Abstract
Iron is an especially important redox-active cofactor in biology because of its ability to mediate reactions with atmospheric O2. Iron-dependent oxygenases exploit this earth-abundant transition metal for the insertion of oxygen atoms into organic compounds. Throughout the astounding diversity of transformations catalyzed by these enzymes, the protein framework directs reactive intermediates toward the precise formation of products, which, in many cases, necessitates the cleavage of strong C–H bonds. In recent years, members of several iron-dependent oxygenase families have been engineered for new-to-nature transformations that offer advantages over conventional synthetic methods. In this Perspective, we first explore what is known about the reactivity of heme-dependent cytochrome P450 oxygenases and nonheme iron-dependent oxygenases bearing the 2-His-1-carboxylate facial triad by reviewing mechanistic studies with an emphasis on how the protein scaffold maximizes the catalytic potential of the iron-heme and iron cofactors. We then review how these cofactors have been repurposed for abiological transformations by engineering the protein frameworks of these enzymes. Finally, we discuss contemporary challenges associated with engineering these platforms and comment on their roles in biocatalysis moving forward.
Keywords: biocatalysis, enzymology, directed evolution, mechanism, oxygenase, cytochrome P450
Graphical Abstract

1. INTRODUCTION
Nature’s finest catalytic machinery is comprised of protein-based enzymes. Constructed mainly of polypeptide chains of the twenty genetically encoded amino acids, enzymes can assume a multiplicity of three-dimensional forms, which is the foundation for the remarkable diversity of chemical transformations that are catalyzed with the efficiency and selectivity required to support a living organism.1 While a great deal of this catalysis can be achieved within the manifold of functional groups provided by the canonical proteinogenic amino acids, whether by simple acid-base reactions or through intermediary covalent linkages, many essential biochemical transformations necessitate catalytic capabilities that exceed what is offered by these residues. The solution comes in the form of metallic or organic cofactors, with which many proteins are equipped to expand their catalytic repertoires. In this context, the interplay between the protein and cofactor is crucially important: the acquired catalytic function stemming from the unique properties of the cofactor is elicited or amplified by the protein environment. Cofactor-protein complexes achieve levels of activity and chemo-, regio-, and stereoselectivity that far surpass that which is produced by either component separately.
Since the emergence of our aerobic atmosphere, redox-active metals have taken on additional functions. Because the direct reaction of molecular oxygen (O2) in its thermodynamically favored triplet ground state with singlet organic compounds is generally spin-forbidden, metallocofactors with unpaired d-electrons, such as iron, provide a channel to mediate such reactions by reductive activation of this atmospherically prevalent molecule.2–4 The resulting metal-oxygen species serve as intermediates in pathways that are central to biology, Complex IV of the electron transport chain in aerobic respiration perhaps being the most notable example. Many other metalloenzymes activate O2 to insert oxygen atoms into the structures of their organic substrates.5 These oxygenases have provided us with powerful catalysts for biosynthesis and synthetic chemistry.
In this Perspective, we discuss the role of iron both in the heme complex and as free ferrous ion, and how its catalytic potential is maximized by proteins to effect oxygenase reactions. Toward this goal, we recount the catalytic cycles of heme-dependent cytochromes P450 and nonheme iron-dependent oxygenases that utilize the 2-His-1-carboxylate facial triad, highlighting throughout how the protein sequence imparts selective catalysis. The knowledge accumulated from decades of rigorous mechanistic studies demonstrates the advantages of using an enzyme to achieve such outcomes, especially in cases where direct comparison can be made to the reactivity of the protein-free cofactor.
From this foundational understanding of how Nature’s catalytic machinery controls reactivity and specific product outcomes, we bring the discussion to contemporary challenges in chemical synthesis by showing how the catalytic capabilities of natural enzymes can lead to discovery and optimization of new-to-nature transformations. Leveraging enzymes for the synthesis of high-value chemicals constitutes a powerful strategy with broad potential.6 While natural evolution has sharpened the myriad native functions of enzymes for specific biological advantages, it would be imprudent to look upon these functions as limits of achievability. Applying directed evolution, it is now feasible, even uncomplicated, to enhance the latent activities of enzymes for non-natural chemistry and further expand our synthetic toolbox,7 just as Nature has expanded hers over millions of years. In other words, the well of innovation is just waiting to be tapped. Recently, a number of iron-dependent oxygenases have been engineered to realize such a goal. Herein, we summarize the efforts of several research groups to push the hemoprotein and nonheme 2-His-1-carboxylate enzymatic platforms into the world of abiological catalysis, and then speculate on the future impact on chemical synthesis.
Eliciting new reactivity from cofactor-dependent enzymes and honing this reactivity by directed evolution is truly at the interface between enzymology and synthetic chemistry. Inspiration drawn from both fields can contribute to transformational advancements in our ability to craft molecules using biocatalysts. While the focus of this Perspective is narrowed to iron-dependent oxygenases, our broader goal is to inspire researchers to examine existing biological cofactors as potential sources of new and useful chemistry, from which fundamental knowledge stands to be extracted and new synthetic tools stand to be developed.
2. HEME
2.1. Structure and Reactivity
Heme cofactors employ the organic porphyrin framework to coordinate iron for a diversity of functions.8 The 18π electrons of the core structure (Figure 1, red substructure), which consists of four pyrrole subunits connected by methine bridges, are thought to be the basis of the cofactor’s aromatic character,9 the key contributor to its absorption properties and the generation of π-cation radicals during the formation of high-valent iron intermediates.10 The resulting planar structure coordinates the iron in a tetradentate fashion and occupies an entire plane of the octahedron. One or both coordination sites perpendicular to this equatorial plane can be occupied by additional axial ligands (X in Figure 1), which often play a crucial role in tuning the electronic properties of the metal (vide infra).11 In the active site of an enzyme, these ligands are contributed by amino acid side-chains.
Figure 1.
Structure of heme B. The bolded red bonds constitute the 18π electrons of the aromatic system in the [18]annulene model. X represents the axially coordinated protein ligand.
Heme’s impressive catalytic repertoire is evident throughout its assortment of biological functions. From oxygen maintenance12,13 and electron transfer14 to decomposition of harmful oxygen species15,16 and facilitating an array of chemically challenging oxidative transformations,17–21 Nature has given this special cofactor a starring role. Heme has also been an inspiration to chemists for decades. Their efforts have not only yielded model complexes that elevated our understanding of hemoproteins, but also afforded a collection of biomimetic catalysts that have addressed a number of synthetic challenges. These synthetic porphyrins have been extensively reviewed elsewhere.19,22–26
Although Nature and humanity together have leveled a great deal of attention on heme, the natural forms of the cofactor by itself have found very limited use in biology as well as industrial pursuits. Thus, the question must be asked: how does the hemoprotein environment tune the cofactor such that the desired reactivity is achieved? Here we strive to answer this question in the context of cytochromes P450, the family of hemoproteins that have been described as ‘a biological blowtorch’.27 These enzymes catalyze a suite of aliphatic and aromatic C–H oxidations,28,29 heteroatom oxidations,30,31 olefin epoxidations,32 C–C desaturations,33,34 and more,18,35 rendering them an ideal subject for a case study on how the polypeptide chain can activate and tune the heme cofactor for highly selective transformations that are not observed in the absence of the protein scaffold.
2.2. C–H Hydroxylation Catalyzed by Cytochromes P450
Cytochromes P450—named for the 450 nm absorption band observed with the reduced, CO-bound state of the heme B cofactor36—are found throughout every kingdom of life.37 P450s coordinate one of the two axial sites of the iron center with a cysteine ligand, leaving the other open for binding of water or O2. In the hydroxylation reaction, the net four-electron reduction of O2 is balanced by the two-electron oxidations of both NAD(P)H and the aliphatic C–H bond undergoing oxygenation (Equation 1).18 Amazingly, the bond dissociation energies (BDE) of the targeted C–H bonds can range as high as 102.9 kcal/mol,38 an energy barrier that is overcome by reactive high-valent iron-oxo intermediates. Throughout the step-by-step mechanism presented below, we highlight the role of the protein scaffold and its contributing ligands in facilitating the formation and directing the reactivity of these reactive intermediates.
| (1) |
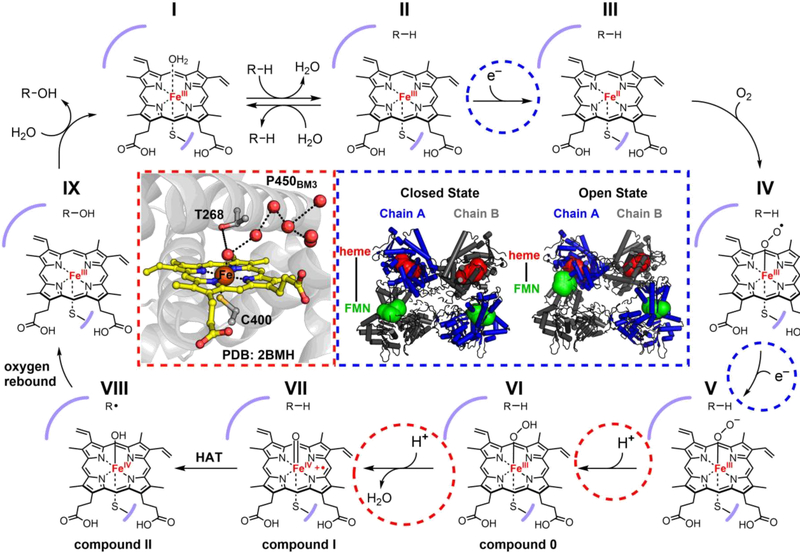
In the resting state of the P450 active site, a water ligand occupies the sixth coordination site of the low-spin (S = 1/2) Fe(III) cofactor (Figure 2, state I).11,18,39 Binding of the substrate then displaces this axially coordinated water, which is important for two main reasons: (1) an axial coordination site is now open for binding of O2, and (2) the change in the coordination environment shifts the iron to a high-spin (S = 5/2) state (state II).11,18 The latter consequence brings the redox potential of the iron into the range of the corresponding cytochrome P450 reductase, which then delivers an electron originating from NAD(P)H through the reduced form of its flavin mononucleotide (FMN) cofactor.40–42 The coupling of this protein-controlled substrate binding event with the reorganization of the ligand sphere effectively prevents entrance into the catalytic cycle in the absence of substrate. It is worthy of note that while this substrate-induced spin-shift strategy is a classic example of the control of electron flow in P450 catalysis, it is not universally employed within the family. Alternative mechanisms have been reviewed elsewhere.43
Figure 2.
Mechanism of P450-catalyzed hydroxylation. The red inset (left) depicts the active site of the P450BM3 heme domain in the substrate-free crystal structure (PDB: 2BMH). The conserved T268 residue sits directly above the heme, in close contact with an ordered water network (red spheres) for the proton-transfer steps. The blue inset (right) shows the conformational shift between the closed and open states of the full-length P450BM3 dimeric complex (models derived from cryo-EM maps EMD: 20785 and EMD: 20786), which brings the heme (red spheres) from one monomer and FMN (green spheres) from the other in close proximity for the electron transfer steps.
Reduction of the heme to Fe(II) triggers binding and reduction of O2 to yield a ferric superoxo species (Figure 2, state IV), an intermediate that, if neglected, will produce harmful reactive oxygen species (ROS).44 Thus, efficient delivery of a second electron and a proton are required at this stage to push the reaction forward toward the formation of the ferric hydroperoxo intermediate known as compound 0 (state VI).45 This sequence, in addition to the initial reduction of the cofactor, necessitates precisely controlled access of the partner P450 reductase to the P450 active site. Complementing the years of rigorous explorations into this process,46 the recently solved single-particle cryo-EM structure of the full-length cytochrome P450 from Bacillus megaterium (P450BM3), a self-sufficient P450 with the partner reductase fused into a single polypeptide chain, is an excellent model of how such control can be imparted by the protein framework.47 This structure provides a clear picture of how the dimeric complex can exist in open and closed states, and how a conformational shift to the former brings the FMN cofactor of one monomer into close proximity with the heme of the other, presenting a mechanism by which electrons can be delivered to the cofactor in a manner controlled at the quaternary level of the protein structure (blue inset). Meanwhile, a conserved threonine residue (T268 in P450BM3) positioned above the heme likely facilitates the proton transfer either by direct interaction with distal oxygen of the ferric peroxo intermediate (state V), or by extending its access to a nearby water network (red inset).48–50
This conserved threonine residue also plays a crucial role in the subsequent O–O bond scission step by assisting the delivery of a second proton to the distal oxygen of compound 0 (Figure 2, red inset), which results in release of water and formation of compound I (state VII). Mutation of this threonine residue to alanine in either P450BM3 or P450CAM diminished hydroxylation activity and increased production of H2O2.48,49 The uncoupling of O2 reduction from substrate oxidation highlights the importance of precision proton delivery for efficient O–O bond scission. Moreover, a recent study showed that a glutamic acid substitution allowed these P450s to operate as peroxygenases, whereby substrate oxidation could be achieved through the peroxide shunt pathway.51 These “pull” effects are complemented by the “push” effect exerted by the axial cysteine ligand: the strong σ-donation from the thiolate, which effectively drives electron density into the antibonding O–O orbital, also contributes to productive O–O bond heterolysis.11,19,52
The two additional electrons required for reduction of hydrogen peroxide to water and O–O bond scission are afforded by the iron and the organic porphyrin scaffold, resulting in the formation of the high-valent Fe(IV)-oxo (ferryl) π-cation radical species known as compound I (Figure 2, state VII).10,53 This potent oxidant intermediate facilitates hydrogen atom transfer (HAT) from the substrate C–H bond to the cofactor, generating the Fe(IV)-hydroxide species known as compound II and a substrate radical (state VIII).54 The reaction cycle is completed by transfer of the oxygen ligand to the carbon-centered radical (termed oxygen rebound, state VIII to IX),55 release of the hydroxylated product, and then rebinding of the water, returning the cofactor to its resting state (state IX to I).18
During this sequence, P450s exhibit exquisite control over highly reactive intermediates (e.g., compound I) to achieve productive cleavage of the substrate C–H bond while preventing the oxidation of readily oxidizable amino acids within its own framework, such as tyrosines and tryptophans. The axial cysteine ligand was initially posited to play a crucial role in the prevention of such undesired reactions because it is present in all P450s and chloroperoxidase (CPO),11 the members of the hemoprotein family known to cleave strong C–H bonds. Green, Dawson, and Gray postulated the difference in free energy between the productive C–H activation pathway and nonproductive tyrosine oxidation pathway was largely dependent on the pKa of the resulting compound II, which was increased by the strong electron donation from the thiolate ligand.56,57 In 2013, Green and coworkers validated this hypothesis by trapping and characterizing compound II of Streptomyces coelicolor CYP158 at differing pH values, determining its pKa to be nearly 12 (more than 8 orders of magnitude more basic than the histidine-ligated heme peroxidases).54,56 This change in axial ligand from histidine to cysteine alters the thermodynamic favorability of tyrosine oxidation (via uncoupled proton and electron transfer) over substrate C–H bond cleavage (via HAT) from ~14 kcal/mol to only 3 kcal/mol, bringing the P450-catalyzed C–H oxidation reaction into the range of kinetic control.54 These conclusions were further supported by the correlation of a shorter Fe–S bond length with increased reactivity in P450s and CPOs,58 and then later by the increase in reactivity of a selenocysteine-ligated P450,59 which exhibits even stronger electron donation to the metal center than its sulfur counterpart. It is worthy of mention that other heme-dependent oxygenases, such as tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase, indeed utilize histidine axial ligands to direct high-valent iron intermediates, but activation of their aromatic substrates presents a distinct set of challenges requiring divergent catalytic solutions. The mechanisms of these transformations have been discussed elsewhere.60,61
The decades of P450-centered research summarized above illustrate how the interplay between peptide and cofactor can achieve reactivity that is nonexistent with either individual component. The hydroxylation pathway is just one of many examples: Nature has tuned the P450 peptide-heme interaction for many other catalytic functions. From the perspective of the protein engineer, a question that naturally follows is: can this remarkable catalytic machinery be hijacked for abiological reactions and likewise tuned to achieve useful levels of activity and selectivity?
2.3. New Hemoprotein Activities Emerge
In recent years, our understanding of reactions catalyzed by hemoproteins and the characterization of their reaction intermediates have been leveraged to develop new reactivities, which, combined with engineering techniques such as directed evolution, have furnished a suite of powerful hemoprotein catalysts. The examples recounted below are not intended to serve as a detailed analysis of contributions to synthetic methodology, but rather to summarize that which is currently possible with hemoprotein biocatalysts.
Inspired by the powerful transition metal-catalyzed carbene-transfer reactions developed by synthetic chemists,62 Coelho, Brustad, and coworkers hypothesized that the heme cofactor could form analogous metal-carbenoid intermediates, as they would have an isolobal relationship with compound I.63 The carbene could then be transferred, for example, to an olefin substrate to form a cyclopropane product, similar to native P450-catalyzed epoxidation reactions. Indeed, P450BM3 exhibited activity for the cyclopropanation of styrene with ethyl diazoacetate (EDA) as the carbene precursor, which forms the iron-carbenoid intermediate upon loss of N2.64 Although this reaction is also catalyzed by free hemin in the presence of dithionite, the protein environment enforced a different selectivity of the product configuration. Screening a collection of P450 variants identified P450BM3-CIS, which catalyzed the reaction with 199 total turnovers (TTNs), a 71:29 cis/trans ratio, and up to a 94% enantiomeric excess (% ee)—a significant improvement in both activity and selectivity over the cofactor alone.
Coelho and Brustad then initiated a directed evolution campaign to generate even more efficient catalysts, screening this new function in whole Escherichia coli cells. Their experiments suggested that the cofactor was predominantly active in the Fe(II) state, as the reaction occurred optimally in the presence of dithionite and the absence of O2. Moreover, reduction of the cofactor by the native NADPH-dependent reductase domain was unsuccessful, suggesting that the non-native substrate fails to trigger the necessary shift in the iron’s spin state, as observed in the native reaction sequence (Figure 2). These roadblocks to enzyme-catalyzed cyclopropanation in vivo were circumvented by mutating the axial cysteine ligand, which, as described above, plays a major role in tuning the electronics of the cofactor.65 Indeed, substitution with the less electron-donating serine in P450BM3 (named P411BM3 for the Soret band shift from 450 nm to 411 nm) resulted in a 127 mV increase in the resting-state reduction potential (E°’ Fe[III/II] = −293 mV), bringing it into the range of the reductase domain (E°’ NADP+/NADPH = −320 mV). Exchanging the axial cysteine for serine in P450BM3-CIS (furnishing P411BM3-CIS) abolished the competing epoxidation reaction, even in the presence of O2. However, the P411 enzyme catalyzed the styrene cyclopropanation reaction with up to 67,800 TTN in whole cells.65
These pioneering studies opened the floodgates to a wave of abiological reactions catalyzed by hemoproteins (Figure 3), of which many sport substitutions of key active-site residues distinguished by mechanistic research. A pool of olefin cyclopropanation catalysts has now been engineered to address several reactivity and selectivity challenges within the context of this valuable transformation.66–78 The concept of carbene transfer to π systems has also been expanded to include alkyne substrates, from which the highly strained cyclopropene and bicyclobutane products are furnished in high yield and with excellent selectivity.79,80 Additionally, hemoproteins have been engineered for insertion of carbenes into N–H,81,82 S–H,68,83 Si–H,84 and B–H bonds.85–87 These advancements recently culminated in P411 enzymes proficient at inserting carbenes into C(sp3)–H bonds,88–90 a reaction with enormous potential to transform the way we construct C–C bonds.
Figure 3.
Summary of abiological carbene-transfer reactions catalyzed by hemoproteins. The red asterisks indicate possible chiral centers in the reaction products; the red and blue spheres indicate the possibility of variable functional groups originating from the diazo-bearing substrates.
Hemoproteins have also been discovered and engineered to act as efficient transferases of nitrene intermediates, similar to carbenes. That hemoproteins could insert nitrene intermediates into C–H bonds was shown as far back as 1985.91 It was not until 28 years later, however, that this reactivity was exploited and improved upon by directed evolution to achieve multiple turnovers (Figure 4). McIntosh, Coelho, and coworkers demonstrated that a cyclopropanating enzyme, P411BM3-CIS, could catalyze an intramolecular C–H amination reaction of the 2,4,6-triethylbenzene-1-sulfonyl azide nitrene precursor (wherein the loss of N2 drives formation of an iron-nitrenoid, analogous to the carbene-transfer reaction) with up to 680 TTN;92 Singh and coworkers engineered P450s for a similar purpose.93 An intermolecular version of this reaction was later established with tosyl azide and 4-ethylanisole.94 Aziridination of olefin substrates was also achieved with engineered P411 catalysts, using the same nitrene precursor.95 Recent advances in synthetic methodology,96 and subsequent discovery of a natural P450 nitrene transferase, BezE,97 however, inspired researchers in our laboratory to turn to hydroxylamine esters as a nitrene source, which could furnish unprotected amine products in a single step. Engineered hemoproteins were active with these nitrene precursors in the asymmetric aminohydroxylation of olefin substrates, the top variants exhibiting impressive levels of activity and selectivity.98 More recently, a lineage of P411s was engineered to insert these unprotected nitrenes directly into primary, secondary, and tertiary C(sp3)–H bonds, an invaluable transformation that currently has no synthetic counterpart.99
Figure 4.
Summary of abiological nitrene-transfer reactions catalyzed by hemoproteins. The red asterisks indicate possible chiral centers in the reaction products.
Throughout this expansion of carbene- and nitrene-transfer reactions catalyzed by hemoproteins, the choice of hemoprotein has also grown. P450s, cytochromes c,84 myoglobins,70 protoglobins,66 and a nitric oxide dioxygenase (NOD)66 have all served as starting points for site-specific mutagenesis or directed evolution to engineer more active and more selective biocatalysts. Furthermore, an assortment of both natural and noncanonical amino acids has been explored as axial ligands to the heme.100 Following the Hilvert Lab’s discovery that an N-methyl-His axial ligand enhanced the native reactivity of ascorbate peroxidase and the peroxidase activity of myoglobin,101,102 Carminati and Fasan demonstrated that a myoglobin variant presenting this noncanonical amino acid to a synthetic iron-2,4-diacetyl deuteroporphyrin IX cofactor catalyzed the cyclopropanation of both electron-rich and electron-poor olefins, of which the latter had remained a challenging substrate for such biocatalytic transformations.75 Interestingly, their experimental observations suggested that the reaction mechanism had been altered, and now proceeded down a stepwise radical pathway rather than the previously hypothesized concerted carbene-transfer pathway. We anticipate more such observations as the hemoprotein platform is pushed in creative new directions to overcome contemporary synthetic challenges.
The studies recounted above demonstrate how iron-carbenoid and iron-nitrenoid intermediates within an enzyme active site can be channeled toward the construction of useful bonds, but these highly reactive species can also escape down reaction pathways that generate undesirable side products. In carbene-transfer reactions, alkylation of the heme cofactor or nearby protein residues is a limiting factor in maximizing catalytic turnovers.103 While this side reaction is also possible in nitrene-transfer reactions, reduction of the nitrene to a primary amine is perhaps the most problematic side pathway, particularly in the context of intermolecular reactions.94,104 In both carbene- and nitrene-transfer reactions, substrates bearing multiple modes or sites of reactivity further exacerbate this problem, as additional pathways of intermediate decay can result in diminished chemo-, regio-, and stereoselectivity. While these obstacles should always be considered when embarking on a new reaction, it is worth recognizing the benefits imparted by a mutable protein scaffold: in many of the carbene-transfer and virtually all of the nitrene-transfer reactions described to date, the free heme cofactor fails to produce measurable quantities of the desired products. And, in the cases where activity is observed, the TTNs and enantiomeric excesses pale in comparison to those with the engineered hemoproteins. In summary, the laboratory-evolved protein architecture plays a crucial role in directing reactive intermediates along the desired reaction pathways, much the same way that the natural versions of these cytochromes P450 direct compounds I and II.
3. NONHEME IRON
3.1. Reactions of Ferrous Iron with Oxygen Species
Ferrous iron alone can also be a catalyst in reactions with various oxygen species. Although the oxidation of Fe(II) to Fe(III) in the presence of O2 in aqueous solutions generally leads to the undesired precipitation of ferric hydroxide complexes, especially in alkaline solutions, other conditions that promote useful iron-catalyzed reactions have been described. In the Fenton reaction, for instance, Fe(II) disproportionates H2O2 to HO• and HOO•, and produces H2O as a byproduct.105 The resulting radical species can then oxidize organic compounds, which is the basis of the reaction’s application to the purification of groundwater and soils contaminated with hydrocarbons.106 Fe(II) in the presence of peroxy acids is converted to the Fe(IV)-oxo (ferryl) intermediate, which was demonstrated to catalyze C–H oxidation reactions that later helped establish the stepwise model (HAT and oxygen rebound) for many biological oxidations.107,108 Reaction of aqueous Fe(II) with ozone (O3) also produces the reactive ferryl species.109 Under these conditions, ketones and other byproducts were generated from one- and two-electron oxidations of cyclic alcohols.110 In the Gif oxygenation systems, these ferryl species are formed directly from O2 in the presence of reductants (Zn or NaS) and pyridine.111–113 While the culmination of this body of work has indeed yielded catalytic systems that can functionalize strong C–H bonds, many of these fundamentally important reactions suffer from unproductive oxidation and precipitation of the catalyst, a general lack of control over intermediary radical species, and limited regio- and stereoselectivity, hindering their applications more broadly.
Nonheme iron-dependent oxygenases, however, effect similar C–H functionalization reactions efficaciously by protecting the ferrous cofactor from unproductive oxidation, tuning reactivity with O2, and positioning substrates such that reactive intermediates are guided down the desired pathway. Here, we narrow our focus to mononuclear Fe(II)-dependent oxygenases that employ the 2-His-1-carboxylate iron-binding motif. These enzymes catalyze a remarkable diversity of oxidative transformations stemming from this ostensibly simple, earth-abundant metal, providing another example of how the protein environment can modulate cofactor reactivity and maximize its catalytic potential.
3.2. C–H Oxidation Reactions Catalyzed by 2-His-1-Carboxylate Enzymes
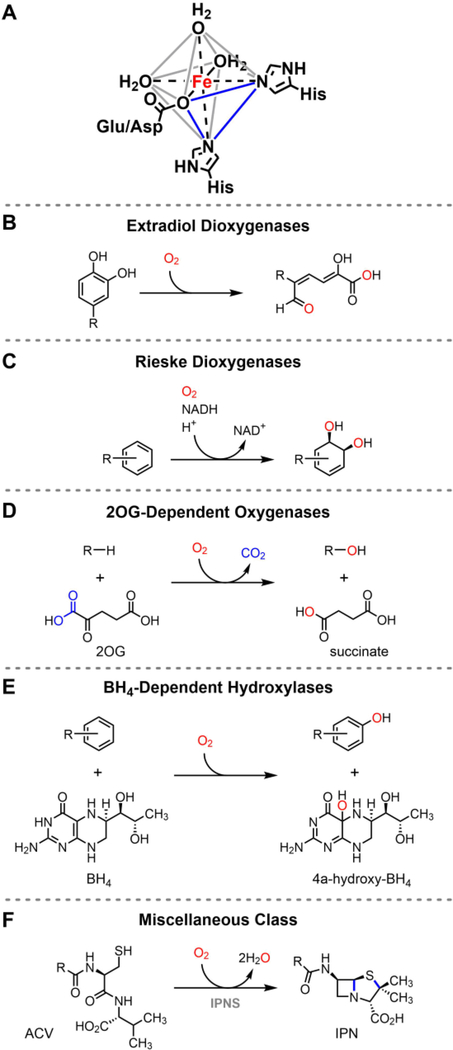
2-His-1-carboxylate enzymes activate O2 for a diversity of C–H oxidation reactions with an iron-binding motif comprised of two histidines and one glutamate or aspartate.2,114–116 This facial triad of protein ligands occupies the vertices of one face of the octahedral iron, leaving open three coordination sites for waters, substrates, co-substrates, and various species of oxygen (Figure 5A). Because of the divergence in requirements for additional co-substrates and cofactors, enzymes that utilize this motif are divided into the following five families: the extradiol dioxygenases (Figure 5B),117 the Rieske dioxygenases (Figure 5C),118 the 2-oxoglutarate (2OG)-dependent oxygenases (Figure 5D),119 the tetrahydrobiopterin (BH4)-dependent hydroxylases (Figure 5E),120 and a miscellaneous class that includes isopenicillin N-synthase (IPNS),121,122 (S)-2-hydroxypropyl-1-phosphonate epoxidase (HppE),123 2-hydroxyethylphosphonate dioxygenase (HEPD),124 methylphosphonate synthase (MPnS) (Figure 5F),125 and 1-aminocyclopropane-1-carboxylate oxidase (ACCO).126 The references logged above provide a more comprehensive discussion of each individual family, as the staggeringly large catalogue of chemical transformations described by these enzymes is outside the scope of this Perspective. Biomimetic nonheme iron model complexes have also been reviewed extensively.127–129
Figure 5.
A, The 2-His-1-carboxylate facial triad iron-binding motif. The vertices of one face of the octahedron are occupied by the three protein ligands (blue triangle). The general reactions catalyzed by each of the 2-His-1-carboxylate enzyme families are shown in B-F. The conversion of δ-(L-α-aminoadipoyl)-l-cysteinyl-D-valine (ACV) to isopenicillin N by the oxidase IPNS is a representative example in the miscellaneous class, which includes oxygenases, oxidases, and peroxidases.
Despite the catalytic diversity of 2-His-1-carboxylate enzymes, a general strategy to regulate entry into the reaction cycle has emerged. The ferrous resting state of the cofactor renders it, in principle, highly susceptible to unproductive oxidation, necessitating a mechanism to protect the active site during periods of inactivity. Similar to P450s (vide supra), the binding of substrates triggers the dissociation of one or more coordinated water molecules, constituting a protein-controlled shift in the ligand sphere to promote a five-coordinate Fe(II) center, which opens a site for binding of O2.2,115 The displacement of water with anionic ligands is also thought to decrease the Fe(III/II) reduction potential for productive oxidation of the cofactor.115 Whereas the substrates or co-substrates of the extradiol dioxygenases, 2OG-dependent oxygenases, and IPNS fill this role by directly coordinating to the iron,117,119,130 the BH4-dependent hydroxylases and Rieske dioxygenases, whose substrates contribute no additional anionic ligands, effect a similar outcome by bidentate coordination of the protein’s carboxylate ligand.118,120
The steps following substrate binding and O2 activation in each of the 2-His-1-carboxylate families diverge, giving rise to an assortment of structurally and electronically distinct intermediates. Nevertheless, a unifying theme concerning the protein’s influence over the reaction progression is clear: the polypeptide scaffold binds the substrate, co-substrate, or additional cofactor involved in the subsequent step in an orientation that encourages productive interaction with the intermediary iron-bound oxygen species. In IPNS, the end-on ferric superoxide initiates a HAT from the substrate C–H bond neighboring the iron-coordinated thiolate, the committed step leading to C–N and C–S bond formation.122 The juxtaposition of the implicated atoms was shown clearly in the NO-bound crystal structure (PDB: 1BLZ), wherein the distal oxygen of the O2 surrogate is in close proximity to the target carbon (Figure 6).130 Similar observations were made in the NO-bound crystal structures of the 2OG-dependent enzymes clavaminate synthase 1 (CAS1, PDB: 1GVG) and WelO5 (PDB: 5IQV).131,132 In these cases, however, the distal oxygen of the O2 surrogate is poised to attack C2 of the co-substrate 2OG, which ultimately provokes O–O bond scission and ferryl formation (Figure 7). In the BH4-dependent hydroxylases, a bridged Fe(II)–O–O–BH4 intermediate is thought to precede ferryl formation and substrate oxidation, again necessitating a propinquity of the two cofactors.133,134 Indeed, this was observed in a crystal structure of the phenylalanine hydroxylase ternary complex with the 3-(2-thienyl)-l-alanine (THA) substrate analogue (PDB: 1KW0, Figure 8).135 The Rieske and extradiol dioxygenases leverage a side-on binding mode of O2 to affect product formation. In the former family, the orientation of the substrate results in syn-hydroxylation of the aromatic system, as was revealed by the crystal structures of naphthalene dioxygenase (NDO, PDB: 1O7N) and carbazole 1,9a-dioxygenase (CARDO, PDB: 3VMI) bearing the intermediate state (Figure 9).136,137 In the latter family, a single crystal structure depicting multiple states of the homoprotocatechuate 2,3-dioxygenase (2,3-HPCD, PDB: 2IGA) reaction cycle demonstrates how the cofactor-substrate relationship promotes substrate-driven reduction of O2 and eventually cleavage of the aromatic ring (Figure 10).138 In a more recent study on 3-hydroxyanthranilate-3,4-dioxygenase (HAO), a close relative of the extradiol dioxygenases, an astonishing seven states of the reaction cycle were captured by x-ray crystallography.139 Although this enzyme does not appear to bind O2 in the same side-on orientation, the protein scaffold still guides the reaction through a similar course of events.
Figure 6.
Abbreviated mechanism of the conversion of L-δ-aminoadipoyl-l-Cys-D-Val (ACV, green) to isopenicillin N (IPN) catalyzed by IPNS. The crystal structure of the NO-bound complex (right, PDB: 1BLZ), a mimic of the HAT-initiating ferric superoxide intermediate, demonstrates how the protein-enforced orientation of the iron-bound substrate results in productive reactivity.
Figure 7.
Established mechanism of aliphatic C–H hydroxylation catalyzed by 2OG-dependent oxygenases. The NO-bound crystal structure of CAS1 (left, PDB: 1GVG) shows a close proximity of the distal oxygen of the ferric superoxide intermediate to C2 of 2OG (yellow), leading to oxidative cleavage of the co-substrate to succinate and CO2, and ultimately hydroxylation of the primary substrate.
Figure 8.
Abbreviated mechanism of the conversion of L-phenylalanine to L-tyrosine catalyzed by phenylalanine hydroxylase. The BH4-bound crystal structure with the 3-(2-thienyl)-l-alanine (THA, green) substrate analogue (right, PDB: 1KW0) shows how the protein orients the two cofactors in such a way to achieve reductive cleavage of O2 and oxidation of the aromatic substrate.
Figure 9.
A side-on binding orientation of O2 to the iron cofactor in the Rieske dioxygenase family, as observed in the crystal structure of NDO (left, PDB: 1O7N) with the indole substrate analogue (green), results in syn-hydroxylation of aromatic substrates.
Figure 10.
Abbreviated mechanism of the cleavage of catechol substrates catalyzed by members of the extradiol ring-cleaving dioxygenase family. The side-on binding mode of O2 observed in the crystal structure of 2,3-HPCD (right, PDB: 2IGA) positions the intermediate close to the correct carbon of the 4-nitrocatechol substrate (4NC, green), which results in substrate-driven reduction of O2 and ultimately insertion of both oxygens into product structure. Remarkably, multiple states of this reaction cycle were captured in different subunits of the asymmetric unit in the same crystal sample.
Several of the 2-His-1-carboxylate families form high-valent ferryl intermediates for the cleavage of strong C–H bonds.140 Unlike those formed free in solution, these enzymatic intermediates are channeled with precise stereoelectronic control. 2OG-dependent oxygenases, in particular, are a testament to such precise channeling, as ferryl-mediated C–H activation leads to an astounding diversity of transformations that, in most instances, are catalyzed with exceptional selectivity.141 In this family, the reduction of O2 and oxidative conversion of 2OG to succinate and CO2 results in ferryl formation,142–152 which then effects C–H bond cleavage to yield ferric-hydroxide and substrate-centered radical intermediates, a key branchpoint in 2OG-dependent oxygenase catalysis. The origin of chemoselectivity stemming from this state has been studied extensively by dissecting and comparing the mechanisms employed by the hydroxylase and halogenase subfamilies. In the hydroxylation pathway, oxygen rebound furnishes the product and regenerates the Fe(II) cofactor for subsequent turnovers (Figure 7), similar to P450-cataylzed hydroxylation reactions.18,119 In the halogenation pathway, a cis-coordinated halogen is transferred (Cl• or Br•) in lieu of the hydroxyl group (Figure 11). This transfer is first enabled by substitution of the iron-binding carboxylate ligand of the facial triad for an alanine or glycine, as demonstrated by the substrate-free crystal structure of the carrier-protein-dependent chlorinase SyrB2 (PDB: 2FCV and 2FCT), wherein the halide coordinates directly to the cofactor.153 Further dissection of the SyrB2 reaction placed crucial importance on the substrate-cofactor disposition to achieve a selective halogenation outcome (Figure 11B).154–156 More recently, structural studies on the chlorinase WelO5 presented an alternative strategy to effect halogenation: a protein-controlled rearrangement of the ferryl intermediate properly juxtaposes the substrate and cofactor for selective halogen transfer (Figure 11C).132 In both cases, subtle yet precise effects of the protein environment seem to dictate the preference for rather disparate outcomes, a premise that is also relevant to the desaturation,157 epoxidation,152,158 cyclization,150,159,160 epimerization,148 and endoperoxidation161 reactions catalyzed by this family.
Figure 11.
Comparative models showing the generalized protein-dictated orientations of the substrate and ferryl intermediate in 2OG-dependent enzyme catalysis. The inline ferryl with substrate bound directly above the oxo is the typical configuration for hydroxylation (A). Halogenation is achieved by binding the substrate such that rebound of the halogen radical is favored (B), or by reorientation of the ferryl to disfavor oxygen rebound (C). The blue X represents an iron-coordinated aspartate, glutamate, or halide; the substrate is depicted as R–H; the gray R–H and iron-oxo bond are shown to illustrate the change in position of the respective species, not to be interpreted as being present in the complex.
3.3. The Facial Triad Supports Abiological Activity
The astonishing catalytic repertoire of nonheme iron-dependent oxygenases, oxidases, and peroxidases bearing the 2-His-1-carboxylate facial triad has inspired researchers to investigate whether this enzymatic platform could be extended to useful abiological transformations. While this vision has indeed proven to be possible, the collection of engineered catalysts is still in its infancy. However, the future is nonetheless bright for this family of enzymes, and we anticipate that the progress summarized below is just the beginning.
Members of the 2OG-dependent halogenase subfamily hold great biocatalytic potential within the confines of their native catalytic function, as there is currently a paucity of methods to directly halogenate aliphatic C–H bonds with a high degree of selectivity.162–166 Our understanding of these enzymes also led some researchers to investigate whether other anionic species that would likely coordinate to the cofactor could be coupled to intermediate substrate radicals via an analogous mechanism. Indeed, trace levels of nitration and azidation activity were detected in SyrB2 preparations with the native substrate and substrate analogues, constituting an important abiological addition to the growing number of enzymatic C–H functionalization reactions (Figure 12A).167 In recent years, a few other halogenases that operate on freestanding amino acids and other small molecules have also been shown to catalyze the azidation reaction.168,169 Although direct azidation of amino acids may prove to be a useful tool for bioorthogonal chemistry applications,170 these enzymes have yet to be engineered for this specific purpose. During future directed evolution campaigns, the second-sphere residues analogous to those implicated in ferryl redirection by the studies of native and engineered halogenases,132,171 as depicted in Figure 11C, are sensible targets for site-saturation mutagenesis and screening to enhance these activities.
Figure 12.
Abiological azidation and nitration (A), nitrile formation (B), and nitrene-transfer (C) reactions catalyzed by 2-His-1-carboxylate enzymes. S—SyrB1 represents the carrier-protein appended to the substrate by phosphopantetheine; NOG = N-oxalylglycine, a structural mimic of 2OG.
Azide-bearing amino acids have also been explored as substrates for 2OG-dependent oxygenases. In a recent study, the native hydroxylases PolL and LdoA catalyzed the oxidative conversion of these azidated substrates to nitrile products (Figure 12B).172 Further investigation demonstrated the requirement of both O2 and 2OG, suggesting that the reaction still proceeds by ferryl formation and HAT, and that the azido moiety acts as an assisting group. A similar finding was also reported in the Rieske dioxygenase family, wherein toluene dioxygenase (TDO) converted a benzyl azide to a benzyl nitrile.173 In both cases, iron-nitrenoid intermediates were proposed as a possible branchpoint after substrate hydroxylation.
Goldberg, Knight, and coworkers recently demonstrated that similar iron-nitrenoid intermediates could effect nitrene-transfer reactions (Figure 12C) analogous to those catalyzed by engineered hemoproteins (vide supra).174 Activity for the intermolecular aziridination of styrene and the intramolecular benzylic C–H insertion of 2-ethylbenzenesulfonyl azide was detected with the 2OG-dependent ethylene-forming enzyme (EFE). EFE variants exhibiting improvements in both activity and stereoselectivity were obtained by directed evolution, establishing that this family of enzymes can also be tuned for abiological catalysis. A subsequent study by Vila, Steck, and coworkers suggests that nitrene transferase activity may be a common side reactivity of 2-His-1-carboxylate enzymes, as various 2OG-dependent oxygenases and Rieske dioxygenases were also shown to facilitate similar nitrene-transfer reactions.175
The presence of multiple iron coordination sites left unoccupied by protein ligands creates attractive opportunities for engineering this enzymatic platform using exogenous ligands to tune the reactivity of the cofactor (Figure 5A). Indeed, Goldberg and Knight discovered the native co-substrate, 2OG, of EFE enhanced the activity of the aziridination and C–H insertion reactions with both the native and engineered versions of the enzyme.174 Other anionic ligands, such as acetate and N-oxalylglycine (NOG), further boosted the activity of both reactions and improved the chemoselectivity of the C–H insertion reaction. Although they did not determine the mechanisms of these enhancements, their observations demonstrate that exogenous ligands are a further handle for tuning novel reactivities. This expanded access to the iron provides an additional, flexible component upon which the protein scaffold can draw to guide reactive intermediates down new pathways.
4. ENGINEERING THE FUTURE
4.1. From Comprehension to Application
The machinery of natural enzymes has fueled the imagination of researchers for decades. But is an understanding of mechanism a prerequisite to engineering an abiological function? Luckily, the answer is no. While the disparities between our understanding of the chemistry occurring at the enzyme active site and the astounding complexity of interactions that contribute to protein folding, dynamics, and catalysis preclude us from designing most new biocatalysts de novo (at least for the time being), we can engineer new enzymes from existing scaffolds even if the intricacies of such scaffolds cannot be fully deconstructed. Nevertheless, knowledge gained from mechanistic studies, while not strictly required, can certainly assist in discovering new reactivities and streamlining engineering processes such that desired catalytic enhancements can be achieved on reasonable timescales. To bridge the gap between comprehension and application, we introduce the challenges of engineering natural enzymes for abiological chemistry, and then provide examples of how mechanistic knowledge of both natural and laboratory-evolved enzymes has guided protein engineers in tuning iron-dependent oxygenases.
From controlling the flow of electrons and protons to protecting the active-site architecture from oxidative inactivation, natural evolution has honed iron-dependent oxygenases into sophisticated molecular machines. The driving force behind this process is the selective advantage conferred by the products, which promote the survival of an organism bearing mutations that enhance the reaction outcome. Over time, this selective pressure can furnish a highly refined enzyme. In the context of evolving a biocatalyst for an abiological reaction, the protein engineer must first discover an enzyme exhibiting measurable activity for the desired reaction, generate mutants of this parent enzyme, and then foster the desired outcome by compounding beneficial mutations and rejecting mutations that fail to yield improvements.176 In principle, this process could produce enzymes comparable to the products of natural evolution. In practice, however, time constitutes a major limitation: screening the entire landscape of possible mutations in the context of even the smallest of proteins is impracticable. Moreover, many abiological reactions cannot be coupled to the fitness of an organism, or the products fail to produce a distinctive fluorogenic handle that would allow for rapid detection. Most efforts necessitate use of more time-intensive analytical techniques that decrease capacity to screen sequences (e.g., liquid and gas chromatography). Consequently, we resort to strategies to create smarter, experimentally tractable libraries that allow us to climb local fitness peaks (e.g., site-saturation mutagenesis).177 Although such a process will likely miss out on some of the characteristics that make natural enzymes so powerful, the results are nonetheless impressive.
An understanding of natural enzyme mechanism, even one that is rudimentary, can point the engineer to specific residues or regions of a protein structure that are important to catalysis.178 This knowledge can then be applied to the design of libraries for engineering. In the context of iron-dependent oxygenases, this is clearly exemplified in the transformation of P450BM3 to P411BM3 (vide supra): although the entirety of the contemporary P450 C–H activation model was likely superfluous, knowledge of the axial ligand’s electronic effects on the heme cofactor was pivotal to the discovery of P450BM3 variants that exhibited redox potentials suited for cyclopropanation in whole cells.65 Likewise, crystal structures of the P450BM3 heme domain and engineered variants thereof have aided numerous other directed evolution campaigns in the exploration of the platform’s ability to catalyze abiological reactions.65,94,179
Mechanistic investigations of laboratory-evolved variants can also confer useful information for the design of smarter libraries. A recent study of Rhodothermus marinus cytochrome c engineered for C–Si and C–B bond formation yielded a crystal structure with an iron porphyrin-bound carbene intermediate.180 The structure revealed how mutations to a surface loop region rendered it more flexible than that of the native enzyme, an observation that was leveraged to engineer a C–B bond-forming enzyme capable of accepting diverse trifluorodiazo alkane carbene precursors to furnish the corresponding α-trifluoromethylated organoborane products.87 Site-saturation libraries focused on this region yielded multiple beneficial mutations, and the final variant maintained levels of activity and selectivity with a trifluorodiazo alkane substrate scope comparable to the model reaction. Computational analysis of the engineered enzyme supported the original mechanism-driven hypothesis: the heme-binding pocket positioned the iron-carbenoid intermediate such that the pro-R face was exposed to the putative borane-binding pocket, while the variable alkyl substituent was arranged to have a minimal effect on the reaction outcome.87
Although the finer details of our mechanistic knowledge oftentimes lack a direct application to the engineering process, it is difficult to imagine a world where we climb to the same peaks of ingenuity without the contributions from mechanistic enzymology. It is even conceivable that some of these recent innovations, and those still to be described, already exist in the biological world, which will render their eventual discoveries all the more enchanting.
4.2. Iron-Dependent Oxygenases in Organic Synthesis
We are optimistic that applications of iron-dependent biocatalysts as real-world synthetic tools will grow, as the expansion of methods to selectively functionalize C–H bonds will certainly have an impact on both the de novo synthesis and late-stage modification of drug compounds and potentially therapeutic natural products. The native oxidative functions of P450s have already been applied to such goals, whether in the hydroxylation of steroids and other useful organic molecules,181,182 or more recently in the chemoenzymatic syntheses of nigelladine A and several meroterpenoid natural products.183,184 Likewise, native 2OG-dependent hydroxylases and halogenases have been utilized by the Renata Lab and others in the synthesis and tailoring of natural product compounds.185–195 A few examples of the abiological transformations enabled by these platforms have already surfaced: the cyclopropyl core structures of the approved drugs levomilnacipran, ticagrelor, tranylcypromine, and tasimelteon, as well as a TRPV1 antagonist drug candidate, have been constructed with engineered P450s and globins.196–198
Enzyme engineers ultimately aspire to see the products of their creativity and labor used directly in the manufacture of valuable compounds. Industrial-scale processes, however, require much more than exciting new reactivity. Space-time yield (g L−1 h−1) of the desired product is a key metric of overall performance, to which the activity, specificity, and stability of the enzyme are all contributing factors. From a protein engineering standpoint, advances in both experimental technologies and computational methods—to effectively increase screening capacity and narrow library sizes, respectively—will better equip us to navigate complex fitness landscapes and reach these requisite fitness peaks. However, other factors in the process must also be considered, including substrate and product titers, solvent compatibility with upstream and downstream steps, and isolation of the products in pure form. The challenges associated with incorporating biocatalysts into industrial processes and realistic approaches moving forward have been the subjects of several recent and comprehensive reviews.199–202
5. CONCLUDING REMARKS
Until the day when computational methods and de novo protein design can furnish biocatalysts capable of fulfilling our demands, leveraging our understanding of natural enzymes toward discovery and evolution of new functions remains a fruitful approach to the development of useful synthetic tools. Nature’s catalytic machinery has energized the scientific community for centuries, and engineered versions of enzymes have now expanded the pool from which to draw inspiration. As in all disciplines of science, collaboration is crucial. The convergence of enzymology, synthetic chemistry, computation, and engineering is the motor that drives forward the field of biocatalysis.
ACKNOWLEDGEMENTS
This work was supported by the US Army Research Office Institute for Collaborative Biotechnologies contract W911NF-19-D-0001 and the Joseph J. Jacobs Institute for Molecular Engineering for Medicine. N.P.D. was supported by the Ruth L. Kirschstein NIH Postdoctoral Fellowship (F32GM131620). We thank Professor Haoming Zhang for providing the open- and closed-state coordinates resulting from the full-length P450BM3 cryo-EM structures. We also thank Dr. S. V. Athavale, Dr. D. C. Miller, and Dr. Z. Liu for providing helpful comments on the manuscript.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Silverman RB Enzymes as Catalysts In Organic Chemistry of Enzyme-Catalyzed Reactions (Second Edition); Academic Press: San Diego, 2002, p 1–38. [Google Scholar]
- (2).Solomon EI; Brunold TC; Davis MI; Kemsley JN; Lee SK; Lehnert N; Neese F; Skulan AJ; Yang YS; Zhou J Geometric and Electronic Structure/Function Correlations in Non-Heme Iron Enzymes. Chem. Rev. 2000, 100, 235–349. [DOI] [PubMed] [Google Scholar]
- (3).Decker A; Solomon EI Dioxygen Activation by Copper, Heme and Non-Heme Iron Enzymes: Comparison of Electronic Structures and Reactivities. Curr. Opin. Chem. Biol. 2005, 9, 152–163. [DOI] [PubMed] [Google Scholar]
- (4).Pau MYM; Lipscomb JD; Solomon EI Substrate Activation for O2 Reactions by Oxidized Metal Centers in Biology. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 18355–18362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Groves JT The Bioinorganic Chemistry of Iron in Oxygenases and Supramolecular Assemblies. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 3569–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Drauz K; Gröger H; May O Introduction – Principles and Historical Landmarks of Enzyme Catalysis in Organic Synthesis In Enzyme Catalysis in Organic Synthesis (Third Edition); Wiley-VCH: Weinheim, Germany, 2012, p 1–42. [Google Scholar]
- (7).Chen K; Arnold FH Engineering New Catalytic Activities in Enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar]
- (8).Munro AW; Girvan HM; McLean KJ; Cheesman MR; Leys D Heme and Hemoproteins In Tetrapyrroles: Birth, Life and Death; Springer New York: New York, NY, 2009, p 160–183. [Google Scholar]
- (9).Lash TD Origin of Aromatic Character in Porphyrinoid Systems. J. Porphyr. Phthalocyanines 2011, 15, 1093–1115. [Google Scholar]
- (10).Dolphin D; Forman A; Borg DC; Fajer J; Felton RH Compounds I of Catalase and Horse Radish Peroxidase: π-Cation Radicals. Proc. Natl. Acad. Sci. U.S.A. 1971, 68, 614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dawson J Probing Structure-Function Relations in Heme-Containing Oxygenases and Peroxidases. Science 1988, 240, 433–439. [DOI] [PubMed] [Google Scholar]
- (12).Hardison RC A Brief History of Hemoglobins: Plant, Animal, Protist, and Bacteria. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ordway GA; Garry DJ Myoglobin: An Essential Hemoprotein in Striated Muscle. J. Exp. Biol. 2004, 207, 3441–3446. [DOI] [PubMed] [Google Scholar]
- (14).Liu J; Chakraborty S; Hosseinzadeh P; Yu Y; Tian S; Petrik I; Bhagi A; Lu Y Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Chelikani P; Fita I; Loewen PC Diversity of Structures and Properties Among Catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Welinder KG Superfamily of Plant, Fungal and Bacterial Peroxidases. Curr. Opin. Struct. Biol. 1992, 2, 388–393. [Google Scholar]
- (17).Alderton WK; Cooper CE; Knowles RG Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Meunier B; de Visser SP; Shaik S Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes. Chem. Rev. 2004, 104, 3947–3980. [DOI] [PubMed] [Google Scholar]
- (19).Huang X; Groves JT Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sono M; Roach MP; Coulter ED; Dawson JH Heme-Containing Oxygenases. Chem. Rev. 1996, 96, 2841–2888. [DOI] [PubMed] [Google Scholar]
- (21).Poulos TL Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Baglia RA; Zaragoza JPT; Goldberg DP Biomimetic Reactivity of Oxygen-Derived Manganese and Iron Porphyrinoid Complexes. Chem. Rev. 2017, 117, 13320–13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Momenteau M; Reed CA Synthetic Heme-Dioxygen Complexes. Chem. Rev. 1994, 94, 659–698. [Google Scholar]
- (24).Costas M Selective C–H oxidation catalyzed by metalloporphyrins. Coord. Chem. Rev. 2011, 255, 2912–2932. [Google Scholar]
- (25).Sahu S; Goldberg DP Activation of Dioxygen by Iron and Manganese Complexes: A Heme and Nonheme Perspective. J. Am. Chem. Soc. 2016, 138, 11410–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fujii H Electronic Structure and Reactivity of High-Valent Oxo Iron Porphyrins. Coord. Chem. Rev. 2002, 226, 51–60. [Google Scholar]
- (27).Schlichting I; Berendzen J; Chu K; Stock AM; Maves SA; Benson DE; Sweet RM; Ringe D; Petsko GA; Sligar SG The Catalytic Pathway of Cytochrome P450cam at Atomic Resolution. Science 2000, 287, 1615–1622. [DOI] [PubMed] [Google Scholar]
- (28).de Visser SP; Shaik S A Proton-Shuttle Mechanism Mediated by the Porphyrin in Benzene Hydroxylation by Cytochrome P450 Enzymes. J. Am. Chem. Soc. 2003, 125, 7413–7424. [DOI] [PubMed] [Google Scholar]
- (29).Newcomb M; Toy PH Hypersensitive Radical Probes and the Mechanisms of Cytochrome P450-Catalyzed Hydroxylation Reactions. Acc. Chem. Res. 2000, 33, 449–455. [DOI] [PubMed] [Google Scholar]
- (30).Seto Y; Guengerich FP Partitioning Between N-Dealkylation and N-Oxygenation in the Oxidation of N,N-Dialkylarylamines Catalyzed by Cytochrome P450 2B1. J. Biol. Chem. 1993, 268, 9986–9997. [PubMed] [Google Scholar]
- (31).Goto Y; Matsui T; Ozaki S.-i.; Watanabe Y; Fukuzumi S Mechanisms of Sulfoxidation Catalyzed by High-Valent Intermediates of Heme Enzymes: Electron-Transfer vs Oxygen-Transfer Mechanism. J. Am. Chem. Soc. 1999, 121, 9497–9502. [Google Scholar]
- (32).Anzai Y; Li S; Chaulagain MR; Kinoshita K; Kato F; Montgomery J; Sherman DH Functional Analysis of MycCI and MycG, Cytochrome P450 Enzymes Involved in Biosynthesis of Mycinamicin Macrolide Antibiotics. Chem. Biol. 2008, 15, 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Rettie A; Rettenmeier A; Howald W; Baillie T Cytochrome P-450—Catalyzed Formation of Δ4-VPA, a Toxic Metabolite of Valproic Acid. Science 1987, 235, 890–893. [DOI] [PubMed] [Google Scholar]
- (34).Kramlinger VM; Nagy LD; Fujiwara R; Johnson KM; Phan TTN; Xiao Y; Enright JM; Toomey MB; Corbo JC; Guengerich FP Human Cytochrome P450 27C1 Catalyzes 3,4-Desaturation of Retinoids. FEBS Lett. 2016, 590, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Belin P; Le Du MH; Fielding A; Lequin O; Jacquet M; Charbonnier J-B; Lecoq A; Thai R; Courçon M; Masson C; Dugave C; Genet R; Pernodet J-L; Gondry M Identification and Structural Basis of the Reaction Catalyzed by CYP121, an Essential Cytochrome P450 in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 7426–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Omura T; Sato R The Carbon Monoxide-Binding Pigment of Liver Microsomes: I. Evidence for its Hemoprotein Nature. J. Biol. Chem. 1964, 239, 2370–2378. [PubMed] [Google Scholar]
- (37).Nelson DR The Cytochrome P450 Homepage. Hum. Genet. 2009, 4, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Chen MM; Coelho PS; Arnold FH Utilizing Terminal Oxidants to Achieve P450-Catalyzed Oxidation of Methane. Adv. Synth. Catal. 2012, 354, 964–968. [Google Scholar]
- (39).Vallee BL; Williams RJ Metalloenzymes: The Entatic Nature of Their Active Sites. Proc. Natl. Acad. Sci. U.S.A. 1968, 59, 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sevrioukova IF; Li H; Zhang H; Peterson JA; Poulos TL Structure of a Cytochrome P450–Redox Partner Electron-Transfer Complex. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Sevrioukova I; Shaffer C; Ballou DP; Peterson JA Equilibrium and Transient State Spectrophotometric Studies of the Mechanism of Reduction of the Flavoprotein Domain of P450BM-3. Biochemistry 1996, 35, 7058–7068. [DOI] [PubMed] [Google Scholar]
- (42).Daff SN; Chapman SK; Holt RA; Govindaraj S; Poulos TL; Munro AW Redox Control of the Catalytic Cycle of Flavocytochrome P-450 BM3. Biochemistry 1997, 36, 13816–13823. [DOI] [PubMed] [Google Scholar]
- (43).Hlavica P Control by Substrate of the Cytochrome P450-Dependent Redox Machinery: Mechanistic Insights. Curr. Drug Metab. 2007, 8, 594–611. [DOI] [PubMed] [Google Scholar]
- (44).Zangar RC; Davydov DR; Verma S Mechanisms That Regulate Production of Reactive Oxygen Species by Cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [DOI] [PubMed] [Google Scholar]
- (45).Baek HK; Van Wart HE Elementary Steps in the Formation of Horseradish Peroxidase Compound I: Direct Observation of Compound 0, a New Intermediate with a Hyperporphyrin Spectrum. Biochemistry 1989, 28, 5714–5719. [DOI] [PubMed] [Google Scholar]
- (46).Waskell L; Kim J-JP Electron Transfer Partners of Cytochrome P450 In Cytochrome P450; Springer, Cham: Switzerland, 2015, p 33–68. [Google Scholar]
- (47).Su M; Chakraborty S; Osawa Y; Zhang H Cryo-EM Reveals the Architecture of the Dimeric Cytochrome P450 CYP102A1 Enzyme and Conformational Changes Required for Redox Partner Recognition. J. Biol. Chem. 2020, 295, 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Yeom H; Sligar SG; Li H; Poulos TL; Fulco AJ The Role of Thr268 in Oxygen Activation of Cytochrome P450BM-3. Biochemistry 1995, 34, 14733–14740. [DOI] [PubMed] [Google Scholar]
- (49).Raag R; Martinis SA; Sligar SG; Poulos TL Crystal Structure of the Cytochrome P-450CAM Active Site Mutant Thr252Ala. Biochemistry 1991, 30, 11420–11429. [DOI] [PubMed] [Google Scholar]
- (50).Li H; Poulos TL Modeling Protein-Substrate Interactions in the Heme Domain of Cytochrome P450BM-3. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1995, 51, 21–32. [DOI] [PubMed] [Google Scholar]
- (51).Shoji O; Fujishiro T; Nishio K; Kano Y; Kimoto H; Chien S-C; Onoda H; Muramatsu A; Tanaka S; Hori A; Sugimoto H; Shiro Y; Watanabe Y A Substrate-Binding-State Mimic of H2O2-Dependent Cytochrome P450 Produced by One-Point Mutagenesis and Peroxygenation of Non-Native Substrates. Catal. Sci. Technol. 2016, 6, 5806–5811. [Google Scholar]
- (52).Dawson JH; Sono M Cytochrome P-450 and Chloroperoxidase: Thiolate-Ligated Heme Enzymes. Spectroscopic Determination of Their Active-Site Structures and Mechanistic Implications of Thiolate Ligation. Chem. Rev. 1987, 87, 1255–1276. [Google Scholar]
- (53).Green MT Evidence for Sulfur-Based Radicals in Thiolate Compound I Intermediates. J. Am. Chem. Soc. 1999, 121, 7939–7940. [Google Scholar]
- (54).Yosca TH; Rittle J; Krest CM; Onderko EL; Silakov A; Calixto JC; Behan RK; Green MT Iron(IV)hydroxide pKa and the Role of Thiolate Ligation in C–H Bond Activation by Cytochrome P450. Science 2013, 342, 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Groves JT Key Elements of the Chemistry of Cytochrome P-450: The Oxygen Rebound Mechanism. J. Chem. Educ. 1985, 62, 928–931. [Google Scholar]
- (56).Green MT; Dawson JH; Gray HB Oxoiron(IV) in Chloroperoxidase Compound II is Basic: Implications for P450 Chemistry. Science 2004, 304, 1653–1656. [DOI] [PubMed] [Google Scholar]
- (57).Green MT C-H Bond Activation in Heme Proteins: The Role of Thiolate Ligation in Cytochrome P450. Curr. Opin. Chem. Biol. 2009, 13, 84–88. [DOI] [PubMed] [Google Scholar]
- (58).Krest CM; Silakov A; Rittle J; Yosca TH; Onderko EL; Calixto JC; Green MT Significantly Shorter Fe–S Bond in Cytochrome P450-I is Consistent with Greater Reactivity Relative to Chloroperoxidase. Nat. Chem. 2015, 7, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Onderko EL; Silakov A; Yosca TH; Green MT Characterization of a Selenocysteine-Ligated P450 Compound I Reveals Direct Link Between Electron Donation and Reactivity. Nat. Chem. 2017, 9, 623–628. [DOI] [PubMed] [Google Scholar]
- (60).Basran J; Booth ES; Lee M; Handa S; Raven EL Analysis of Reaction Intermediates in Tryptophan 2,3-Dioxygenase: A Comparison with Indoleamine 2,3-Dioxygenase. Biochemistry 2016, 55, 6743–6750. [DOI] [PubMed] [Google Scholar]
- (61).Geng J; Weitz AC; Dornevil K; Hendrich MP; Liu A Kinetic and Spectroscopic Characterization of the Catalytic Ternary Complex of Tryptophan 2,3-Dioxygenase. Biochemistry 2020, 59, 2813–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Davies HML; Manning JR Catalytic C–H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature 2008, 451, 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Hoffmann R Building Bridges Between Inorganic and Organic Chemistry (Nobel Lecture). Angew. Chem. Int. Ed. 1982, 21, 711–724. [Google Scholar]
- (64).Coelho PS; Brustad EM; Kannan A; Arnold FH Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes. Science 2013, 339, 307–310. [DOI] [PubMed] [Google Scholar]
- (65).Coelho PS; Wang ZJ; Ener ME; Baril SA; Kannan A; Arnold FH; Brustad EM A Serine-Substituted P450 Catalyzes Highly Efficient Carbene Transfer to Olefins in vivo. Nat. Chem. Biol. 2013, 9, 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Knight AM; Kan SBJ; Lewis RD; Brandenberg OF; Chen K; Arnold FH Diverse Engineered Heme Proteins Enable Stereodivergent Cyclopropanation of Unactivated Alkenes. ACS Cent. Sci. 2018, 4, 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Brandenberg OF; Prier CK; Chen K; Knight AM; Wu Z; Arnold FH Stereoselective Enzymatic Synthesis of Heteroatom-Substituted Cyclopropanes. ACS Catal. 2018, 8, 2629–2634. [Google Scholar]
- (68).Chen K; Zhang S-Q; Brandenberg OF; Hong X; Arnold FH Alternate Heme Ligation Steers Activity and Selectivity in Engineered Cytochrome P450-Catalyzed Carbene-Transfer Reactions. J. Am. Chem. Soc. 2018, 140, 16402–16407. [DOI] [PubMed] [Google Scholar]
- (69).Brandenberg OF; Chen K; Arnold FH Directed Evolution of a Cytochrome P450 Carbene Transferase for Selective Functionalization of Cyclic Compounds. J. Am. Chem. Soc. 2019, 141, 8989–8995. [DOI] [PubMed] [Google Scholar]
- (70).Bordeaux M; Tyagi V; Fasan R Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts. Angew. Chem. Int. Ed. 2015, 54, 1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Tinoco A; Steck V; Tyagi V; Fasan R Highly Diastereo- and Enantioselective Synthesis of Trifluoromethyl-Substituted Cyclopropanes via Myoglobin-Catalyzed Transfer of Trifluoromethylcarbene. J. Am. Chem. Soc. 2017, 139, 5293–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chandgude AL; Fasan R Highly Diastereo- and Enantioselective Synthesis of Nitrile-Substituted Cyclopropanes by Myoglobin-Mediated Carbene Transfer Catalysis. Angew. Chem. Int. Ed. 2018, 57, 15852–15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Chandgude AL; Ren X; Fasan R Stereodivergent Intramolecular Cyclopropanation Enabled by Engineered Carbene Transferases. J. Am. Chem. Soc. 2019, 141, 9145–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Vargas DA; Khade RL; Zhang Y; Fasan R Biocatalytic Strategy for Highly Diastereo- and Enantioselective Synthesis of 2,3-Dihydrobenzofuran-Based Tricyclic Scaffolds. Angew. Chem. Int. Ed. 2019, 58, 10148–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Carminati DM; Fasan R Stereoselective Cyclopropanation of Electron-Deficient Olefins with a Cofactor Redesigned Carbene Transferase Featuring Radical Reactivity. ACS Catal. 2019, 9, 9683–9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Ren X; Chandgude AL; Fasan R Highly Stereoselective Synthesis of Fused Cyclopropane-γ-Lactams via Biocatalytic Iron-Catalyzed Intramolecular Cyclopropanation. ACS Catal. 2020, 10, 2308–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Renata H; Wang ZJ; Kitto RZ; Arnold FH P450-Catalyzed Asymmetric Cyclopropanation of Electron-Deficient Olefins Under Aerobic Conditions. Catal. Sci. Technol. 2014, 4, 3640–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Wittmann BJ; Knight AM; Hofstra JL; Reisman SE; Jennifer Kan SB; Arnold FH Diversity-Oriented Enzymatic Synthesis of Cyclopropane Building Blocks. ACS Catal. 2020, 10, 7112–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Chen K; Huang X; Kan SBJ; Zhang RK; Arnold FH Enzymatic Construction of Highly Strained Carbocycles. Science 2018, 360, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Chen K; Arnold FH Engineering Cytochrome P450s for Enantioselective Cyclopropenation of Internal Alkynes. J. Am. Chem. Soc. 2020, 142, 6891–6895. [DOI] [PubMed] [Google Scholar]
- (81).Wang ZJ; Peck NE; Renata H; Arnold FH Cytochrome P450-Catalyzed Insertion of Carbenoids into N-H Bonds. Chem. Sci. 2014, 5, 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Sreenilayam G; Fasan R Myoglobin-Catalyzed Intermolecular Carbene N–H Insertion with Arylamine Substrates. ChemComm 2015, 51, 1532–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Tyagi V; Bonn RB; Fasan R Intermolecular Carbene S–H Insertion Catalysed by Engineered Myoglobin-Based Catalysts. Chem. Sci. 2015, 6, 2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kan SBJ; Lewis RD; Chen K; Arnold FH Directed Evolution of Cytochrome c for Carbon-Silicon Bond Formation: Bringing Silicon to Life. Science 2016, 354, 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Kan SBJ; Huang XY; Gumulya Y; Chen K; Arnold FH Genetically Programmed Chiral Organoborane Synthesis. Nature 2017, 552, 132–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Chen K; Huang X; Zhang S-Q; Zhou AZ; Kan SBJ; Hong X; Arnold FH Engineered Cytochrome c-Catalyzed Lactone-Carbene B–H Insertion. Synlett 2019, 30, 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Huang X; Garcia-Borràs M; Miao K; Kan SBJ; Zutshi A; Houk KN; Arnold FH A Biocatalytic Platform for Synthesis of Chiral α-Trifluoromethylated Organoborons. ACS Cent. Sci. 2019, 5, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Zhang RK; Chen K; Huang X; Wohlschlager L; Renata H; Arnold FH Enzymatic Assembly of Carbon–Carbon Bonds via Iron-Catalysed sp3 C–H Functionalization. Nature 2019, 565, 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Zhang J; Huang X; Zhang RK; Arnold FH Enantiodivergent α-Amino C–H Fluoroalkylation Catalyzed by Engineered Cytochrome P450s. J. Am. Chem. Soc. 2019, 141, 9798–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Zhou AZ; Chen K; Arnold FH Enzymatic Lactone-Carbene C–H Insertion to Build Contiguous Chiral Centers. ACS Catal. 2020, 10, 5393–5398. [Google Scholar]
- (91).Svastits EW; Dawson JH; Breslow R; Gellman SH Functionalized Nitrogen Atom Transfer Catalyzed by Cytochrome P-450. J. Am. Chem. Soc. 1985, 107, 6427–6428. [Google Scholar]
- (92).McIntosh JA; Coelho PS; Farwell CC; Wang ZJ; Lewis JC; Brown TR; Arnold FH Enantioselective Intramolecular C-H Amination Catalyzed by Engineered Cytochrome P450 Enzymes In Vitro and In Vivo. Angew. Chem. Int. Ed. 2013, 52, 9309–9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Singh R; Bordeaux M; Fasan R P450-Catalyzed Intramolecular sp3 C–H Amination with Arylsulfonyl Azide Substrates. ACS Catal. 2014, 4, 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Prier CK; Zhang RJK; Buller AR; Brinkmann-Chen S; Arnold FH Enantioselective, Intermolecular Benzylic C-H Amination Catalysed by an Engineered Iron-haem Enzyme. Nat. Chem. 2017, 9, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Farwell CC; Zhang RK; McIntosh JA; Hyster TK; Arnold FH Enantioselective Enzyme-Catalyzed Aziridination Enabled by Active-Site Evolution of a Cytochrome P450. ACS Cent. Sci. 2015, 1, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Sabir S; Kumar G; Jat JL O-Substituted Hydroxyl Amine Reagents: An Overview of Recent Synthetic Advances. Org. Biomol. Chem. 2018, 16, 3314–3327. [DOI] [PubMed] [Google Scholar]
- (97).Tsutsumi H; Katsuyama Y; Izumikawa M; Takagi M; Fujie M; Satoh N; Shin-ya K; Ohnishi Y Unprecedented Cyclization Catalyzed by a Cytochrome P450 in Benzastatin Biosynthesis. J. Am. Chem. Soc. 2018, 140, 6631–6639. [DOI] [PubMed] [Google Scholar]
- (98).Cho I; Prier CK; Jia Z-J; Zhang RK; Görbe T; Arnold FH Enantioselective Aminohydroxylation of Styrenyl Olefins Catalyzed by an Engineered Hemoprotein. Angew. Chem. Int. Ed. 2019, 58, 3138–3142. [DOI] [PubMed] [Google Scholar]
- (99).Jia Z-J; Gao S; Arnold FH Enzymatic Primary Amination of Benzylic and Allylic C(sp3)–H Bonds. J. Am. Chem. Soc. 2020, 142, 10279–10283. [DOI] [PubMed] [Google Scholar]
- (100).Moore EJ; Fasan R Effect of Proximal Ligand Substitutions on the Carbene and Nitrene Transferase Activity of Myoglobin. Tetrahedron 2019, 75, 2357–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Green AP; Hayashi T; Mittl PRE; Hilvert D A Chemically Programmed Proximal Ligand Enhances the Catalytic Properties of a Heme Enzyme. J. Am. Chem. Soc. 2016, 138, 11344–11352. [DOI] [PubMed] [Google Scholar]
- (102).Pott M; Hayashi T; Mori T; Mittl PRE; Green AP; Hilvert D A Noncanonical Proximal Heme Ligand Affords an Efficient Peroxidase in a Globin Fold. J. Am. Chem. Soc. 2018, 140, 1535–1543. [DOI] [PubMed] [Google Scholar]
- (103).Renata H; Lewis RD; Sweredoski MJ; Moradian A; Hess S; Wang ZJ; Arnold FH Identification of Mechanism-Based Inactivation in P450-Catalyzed Cyclopropanation Facilitates Engineering of Improved Enzymes. J. Am. Chem. Soc. 2016, 138, 12527–12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Farwell CC; McIntosh JA; Hyster TK; Wang ZJ; Arnold FH Enantioselective Imidation of Sulfides via Enzyme-Catalyzed Intermolecular Nitrogen-Atom Transfer. J. Am. Chem. Soc. 2014, 136, 8766–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Fenton HJH LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc., Trans. 1894, 65, 899–910. [Google Scholar]
- (106).Bryant JD; Wilson JT Fenton’s in-situ Reagent Chemical Oxidation of Hydrocarbon Contamination in Soil and Groundwater. Remediation 1999, 9, 13–25. [Google Scholar]
- (107).Groves JT; Van der Puy M Stereospecific Aliphatic Hydroxylation by Iron-Hydrogen Peroxide. Evidence for a Stepwise Srocess. J. Am. Chem. Soc. 1976, 98, 5290–5297. [Google Scholar]
- (108).Groves JT High-Valent Iron in Chemical and Biological Oxidations. J. Inorg. Biochem. 2006, 100, 434–447. [DOI] [PubMed] [Google Scholar]
- (109).Loegager T; Holcman J; Sehested K; Pedersen T Oxidation of Ferrous Ions by Ozone in Acidic Solutions. Inorg. Chem. 1992, 31, 3523–3529. [Google Scholar]
- (110).Pestovsky O; Bakac A Reactivity of Aqueous Fe(IV) in Hydride and Hydrogen Atom Transfer Reactions. J. Am. Chem. Soc. 2004, 126, 13757–13764. [DOI] [PubMed] [Google Scholar]
- (111).Barton DHR; Gastiger MJ; Motherwell WB A New Procedure for the Oxidation of Saturated Hydrocarbons. J. Chem. Soc., Chem. Commun. 1983, 41–43. [Google Scholar]
- (112).Barton DHR; Doller D The Selective Functionalization of Saturated Hydrocarbons: Gif Chemistry. Acc. Chem. Res. 1992, 25, 504–512. [Google Scholar]
- (113).Stavropoulos P; Çelenligil-Çetin R; Tapper AE The Gif Paradox. Acc. Chem. Res. 2001, 34, 745–752. [DOI] [PubMed] [Google Scholar]
- (114).Hegg EL; Jr LQ The 2-His-1-Carboxylate Facial Triad — An Emerging Structural Motif in Mononuclear Non-Heme Iron(II) Enzymes. Eur. J. Biochem. 1997, 250, 625–629. [DOI] [PubMed] [Google Scholar]
- (115).Koehntop KD; Emerson JP; Que L Jr. The 2-His-1-Carboxylate Facial Triad: A Versatile Platform for Dioxygen Activation by Mononuclear Non-Heme Iron(II) Enzymes. J. Biol. Inorg. Chem. 2005, 10, 87–93. [DOI] [PubMed] [Google Scholar]
- (116).Kal S; Que L Dioxygen Activation by Nonheme Iron Enzymes with the 2-His-1-Carboxylate Facial Triad That Generate High-Valent Oxoiron Oxidants. J. Biol. Inorg. Chem. 2017, 22, 339–365. [DOI] [PubMed] [Google Scholar]
- (117).Lipscomb JD Mechanism of Extradiol Aromatic Ring-Cleaving Dioxygenases. Curr. Opin. Struct. Biol. 2008, 18, 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Barry SM; Challis GL Mechanism and Catalytic Diversity of Rieske Non-Heme Iron-Dependent Oxygenases. ACS Catal. 2013, 3, 2362–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Martinez S; Hausinger RP Catalytic Mechanisms of Fe(II)- and 2-Oxoglutarate-dependent Oxygenases. J. Biol. Chem. 2015, 290, 20702–20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Fitzpatrick PF Mechanism of Aromatic Amino Acid Hydroxylation. Biochemistry 2003, 42, 14083–14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Baldwin JE; Bradley M Isopenicillin N Synthase: Mechanistic Studies. Chem. Rev. 1990, 90, 1079–1088. [Google Scholar]
- (122).Tamanaha E; Zhang B; Guo Y; Chang WC; Barr EW; Xing G; St Clair J; Ye S; Neese F; Bollinger JM Jr.; Krebs C Spectroscopic Evidence for the Two C-H-Cleaving Intermediates of Aspergillus nidulans Isopenicillin N Synthase. J. Am. Chem. Soc. 2016, 138, 8862–8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Wang C; Chang WC; Guo YS; Huang H; Peck SC; Pandelia ME; Lin GM; Liu HW; Krebs C; Bollinger JM Evidence that the Fosfomycin-Producing Epoxidase, HppE, is a Non-Heme-Iron Peroxidase. Science 2013, 342, 991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Peck SC; Wang C; Dassama LMK; Zhang B; Guo Y; Rajakovich LJ; Bollinger JM; Krebs C; van der Donk WA O–H Activation by an Unexpected Ferryl Intermediate during Catalysis by 2-Hydroxyethylphosphonate Dioxygenase. J. Am. Chem. Soc. 2017, 139, 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Born DA; Ulrich EC; Ju K-S; Peck SC; van der Donk WA; Drennan CL Structural Basis for Methylphosphonate Biosynthesis. Science 2017, 358, 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Mirica LM; Klinman JP The Nature of O2 Activation by the Ethylene-Forming Enzyme 1-Aminocyclopropane-1-Carboxylic Acid Oxidase. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 1814–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Puri M; Que L Toward the Synthesis of More Reactive S = 2 Non-Heme Oxoiron(IV) Complexes. Acc. Chem. Res. 2015, 48, 2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Oloo WN; Que L Bioinspired Nonheme Iron Catalysts for C–H and C═C Bond Oxidation: Insights into the Nature of the Metal-Based Oxidants. Acc. Chem. Res. 2015, 48, 2612–2621. [DOI] [PubMed] [Google Scholar]
- (129).Kal S; Xu S; Que L Jr. Bio-inspired Nonheme Iron Oxidation Catalysis: Involvement of Oxoiron(V) Oxidants in Cleaving Strong C−H Bonds. Angew. Chem. Int. Ed. 2020, 59, 7332–7349. [DOI] [PubMed] [Google Scholar]
- (130).Roach RL; Clifton IJ; Hensgens CMH; Shibata N; Schofield CJ; Hajdu J; Baldwin JE Structure of Isopenicillin N Synthase Complexed with Substrate and the Mechanism of Penicillin Formation. Nature 1997, 387. [DOI] [PubMed] [Google Scholar]
- (131).Zhang Z; Ren J.-s.; Harlos K; McKinnon CH; Clifton IJ; Schofield CJ Crystal Structure of a Clavaminate Synthase-Fe(II)-2-Oxoglutarate-Substrate-NO Complex: Evidence for Metal Centered Rearrangements. FEBS Lett. 2002, 517, 7–12. [DOI] [PubMed] [Google Scholar]
- (132).Mitchell AJ; Zhu Q; Maggiolo AO; Ananth NR; Hillwig ML; Liu X; Boal AK Structural Basis for Halogenation by Iron- and 2-Oxo-Glutarate-Dependent Enzyme WelO5. Nat. Chem. Biol. 2016, 12, 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Bassan A; Blomberg MRA; Siegbahn PEM Mechanism of Dioxygen Cleavage in Tetrahydrobiopterin-Dependent Amino Acid Hydroxylases. Chem. Eur. J. 2003, 9, 106–115. [DOI] [PubMed] [Google Scholar]
- (134).Olsson E; Martinez A; Teigen K; Jensen VR Formation of the Iron–Oxo Hydroxylating Species in the Catalytic Cycle of Aromatic Amino Acid Hydroxylases. Chem. Eur. J. 2011, 17, 3746–3758. [DOI] [PubMed] [Google Scholar]
- (135).Andreas Andersen O; Flatmark T; Hough E Crystal Structure of the Ternary Complex of the Catalytic Domain of Human Phenylalanine Hydroxylase with Tetrahydrobiopterin and 3-(2-Thienyl)-l-alanine, and its Implications for the Mechanism of Catalysis and Substrate Activation. J. Mol. Biol. 2002, 320, 1095–1108. [DOI] [PubMed] [Google Scholar]
- (136).Karlsson A; Parales JV; Parales RE; Gibson DT; Eklund H; Ramaswamy S Crystal Structure of Naphthalene Dioxygenase: Side-on Binding of Dioxygen to Iron. Science 2003, 299, 1039–1042. [DOI] [PubMed] [Google Scholar]
- (137).Ashikawa Y; Fujimoto Z; Usami Y; Inoue K; Noguchi H; Yamane H; Nojiri H Structural Insight Into the Substrate- and Dioxygen-Binding Manner in the Catalytic Cycle of Rieske Nonheme Iron Oxygenase System, Carbazole 1,9a-Dioxygenase. BMC Struct. Biol. 2012, 12, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Kovaleva EG; Lipscomb JD Crystal Structures of Fe(II) Dioxygenase Superoxo, Alkylperoxo, and Bound Product Intermediates. Science 2007, 316, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Wang Y; Liu KF; Yang Y; Davis I; Liu A Observing 3-Hydroxyanthranilate-3,4-Dioxygenase in Action Through a Crystalline Lens. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 19720–19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Krebs C; Galonić Fujimori D; Walsh CT; Bollinger JM Jr. Non-Heme Fe(IV)-Oxo Intermediates. Acc. Chem. Res. 2007, 40, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Herr CQ; Hausinger RP Amazing Diversity in Biochemical Roles of Fe(II)/2-Oxoglutarate Oxygenases. Trends Biochem. Sci. 2018, 43, 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Price JC; Barr EW; Tirupati B; Bollinger JM Jr.; Krebs C The First Direct Characterization of a High-Valent Iron Intermediate in the Reaction of an α-Ketoglutarate-Dependent Dioxygenase: A High-Spin Fe(IV) Complex in Taurine/α-Ketoglutarate Dioxygenase (TauD) from Escherichia coli. Biochemistry 2003, 42, 7497–7508. [DOI] [PubMed] [Google Scholar]
- (143).Price JC; Barr EW; Glass TE; Krebs C; Bollinger JM Jr. Evidence for Hydrogen Abstraction from C1 of Taurine by the High-Spin Fe(IV) Intermediate Detected During Oxygen Activation by Taurine: α-Ketoglutarate Dioxygenase (TauD). J. Am. Chem. Soc. 2003, 125, 13008–13009. [DOI] [PubMed] [Google Scholar]
- (144).Hoffart LM; Barr EW; Guyer RB; Bollinger JM Jr.; Krebs C Direct Spectroscopic Detection of a C-H-Cleaving High-Spin Fe(IV) Complex in a Prolyl-4-Hydroxylase. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 14738–14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Galonić DP; Barr EW; Walsh CT; Bollinger JM Jr.; Krebs C Two Interconverting Fe(IV) Intermediates in Aliphatic Chlorination by the Halogenase CytC3. Nat. Chem. Biol. 2007, 3, 113–116. [DOI] [PubMed] [Google Scholar]
- (146).Matthews ML; Krest CM; Barr EW; Vaillancourt FH; Walsh CT; Green MT; Krebs C; Bollinger JM Jr. Substrate-Triggered Formation and Remarkable Stability of the C-H Bond-Cleaving Chloroferryl Intermediate in the Aliphatic Halogenase, SyrB2. Biochemistry 2009, 48, 4331–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Grzyska PK; Appelman EH; Hausinger RP; Proshlyakov DA Insight Into the Mechanism of an Iron Dioxygenase by Resolution of Steps Following the Fe(IV)=O Species. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 3982–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Chang W.-c.; Guo Y; Wang C; Butch SE; Rosenzweig AC; Boal AK; Krebs C; Bollinger JM Jr. Mechanism of the C5 Stereoinversion Reaction in the Biosynthesis of Carbapenem Antibiotics. Science 2014, 343, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Mitchell AJ; Dunham NP; Martinie RJ; Bergman JA; Pollock CJ; Hu K; Allen BD; Chang WC; Silakov A; Bollinger JM Jr.; Krebs C; Boal AK Visualizing the Reaction Cycle in an Iron(II)- and 2-(Oxo)-glutarate-Dependent Hydroxylase. J. Am. Chem. Soc. 2017, 139, 13830–13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Pan J; Bhardwaj M; Zhang B; Chang W. c.; Schardl CL; Krebs C; Grossman RB; Bollinger JM Jr. Installation of the Ether Bridge of Lolines by the Iron- and 2-Oxoglutarate-Dependent Oxygenase, LolO: Regio- and Stereochemistry of Sequential Hydroxylation and Oxacyclization Reactions. Biochemistry 2018, 57, 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Pan J; Wenger ES; Matthews ML; Pollock CJ; Bhardwaj M; Kim AJ; Allen BD; Grossman RB; Krebs C; Bollinger JM Evidence for Modulation of Oxygen Rebound Rate in Control of Outcome by Iron(II)- and 2-Oxoglutarate-Dependent Oxygenases. J. Am. Chem. Soc. 2019, 141, 15153–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Li J; Liao H-J; Tang Y; Huang J-L; Cha L; Lin T-S; Lee JL; Kurnikov IV; Kurnikova MG; Chang W.-c.; Chan N-L; Guo Y Epoxidation Catalyzed by the Nonheme Iron(II)- and 2-Oxoglutarate-Dependent Oxygenase, AsqJ: Mechanistic Elucidation of Oxygen Atom Transfer by a Ferryl Intermediate. J. Am. Chem. Soc. 2020, 142, 6268–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Blasiak LC; Vaillancourt FH; Walsh CT; Drennan CL Crystal Structure of the Non-Haem Iron Halogenase SyrB2 in Syringomycin Biosynthesis. Nature 2006, 440, 368–371. [DOI] [PubMed] [Google Scholar]
- (154).Matthews ML; Neumann CS; Miles LA; Grove TL; Booker SJ; Krebs C; Walsh CT; Bollinger JM Jr. Substrate Positioning Controls the Partition Between Halogenation and Hydroyxlation in the Aliphatic Halogenase, SyrB2. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 17723–17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Wong SD; Srnec M; Matthews ML; Liu LV; Kwak Y; Park K; Bell CB 3rd; Alp EE; Zhao J; Yoda Y; Kitao S; Seto M; Krebs C; Bollinger JM Jr.; Solomon EI Elucidation of the Fe(IV)=O Intermediate in the Catalytic Cycle of the Halogenase SyrB2. Nature 2013, 499, 320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Martinie RJ; Livada J; Chang W.-c.; Green MT; Krebs C; Bollinger JM Jr.; Silakov A Experimental Correlation of Substrate Position with Reaction Outcome in the Aliphatic Halogenase, SyrB2. J. Am. Chem. Soc. 2015, 137, 6912–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Dunham NP; Chang W.-c.; itchell AJ; Martinie RJ; Zhang B; Bergman JA; Rajakovich LJ; Wang B; Silakov A; Krebs C; Boal AK; Bollinger JM Jr. Two Distinct Mechanisms for C-C Desaturation by Iron(II)- and 2-(Oxo)glutarate-Dependent Oxygenases: Importance of Alpha-Heteroatom Assistance. J. Am. Chem. Soc. 2018, 140, 7116–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Ushimaru R; Ruszczycky MW; Liu H.-w. Changes in Regioselectivity of H Atom Abstraction during the Hydroxylation and Cyclization Reactions Catalyzed by Hyoscyamine 6β-Hydroxylase. J. Am. Chem. Soc. 2019, 141, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Chang W.-c.; Yang Z-J; Tu Y-H; Chien T-C Reaction Mechanism of a Nonheme Iron Enzyme Catalyzed Oxidative Cyclization via C–C Bond Formation. Org. Lett. 2019, 21, 228–232. [DOI] [PubMed] [Google Scholar]
- (160).Borowski T; de Marothy S; Broclawik E; Schofield CJ; Siegbahn PEM Mechanism for Cyclization Reaction by Clavaminic Acid Synthase. Insights From Modeling Studies. Biochemistry 2007, 46, 3682–3691. [DOI] [PubMed] [Google Scholar]
- (161).Dunham NP; Del Río Pantoja JM; Zhang B; Rajakovich LJ; Allen BD; Krebs C; Boal AK; Bollinger JM Hydrogen Donation but not Abstraction by a Tyrosine (Y68) During Endoperoxide Installation by Verruculogen Synthase (FtmOx1). J. Am. Chem. Soc. 2019, 141, 9964–9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Liu W; Groves JT Manganese Porphyrins Catalyze Selective C−H Bond Halogenations. J. Am. Chem. Soc. 2010, 132, 12847–12849. [DOI] [PubMed] [Google Scholar]
- (163).Liu W; Huang X; Cheng M-J; Nielsen RJ; Goddard WA; Groves JT Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin. Science 2012, 337, 1322–1325. [DOI] [PubMed] [Google Scholar]
- (164).Schmidt VA; Quinn RK; Brusoe AT; Alexanian EJ Site-Selective Aliphatic C–H Bromination Using N-Bromoamides and Visible Light. J. Am. Chem. Soc. 2014, 136, 14389–14392. [DOI] [PubMed] [Google Scholar]
- (165).Liu W; Groves JT Manganese Catalyzed C–H Halogenation. Acc. Chem. Res. 2015, 48, 1727–1735. [DOI] [PubMed] [Google Scholar]
- (166).Margrey KA; Czaplyski WL; Nicewicz DA; Alexanian EJ A General Strategy for Aliphatic C–H Functionalization Enabled by Organic Photoredox Catalysis. J. Am. Chem. Soc. 2018, 140, 4213–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Matthews ML; Chang WC; Layne AP; Miles LA; Krebs C; Bollinger JM Jr. Direct Nitration and Azidation of Aliphatic Carbons by an Iron-Dependent Halogenase. Nat. Chem. Biol. 2014, 10, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Neugebauer ME; Sumida KH; Pelton JG; McMurry JL; Marchand JA; Chang MCY A family of Radical Halogenases for the Engineering of Amino-Acid-Based Products. Nat. Chem. Biol. 2019, 15, 1009–1016. [DOI] [PubMed] [Google Scholar]
- (169).Kim CY; Mitchell AJ; Glinkerman CM; Li F-S; Pluskal T; Weng J-K The Chloroalkaloid (−)-Acutumine is Biosynthesized via a Fe(II)- and 2-Oxoglutarate-Dependent Halogenase in Menispermaceae Plants. Nat. Commun. 2020, 11, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Jewett JC; Bertozzi CR Cu-Free Click Cycloaddition Reactions in Chemical Biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Mitchell AJ; Dunham NP; Bergman JA; Wang B; Zhu Q; Chang W.-c.; Liu X; Boal AK Structure-Guided Reprogramming of a Hydroxylase to Halogenate its Small Molecule Substrate. Biochemistry 2017, 56, 441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Davidson M; McNamee M; Fan R; Guo Y; Chang W -c. Repurposing Nonheme Iron Hydroxylases To Enable Catalytic Nitrile Installation through an Azido Group Assistance. J. Am. Chem. Soc. 2019, 141, 3419–3423. [DOI] [PubMed] [Google Scholar]
- (173).Vila MA; Pazos M; Iglesias C; Veiga N; Seoane G; Carrera I Toluene Dioxygenase-Catalysed Oxidation of Benzyl Azide to Benzonitrile: Mechanistic Insights for an Unprecedented Enzymatic Transformation. ChemBioChem 2016, 17, 291–295. [DOI] [PubMed] [Google Scholar]
- (174).Goldberg NW; Knight AM; Zhang RK; Arnold FH Nitrene Transfer Catalyzed by a Non-Heme Iron Enzyme and Enhanced by Non-Native Small-Molecule Ligands. J. Am. Chem. Soc. 2019, 141, 19585–19588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Vila MA; Steck V; Rodriguez Giordano S; Carrera I; Fasan R C−H Amination via Nitrene Transfer Catalyzed by Mononuclear Non-Heme Iron-Dependent Enzymes. ChemBioChem 2020, 21, 1981–1987. [DOI] [PubMed] [Google Scholar]
- (176).Hammer SC; Knight AM; Arnold FH Design and Evolution of Enzymes for Non-Natural Chemistry. Curr. Opin. Green Sustain. Chem. 2017, 7, 23–30. [Google Scholar]
- (177).Reetz MT; Carballeira JD Iterative Saturation Mutagenesis (ISM) for Rapid Directed Evolution of Functional Enzymes. Nat. Protoc. 2007, 2, 891–903. [DOI] [PubMed] [Google Scholar]
- (178).Renata H; Wang ZJ; Arnold FH Expanding the Enzyme Universe: Accessing Non-Natural Reactions by Mechanism-Guided Directed Evolution. Angew. Chem. Int. Ed. 2015, 54, 3351–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Haines DC; Tomchick DR; Machius M; Peterson JA Pivotal Role of Water in the Mechanism of P450BM-3. Biochemistry 2001, 40, 13456–13465. [DOI] [PubMed] [Google Scholar]
- (180).Lewis RD; Garcia-Borràs M; Chalkley MJ; Buller AR; Houk KN; Kan SBJ; Arnold FH Catalytic Iron-Carbene Intermediate Revealed in a Cytochrome c Carbene Transferase. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 7308–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Zhang X; Peng Y; Zhao J; Li Q; Yu X; Acevedo-Rocha CG; Li A Bacterial Cytochrome P450-Catalyzed Regio- and Stereoselective Steroid Hydroxylation Enabled by Directed Evolution and Rational Design. Bioresour. Bioprocess. 2020, 7, 2. [Google Scholar]
- (182).Li R-J; Zhang Z; Acevedo-Rocha CG; Zhao J; Li A Biosynthesis of Organic Molecules via Artificial Cascade Reactions Based on Cytochrome P450 Monooxygenases. Green Synth. Catal. 2020, 1, 52–59. [Google Scholar]
- (183).Loskot SA; Romney DK; Arnold FH; Stoltz BM Enantioselective Total Synthesis of Nigelladine A via Late-Stage C–H Oxidation Enabled by an Engineered P450 Enzyme. J. Am. Chem. Soc. 2017, 139, 10196–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Li J; Li F; King-Smith E; Renata H Merging Chemoenzymatic and Radical-Based Retrosynthetic Logic for Rapid and Modular Synthesis of Oxidized Meroterpenoids. Nat. Chem. 2020, 12, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Zhang X; King-Smith E; Renata H Total Synthesis of Tambromycin by Combining Chemocatalytic and Biocatalytic C−H Functionalization. Angew. Chem. Int. Ed. 2018, 57, 5037–5041. [DOI] [PubMed] [Google Scholar]
- (186).Zwick CR; Renata H A One-Pot Chemoenzymatic Synthesis of (2S, 4R)-4-Methylproline Enables the First Total Synthesis of Antiviral Lipopeptide Cavinafungin B. Tetrahedron 2018, 74, 6469–6473. [Google Scholar]
- (187).Zwick CR; Renata H Remote C–H Hydroxylation by an α-Ketoglutarate-Dependent Dioxygenase Enables Efficient Chemoenzymatic Synthesis of Manzacidin C and Proline Analogs. J. Am. Chem. Soc. 2018, 140, 1165–1169. [DOI] [PubMed] [Google Scholar]
- (188).Zhang X; Renata H Efficient chemoenzymatic synthesis of (2S,3R)-3-hydroxy-3-methylproline, a key fragment in polyoxypeptin A and FR225659. Tetrahedron 2019, 75, 3253–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Hayashi T; Ligibel M; Sager E; Voss M; Hunziker J; Schroer K; Snajdrova R; Buller R Evolved Aliphatic Halogenases Enable Regiocomplementary C−H Functionalization of a Pharmaceutically Relevant Compound. Angew. Chem. Int. Ed. 2019, 58, 18535–18539. [DOI] [PubMed] [Google Scholar]
- (190).Zwick CR; Sosa MB; Renata H Characterization of a Citrulline 4-Hydroxylase from Nonribosomal Peptide GE81112 Biosynthesis and Engineering of Its Substrate Specificity for the Chemoenzymatic Synthesis of Enduracididine. Angew. Chem. Int. Ed. 2019, 58, 18854–18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Doyon TJ; Perkins JC; Baker Dockrey SA; Romero EO; Skinner KC; Zimmerman PM; Narayan ARH Chemoenzymatic o-Quinone Methide Formation. J. Am. Chem. Soc. 2019, 141, 20269–20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Duewel S; Schmermund L; Faber T; Harms K; Srinivasan V; Meggers E; Hoebenreich S Directed Evolution of an FeII-Dependent Halogenase for Asymmetric C(sp3)–H Chlorination. ACS Catal. 2020, 10, 1272–1277. [Google Scholar]
- (193).Zhang X; King-Smith E; Dong L-B; Yang L-C; Rudolf JD; Shen B; Renata H Divergent Synthesis of Complex Diterpenes Through a Hybrid Oxidative Approach. Science 2020, 369, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Mailyan AK; Chen JL; Li W; Keller AA; Sternisha SM; Miller BG; Zakarian A Short Total Synthesis of [15N5]-Cylindrospermopsins from 15NH4Cl Enables Precise Quantification of Freshwater Cyanobacterial Contamination. J. Am. Chem. Soc. 2018, 140, 6027–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Baker Dockrey SA; Lukowski AL; Becker MR; Narayan ARH Biocatalytic Site- and Enantioselective Oxidative Dearomatization of Phenols. Nat. Chem. 2018, 10, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Wang ZJ; Renata H; Peck NE; Farwell CC; Coelho PS; Arnold FH Improved Cyclopropanation Activity of Histidine-Ligated Cytochrome P450 Enables the Enantioselective Formal Synthesis of Levomilnacipran. Angew. Chem. Int. Ed. 2014, 53, 6810–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Hernandez KE; Renata H; Lewis RD; Kan SBJ; Zhang C; Forte J; Rozzell D; McIntosh JA; Arnold FH Highly Stereoselective Biocatalytic Synthesis of Key Cyclopropane Intermediate to Ticagrelor. ACS Catal. 2016, 6, 7810–7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Bajaj P; Sreenilayam G; Tyagi V; Fasan R Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity. Angew. Chem. Int. Ed. 2016, 55, 16110–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Truppo MD Biocatalysis in the Pharmaceutical Industry: The Need for Speed. ACS Med. Chem. Lett. 2017, 8, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Sheldon RA; Woodley JM Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [DOI] [PubMed] [Google Scholar]
- (201).Hauer B Embracing Nature’s Catalysts: A Viewpoint on the Future of Biocatalysis. ACS Catal. 2020, 10, 8418–8427. [Google Scholar]
- (202).Wu S; Snajdrova R; Moore JC; Baldenius K; Bornscheuer U Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2020, 59, 2–34. [DOI] [PMC free article] [PubMed] [Google Scholar]