To the Editor:
Respiratory syncytial virus (RSV) infection is a major cause of severe respiratory disease in infants and in immunocompromised and older adults. RSV infects virtually all children by 2–3 years of age, resulting in nearly 3 million hospitalizations and 100,000 in-hospital deaths annually, mostly in developing countries (1). There is no approved vaccine against RSV infection. Passive prophylaxis with the anti-RSV antibody palivizumab is the only intervention licensed for the prevention of severe RSV disease in high-risk individuals (2, 3). RSV-specific serum IgG antibodies are present in most children and adults, reflecting the universality of RSV infection throughout life. Neutralizing antibodies remain a commonly accepted measure of protective immunity in vaccine trials (4). However, IgG antibodies might influence the course of RSV disease, not only by acting as neutralizing antibodies but also by activating effector functions through the receptors for the Fc portion of IgG (FcγRs) (5, 6). These receptors are widely expressed in myeloid and B cells. Whether T cells express FcγRs is still controversial, but recent studies strongly suggest that a minor fraction of T cells express FcγRII (CD32) (7–10). We show in the present study that severe RSV infection in infants is associated with a marked upregulation of CD32 on T cells. Moreover, we found that CD32 ligation improves the activation of CD4+ and CD8+ T cells from hospitalized infants.
Our study included 89 infants (median age, 6 mo [interquartile range, 3–10.5]; male, 58%) admitted to “Pedro de Elizalde” Children’s Hospital, Buenos Aires, Argentina, with RSV infection confirmed by direct immunofluorescence of nasopharyngeal aspirates. The local institutional review board approved the study, and written informed consent was obtained from parents. All infants had a clinical disease severity score (modified Tal score) greater than or equal to 7 and needed O2. Those admitted to the pediatric ICU required mechanical ventilation (n = 5). Blood samples were collected at enrollment, usually 2–3 days after the onset of symptoms. Age- and sex-matched infants admitted for scheduled surgery were included as healthy control subjects (n = 43). They had no airway infections for a 4-week period before the study or any episode of severe RSV infection in their past. Peripheral blood mononuclear cells were obtained from blood samples (0.4–0.6 ml) by using Ficoll-Hypaque gradient (GE Healthcare Life Sciences). CD4+, CD8+, and/or CD3+ T cells were sorted with a FACSAria Fusion flow cytometer (BD Biosciences). Purity was >96%. To perform real-time qRT-PCR, total RNA was extracted using the PureLink-RNA Mini Kit (Thermo Fisher). CD32a and CD32b isoforms were quantified as described previously (9). Antibody-dependent enhancement assays were performed using RSV (subtype A, strain Long) expanded in HEp-2 cells (American Type Culture Collection) and purified by ultracentrifugation on a 20% sucrose layer. Phytohemagglutinin (PHA)-stimulated isolated T cells (1 × 106/ml, 4 μg/ml; Sigma-Aldrich) were challenged with RSV (multiplicity of infection, 0.5) previously preincubated or not with subneutralizing concentrations of intravenous immunoglobulin (2 μg/ml; Universidad Nacional de Córdoba) for 2 days. The percentage of infection was determined by flow cytometry. T-cell functional assays were performed using sorted T cells (1 × 106/ml) incubated with anti-CD32 monoclonal antibody (30 μg/ml; STEMCELL Technologies). Cross-linking of CD32 was induced by antimouse IgG F(ab′)2 (50 μg/ml; Jackson ImmunoResearch). Next, cells were stimulated with PHA and cultured for 3 days. Cytokines were quantified in cell supernatants (BioLegend). Degranulation of CD8+ T cells was evaluated by flow cytometry. Statistical analysis was achieved using GraphPad Prism version 7 software. P < 0.05 was considered statistically significant.
We first analyzed the cell surface expression of CD32 in circulating CD4+ and CD8+ T cells from RSV-infected infants and healthy control subjects by flow cytometry. We found a higher expression of CD32 on both CD4+ and CD8+ T cells from RSV-infected infants compared with healthy control subjects (Figure 1A). Because there are no commercially available antibodies able to distinguish between activating CD32a and inhibitory CD32b isoforms, we evaluated the relative expression of both isoforms by RT-qPCR. mRNA levels of CD32a were markedly higher than CD32b in T cells from both groups (Figure 1B). We then analyzed whether CD32 expression might enable IgG antibodies to enhance RSV infection, a phenomenon well characterized in macrophages and natural killer cells (6). As shown in Figure 1C, subneutralizing doses of intravenous immunoglobulin failed to increase T-cell infection by RSV, perhaps reflecting its lower expression of FcγRs than found in macrophages and natural killer cells (5). The distribution of CD32+ cells within T-cell subsets showed marked differences between CD4+ and CD8+ T cells. Major changes were found in the naive and central memory T-cell subsets (Figure 2A). We hypothesized that the expression of CD32a by T cells from infants with RSV infection might provide a stimulatory signal able to promote T-cell activation. Figure 2B shows that CD32 ligation significantly enhanced the production of IL-2, IFN-γ, TNF-α, IL-4, IL-6, and IL-10 triggered by PHA, but it decreased IL-17 production by CD4+ T cells. Cytokines were not detected without PHA stimulation (data not shown). CD32 ligation was also able to enhance CD8+ T-cell degranulation induced by PMA/ionomycin, measured as CD107a expression (Figure 2C). Finally, because patients with RSV infection show substantial interindividual variation regarding CD32 expression on T cells, we analyzed whether CD32 expression was related to the clinical course of infection. Indeed, we found that the frequency of either CD32+CD4+ or CD32+CD8+ T cells negatively correlated with disease severity (Figure 2D), suggesting that CD32 expression by T cells might contribute to the proper resolution of RSV infection.
Figure 1.
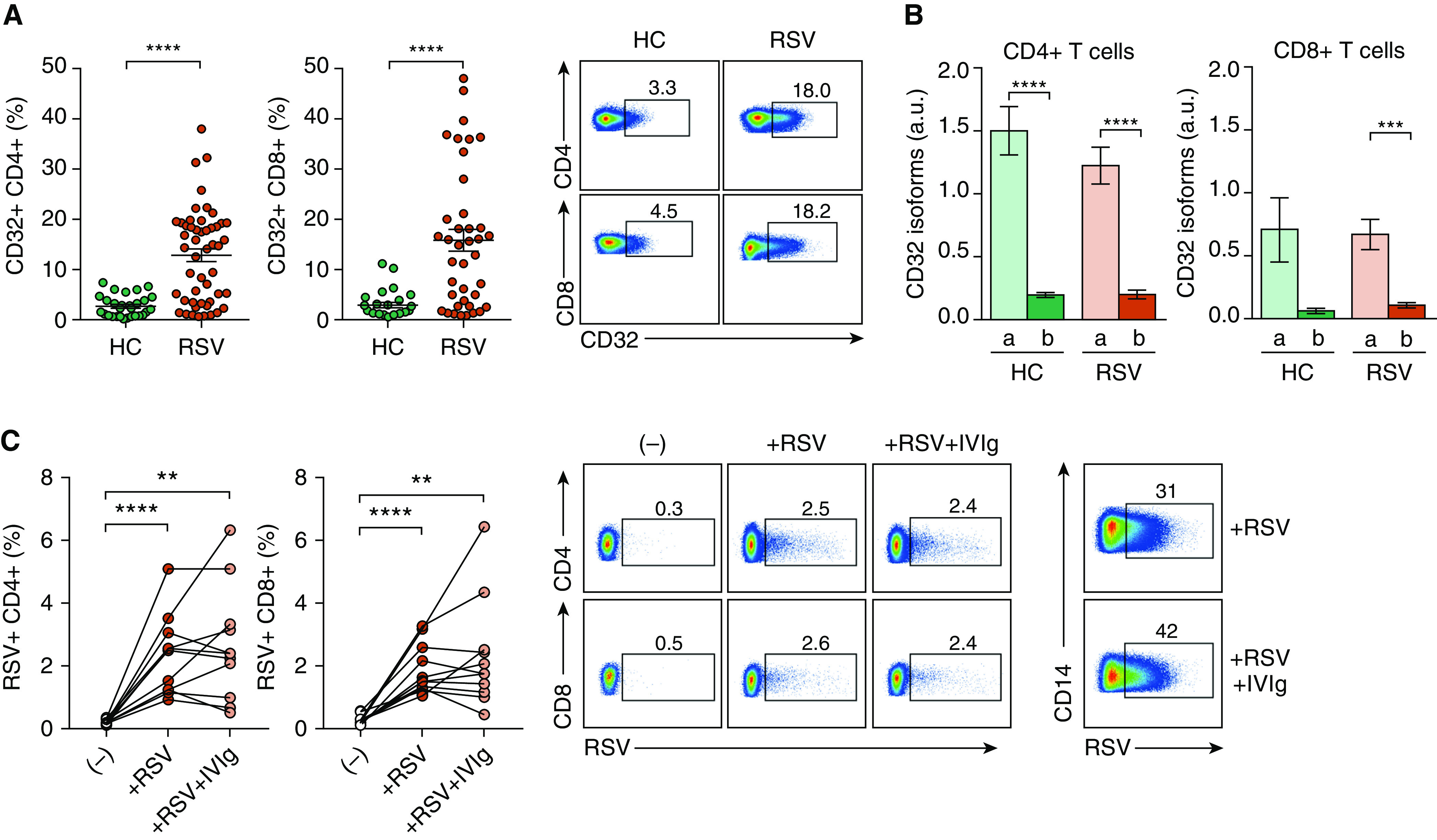
High CD32 expression in T cells from infants with respiratory syncytial virus (RSV) infection. (A) Frequency of CD32+ on gated CD4+ (n = 50) and CD8+ (n = 39) T cells in peripheral blood mononuclear cells from subjects with RSV and healthy control subjects (HC; n = 29) by flow cytometry. Data were acquired using a FACSCanto II flow cytometer (BD Biosciences) and analyzed with FlowJo software. (B) Relative expression of CD32a and CD32b mRNA isoforms in purified CD4+ and CD8+ T cells from children with RSV (n = 19) and HC (n = 14) quantified by RT-qPCR, as described previously (9). GAPDH was used as a housekeeping gene. (C) Sorted T cells (1 × 106/ml) from children with RSV infection (n = 11) were stimulated with phytohemagglutinin (PHA; 4 μg/ml) for 24 hours. Then, cells were challenged with RSV (subtype A, strain Long; multiplicity of infection, 0.5), preincubated or not with a subneutralizing concentration of intravenous immunoglobulin (IVIg; 2 μg/ml). After 2 days, cells were fixed, permeabilized (BD Biosciences), and labeled with a mouse antihuman RSV monoclonal antibody (mAb, EMD Millipore) plus a phycoerythrin goat antimouse mAb (Dako). The percentage of infection was evaluated in CD4+ and CD8+ T cells by flow cytometry. Purified CD14+ monocytes challenged with RSV in the absence or presence of subneutralizing doses of IVIg were used as a positive control for antibody-dependent enhancement of RSV infection (right). Representative dot plots are shown in A (right) and C (right). Mean ± SEM of n donors is shown in A (left and middle) and in B and C (left and middle). **P < 0.01, ***P < 0.001, and ****P < 0.0001. An unpaired t test was used for analysis in A. The Wilcoxon matched-pairs signed-rank test was used for analysis in B. The Friedman test was used for analysis in C. a.u. = arbitrary units.
Figure 2.
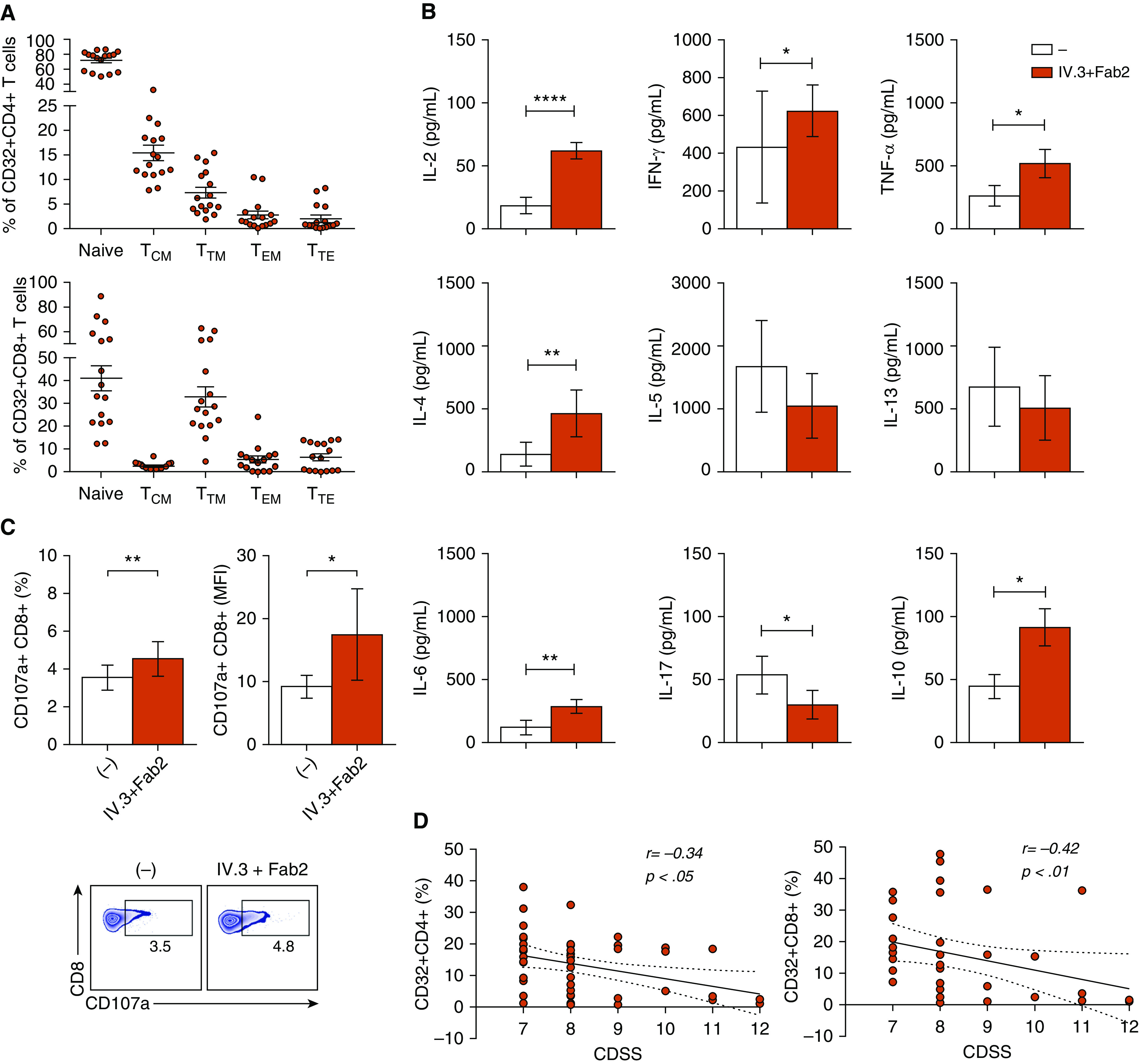
Enhanced response of T cells from infants with RSV infection upon CD32 ligation. (A) Distribution of CD32+ cells into the different subsets of CD4+ and CD8+ T cells from infants with RSV infection (n = 16). T-cell characterization as naive (CD45RA+CD27+CCR7+), central memory (CD45RA−CCR7+CD27+; TCM), transitional memory (CD45RA−CD27+CCR7−; TTM), effector memory (CD45RA−CD27−CCR7−; TEM), and terminally effector memory (CD45RA+CD27−CCR7−; TTE) was performed as described previously (7). The percentage of CD32+ cells in each cell subset analyzed by multiparametric flow cytometry cell is shown. (B) Sorted T cells (1 × 106/ml) were treated for 30 minutes with anti-CD32 mAb (30 μg/ml), and cross-linking of CD32 was then induced by the addition of goat antimouse IgG F(ab′)2 (50 μg/ml). Next, cells were cultured with PHA (4 μg/ml) for 3 days, and cytokine concentrations were quantified by LEGENDplex immunoassay (BioLegend) and/or ELISA (n = 16). (C) Sorted T cells (1 × 106/ml) were incubated with anti-CD32 mAb, and their ligation was induced by the addition of goat antimouse IgG F(ab′)2. Degranulation of CD8+ T cells (n = 9) was evaluated by flow cytometry to measure CD107a membrane expression after stimulation with PMA/ionomycin (Sigma-Aldrich) for 2 hours. Data show the percentage and mean fluorescence intensity (MFI) of CD107a+CD8+ T cells. (D) Correlation between clinical disease severity score (CDSS) and the percentage of CD32+CD4+ T cells and CD32+CD8+ T cells in infected children. CDSS was determined at the time of sampling using a modified Tal score. Correlations were evaluated by using the Spearman rank correlation coefficient test. Representative dot plots are shown in C (bottom). Mean ± SEM of n donors is shown in A–C. *P < 0.05, **P < 0.01, and ****P < 0.0001. The Wilcoxon matched-pairs signed-rank test was used for analysis in B and C.
We have recently reported a dampening of IL-2 function during severe RSV disease that might partially explain the inability of infants to promote a robust memory T-cell response (11). IgG-containing immune complexes are the natural ligands of CD32. Most severe RSV cases occur in infants under 6 months of age, who have high amounts of maternal IgG able to interact with viral particles, leading to the formation of immune complexes (6). It is widely accepted that immune complexes trigger the activation of myeloid cells but inhibit B-cell activation (5, 12). Our present results show that severe RSV disease is associated with a marked upregulation of CD32 expression by either CD4+ or CD8+ T cells. The fact that CD32 ligation provides a stimulatory signal able to promote the activation of T cells might represent a novel pathway through which anti-RSV IgG antibodies might improve T-cell function in response to both natural infection or RSV vaccination.
Supplementary Material
Footnotes
Supported by grants from the National Agency for Promotion of Science and Technology, Argentina (PIDC 0010-2015 [J.G.], PMO BID PICT 2016-0444 [L.A.]); the National Scientific and Technical Research Council (CONICET) (PIP 2015-0223 [L.A.]); and the University of Buenos Aires (20020170100573BA [J.G.]).
Author Contributions: Conception and design: I.S., S.R., J.G., and L.A. Analysis and interpretation: I.S., S.R., M.P.H., V.S., L.D.L., C.D., F.F., J.G., and L.A. Drafting of the manuscript for important intellectual content: I.S., S.R., M.E.P., J.G., and L.A.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Geoghegan S, Erviti A, Caballero MT, Vallone F, Zanone SM, Losada JV, et al. Mortality due to respiratory syncytial virus: burden and risk factors. Am J Respir Crit Care Med. 2017;195:96–103. doi: 10.1164/rccm.201603-0658OC. [DOI] [PubMed] [Google Scholar]
- 2.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, et al. Respiratory Syncytial Virus Network (ReSViNET) Foundation. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18:e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [Published erratum appears in Lancet Infect Dis 18:941.] [DOI] [PubMed] [Google Scholar]
- 3.Han J, Takeda K, Wang M, Zeng W, Jia Y, Shiraishi Y, et al. Effects of anti-G and anti-F antibodies on airway function after respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2014;51:143–154. doi: 10.1165/rcmb.2013-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capella C, Chaiwatpongsakorn S, Gorrell E, Risch ZA, Ye F, Mertz SE, et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis. 2017;216:1398–1406. doi: 10.1093/infdis/jix489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Mohsen M, Kuri-Cervantes L, Grau-Exposito J, Spivak AM, Nell RA, Tomescu C, et al. CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells. Sci Transl Med. 2018;10:eaar6759. doi: 10.1126/scitranslmed.aar6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan AK. Human CD4+ T-cells: a role for low-affinity Fc receptors. Front Immunol. 2016;7:215. doi: 10.3389/fimmu.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holgado MP, Sananez I, Raiden S, Geffner JR, Arruvito L. CD32 ligation promotes the activation of CD4+ T cells. Front Immunol. 2018;9:2814. doi: 10.3389/fimmu.2018.02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornhill JP, Pace M, Martin GE, Hoare J, Peake S, Herrera C, et al. CHERUB investigators. CD32 expressing doublets in HIV-infected gut-associated lymphoid tissue are associated with a T follicular helper cell phenotype. Mucosal Immunol. 2019;12:1212–1219. doi: 10.1038/s41385-019-0180-2. [DOI] [PubMed] [Google Scholar]
- 11.Sananez I, Raiden S, Erra-Díaz F, De Lillo L, Holgado MP, Geffner J, et al. Dampening of IL-2 function in infants with severe respiratory syncytial virus disease. J Infect Dis. 2018;218:75–83. doi: 10.1093/infdis/jiy180. [DOI] [PubMed] [Google Scholar]
- 12.Pandya PH, Wilkes DS. Complement system in lung disease. Am J Respir Cell Mol Biol. 2014;51:467–473. doi: 10.1165/rcmb.2013-0485TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


