanism will suggest that a long interval is required after HL and its treatment for sufficient endothelial damage to occur in order to have significant arterial inflammation that may progress to ASCVD.
Other arterial structural changes occurring soon after anthracycline-based chemotherapy have been reported to contribute anthracycline-induced endothelial dysfunction. Aortic stiffness has been reported within 4 months of treatment with anthracycline-based chemotherapy in a group of oncologic patients.[26] Similar early aortic stiffening (within 6 months of treatment) was confirmed in another study of oncologic patients treated with low dose of anthracycline.[27] Apart from aortic stiffness, flow-mediated dilatation and nitrate-mediated dilatation are 2 sonographic techniques to measure endothelial dysfunction. In a study by Jenei et al, long-term survivors of childhood cancer treated with anthracycline-based chemotherapy experienced marked preclinical vasculopathy characterized by endothelial dysfunction (evaluated by flow-mediated dilatation and nitrate-mediated dilatation) and increased arterial stiffness.[28] Aortic stiffness increases doing follow-up of patients receiving anthracycline-based chemotherapy.[29] From the foregoing discussion, it is evident by subclinical ASCVD can be assessed by different noninvasive imaging techniques. Arterial changes such as aortic stiffness and endothelial dysfunction measured by magnetic resonance imaging and ultrasound scan show that the vascular changes due to anthracycline chemotherapy occur early after the initiation of treatment. These findings support the data in the literature showing that the incidence of ASCVD starts to rise within 5 years of anthracycline-based chemotherapy without plateauing throughout the life cancer survivors.[23,30] While arterial inflammation is a very early step in atherogenesis, our failure to demonstrate significant increased in arterial FDG uptake in our cohort may be due any of several factors including the short-term follow-up and many technical factors that could compromise adequate quantification of arterial FDG uptake.
Patients infected with HIV are at increased risk of ASCVD. Arterial inflammation measured by the intensity of arterial FDG uptake is known to be higher in HIV-infected people compared with age- and gender-matched controls.[15,31] We compared the changes in arterial FDG uptake between HIV-infected and HIV-uninfected patients and found no significant difference in the 2 groups. Similarly, in a sub-group analysis where we compared patients who were smokers and those who were hypertensive versus those who were not, we found no significant difference in the groups based on these 2 variables. An additional factor that may be responsible for the lack of significant differences in these groups relates to the low prevalence of these known cardiovascular risks in our patient population. For example, there were only 15 smokers and 3 patients with systemic hypertension. Our findings regarding sub-group analysis based on the history of diabetes mellitus was a little different. The patients who were diabetic experience significant weight loss and, consequently, a significant decrease in BMI between the pre- and post-chemotherapy FDG PET/CT scans. We found a decline in the SUVmax measured in the ascending aorta between the pre- and post-chemotherapy FDG PET/CT in the diabetic patients while non-diabetic patients experienced a rise in SUVmax of FDG uptake in the ascending aorta. Similarly, we found a significantly higher rise in TBR of FDG uptake in the femoral arterial among diabetic patients compared with non-diabetic patients. We doubt if these significant differences found in 2 of the measured FDG PET parameters are meaningful, especially considering that there were only 2 patients in our study patients who were diabetic at the time of the study.
In this study, we showed the utility of FDG PET/CT obtained for baseline pre-chemotherapy staging and post-chemotherapy re-staging in patients with HL treated with anthracycline-based chemotherapy in the evaluation of arterial inflammation as a risk factor of ASCVD. Evaluation of arterial inflammation in this patient group is essential to evaluate their cardiovascular risk and measure the effectiveness of anti-inflammatory therapy for risk reduction, which has attracted much interest in recent times. Our study has many limitations. Our study population is modest. This is because of the strict selection criteria we applied. Most importantly, we excluded all patients in whom lymphoma involves lymph node groups adjacent to the artery of interest that precluded accurate quantification of arterial FDG uptake without photon spill-over from the lymph nodes. Lymph nodes are generally located around major vessels; hence a large proportion of patients were excluded for this reason. The retrospective design of our study also precludes the application of imaging conditions, including delayed imaging necessary to optimized arterial FDG uptake and improve background activity clearance.[21,32] We failed to demonstrate a significant increase in arterial inflammation following anthracycline-based chemotherapy. We speculate that the short interval between the completion of chemotherapy and the time of follow-up imaging in this study could be a significant contributing factor. Future studies in this area should explore a more extended follow-up interval between the completion of chemotherapy and post-chemotherapy FDG PET/CT.
5. Conclusion
In patients with Hodgkin lymphoma who were treated with anthracycline-based chemotherapy, we found no significant increase in arterial inflammation measured by FDG PET/CT after an average follow-up of about 65 weeks since completion of chemotherapy. The presence of systemic hypertension, diabetes mellitus, and smoking history did not seem to impact on arterial inflammation in our study population.
Acknowledgments
We wish to thank all members of staff at the Department of Nuclear Medicine, Steve Biko Academic Hospital, Pretoria, South Africa, for their support during the conduct of this study.
Author contributions
Conceptualization: Ismaheel O. Lawal, Alfred O. Ankrah, Mike Sathekge.
Data curation: Ismaheel O. Lawal, Gbenga O. Popoola, Alfred O. Ankrah.
Formal analysis: Ismaheel O. Lawal, Akintunde T. Orunmuyi, Gbenga O. Popoola.
Investigation: Ismaheel O. Lawal, Thabo Lengana, Kgomotso M.G. Mokoala.
Project administration: Mike Sathekge.
Writing – original draft: Ismaheel O. Lawal, Akintunde T. Orunmuyi, Gbenga O. Popoola, Mike Sathekge.
Writing – review & editing: Ismaheel O. Lawal, Akintunde T. Orunmuyi, Gbenga O. Popoola, Thabo Lengana, Kgomotso M.G. Mokoala, Alfred O. Ankrah, Mike Sathekge.
Footnotes
Abbreviations: ABVD = adriamycin, bleomycin, vinblastine, dacarbazine, ASCVD = atherosclerotic cardiovascular disease, BMI = body mass index, FDG PET/CT = flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography, HIV = human immunodeficiency virus, HL = Hodgkin lymphoma, SUVmax = maximum standardized uptake value, TBR = target-to-background ratio.
How to cite this article: Lawal IO, Orunmuyi AT, Popoola GO, Lengana T, Mokoala KM, Ankrah AO, Sathekge MM. FDG PET/CT for evaluating systemic arterial inflammation induced by anthracycline-based chemotherapy of Hodgkin lymphoma: A retrospective cohort study. Medicine. 2020;99:48(e23259).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
BMI: body mass index; CD4: cluster of differentiation 4; DM: diabetes mellitus; HIV: human immunodeficiency virus; HTN: systemic hypertension; PET: positron emission tomography.
BMI: body mass index; FBS: fasting blood sugar; FDG: fluorodeoxyglucose; FDG PET/CT: flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography; SUVmax: maximum standardized uptake value; t: paired samples T test; TBR: target-to-background ratio.
P < .05.
BMI: body mass index; FBS: fasting blood sugar; FDG: fluorodeoxyglucose; FDG PET/CT: flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography; HIV: human immunodeficiency virus; SUVmax: maximum standardized uptake value; t: independent samples T test; TBR: target-to-background ratio.
BMI: body mass index; FBS: fasting blood sugar; FDG: fluorodeoxyglucose; SUVmax: maximum standardized uptake value; t: independent samples T test; TBR: target-to-background ratio.
BMI: body mass index; FBS: fasting blood sugar; FDG: fluorodeoxyglucose; SUVmax: maximum standardized uptake value; t: independent samples T test; TBR: target-to-background ratio.
BMI: body mass index; FBS: fasting blood sugar; FDG: fluorodeoxyglucose; SUVmax: maximum standardized uptake value; t: independent samples T test; TBR: target-to-background ratio.
P < .05.
References
- [1].Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med 2012;366:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moding EJ, Advani R, Rosenberg SA, et al. Prognostic factors and patterns of failure in advanced stage Hodgkin lymphoma treated with combined modality therapy. Radiother Oncol 2018;129:507–12. [DOI] [PubMed] [Google Scholar]
- [3]. US Department of Health and Human Services; National Cancer Institute. Cancer incidence and survival among children and adolescents: United States Seer Program 1975–2013. Bethesda, MD, USA: 1–192. [Google Scholar]
- [4].Zamorano JL, Lancellotti P, Muñoz DR, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016;37:2768–801. [DOI] [PubMed] [Google Scholar]
- [5].Wiessman M, Leshem D, Yeshurun M, et al. Dysfunctional endothelial progenitor cells in patients with Hodgkin's lymphoma in complete remission. Cancer Med 2019;8:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005;353:999–1007. [DOI] [PubMed] [Google Scholar]
- [7].van Nimwegan FA, Schaapveld M, Janus CPM, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 2015;175:1007–17. [DOI] [PubMed] [Google Scholar]
- [8].Wong BW, Meredith A, Lin D, et al. The biological role of inflammation in atherosclerosis. Can J Cardiol 2012;28:631–41. [DOI] [PubMed] [Google Scholar]
- [9].Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- [10].Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–505. [DOI] [PubMed] [Google Scholar]
- [11].Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24. [DOI] [PubMed] [Google Scholar]
- [12].Graebe M, Pedersen SF, Borgwardt L, et al. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg 2009;37:714–21. [DOI] [PubMed] [Google Scholar]
- [13].Abdelbaky A, Corsini E, Figueroa AL, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging 2013;6:747–54. [DOI] [PubMed] [Google Scholar]
- [14].Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009;50:1611–20. [DOI] [PubMed] [Google Scholar]
- [15].Lawal IO, Ankrah AO, Popoola GO, et al. Arterial inflammation in young patients with human immunodeficiency virus infection: a cross-sectional study using F-18 FDG PET/CT. J Nucl Cardiol 2019;26:1258–65. [DOI] [PubMed] [Google Scholar]
- [16].Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV event. JACC Cardiovasc Imaging 2013;6:1250–9. [DOI] [PubMed] [Google Scholar]
- [17].Lawal IO, Nyakale NE, Harry LM, et al. The role of F-18 FDG PET/CT in evaluating the impact of HIV infection on tumor burden and therapy outcome in patients with Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 2017;44:2025–33. [DOI] [PubMed] [Google Scholar]
- [18].Kostakoglu L, Cheson BD. Current role of FDG PET/CT in lymphoma. Eur J Nucl Med Mol Imaging 2014;41:1004–27. [DOI] [PubMed] [Google Scholar]
- [19].Lawal I, Lengana T, Ololade K, et al. 1818F-FDG PET/CT in the detection of asymptomatic malignant melanoma recurrence. Nuklearmedizin 2017;56:83–9. [DOI] [PubMed] [Google Scholar]
- [20].Boellaard R, Delgado-Bolton R, Oyen WJG, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;45:328–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bucerius J, Hyafil F, Verberne HJ, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging 2016;43:780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016;17:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maraldo MV, Giusti F, Vogelius IR, et al. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2015;2:e492–502. [DOI] [PubMed] [Google Scholar]
- [24].Takx RAP, MacNabb MH, Emami H, et al. Increased arterial inflammation in individuals with stage 3 chronic kidney disease. Eur J Nucl Med Mol Imaging 2016;43:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Joseph P, Ishai A, Mani V, et al. Short-term changes in arterial inflammation predict long-term changes in atherosclerosis progression. Eur J Nucl Med Mol Imaging 2017;44:141–50. [DOI] [PubMed] [Google Scholar]
- [26].Chaosuwannakit N, D’Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol 2010;28:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Drafts BC, Twomley KM, D’Agostino R, Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jenei Z, Bárdi E, Magyar MT, et al. Anthracycline causes impaired vascular endothelial fumction and aortic stiffness in long term survivors of childhood cancer. Pathol Oncol Res 2013;19:375–83. [DOI] [PubMed] [Google Scholar]
- [29].Daskalaki M, Makris T, Vassilakopoulos T, et al. Effects of anthracyclines on aortic distensibility in patients with lymphomas: a prospective study. Hellenic J Cardiol 2014;55:191–6. [PubMed] [Google Scholar]
- [30].Rugbjerg K, Mellemkjær L, Boice JD, et al. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943–2009. J Natl Cancer Inst 2014;106:dju110. [DOI] [PubMed] [Google Scholar]
- [31].Lawal IO, Ankrah AO, Stoltz AC, et al. Radionuclide imaging of inflammation in atherosclerotic vascular disease among people living with HIV infection: current practice and future perspective. Eur J Hybrid Imaging 2019;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lawal IO, Mokoala KG, Popoola GO, et al. Impact of optimized PET imaging conditions on 18F-FDG uptake quantification in patients with apparently normal aortas. J Nucl Cardiol 2019;Epub ahead of print on August 06. [DOI] [PubMed] [Google Scholar]


