To the Editor:
Clinical, radiographic, and histopathologic criteria continue to emerge in cases of e-cigarette, or vaping, product use–associated lung illness (EVALI) (1, 2). We report a unique case of EVALI with radiographic, cytologic, and electron microscopy (EM) findings most consistent with secondary pulmonary alveolar proteinosis (PAP).
A 20-year-old female patient with chronic tetrahydrocannabinol (THC) vape use presented to the emergency room with 10 days of progressive dyspnea and cough. Her past medical history was significant for major depressive and anxiety disorder since 2015. She had no prior medical history of recurrent respiratory symptoms or prior diagnosis of chronic respiratory disease. She admitted to vaping counterfeit THC-based e-cigarette cartridges daily (50–100 puffs/d) for over a year. More specifically, she admitted purchasing THC-based cartridges from a friend that often did not have standardized labeling, strongly suggestive of noncertified or counterfeit production. She also reported inhaling marijuana using a water pipe with “dabs” on a bong nail as well as regular alcohol use (1–2 drinks per evening) but denied combustible cigarette, smokeless tobacco, or other illicit drug use.
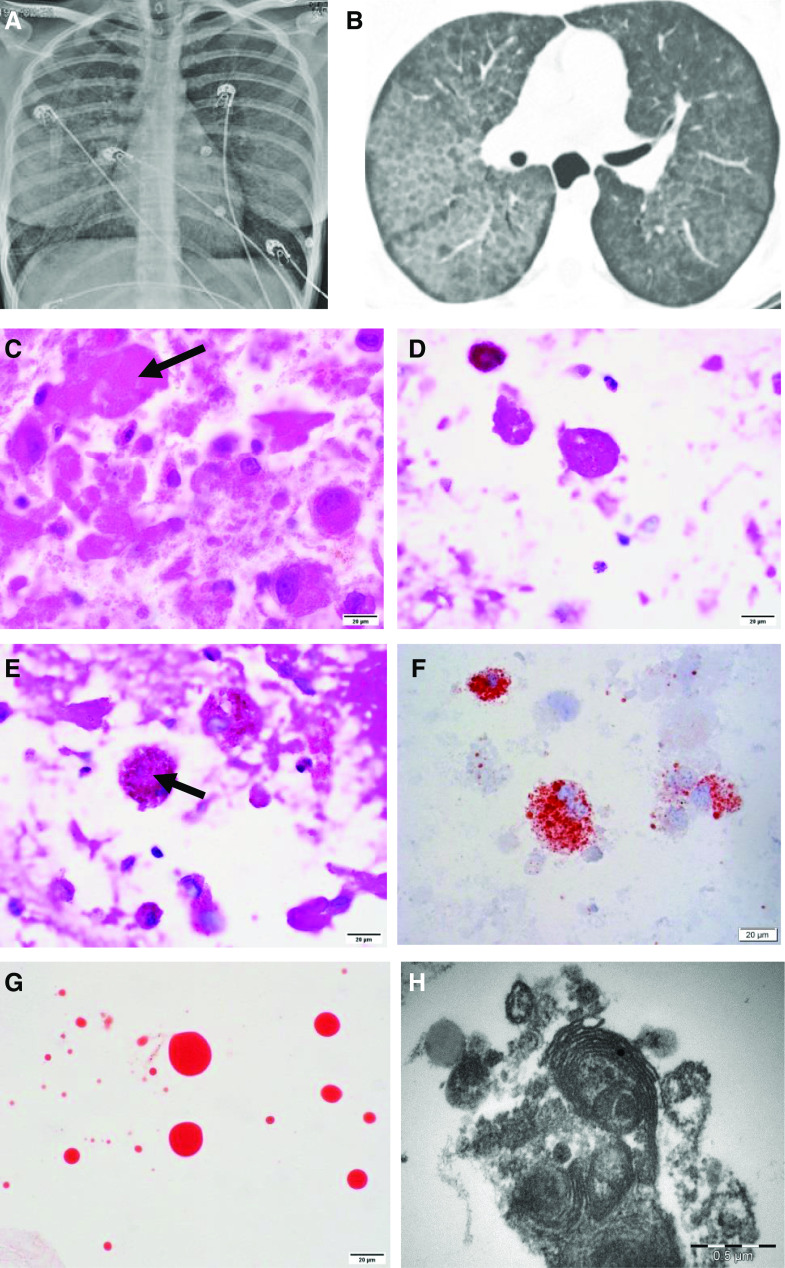
On presentation, her physical exam was significant for hypoxemia, tachypnea, fever, and bilateral lower lobe inspiratory crackles. Her laboratory evaluation revealed leukocytosis with significant neutrophilia and elevated inflammatory markers, but microbiologic culture studies were negative, ruling out infectious etiology (Table 1). Chest X-ray showed bilateral diffuse interstitial opacities. A high-resolution computerized tomographic imaging of the chest demonstrated diffuse bilateral ground glass opacities with subpleural sparing and associated septal thickening, consistent with “crazy paving” (Figures 1A and 1B).
Table 1.
Laboratory Workup at Initial Presentation
| Laboratory Test | Result | Reference Values |
|---|---|---|
| CRP, mg/L | 178 | 0–10 |
| ESR, mm/h | 70 | 0–20 |
| LD, U/L | 904 | 118–225 |
| Platelets, ×109/L | 377 | 160–370 |
| WBC, ×109/L | 20.9 | 4.0–10.0 |
| Neutrophils, ×1,000/μl | 18.2 | 1.6–6.1 |
| Lymphocytes, ×1,000/μl | 1.3 | 1.2–3.7 |
| Hb, g/dl | 10.7 | 11.2–15.7 |
| Hct, % | 33 | 34–45 |
| BUN, mg/dl | 12 | 6–20 |
| Creatinine, mg/dl | 0.5 | 0.5–1.0 |
| Sodium, mmol/L | 134 | 133–145 |
| Postassium, mmol/L | 2.9 | 3.3–5.1 |
| Chloride, mmol/L | 94 | 96–108 |
| Cultures | Negative | NA |
Definition of abbreviations: BUN = blood urea nitrogen; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; Hct = hematocrit; LD = lactate dehydrogenase; NA = not applicable; WBC = white blood cell count.
Figure 1.
(A) Frontal chest X-ray with bilateral perihilar hazy air space opacification. (B) High-resolution chest computed tomographic scan showing interlobular septal thickening forming a crazy paving pattern. (C) Hematoxylin and eosin showing extracellular eosinophilic hyaline material (arrow). (D) Periodic acid–Schiff with diastase–positive extracellular globular material. (E) Periodic acid–Schiff with diastase–positive granular deposit in intracytoplasmic macrophages (arrow). (F) Oil red O stain highlighting intracytoplasmic (macrophages) lipid vacuoles. (G) Oil red O stain–positive extracellular hyaline globules. (C–G) Scale bars, 20 μm. (H) Electron microscopy showing myelin-like whorled membranous structures (lamellar bodies) (scale bar, 0.5 μm).
After admission for noninvasive respiratory support and oxygen supplementation, BAL was performed. BAL cytology showed a neutrophilic infiltrate with numerous foamy macrophages and extracellular granular to globular material in the background. Subsequent Oil red O (ORO) staining showed intracytoplasmic (macrophages) lipid deposition. Periodic acid–Schiff with diastase (PAS-D) was positive for extracellular and amorphous eosinophilic material within the macrophages. Extracellular hyaline globules stained with ORO were also noted. EM showed characteristic lamellar bodies, representing surfactant (see Figures 1C–1H). The patient improved after treatment with antibiotics and steroids, independent of therapeutic whole lung lavage. She was subsequently discharged home, vowing to discontinue vaping.
The radiographic, cytologic, and EM findings (crazy paving, PAS and PAS-D–positive material, and lamellar bodies) are unique in this case of EVALI and have not been previously described. Furthermore, they are most consistent with secondary PAP (3, 4).
Prior case reports of EVALI demonstrate similar clinical presentation and serologic evaluation as the case presented here with dyspnea, hypoxemia, leukocytosis with neutrophilic predominance, and prominent inflammatory markers in the absence of an identifiable infectious cause (5, 6). In addition, our patient showed an elevated lactate dehydrogenase level, which is a nonspecific finding but often present in PAP. Other routine laboratory tests are usually normal in PAP (3).
Radiographically, EVALI presents with a variety of patterns and commonly shows bilateral opacities with subpleural sparing. EVALI may mimic other conditions radiographically, such as hypersensitivity pneumonitis, diffuse alveolar hemorrhage, acute eosinophilic pneumonia, diffuse alveolar damage, organizing pneumonia (OP), lipoid pneumonia, and giant cell interstitial pneumonia (4). PAP is commonly associated with bilateral symmetrical or patchy ground glass opacities, often with mid to lower lung predominance, and with septal thickening, which is also known as crazy paving (3).
A variety of histologic findings have been described in EVALI, including diffuse alveolar damage, OP, and acute fibrinous OP (7, 8). Accumulation of ORO stain–positive macrophages is a well-known, albeit nonspecific, feature of EVALI (8). PAS and PAS-D–positive material and macrophages, as well as lamellar bodies on EM, have not been described in other cases of EVALI and are more commonly associated with PAP. On routine histologic exam, PAP shows well-preserved alveoli filled with granular, eosinophilic, PAS-D–positive material, and minimal associated interstitial inflammation and fibrosis (3).
The vast majority of PAP cases are related to anti–granulocyte–macrophage colony–stimulating factor autoantibodies (primary PAP). Approximately 8% to 9% of PAP cases are classified as secondary, due to underlying conditions such as hematologic diseases, nonhematologic malignancies, immune deficiencies, chronic infection or inflammation, and toxic inhalation exposure. Common treatment for primary PAP is whole lung lavage and high-dose steroids. Conversely, treatment for secondary PAP is treatment of the underlying cause (3). Similar to other cases of EVALI, the patient presented in this case report responded to steroid treatment, forgoing the need for whole lung lavage, which further supports a diagnosis of secondary PAP (3, 9).
Pathogenesis of secondary PAP is thought to be due to impaired alveolar macrophage number or function, including surfactant catabolism (3). Recently, alterations in lung lipid homeostasis with aberrant phospholipids in alveolar macrophages and increased surfactant-associated phospholipids were described in a mouse model of chronic e-cigarette exposure (10). We hypothesize EVALI may represent a severe form of macrophage dysfunction, with subsequent surfactant accumulation in the alveolar spaces and secondary pulmonary proteinosis development. Considering that most patients with EVALI do not present with secondary PAP, further testing is required in eliciting the underling mechanism of EVALI pathogenesis and for identifying susceptible populations more prone to e-cigarette– or vaping-associated lung injury.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Karen Bentley and Karen Vanderbilt of the Electron Microscopy Core Facility at the University of Rochester Medical Center for technical assistance.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202002-0252LE on May 11, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Zou RH, Tiberio PJ, Triantafyllou GA, Lamberty PE, Lynch MJ, Kreit JW, et al. Clinical characterization of e-cigarette, or vaping, product use associated lung injury in 36 patients in Pittsburgh, Pennsylvania. Am J Respir Crit Care Med. 2020;201:1303–1306. doi: 10.1164/rccm.202001-0079LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. 2020;382:903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Trapnell BC. Pulmonary alveolar proteinosis syndrome. Clin Chest Med. 2016;37:431–440. doi: 10.1016/j.ccm.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry TS, Kligerman SJ, Raptis CA, Mann H, Sechrist JW, Kanne JP. Imaging findings of vaping-associated lung injury. AJR Am J Roentgenol. 2020;214:498–505. doi: 10.2214/AJR.19.22251. [DOI] [PubMed] [Google Scholar]
- 5.Triantafyllou GA, Tiberio PJ, Zou RH, Lamberty PE, Lynch MJ, Kreit JW, et al. Vaping-associated acute lung injury: a case series. Am J Respir Crit Care Med. 2019;200:1430–1431. doi: 10.1164/rccm.201909-1809LE. [DOI] [PubMed] [Google Scholar]
- 6.Boloña E, Felix M, Vanegas E, Vera Paz C, Cherrez-Ojeda I. A case of vaping-associated pulmonary illness in South America: highlighting the need for awareness and surveillance programs in the region. Am J Respir Crit Care Med. 2020;201:733–735. doi: 10.1164/rccm.201910-2002LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu MA, Jabre NA, Mogayzel PJ., Jr Vaping-related lung injury in an adolescent. Am J Respir Crit Care Med. 2020;201:481–482. doi: 10.1164/rccm.201909-1786IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhopadhyay S, Mehrad M, Dammert P, Arrossi AV, Sarda R, Brenner DS, et al. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping) Am J Clin Pathol. 2020;153:30–39. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 9.Alzghoul BN, Innabi A, Mukhtar F, Jantz MA. Rapid resolution of severe vaping induced acute lipoid pneumonia following corticosteroid treatment. Am J Respir Crit Care Med. doi: 10.1164/rccm.201909-1826IM. [online ahead of print] 6 Mar 2020; DOI: 10.1164/rccm.201909-1826IM. [DOI] [PubMed] [Google Scholar]
- 10.Madison MC, Landers CT, Gu B-H, Chang C-Y, Tung H-Y, You R, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129:4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.