From the Authors:
We appreciate the comments by Schnedl and colleagues in response to our trial (1) and wish to respond in detail to provide further clarification. They raise several points. First, drawing an analogy to insulin, antibiotics, and beta blockers, they question whether the treatment group was vitamin D deficient. However, the analogy may not be relevant to our trial, which used calcitriol and not vitamin D3. Moreover, vitamin D deficiency/insufficiency is nearly universal in critically ill patients (99–100% in some studies) (2, 3). In our study, 93% of patients at enrollment had 25-hydroxyvitamin D [25(OH)D] levels below 30 ng/ml, and the other 7% had 25(OH)D levels between 30 and 40 ng/ml.
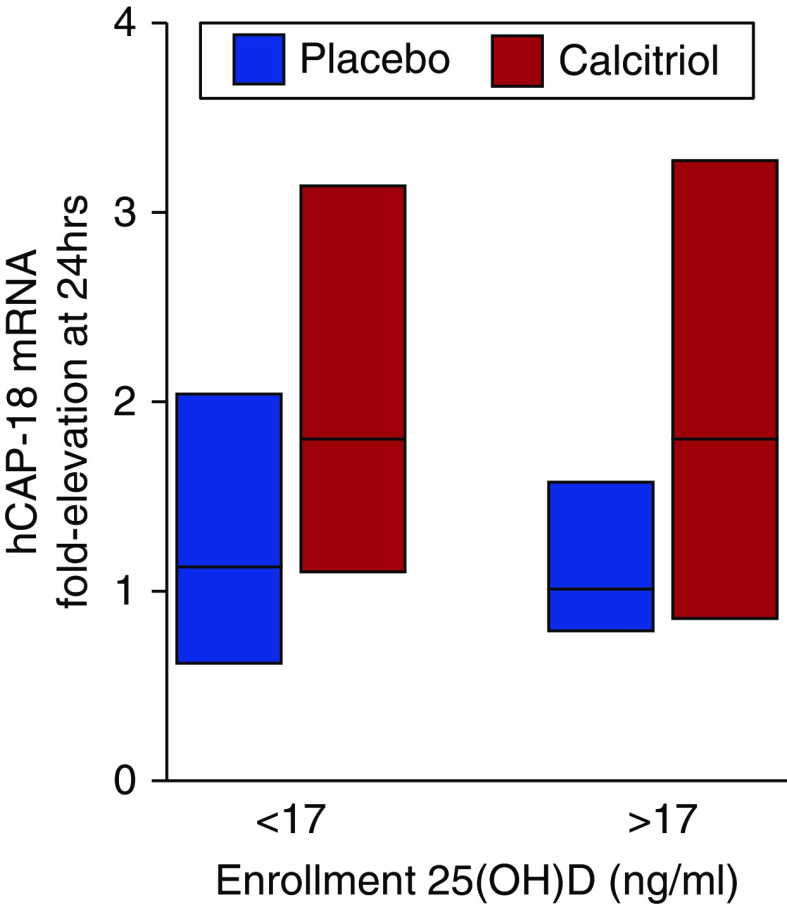
Schnedl and colleagues suggest analyzing whether patients with enrollment 25(OH)D levels below versus above the median had a more potent immunologic response to calcitriol. We did not present subgroup analyses in the original manuscript because of the modest sample size. However, the suggested analysis is shown in Figure 1. Fold elevation in leukocyte human cathelicidin antimicrobial peptide 18 messenger RNA expression was similar in calcitriol-treated versus placebo-treated patients with enrollment 25(OH)D levels below versus above the median level (17 ng/ml).
Figure 1.
Leukocyte hCAP-18 mRNA expression in patients with enrollment 25-hydroxyvitamin D [25(OH)D] levels below (n = 29) versus above (n = 29) the median level of 17 ng/ml. Bars represent median (25th–75th interquartile range). hCAP-18 = human cathelicidin antimicrobial peptide 18. A 1.0-fold elevation is equivalent to no change.
Second, Schnedl and colleagues comment that the interassay coefficients of variation (CVs) for 25(OH)D and 1,25-dihydroxyvitamin D [1,25(OH)D] are “surprisingly high.” We note that our study assessed interassay CV using blinded split sample replicates from intensive care unit patients, rather than manufacturer-reported CVs or CVs from standard reference materials. We further note that our CVs are within the range reported by the National Institutes of Standards and Technology for 25(OH)D measurement (4). Finally, all vitamin D metabolites were measured using immunoaffinity enrichment and liquid chromatography–tandem mass spectrometry, which is widely regarded to be the gold standard method of measurement (5, 6).
Third, Schnedl and colleagues comment that a different drug (vitamin D3 instead of calcitriol), dosing regimen, and patient population should have been studied. Each of these items merits careful consideration.
Drug
There has been an explosion of observational studies on 25(OH)D, in contrast to the limited studies of the active metabolite, 1,25(OH)D. The argument in favor of using vitamin D3 as an immunomodulatory agent is certainly reasonable: by raising local 25(OH)D levels, endogenous 1,25(OH)D production in monocytes and other cells may be enhanced. We note that nearly all preclinical studies (performed both in vitro and in animals) demonstrating immunomodulatory effects of vitamin D metabolites used 1,25(OH)D, rather than precursors such as vitamin D3. Furthermore, the increase in plasma 1,25(OH)D levels induced by high-dose vitamin D3 supplementation in critically ill patients (7) was lower than what we observed with direct calcitriol administration (1). We recognize, however, that plasma 1,25(OH)D levels may not be the best measure of local or intracellular levels, which could conceivably be more effectively increased by strategies focused on circulating 25(OH)D. Which drug is more effective would require a head-to-head comparison, which would only be relevant if both were shown in separate randomized controlled trials to be beneficial.
Schnedl and colleagues also argue in favor of vitamin D3 on the basis of the longer half-life of 25(OH)D (2–3 wk) compared with that of 1,25(OH)D (several hours). However, whether the immunomodulatory effects of oral vitamin D3 are superior to intravenous calcitriol cannot be assessed on the basis of a comparison of their half-lives. Moreover, patients with critical illness often have impaired gastrointestinal absorption and/or difficulty taking oral medications. Even in the absence of impaired absorption, the faster onset of intravenous calcitriol would arguably be preferable to the slower effects of oral vitamin D3 in a critically ill population: plasma 1,25(OH)D levels peak within minutes of administration of the former (8), whereas plasma 25(OH)D levels require several days (9), if not weeks (10), to peak after administration of the latter.
Dosing Regimen
We tested a single dose of calcitriol because of the physiologic nature of the study. The concentrations of 1,25(OH)D used in most in vitro studies, which ranged between 1 and 100 nmol/L, are much higher than physiologic levels in humans and may be higher than can be safely achieved without the development of hypercalcemia. However, immune responses in vitro have also been observed with 1,25(OH)D concentrations as low as 0.1 nmol/L (11–13)—levels that were achieved in the current study [median plasma 1,25(OH)D levels at 6 h were 0.2 (interquartile range [IQR], 0.1–0.3) nmol/L]. In addition, others have evaluated the pharmacokinetics of higher doses of intravenous calcitriol: after a single 10-μg intravenous dose of calcitriol among patients with advanced solid tumors, mean plasma 1,25(OH)D levels peaked at 1.1 (standard deviation, 0.5) nmol/L and were well tolerated (8). Thus, the possibility remains that with higher and repeated dosing of intravenous calcitriol, greater immunomodulatory effects may be observed. Such a strategy may indeed be appropriate for future studies of calcitriol in critically ill patients, as alluded to in the letter by Chapman and Alhajhusain.
Patient Population
Schnedl and colleagues make the argument that we excluded the only patient group likely to benefit from calcitriol: patients with end-stage renal disease. However, the studies we cited on the impaired conversion of 25(OH)D to 1,25(OH)D in the presence of fibroblast growth factor-23 (FGF23) included patients with end-stage renal disease as well as healthy volunteers (14). It is quite likely that conversion of 25(OH)D to 1,25(OH)D is impaired in critically ill patients. We (2) and others (15, 16) have documented elevated plasma levels of FGF23, a potent phosphaturic hormone release from bone, among critically ill patients with and without acute kidney injury. FGF23 inhibits 25-hydroxyvitamin D-1α-hydroxylase and also stimulates 25-hydroxyvitamin D-24-hydroxylase, thereby simultaneously inhibiting the activation and enhancing the catabolism of 25(OH)D, respectively (17, 18). Until recently, these effects were thought to primarily involve the kidneys, but recent data indicate that FGF23 also inhibits extrarenal 25-hydroxyvitamin D-1α-hydroxylase in monocytes (14). Even if conversion of 25(OH)D to 1,25(OH)D is not universally impaired in all patients with critical illness, additional pharmacologic augmentation of 1,25(OH)D may prove to be clinically beneficial. Only additional randomized trials will provide the answer.
Chapman and Alhajhusain also question whether clinical outcomes were different in “responders” versus “nonresponders,” as defined by changes in plasma 1,25(OH)D levels from 0 to 6 hours. Every patient who received calcitriol had an increase in plasma 1,25(OH)D levels at 6 hours (range, 14–225 pg/ml on an absolute basis and 1.6- to 84-fold on a relative basis). We found a graded relation between fold elevation in 1,25(OH)D levels (0–6 h) and fold elevation in human cathelicidin antimicrobial peptide 18 and interleukin 10 leukocyte mRNA expression (0–24 h) (Figures 4B and E1A from Reference 1). Whether clinical outcomes differ between patients who demonstrate a greater versus lesser increase in plasma 1,25(OH)D levels in response to calcitriol is an interesting question, but our study was not powered to evaluate clinical outcomes, particularly among subgroups. Nonetheless, we analyzed hospital mortality and length of stay among patients who received calcitriol and had an absolute increase in 1,25(OH)D levels above versus below the median. We found similar rates of hospital mortality (18 and 22%; P > 0.99) and length of stay (median, 20 [IQR, 16–28] and 21 [IQR, 13–38] d; P = 0.95), which were also similar to rates in the placebo group.
Footnotes
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–541. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leaf DE, Wolf M, Waikar SS, Chase H, Christov M, Cremers S, Stern L. FGF-23 levels in patients with AKI and risk of adverse outcomes. Clin J Am Soc Nephrol. 2012;7:1217–1223. doi: 10.2215/CJN.00550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204:37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedner M, Lippa KA, Tai SS. An assessment of 25-hydroxyvitamin D measurements in comparability studies conducted by the Vitamin D Metabolites Quality Assurance Program. Clin Chim Acta. 2013;426:6–11. doi: 10.1016/j.cca.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 6.Lai JK, Lucas RM, Banks E, Ponsonby AL Ausimmune Investigator Group. Variability in vitamin D assays impairs clinical assessment of vitamin D status. Intern Med J. 2012;42:43–50. doi: 10.1111/j.1445-5994.2011.02471.x. [DOI] [PubMed] [Google Scholar]
- 7.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Münch A, Warnkross H, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 8.Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, Schwartz J, Brattain MG, Hutson A, French R, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13:1216–1223. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- 9.Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, Stojakovic T, Schnedl C, Dobnig H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98:4339–4345. doi: 10.1210/jc.2012-4287. [DOI] [PubMed] [Google Scholar]
- 11.Daniel C, Schlauch T, Zügel U, Steinmeyer A, Radeke HH, Steinhilber D, Stein J. 22-ene-25-oxa-vitamin D: a new vitamin D analogue with profound immunosuppressive capacities. Eur J Clin Invest. 2005;35:343–349. doi: 10.1111/j.1365-2362.2005.01492.x. [DOI] [PubMed] [Google Scholar]
- 12.Griffin MD, Xing N, Kumar R. Gene expression profiles in dendritic cells conditioned by 1alpha,25-dihydroxyvitamin D3 analog. J Steroid Biochem Mol Biol. 2004;89-90:443–448. doi: 10.1016/j.jsbmb.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Széles L, Keresztes G, Töröcsik D, Balajthy Z, Krenács L, Póliska S, Steinmeyer A, Zuegel U, Pruenster M, Rot A, et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 14.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28:46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bech A, Blans M, Telting D, de Boer H. Incidence and aetiology of renal phosphate loss in patients with hypophosphatemia in the intensive care unit. Intensive Care Med. 2013;39:1785–1791. doi: 10.1007/s00134-013-2970-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Hsu R, Hsu CY, Kordesch K, Nicasio E, Cortez A, McAlpine I, Brady S, Zhuo H, Kangelaris KN, et al. FGF-23 and PTH levels in patients with acute kidney injury: A cross-sectional case series study. Ann Intensive Care. 2011;1:21. doi: 10.1186/2110-5820-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, et al. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J. 2005;390:325–331. doi: 10.1042/BJ20041799. [DOI] [PMC free article] [PubMed] [Google Scholar]