ABSTRACT
Background
This study was designed to investigate whether COVID-19 patients with recently received immunotherapy or other anti-cancer treatments had more severe symptoms and higher mortality.
Methods
A literature search was performed using the electronic platforms to obtain relevant research studies published up to June 28, 2020. Odds ratio (OR) and 95% confidence intervals (CI) of research endpoints in each study were calculated and merged. Statistical analyses were performed with Stata 12.0 (Stata Corp LP, College Station, TX).
Results
A total of 17 studies comprising 3581 cancer patients with COVID-19 were included in this meta-analysis. SARS-CoV-2-infected cancer patients who recently received anti-cancer treatment did not observe a higher risk of exacerbation and mortality (All p-value >0.05). We also found that surgery, targeted therapy, chemotherapy, immunotherapy, and radiotherapy were not associated with increased risk of exacerbation and mortality (All p-value >0.05). Chemotherapy within 28 d increased the risk of death events (OR 1.45, 95% CI 1.10–1.91, P = .008, p-value = 0.015 for test of interaction), and immunotherapy within 90 d increased the risk of exacerbation (OR 2.53,95%1.30–4.91, P = .006, p-value = 0.170 for test of interaction).
Conclusion
Cancer patients recently under anti-cancer treatment before diagnosed with COVID-19, including surgery, targeted therapy, immunotherapy, and radiotherapy, were not associated with increased risk of exacerbation and mortality. Chemotherapy within 28 d increased the risk of mortality, and chemotherapy was not associated with increased risk of severe COVID-19. The role of anti-cancer therapy in cancer patients with COVID-19 still needs further exploration, especially chemotherapy and immunotherapy.
KEYWORDS: COVID-19, cancer, SARS-CoV-2, meta-analysis, anti-cancer therapy
1. Introduction
COVID-19 is a life-threatening disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoX-2).1,2 Since the COVID-19 outbreak in China at the end of 2019 and until the 7 July 2020, the pandemic has infected more than 11 million people worldwide. Due to routine anti-cancer treatments and examination in the hospital, patients with cancer are at higher risk of contracting COVID-19.3–5 Besides, patients with cancer patients are more susceptible to infections due to overall poor health status and systemic immunosuppressive states, which has attracted increasing attention from clinicians.6–10 Currently, a key question in oncology practice amidst the COVID-19 pandemic is whether anti-cancer therapy affects COVID-19 severity and mortality.
Patients with cancer recently received anti-cancer treatments have been generally assumed to be at a higher risk of exacerbation.11,12 Early studies suggested that anti-tumor treatment within 14 d before COVID-19 diagnosis increased the risk of developing severe events.13 However, recently published studies have reached different or even opposite conclusions.14–16 Oncologists hold different notions and continue to receive mixed messages regarding whether cancer patients should receive anti-tumor treatments as usual.17–19 In light of this, a systematic review is needed to summarize the best available evidence of the impact of anti-cancer therapy in cancer patients with COVID-19.
Knowledge of anti-cancer therapy’s role is essential for risk assessment, monitoring, disease prevention, and control in cancer patients with COVID-19. To date, there has been no systematic review that comprehensively explores the role of anti-cancer treatment in cancer patients with COVID-19 to guide clinical practice better. Therefore, we performed a meta-analysis of the available studies to explore whether COVID-19 patients with recent immunotherapy or other anti-cancer treatments had more severe symptoms and higher mortality.
2. Materials and methods
2.1. Search strategy
This study followed the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.20PubMed, Embase, Web of Science, and the Cochrane Library were systematically searched for relevant articles published before June 28, 2020. The search strategy included the following specific terms: “2019-nCoV” or “Coronavirus” or “COVID-19” or “SARS-CoV-2” or “2019-nCoV” or “Wuhan Coronavirus” and “cancer” or“tumor” or “carcinoma” or “ malignancy” and “anti-cancer treatment” or “anti-tumor treatment” or “surgery” or “chemotherapy” or “targeted therapy” or “radiotherapy” or “immunotherapy.” Besides, the references of the relevant reviews and original articles were manually searched to find out more potential eligible studies. The above process was performed independently by two reviewers.
2.2. Inclusion and exclusion criteria
The inclusion criteria of the study are as follows: (1) Types of Studies: published studies reported the relationship between anti-tumor treatment and cancer patients infected by SARS-CoV-2; (2) Exposure intervention: COVID-19 patients received anti-cancer treatment (surgery, chemotherapy, targeted therapy, radiotherapy) within 40 d, and the time interval of immunotherapy was relaxed to six months;21,22(3) Outcome indicator: the odds ratios (OR) with 95% confidence intervals (CI) for each type of anti-cancer therapy.
The exclusion criteria: (1) Reviews, summaries of meetings or discussions; (2) Insufficient data information provided; (3) Duplicate publications; (4) The sample size was less than 25; (5) Patients received immunotherapy more than 180 d or other anti-tumor therapies more than 40 d before diagnosed as COVID-19.
2.3. Quality assessment and data extraction
Two investigators independently and separately conducted the data extraction and quality assessment, and any discrepancies were resolved by discussion or by consulting a third reviewer. The extracted data included as follows: (1) publication data were encompassing the first author surname, the year of publication, country of the population, sample size, study design, and population size; (2) clinicopathological data such as age, gender, severe events, death events; (3) statistical data including OR and corresponding 95% CI. If the univariate and multivariate analysis were both reported, we selected the multivariate analysis. If the OR was not presented directly, available data from original articles were used to estimate the OR.
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS) tool.23 The NOS consists of three parts: selection, comparability, and exposure. A study was considered of high quality if it had a NOS score of ≥7.Higher NOS scores indicated higher literature quality.
2.4. Statistical analysis
The meta-analysis was performed using Stata12.0 software (Stata Corp, College Station, Texas). We calculated odds ratios (OR) and corresponding 95% confidence intervals (CI) in each included study. Then, the OR and 95% CI were used to measure the association between anti-tumor treatment and severe and death events. Heterogeneity was assessed with the Cochran Q statistic and the I2 statistic. For moderate heterogeneity (I2 ≥ 50%), random rather than fixed-effects models were used. Results were considered significant statistically when the p-value was less than 0.05. We also tested for interaction between the subgroups and considered the interaction test significant when its p-value was <0.05.24 Publication bias was assessed using the Begg funnel plot and Egger test linear regression test (where at least five studies were available). If P < .05 indicates obvious publication bias.
3. Results
3.1. Study selection
Figure 1 illustrates the search process and the final selection of relevant studies. Our search found 1,106 studies from PubMed, Cochrane Library, Web of Science, and Embase databases, and 2 studies were retrieved from reference lists. After removing duplicates, 208 references were screened for titles and abstracts. Of these, 168 articles were excluded after the first screening based on abstracts or titles, leaving 40 articles for full-text review. After a full-text review, 23 articles were excluded due to the violation of inclusion criteria and the remaining 17 articles were eligible for inclusion in the current analysis.3,13,15,16,25–37
Figure 1.
PRISMA flow diagram of the meta-analysis.
3.2. Study characteristics and quality assessment
Seventeen relevant studies were retrieved, including 15 retrospective studies and 2 prospective studies, comprising 3,581 cancer patients infected by SARS-CoV-2.3,13,15,16,25–37 Detailed clinical characteristics of the study patients are reported in Table 1. Seven studies were performed in China,3,9,13,15,25,26,32 five in the USA,16,27–30 two in Spain,33,37 one in France,36 and the other two in Italy.31,35 Four of the included studies reported data on patients who diagnosis with COVID-19 while they received anti-cancer therapy.31,34,35,37 Additionally, patients who recently received anti-cancer treatment before the diagnosis of COVID-19 within 4 weeks and 30 d were reported in four studies and six studies, respectively. The anti-cancer treatment characteristics are summarized in Supplementary Tables 1 and 2. All articles are of high quality because of NOS score no less than 7.
Table 1.
Main characteristics of the included studies in meta-analysis.
| Study | Year | Country | Sample | Male | Median (IQR) |
Study type | Outcomes |
Time interval between recent anti-cancer treatment and diagnosis of COVID-19 |
NOS | |
|---|---|---|---|---|---|---|---|---|---|---|
| /Mean age (years) |
Non-severe (survivor) |
Severe (death) |
||||||||
| J.Ma | 2020 | China | 37 | 20 | 62.0(11.0) | Retrospective | 17 | 20 | 30 d | 8 |
| L.Zhang | 2020 | China | 28 | 17 | 65.0(56.0–70.0) | Retrospective | 13 | 15 | 14 d; 30 d; | 7 |
| M.Dai | 2020 | China | 105 | 57 | 64.0(14.0) | Retrospective | 65 | 40 | 40 d | 8 |
| V.Mehta | 2020 | USA | 218 | 127 | 69.0(10.0–92.0) | Retrospective | 157 | 61 | 30 d | 8 |
| F.Yang | 2020 | China | 52 | 28 | 63.0(34.0–98.0) | Retrospective | 33 | 19 | 30 d | 8 |
| K.Yang | 2020 | China | 205 | 100 | 63.0(14.0–96.0) | Retrospective | 165 | 40 | 4 weeks | 8 |
| H. Zhang | 2020 | China | 107 | 60 | 66.0(37.0–98.0) | Retrospective | 51 | 56 | 0 | 8 |
| N.Kuderer | 2020 | USA | 928 | 468 | 66.0 (57.0–76.0) | Retrospective | 807 | 121 | 4 weeks | 8 |
| E.Stroppa | 2020 | Italy | 25 | 20 | 71.6(50.0–84.0) | Retrospective | 16 | 9 | 0 | 8 |
| R.Yarza | 2020 | Spain | 63 | 29 | 66.0(63.4–68.8) | Retrospective | 47 | 16 | 4 weeks | 9 |
| F.Martín | 2020 | Spain | 34 | 15 | 72.5 (35.0–94.0) | Retrospective | 23 | 11 | 0 | 7 |
| L.Lee | 2020 | USA | 800 | 449 | 69.0(59.0–76.0) | Prospective | 412 | 226 | 4 weeks | 9 |
| J.Tian | 2020 | China | 232 | 119 | 64.0(58.0–69.0) | Retrospective | 84 | 148 | 1 weeks; 1–2 weeks; 2–3 weeks; >3 weeks; |
9 |
| M.Garassino | 2020 | Italy | 200 | 141 | 68.0(61.8–75.0) | Prospective | 125 | 66 | 0 | 9 |
| E.Robilotti | 2020 | USA | 423 | 212 | NA | Retrospective | 372 | 51 | 30 d;90 d# | 8 |
| S.Assaad | 2020 | France | 55 | 144 | 63.8 | Retrospective | 8 | 47 | 30 d | 8 |
| J.Luo | 2020 | USA | 69 | 36 | 69.0 (31.0–91.0) | Retrospective | 41 | 24 | 42 d; 90 d; 180 d | 8 |
Abbreviation: NOS, Newcastle–Ottawa Scale; #: Immunotherapy within 90 d.
3.3. Relationship between anti-cancer therapy and the risk of exacerbation and mortality
Five studies provided the data in terms of anti-cancer therapy and the risk of exacerbation in cancer patients with COVID-19.3,9,13,25,26 With no obvious heterogeneity (I2 = 22.3%, P = .266) among these studies, so a fixed-effect pattern was used for assessment. No correlations were observed between anti-cancer therapy and the risk of exacerbation (OR 1.54, 95% CI 0.96–2.49, P = .074). Nine studies were evaluated for anti-cancer therapy and the risk of death events.16,26,27,31,32,34–37 A random-effects model was used since the heterogeneity test suggested obvious heterogeneity (I2 = 68.3%, P = .001). The result showed no significant correlation between anti-cancer therapy and the risk of mortality in cancer patients with COVID-19 (OR 1.33, 95% CI 0.84–2.10, P = .229) (Figure 2).
Figure 2.
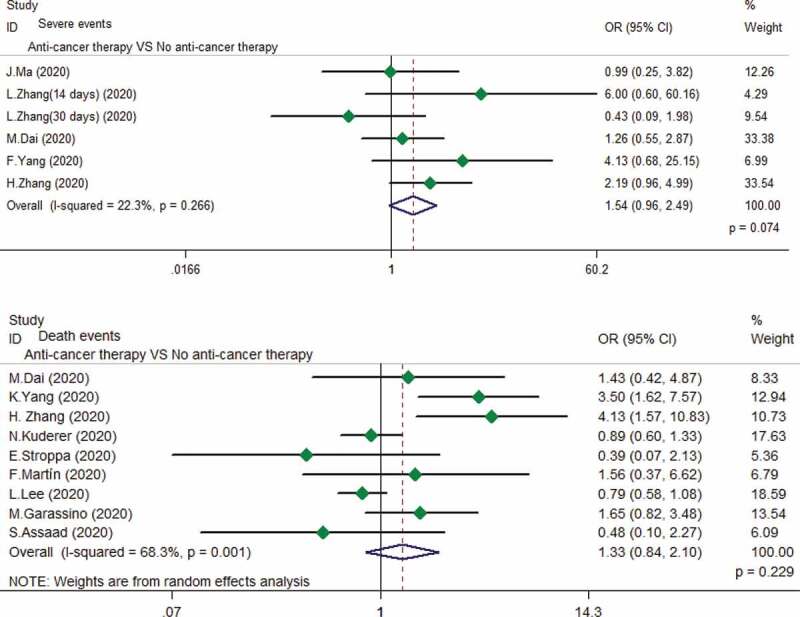
Relationship between anti-cancer therapy and the risk of exacerbation and mortality in cancer patients with COVID-19.
3.4. Relationship between different anti-cancer therapy and the risk of exacerbation
Five anti-cancer treatments, including surgery, chemotherapy, targeted therapy, immunotherapy, and radiotherapy, were analyzed to determine the relationship between different anti-cancer therapy and the risk of exacerbation. As shown in Figure 3, surgery, chemotherapy, and immunotherapy are not associated with severe events in cancer patients infected by SARS-CoV-2 (All P-value >0.05). Only one study reported on targeted therapy and radiotherapy, and we do not observe patients who have recently received targeted therapy or radiotherapy at higher risk of exacerbation (Table 2).26
Figure 3.

Relationship between different anti-cancer treatments and the risk of exacerbation in cancer patients with COVID-19.
Table 2.
The results of meta-analysis.
| No.of studies | OR (95%CI) | P-value | Heterogeneity |
Model used | Begg’s test | Egger’s test |
P-value of interaction test |
||
|---|---|---|---|---|---|---|---|---|---|
| I2 | Ph | ||||||||
| Severe events | |||||||||
| Anti-cancer therapy | 6 | 1.54 (0.96–2.49) | 0.074 | 22.3% | 0.266 | Fixed | 0.707 | 0.791 | |
| Surgery# | 4 | 1.01 (0.35–2.87) | 0.986 | 63.3% | 0.043 | Random | NA | NA | |
| Chemotherapy | 7 | 0.91 (0.67–1.24) | 0.555 | 23.7% | 0.248 | Fixed | 0.764 | 0.658 | 0.168 |
| Within 28 d | 4 | 0.66 (0.38–1.15) | 0.146 | 49.5% | 0.114 | Fixed | NA | NA | |
| Within 40 d | 3 | 1.05 (0.73–1.50) | 0.807 | 0 | 0.953 | Fixed | 0.624 | 0.534 | |
| Targetedtherapy | 1 | 0.17 (0.01–3.22) | 0.229 | NA | NA | NA | NA | NA | |
| Radiotherapy | 1 | 0.45 (0.11–1.73) | 0.243 | NA | NA | NA | NA | NA | |
| Immunotherapy | 8 | 1.54 (0.98–2.43) | 0.061 | 14.9% | 0.313 | Fixed | 0.368 | 0.144 | 0.170 |
| Within 28 d | 2 | 0.56 (0.15–2.03) | 0.376 | 0 | 0.355 | Fixed | NA | NA | |
| Within 42 d | 3 | 1.18 (0.45–3.07) | 0.734 | 7.30% | 0.340 | Fixed | NA | NA | |
| Within 90 d | 2 | 2.53 (1.30–4.91) | 0.006 | 0 | 0.664 | Fixed | NA | NA | |
| Within 180 d | 1 | 1.20 (0.41–3.48) | 0.738 | NA | NA | Fixed | NA | NA | |
| Death events | |||||||||
| Anti-cancer therapy | 9 | 1.33 (0.84–2.10) | 0.229 | 68.3% | 0.001 | Random | 0.754 | 0.284 | |
| Surgery | 4 | 1.17 (0.65–2.08) | 0.604 | 0 | 0.488 | Fixed | NA | NA | 0.275 |
| Within 28 d | 3 | 1.04 (0.56–1.92) | 0.902 | 0 | 0.539 | Fixed | NA | NA | |
| Within 40 d | 1 | 2.90 (0.51–16.34) | 0.229 | NA | NA | NA | NA | NA | |
| Chemotherapy | 9 | 1.28 (0.99–1.66) | 0.056 | 40.6% | 0.096 | Fixed | 0.754 | 0.810 | 0.015* |
| Within 28 d | 6 | 1.45 (1.10–1.91) | 0.008* | 6.5% | 0.375 | Fixed | 0.573 | 0.220 | |
| Within 40 d | 3 | 0.56 (0.27–1.13) | 0.105 | 0.1% | 0.367 | Fixed | NA | NA | |
| Targetedtherapy | 5 | 1.16 (0.72–1.85) | 0.546 | 43.4% | 0.132 | Fixed | 0.806 | 0.673 | 0.545 |
| Within 28 d | 3 | 1.52 (0.55–4.16) | 0.417 | 71.0% | 0.032 | Random | NA | NA | |
| Within 40 d | 2 | 0.81 (0.14–4.84) | 0.820 | 0 | 0.990 | Fixed | NA | NA | |
| Radiotherapy | 4 | 0.81 (0.53–1.23) | 0.317 | 0 | 0.518 | Fixed | NA | NA | 0.545 |
| Within 28 d | 2 | 0.89 (0.53–1.49) | 0.615 | 47.6% | 0.167 | Fixed | NA | NA | |
| Within 40 d | 2 | 0.68 (0.34–1.38) | 0.284 | 0 | 0.916 | Fixed | NA | NA | |
| Immunotherapy | 11 | 1.00 (0.65–1.53) | 0.983 | 16.3% | 0.289 | Fixed | 0.436 | 0.976 | 0.690 |
| Within 28 d | 5 | 0.81 (0.47–1.42) | 0.463 | 48.2% | 0.103 | Fixed | 0.624 | 0.787 | |
| Within 42 d | 4 | 1.20 (0.47–3.10) | 0.705 | 0 | 0.430 | Fixed | NA | NA | |
| Within 90 d | 1 | 1.38 (0.28–6.92) | 0.694 | NA | NA | Fixed | NA | NA | |
| Within 180 d | 1 | 1.67 (0.47–5.90) | 0.427 | NA | NA | Fixed | NA | NA | |
Abbreviation: NA: not available; #: All within 40 d; *: P < 0.05.
3.5. Relationship between different anti-cancer therapy and the risk of mortality
Associations between different anti-cancer therapies and the risk of mortality are shown in Figure 4. No statistically significant correlation was shown between anti-cancer therapy (including surgery, chemotherapy, targeted therapy, immunotherapy, and radiotherapy) and the risk of death events in cancer patients with COVID-19 (All P-value >0.05).16,26–29,31–33,35,36
Figure 4.

Relationship between different anti-cancer treatments and the risk of death events in cancer patients with COVID-19.
3.6. Subgroup analysis
To further verify the correlation of different anti-cancer therapies and the risk of exacerbation and mortality, subgroup analysis was conducted. The results of the subgroup analysis are presented in Table 2. The subgroup analysis results further support the results of surgery, targeted therapy, and radiotherapy. In the result of subgroup analysis, we further observed that chemotherapy within 28 d increased the risk of death events (OR 1.45, 95% CI 1.10–1.91, P = .008, p-value = 0.015 for test of interaction), and immunotherapy within 90 d increased the risk of exacerbation (OR 2.53,95%1.30–4.91, P = .006, p-value = 0.170 for test of interaction). Subgroup analyses showed no statistically significant tests of interaction, except for the subgroup of the association between chemotherapy and the risk of mortality.
3.7. Publication bias
Table 2 shows the results of publication bias, which were evaluated by funnel plots and Eggers test. The publication bias showed no significant publication bias in the included studies (All p > .05).
4. Discussion
Oncology patients are considered more susceptible to SARS-CoV-2 infections due to their immunocompromised status caused by both cancer and various anti-cancer treatments such as chemotherapy, immunotherapy, and radiotherapy.38–41 Given this, with the increasing risk of the COVID-19 pandemic, the management of oncology patients undergoing anti-cancer therapy must be adequately balanced.42 Currently, there is a lack of knowledge to evaluate the risks and benefits of anti-cancer treatment in cancer patients with COVID-19.43,44
Several previous studies have shown that SARS-CoV-2-infected cancer patients who underwent recent anti-cancer treatment had a higher risk of clinically severe events than those not receiving treatment.13 Liang first reported that patients who underwent chemotherapy or surgery in the past month had a numerically higher risk of clinically severe events than did those not receiving chemotherapy or surgery by analyzed data from 18 cancer patients with COVID-19.5 Other scholars only found that patients receiving chemotherapy within 4 weeks before symptom onset were risk factors for death during admission to hospital.32 However, some recent studies have argued that anti-cancer therapy did not affect the severity of COVID-19 among these cancer patients.25 Due to the different study endpoints, experimental design, and sample size, the real impact of anti-cancer treatment on cancer patients with COVID-19 is still unclear. Therefore, we conducted a systematic review and comprehensive meta-analysis to evaluate the relationship between anti-cancer therapy and the risk of exacerbation and mortality in cancer patients with COVID-19.
Compared with patients who had not received anti-cancer therapy within 40 d of testing positive for COVID-19, those who had received anti-cancer therapy did not suffer increased COVID −19 severity and mortality when analyzed by our meta-analysis. To elucidate this relationship in greater detail, we analyzed the role of different anti-cancer treatments, including surgery, targeted therapy, chemotherapy, immunotherapy, and radiotherapy. The result of different anti-cancer treatments obtained further support the above research findings. The time effect should be kept in consideration when interpreting the results. According to the current data, we divided the time interval between recently anti-cancer treatment (surgery, targeted therapy, chemotherapy, and radiotherapy) and diagnosis of COVID-19 into within 28 d and 40 d for subgroup analysis. Subgroup analysis showed that different anti-cancer treatments (surgery, targeted therapy, and radiotherapy) were not associated with increased risk of exacerbation and mortality, in addition to chemotherapy.
Contrary to early reports, receipt of chemotherapy within 40 d before COVID-19 diagnosis was not associated with a higher risk of death and severe events from our study.13,34 Nevertheless, in our subgroup analysis, we observed an increased risk of death events related to chemotherapy within 28 d only. Interaction test results indicated that the association between chemotherapy and the risk of mortality was significantly modified by time interval. These differences might be explained by Tian et al.’s findings.15 Tian et al. found that risk of COVID-19 severity and death was highest for patients with last chemotherapy treatment within 2 weeks of admission, and decreased as the time interval since last chemotherapy increased, with significantly reduced risk when the last treatment was at least 3 weeks before hospital admission.15 Therefore, the time interval between the last chemotherapy and the diagnosis of COVID-19 should be more accurate analysis and fully considered in future studies, especially time interval of fewer than 21 d.
A recent study by Luo et al. suggested that lung cancer patients’ immunotherapy were not more likely to develop severe COVID-19 and death than those who were not received.28 Our meta-analysis show suggested that immunotherapy not associated with increased risk of exacerbation and mortality in cancer patients with COVID-19, similar to the study by Luo et al. Given the dissipating pharmacodynamic impact of immune checkpoint inhibitors, we relaxed the time interval’s inclusion criteria to six months.22,45–47 Based on the available evidence, subgroup analysis was also conducted according to different time intervals (including 28 d, 42 d, 90 d, and 180 d). Subgroup analysis showed that immunotherapy within 90 d increased the risk of severe events. However, no statistically significant tests of interaction were observed in the subgroup of immunotherapy. Therefore, our findings do not prove an evident influence of immunotherapy on the development of severe COVID-19. Recently, Wu et al. observed a higher proportion of severe COVID-19 in cancer patients who received ≥3 cycles of immunotherapy.48 These findings may explain our results on immunotherapy, and future research should be considered the number of immunotherapy cycles received.
To the best of our knowledge, this is the first meta-analysis evaluating the effect of recent anti-cancer treatment before diagnosed with COVID-19 for cancer patients. For the pooled result, we found that cancer patients recently under anti-cancer treatment days before diagnosed with COVID-19, including surgery, targeted therapy, immunotherapy, and radiotherapy, were not associated with increased risk of severe and death events. We also found that chemotherapy within 28 d increased the risk of mortality, and chemotherapy was not associated with increased risk of severe COVID-19. Because of the limited number of patients and potential confounding factors, whether immunotherapy and chemotherapy will be harmful to the patient during the epidemic deserve further evaluation.
The results of this study should be interpreted with caution due to several limitations. Firstly, the major limitation of the current study is the small sample size, which may render the results underpowered. Secondly, the pooled results of anti-cancer therapy only come from one study. Thirdly, due to insufficient information in the included study, the different time interval delimitations are not precise and uniform. Finally, the quality of different studies was different, which might lead to bias.
5. Conclusion
Cancer patients recently under anti-cancer treatment before diagnosed with COVID-19, including surgery, targeted therapy, immunotherapy, and radiotherapy, were not associated with increased risk of exacerbation and mortality. Chemotherapy within 28 d increased the risk of mortality, and chemotherapy was not associated with increased risk of severe COVID-19. Given the limitations of the current study, these findings should be interpreted cautiously but deserve further evaluation in subsequent studies, especially chemotherapy and immunotherapy.
Supplementary Material
Funding Statement
No sources of funding were used to conduct this study.
Authors’ contributions
Concept and design: Bolin Wang. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript: All authors. Administrative, technical, or material support: All authors. Supervision: Yan Huang.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–9. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y,et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X.. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020. doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in cancer patients of a tertiary hospital in Wuhan. medRxiv. 2020:2020.02.22.20025320 [Google Scholar]
- 5.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addeo A, Obeid M, Friedlaender A. COVID-19 and lung cancer: risks, mechanisms and treatment interactions. J Immunother Cancer. 2020;8(1):e000892. doi: 10.1136/jitc-2020-000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidaway P. COVID-19 and cancer: what we know so far. Nat Rev Clin Oncol. 2020;17:336. doi: 10.1038/s41571-020-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, et al. Histopathologic changes and SARS-CoV-2 Immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrag D, Hershman DL, Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323(20):2005. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 11.Moujaess E, Kourie HR, Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit Rev Oncol Hematol. 2020;150:102972. doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 2020;22:53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson A, Gyawali B, Booth CM. Risk of COVID-19 in patients with cancer. JAMA Oncol. 2020;6(9):1471. doi: 10.1001/jamaoncol.2020.2586. [DOI] [PubMed] [Google Scholar]
- 15.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu C-Y, Desai A, de Lima Lopes G, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Malviya R, Kumar V, Gupta R, Awasthi R. Severity and risk of COVID-19 in cancer patients: an evidence-based learning. Dermatol Ther. 2020;e13778. doi: 10.1111/dth.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang W, Hu J, Zhang H, Xie C. The management of patients with lung cancer during the outbreak of coronavirus disease 2019. J Thorac Oncol. 2020;15:e106–e7. doi: 10.1016/j.jtho.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B, Zhu J, Wu XY, Gao XH. Should patients stop their radiotherapy or chemotherapy during the COVID-19 pandemic. Am J Cancer Res. 2020;10:1518–1521. [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 22.Fairfax BP, Taylor CA, Watson RA, Nassiri I, Danielli S, Fang H, Mahé EA, Cooper R, Woodcock V, Traill Z. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26:193–199. doi: 10.1038/s41591-019-0734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee LY, Cazier J-B, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J, Rizvi H, Preeshagul IR, Egger JV, Hoyos D, Bandlamudi C, McCarthy CG, Falcon CJ, Schoenfeld AJ, Arbour KC, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, Pradhan K, Thota R, Reissman S, Sparano JA, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, Bogler Y, Caldararo M, Figueroa CJ, Glickman MS, et al. Determinants of severity in cancer patients with COVID-19 illness. medRxiv. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroppa EM, Toscani I, Citterio C, Anselmi E, Zaffignani E, Codeluppi M, Cavanna L. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol. 2020;16(20):1425–1432. doi: 10.2217/fon-2020-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarza R, Bover M, Paredes D, Lopez-Lopez F, Jara-Casas D, Castelo-Loureiro A, Baena J, Mazarico JM, Folgueira MD, Meléndez-Carmona MÁ, et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Wang L, Chen Y, Wu Q, Chen G, Shen X, Wang Q, Yan Y, Yu Y, Zhong Y, et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;126(17):4023–4031. doi: 10.1002/cncr.33042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garassino MC, Whisenant JG, Huang L-C, Trama A, Torri V, Agustoni F, Baena J, Banna G, Berardi R, Bettini AC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assaad S, Avrillon V, Fournier M-L, Mastroianni B, Russias B, Swalduz A, Cassier P, Eberst L, Steineur M-P, Kazes M, et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martín‐Moro F, Marquet J, Piris M, Michael BM, Saez AJ, Corona M, Jiménez C, Astibia B, García I, Rodríguez E, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190(1):e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43:452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.a BW, b YH . Which type of cancer patients are more susceptible to the SARS-COX-2: evidence from a meta-analysis and bioinformatics analysis. Critical reviews in oncology/hematology. 2020. [DOI] [PMC free article] [PubMed]
- 40.Davis AP, Boyer M, Lee JH, Kao SC. COVID-19: the use of immunotherapy in metastatic lung cancer. Immunotherapy. 2020;12:545–548. doi: 10.2217/imt-2020-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsamakis K, Gavriatopoulou M, Schizas D, Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E, Sioulas A, Tsamakis C, et al. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20:441–447. doi: 10.3892/ol.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rassy E, Khoury-Abboud RM, Ibrahim N, Kattan C, Assi T, Kattan J. What the oncologist needs to know about COVID-19 infection in cancer patients. Future Oncol. 2020;16:1153–1156. doi: 10.2217/fon-2020-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Haar J, Hoes LR, Coles CE, Seamon K, Frohling S, Jager D, Valenza F, de Braud F, De Petris L, Bergh J, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 45.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen YJ, Chitre AS, Chiang EY, Iftikhar H, O’Gorman WE, Au-Yeung A, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579(7798):274–278. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 48.Wu Q, Chu Q, Zhang H, Yang B, He X, Zhong Y, Yuan X, Chua MLK, Xie C. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun (Lond). 2020;40:374–379. doi: 10.1002/cac2.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


