Abstract
The selective functionalization of strong, typically inert carbon-hydrogen (C–H) bonds in organic molecules is changing synthetic chemistry. However, the undirected functionalization of primary C–H bonds without competing functionalization of secondary C–H bonds is rare. The borylation of alkyl C–H bonds has occurred previously with this selectivity, but slow rates required the substrate to be the solvent or in large excess. We report an iridium catalyst ligated by 2-methylphenanthroline with activity that enables, with the substrate as limiting reagent, undirected borylation of primary C–H bonds and, when primary C–H bonds are absent or blocked, borylation of strong secondary C–H bonds. Reactions at the resulting carbon-boron bond show how these borylations can lead to the installation of a wide range of carbon-carbon and carbon-heteroatom bonds at previously inaccessible positions of organic molecules.
The installation of functional groups at the positions of unreactive C–H bonds in organic molecules has been a longstanding goal of synthetic chemistry (1). The reaction at such C–H bonds without the assistance of a nearby directing group (2) is arguably the greatest challenge. Many reactions, both catalyzed and uncatalyzed, are known to occur at benzylic, allylic, secondary, and tertiary C–H bonds (3), but undirected functionalizations of primary C–H bonds, which are stronger and less electron rich, are much less developed.
Primary alkyl C–H bonds are stronger than secondary or tertiary C–H bonds and are much stronger than C–H bonds located alpha to heteroatoms or alpha to an aryl ring. Thus, primary C–H bonds are the least reactive toward reagents, chemical catalysts, or enzymes that abstract hydrogen atoms or a hydride to generate alkyl radical or carbocation intermediates. Yet a catalyst can change the site at which chemical reactions occur (4). The insertions of a particular class of carbene can occur preferentially into primary C–H bonds over secondary C–H bonds with sterically hindered catalysts (5), but only one class of reaction—the borylation of C–H bonds—has been reported to occur without a directing group with exclusive selectivity for functionalization of a primary C–H bond (6). Although unusually selective and having potential synthetic value because of the many types of products that can be formed from alkylboronates, the borylation of primary C–H bonds has typically required the substrate to be the solvent or in large excess and has not occurred in the presence of potentially reactive functional groups (6–11).
Here, we report iridium-catalyzed borylations of primary C–H bonds in a wide range of structures and the borylation of secondary C–H bonds of saturated carbocycles and heterocycles at strong C–H bonds positioned beta to the heteroatom. The use of 2-methylphenanthroline (2-mphen) as ligand accelerated the reaction rate by almost two orders of magnitude relative to the most widely used catalysts containing substituents on bipyridine or phenanthroline ligands at more distal positions, and this rate enhancement enabled reactions to occur with the alkyl substrate as limiting reagent in an inert solvent lacking methyl C–H bonds.
Development of undirected borylation of alkyl C–H bonds in an inert solvent
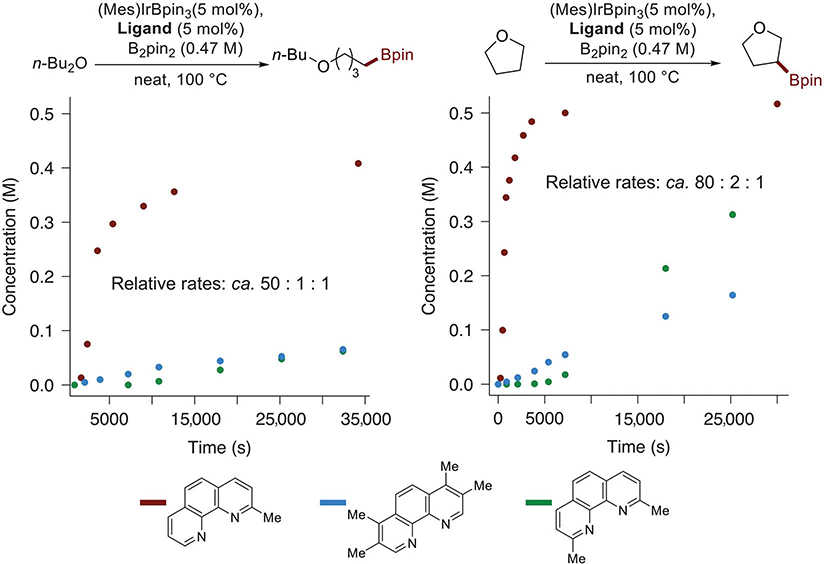
To achieve the borylation of alkyl C–H bonds without requiring a large excess of the substrate, we investigated the rates of the borylation of alkyl C–H bonds with bis-pinacolatodiboron (B2pin2) as reagent catalyzed by iridium complexes of phenanthroline ligands substituted with methyl groups at the 2-position and at the 2- and 9-positions and compared these rates to those of the catalyst containing 3,4,7,8-tetramethylphenanthroline (tmphen) that had previously generated the most active catalyst for the borylation of aryl and primary alkyl C–H bonds. We recently showed, for the silylation of aryl C–H bonds, that catalysts containing 2-mphen and 2,9-dimethylphenanthroline (2,9-dmphen) as ligand are more active than those containing tmphen (12, 13). However, the iridium catalysts generated from 2-mphen and 2,9-dmphen proved to be one and two orders of magnitude less active than those containing tmphen for the borylation of arenes with B2pin2 (see supplementary materials, fig. S5).
Despite these relative rates for the borylation of arenes, the results in Fig. 1 show that the catalyst coordinated by 2-mphen is much more active than that coordinated by tmphen for the borylation exclusively at the primary C–H bonds and secondary C–H bonds beta to oxygen in the linear and cyclic ethers, respectively. The initial rate of the reaction of B2pin2 with neat dibutyl ether catalyzed by the combination of [Ir(mesitylene)(Bpin)3] and 2-mphen was ~40 times greater than that of the reaction of B2pin2 with dibutyl ether catalyzed by the combination of [Ir(mesitylene)(Bpin)3] and tmphen or 2,9-dmphen after short induction periods, and the initial rate of the reaction of B2pin2 with neat tetrahydrofuran (THF) catalyzed by [Ir(mesitylene)(Bpin)3] and 2-mphen was 80 times greater than that of the reaction of THF catalyzed by [Ir(mesitylene)(Bpin)3] and tmphen or 2,9-dmphen.
Fig. 1. Profiles of the Ir-catalyzed reactions of THF and dibutyl ether with B2pin2 with variously substituted phenanthrolines as ligands.
The reactions are catalyzed by the combination of 5 mol % [Ir(mesitylene)(Bpin)3] and 5 mol % 2-mphen (red), tmphen (blue), or 2,9-dmphen (green). The relative rates were estimated from the slope of the curves for the initial rates of reactions with 2-mphen (red) versus those for the reactions with the other two ligands after the induction period. n-Bu2O, n-dibutyl ether; Me, methyl.
These large rate increases with the catalyst containing 2-mphen implied that the same reactions could occur in good yields at acceptable rates with the substrate as the limiting reagent at much lower concentrations in an inert solvent (8, 14). To test the potential of achieving the borylation on a purely unactivated alkane in an inert solvent, we conducted the reaction of dodecane in cycloalkanes. We found that the reaction of dodecane in cyclooctane at 100°C formed products from borylation at one or both of the terminal positions. The ratio of products from borylation of substrate to borylation of solvent was more than 60:1. We also performed a competition experiment involving the reaction of cyclohexane and cyclooctane together at 100°C, which afforded the corresponding borylated products in a 4:1 ratio (for details on the reactivity of these cycloalkanes and saturated heterocycles, see supplementary materials). Thus, the reactions in the remainder of the study were conducted in cyclooctane as solvent.
Although the product from the borylation of dodecane in cyclooctane was observed, monitoring of the reaction over time showed that the rate decreased more rapidly than expected for a typical first-order process, and only 34% of the methyl groups of the substrate converted to alkylboronate units. We considered that the pinacolborane (HBpin) by-product could inhibit the reaction and conducted two experiments to test this hypothesis. First, we conducted the reaction of dodecane with B2pin2 initiated with 2 equiv of added HBpin. Qualitatively, the reaction with the added HBpin was slower than the one without added HBpin (see supplementary materials for details), and this result is consistent with inhibition by the HBpin formed as a by-product.
Second, we conducted the reaction at 100°C in a vessel that was open to a flow of nitrogen. Under these conditions, volatile side products could evaporate from the system. Indeed, the reactions of alkanes with B2pin2 (3 equiv) catalyzed by [Ir(OMe)(COD)]2 and 2-mphen at 100°C in cyclooctane under these conditions occurred to high conversion and yield of products from borylation of the methyl groups. We propose, on the basis of the observation of B2pin3 by 11B nuclear magnetic resonance (NMR) spectroscopy (fig. S11), that the HBpin by-product disproportionates to B2pin3 and BH3 (15, 16), driven by the elimination of BH3 in the flow of nitrogen. Under these conditions, the reaction of dodecane in cyclooctane fully converted the linear alkane and formed a 6:1 ratio of products from reaction at one and two methyl groups, respectively; the 1-boryldodecane was isolated in 65% yield (Fig. 2). Likewise, the reaction of pentylcyclohexane, a hydrocarbon that contains just one set of primary C–H bonds, along with many inequivalent secondary and tertiary C–H bonds, gave a single product under these conditions in high yield from borylation at the methyl group. The remaining mass balance in this case was a small amount of unreacted alkane.
Fig. 2. Borylation of the primary C–H bonds of alkyl groups and secondary C–H bonds of cyclic compounds.
(A) Borylation of primary C–H bonds of acyclic alkanes, ethers, silyl ethers, and protected amines. (B) Borylation of the methyl C–H bonds of alcohols, including the natural terpene menthol. (C) Borylation of the secondary C–H bonds of carbocycles. tBu, tert-butyl; d.r., diastereomeric ratio. (D) Borylation of the methylene C–H bonds beta to oxygen in tetrahydrofurans and tetrahydropyrans and beta to nitrogen in azetidine, pyrrolidine, piperidine, and azepine derivatives. Assay yields were measured by 1H NMR spectroscopy, with isolated yields in every example given in parentheses. The difference in yields determined by NMR spectroscopy and by isolation typically resulted from the difficulty of separating the products from the alkyl reactants, unreacted diboron reagent, and boron-containing side products owing to their similar polarities. In no case did a reaction form >5% of any other product from reaction with the substrate. Yields marked with an asterisk refer to the yield of the corresponding alcohol isolated after oxidation in cases for which the alkylboronate could not be separated from other reaction components. Piv, pivaloyl.
Scope of the borylation of alkyl C–H bonds
With conditions for the borylation of primary alkyl C–H bonds with the substrate as limiting reagent, we tested the scope of the reactions of B2pin2 catalyzed by [Ir(OMe)(COD)]2 and 2-mphen with substrates that contain primary C–H bonds (Fig. 2). Substrates containing ether, silyl ether, imide, carbamate, amine, ketal, and acetal functionality underwent borylation at primary C–H bonds or unactivated secondary C–H bonds when primary C–H bonds were absent or were blocked by steric hindrance. These reactions occurred without direction by the existing functional groups.
As shown in Fig. 2A, alkanes (1 and 3), ethers (2, 4, 5, and 6), amines and amine derivatives (7 to 11), and acetals (12 and 13) containing methyl groups all underwent the borylation at the primary C–H bond. Although most of the reactions at primary C–H bonds were conducted in a system open to a flow of nitrogen, some of the reactions at methyl groups, particularly in substrates containing an electron withdrawing group, occurred to high conversion in a closed system. Dodecane (1) and dioctyl ether (2) formed a mixture of products from the borylation of one or two methyl groups at the chain ends, but tert-butyl octyl ether formed a single product from borylation of the less hindered methyl group (4), and the triisopropylsilyl (TIPS) tetradecyl ether formed a single alcohol product with the boryl group at the ω-position after cleavage of the silyl ether (5). Ethyl butyl ether (6) formed products from borylation at the primary C–H bonds in the ethyl and butyl groups in 71% combined yield with a 90:10 ratio favoring reaction at the primary C–H bond of the ethyl group. This ratio is comparable to that (87:13) observed from the reaction of the neat ether with an iridium catalyst ligated by tmphen (14). N-Hexyl piperidine (11) reacted like the alkyl cyclohexane, forming a single product from reaction at the methyl group. N-Propyl and N-octyl aliphatic imides (7 and 8) each formed a single product from reaction at the methyl group, but the 2-octyl imide (9) formed a mixture of two products from borylation at one or the other methyl group in a 3:2 ratio favoring reaction at the β methyl group over the w methyl group, likely reflecting the electronic effect of the imide on the functionalization process. N-Boc dibutyl amine (Boc = tert-butyl carbamoyl) (10) gave a high yield of product from monoborylation at the n-butyl group without reaction at the more hindered tert-butyl group in the carbamate unit. Although aldehydes and ketones were not tolerated, the neopentane glycol acetals of octanal (12) reacted at the terminal methyl group of the alkyl chain, and the analogous ketal of 2-hexanone (13) reacted at the less hindered of the two methyl groups on the hexyl chain. Neither substrate underwent reaction at the more hindered geminal dimethyl groups.
Alkylarenes also underwent reactions at methyl groups when the aryl C–H bonds contained an ortho substituent (14 and 15). 1,4-Diisopropyl benzene underwent borylation to form a mixture of products from monoborylation and diborylation (mono:di = 3:1) at the alkyl substituents. 3-Bromo-1-isopropylbenzene reacted at the mutually meta C–H bond on the aryl ring and at one methyl C–H bond on the isopropyl substituent to give the product from two distinct borylation processes in good yield. As shown below, these two boryl groups could be used independently for derivatization.
Primary, secondary, and tertiary alcohols (16 to 18) also underwent borylation of a C–H bond after initial borylation of the hydroxyl group (Fig. 2B). The alcohol was first mixed with HBpin and, after conversion of the alcohol to the borate, addition of B2pin2 and catalyst led to reaction at a primary C–H bond, enabling formation of the w-functionalized product of hexadecanol and borylation of the methyl group on the cyclohexyl ring of menthol. In addition to reactions at C–H bonds of these representative primary and secondary alcohols, this sequence led to borylation of the primary C–H bond of the tertiary alcohol butyl cyclohexanol. When this reaction of 18 was conducted on a larger 3.5-mmol scale, the isolated yield (48%) was comparable to that of the small-scale reaction (0.25 mmol, 56%).
The borylation of alkyl C–H bonds also occurred at the secondary positions of many saturated carbocycles (Fig. 2C) and heterocycles (Fig. 2D). The reactions of carbocycles occurred at the most sterically accessible C–H bond. For example, the borylation occurred at the C–H bond trans to the substituent of a cyclopropane carboxylate (19). The reaction of this cyclopropane catalyzed by 2-mphen is approximately five times faster than that catalyzed by the complex of 2,9-dmphen (fig. S6) (17). This higher reactivity enabled the borylations of less-reactive carbocycles, such as cyclobutane (20) and cyclopentanes (21 to 23). The acetals of cyclobutanone and cyclopentanone reacted at the most sterically accessible C–H bond located beta to the fusion of the spirocycle (20 and 21). The reaction occurred at these methylene C–H bonds over the geminal dimethyl substituents on the acetal. Two cyclopentanes containing nitrogen atoms also reacted (22 and 23). These reactions of the fused octahydrocyclopenta[c]pyrrole and the imidocyclopentane also occurred at the less hindered methylene units.
This catalyst also enabled the borylation of a wide range of saturated heterocycles at the position beta to the heteroatom (Fig. 2D) with the heterocycle as limiting reagent. Reactions that functionalize the C–H bonds beta to the heteroatom in such heterocycles are much less common than those that functionalize the weaker C–H bonds alpha to these atoms (18). In general, the reactions of these heterocycles are faster than those of C–H bonds farther from a heteroatom, allowing these reactions to occur in a closed system. For example, borylations at the C–H bonds beta to oxygen in furans (24 to 26), dihydrofuranone (27), and tetrahydropyrans (28 and 29) occurred in good yield under these conditions. 2-Substituted tetrahydrofurans reacted selectively at the 4-position, which is beta to the oxygen, as did the 3-methyl dihydrofuranone. Although reaction of the 2-substituted tetrahydrofurans was not diastereoselective, the reaction of the more conformationally defined 3-substituted pivaloyl tetrahydropyran occurred at the 5-position with 3:1 diastereoselectivity in favor of the cis isomer. Formation of the cis isomer and the greater stability of 3-pivaloyltetrahydropyran with the substituent in an equatorial position implies that the reaction occurs preferentially at an equatorial C–H bond over an axial C–H bond. The origin of the regioselectivity for reactions of these heterocycles is being studied, but one recent paper reporting computational studies of our prior borylation of THF as solvent (19) suggests that cleavage of the C–H bond alpha to the heteroatom occurs but is reversible and does not lead to product because the barrier to form the B–C bond at this position is high.
Saturated nitrogen heterocycles are among the most common units in pharmaceuticals, and the importance of functionalizing the C–H bonds of such heterocycles has been emphasized recently (20). The major products of the borylations we report result from functionalization beta to nitrogen with N-substituted azetidine (30), pyrrolidine (31), and piperidine (32 and 33) in good yields and even in acceptable yield with the larger-ring N-trifluoroacetyl azepane (34). The selectivity for reaction at the position beta to nitrogen versus gamma to nitrogen in the piperidine derivatives was 6:1 in both cases. The products from functionalizations of pyrrolidines resulted exclusively from reaction beta to nitrogen and were isolated in pure form; the product from borylation of pyrrolidine itself was obtained in good yield on a 2.5-mmol scale. These reactions also occurred with versions of these heterocycles containing substituents on the ring (35 and 36). For example, 2-trifluoromethyl pyrrolidine reacted at the more sterically accessible of the methylene units beta to nitrogen, and tert-butyl N-Boc proline reacted at the same position.
Transformations of the products from C–H borylation
The boryl groups in the products of these reactions are poised for conversion to a range of other functional groups. Alkylboronic esters are known to undergo oxidation (21, 22), amination (23), halogenation (24), arylation (25), vinylation, and homologation (25), among other transformations. To showcase this flexibility, we first conducted transformations of the borylated aryl and alkyl C–H bonds of 3-bromo isopropyl benzene (Fig. 3).
Fig. 3. Derivatization of the products from C–H borylation at alkyl C–H bonds.
Borylation of an alkylarene at the aryl and primary alkyl C–H bonds, followed by derivatization specifically at the aryl C–B bond or C–Br bond, and then at the alkyl C–B bond. brs, based on recovered starting material; NBS, N-bromosuccinimide.
The bromide, arylboronate, and alkylboronate all react independently under appropriate conditions. Both C–B bonds are stable when the C–Br bond reacts with boronic acid under Suzuki coupling conditions [Pd(dppf)Cl2 (5 mol%) and K3PO4] to form the biaryl 37 (Fig. 3, top). The reactions at the left and bottom of Fig. 3 show that the aryl C–B bond can react selectively over the alkyl C–B bond. Deboronation to form the aryl C–H or C–D bond (38 and 39) using an iridium catalyst (26) provided labeled and unlabeled product from formal selective borylation at the alkyl C–H bond. Likewise, selective reaction at the aryl C–B bond enabled copper-mediated halogenation (27) and palladium-catalyzed Suzuki coupling (28) to occur selectively at the arylboronate unit, giving the aryl chloride 40 and the biaryls 41 to 43 containing the intact alkyl C–B unit. Coppercatalyzed oxidation (29) converted the arylboronate unit to the phenol 44, maintaining the alkylboronate unit, while oxidation under standard conditions of basic hydrogen peroxide formed the diol 45.
After transformation of the aryl-boron bond, amination at the aryl bromide or transformation of the alkyl-boron bond could be conducted (Fig. 3, right). For example, coupling of cyclohexylamine with the aryl bromide formed the aniline derivative 46 without affecting the alkyl-boron linkage, and subsequent oxidation of the C–B bond formed product 47, containing a new C–O, C–N, and C–C bond from the initial product of C–H bond functionalization at both the alkyl and aryl C–H bonds. Transformation of the alkylboronate unit to a furyl group gave product 48, containing two different C–C bonds at the two C–H bonds that had undergone borylation, and olefination at the alkyl C–B bond gave product 49, resulting from installation of two different types of C–C bonds. Amination and protection with Boc for the purpose of isolation led to product 50, in which the primary C–H bond was converted to a new C–N bond, and the overall process led to formation of one C–C and one C–N bond by C–H bond functionalization and selective derivatizations at the C–B bonds.
In a similar vein, the products from borylation of heterocycles at a secondary C–H bond can undergo multiple derivatizations (30). As shown in Fig. 4, the 3-boryl pyrrolidine 31 underwent cleavage of the pinacolate by fluoride to form the solid, more reactive trifluoroborate analog of the pinacolboronate 51, coupling under metallaphotoredox conditions (31) to form the arylpyrrolidine 52, heteroarylation (25) to generate the furanyl pyrrolidine 53, amination to form the N-Boc amine 54, and oxidation to generate the alcohol 55. Fluorination to form 56 was accomplished by oxidation and deoxyfluorination.
Fig. 4. Derivatization of the B–C bond of an N-Boc 3-borylpyrrolidine.
DAST, diethylaminosulfur trifluoride.
The ability to conduct these reactions with the substrate as limiting reagent enables the borylation of natural products or medicinally relevant synthetic structures containing many C–H bonds and functional groups. For example, dehydroabietic acid contains a carboxylate group along with many aryl and alkyl C–H bonds. After conversion of the carboxylic acid to the tert-butyl ester 57, this molecule underwent borylation exclusively at the methyl C–H bond of the isopropyl group over the aryl C–H bonds, the other three more hindered methyl C–H bonds, and the methylene or methine C–H bonds (Fig. 5). Because of the remoteness of this group from the existing stereogenic center, two diastereomers are formed in equal amounts, but the overall yield was sufficient to provide material to create a series of derivatives of 58. The product 58 from borylation at the methyl group underwent oxidation to form the corresponding alcohol product 59, which would be difficult to obtain by direct oxidation. This intermediate also underwent halogenation (60) to place bromine at the position of the strongest C–H bond of the isopropyl group rather than the weaker benzylic C–H bond, which would be the site of reactivity under conditions of radical bromination. Suzuki cross-coupling formed product 61, containing an aryl group at the original C–H bond that would otherwise be inaccessible, and homologation led to chain extension to form an alkylboronate (62) that could be derivatized in a similar fashion to create an analogous homologated series of products. Amination at the C–B bond similarly formed a product (63) that would otherwise be inaccessible.
Fig. 5.
Borylation and derivatization of tert-butyl ester of dehydroabietic acid.
Mechanistic studies
To gain preliminary insight into the mechanism of the C–H bond functionalization process, we measured the kinetic isotope effect (KIE) for substrates that react at primary and secondary C–H bonds. The KIE value determined from reaction with a mixture of THF and THF-d8 was 2.1 ± 0.1, and the value for the reaction of a mixture of octane and octane-d18 was 3.4 ± 0.2. Whereas the value for the reaction of THF could be interpreted as primary kinetic or equilibrium, the value for the reaction with octane is clearly a primary KIE. Thus, cleavage of the primary C–H bond is irreversible, and the energy of this transition state is higher than that of the subsequent B–C bond formation. The rate of the reaction of tert-butyl octyl ether increased with increasing concentration of the substrate (fig. S15), further implying that the reaction with this catalyst is due to an increase in the rate of the C–H bond cleavage step.
The high selectivity for functionalization of primary over secondary C–H bonds is consistent with prior studies on the borylation of C–H bonds. Prior studies on the origins of the selectivity of the borylation of primary C–H bonds of alkanes catalyzed by Cp*Rh(C6Me6) showed that the exclusive selectivity for reaction of primary over secondary C–H bonds resulted from a combination of 7:1 selectivity for the cleavage of primary over secondary C–H bonds, which was determined experimentally by hydrogen–deuterium exchange, and a 9 kcal/mol difference in energy for the formation of the B–C bond, which was computed by density functional theory (DFT) (32). A small KIE of 2.0 for the reaction of octane catalyzed by this rhodium system implied that the C–H bond cleavage step is reversible and the primary influence of the selectivity results from that for B–C bond formation. In contrast, the KIE for reaction of octane catalyzed by the iridium system reported here implies that the selectivity results from C–H bond cleavage. Thus, the high selectivity for reaction of the primary C–H bonds of octane versus the secondary C–H bonds in linear octane or the cyclooctane solvent likely reflects the selectivity from C–H bond cleavage instead of reductive elimination to form the B–C bond from the primary or secondary alkyl intermediates. The high reactivity of the C–H bonds beta to the heteroatoms in both five- and six-membered saturated heterocycles, so far, is specific to the borylation of C–H bonds. One set of detailed DFT studies has previously suggested that the higher reactivity at this type of C–H bond than at C–H bonds in saturated carbocycles results from attractive noncovalent interactions between the substrate and the boryl ligands (14), and a second study suggests that the selectivity for reaction at the beta position over the alpha position results from a high barrier for reductive elimination from the a-alkoxy (and presumably amino) alkyl intermediate (19). The ability to run reactions with the cyclic ethers and amines in a closed system without prohibitive inhibition by the HBpin side product presumably results from the favorable rates for reaction beta to the heteroatom.
The origin of the accelerating effect of the 2-mphen ligand is particularly intriguing. To gain initial information on the origin of this effect, we carefully monitored the profile of the reaction catalyzed by the iridium complex that would be analogous to the active catalyst in the reactions of arenes studied previously. To do so, we added 2-mphen to [(mes)Ir(Bpin)3] (mes = mesitylene) in the presence of B2pin2 in THF-d8 at room temperature. This combination of ligand and iridium complex has been shown to generate the active form of the catalyst (ligand)Ir(Bpin)3 with other ligands, and this process generated a 2-mphen-ligated Ir boryl complex in ~80% yield in the presence of B2pin2 (fig. S14). In contrast to reactions of arenes, the reaction of THF-d8 catalyzed by this preformed complex occurred with a marked induction period, implying that this complex is not the active catalyst (fig. S13).
We considered that the induction period could involve modification of the 2-mphen ligand to generate the active catalyst, although borylations of bipyridine and phenanthroline ligands have previously been shown to deactivate, rather than activate, the catalyst (33). Because multiple modifications of the 2-mphen were observed by gas chromatography–mass spectrometry and liquid chromatography–mass spectrometry, we assessed the potential that modification of the ligand activates the catalyst and gained information about the important position of such modification by monitoring the profile of the reaction with 2-mphen-d3 in which the methyl group is deuterated and 2-mphen-d7 in which the phenanthroline ring is deuterated. If modification of the ligand leads to the active catalyst, then an isotope effect on this process at the ring or at the methyl group should manifest as a change in the length of the induction period. These experiments showed that the induction period is longer for the reaction with the ligand containing deuterium at the methyl group but unchanged for reaction with the ligand containing deuterium at the ring (fig. S16). This effect of the position of deuterium on the extent of the induction period implies that a change to the methyl group leads to the active catalyst. Further studies are needed to identify the precise structure of the ligand in the active catalyst and to inhibit detrimental changes to its structure, but it is possible that borylation at the methyl group triggers the high activity.
Outlook
The system we report enables the introduction of a range of functional groups at the strongest alkyl C–H bonds in organic molecules in an undirected fashion and at the C–H bonds beta to the heteroatom in saturated heterocycles with the substrate as limiting reagent. While the combination of high reactivity and high selectivities for these C–H bonds, along with the synthetic utility of the resulting products, has not been observed previously, further developments are needed to achieve the full potential of this class of C–H bond functionalization. Clearly, the yields of the reactions in many cases are modest and require catalysts with greater activity and stability. Furthermore, a combination of a catalyst and inert medium that more readily dissolves larger and more polar substrates than cyclooctane is needed. We anticipate that studies on the true active catalyst will enable the pinpointing of features that lead to high activity and enable design or selection of even more active catalysts. Such systems should make the undirected borylation of primary alkyl C–H bonds a synthetic method that greatly expands the utility of undirected C–H borylation beyond the commonly practiced undirected borylation of aryl and heteroaryl C–H bonds.
Supplementary Material
ACKNOWLEDGMENTS
R.O. thanks C. Karmel for helpful discussions.
Funding: We thank the National Institutes of Health (NIH) (R35GM130387) for support of this work. R.O. is a Swiss National Science Foundation postdoctoral fellow. We thank the College of Chemistry’s NMR (CoC-NMR) facility for resources provided, and we thank their staff for assistance. Instruments in CoC-NMR are supported in part by the NIH (S10OD024998).
Footnotes
Competing interests: The authors declare no competing interests.
SUPPLEMENTARY MATERIALS
Data and materials availability:
Characterization of unknown compounds and more detailed mechanistic studies are provided in the supplementary materials.
REFERENCES AND NOTES
- 1.Arndtsen BA, Bergman RG, Mobley TA, Peterson TH, Acc. Chem. Res. 28, 154–162 (1995). [Google Scholar]
- 2.Lyons TW, Sanford MS, Chem. Rev. 110, 1147–1169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartwig JF, J. Am. Chem. Soc. 138, 2–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartwig JF, Acc. Chem. Res. 50, 549–555 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao K et al. , Nat. Chem. 10, 1048–1055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Schlecht S, Semple TC, Hartwig JF, Science 287, 1995–1997 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Murphy JM, Lawrence JD, Kawamura K, Incarvito C, Hartwig JF, J. Am. Chem. Soc. 128, 13684–13685 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Liskey CW, Hartwig JF, J. Am. Chem. Soc. 134, 12422–12425 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Ohmura T, Torigoe T, Suginome M, Chem. Commun. 50, 6333–6336 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Ohmura T, Torigoe T, Suginome M, J. Am. Chem. Soc. 134, 17416–17419 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Jones MR, Fast CD, Schley ND, J. Am. Chem. Soc. 142, 6488–6492 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, Hartwig JF, J. Am. Chem. Soc. 137, 592–595 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Karmel C, Chen Z, Hartwig JF, J. Am. Chem. Soc. 141, 7063–7072 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Liskey CW, Hartwig JF, J. Am. Chem. Soc. 136, 8755–8765 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Brown HC, Gupta SK, J. Am. Chem. Soc. 93, 1816–1818 (1971). [Google Scholar]
- 16.Westcott SA, Blom HP, Marder TB, Baker RT, Calabrese JC, Inorg. Chem. 32, 2175–2182 (1993). [Google Scholar]
- 17.Liskey CW, Hartwig JF, J. Am. Chem. Soc. 135, 3375–3378 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW, Chemistry 18, 10092–10142 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Zhong R-L, Sakaki S, J. Am. Chem. Soc. 141, 9854–9866 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Vitaku E, Smith DT, Njardarson JT, J. Med. Chem. 57, 10257–10274 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Zweifel GT, Brown HC, Org. React. 13, 22 (1963). [Google Scholar]
- 22.Brown HC, Boranes in Organic Chemistry (Cornell Univ. Press, 1972). [Google Scholar]
- 23.Edelstein EK, Grote AC, Palkowitz MD, Morken JP, Synlett 29, 1749–1752 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larouche-Gauthier R, Elford TG, Aggarwal VK, J. Am. Chem. Soc. 133, 16794–16797 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Scott HK, Aggarwal VK, Chemistry 17, 13124–13132 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Kallepalli VA et al. , J. Org. Chem. 80, 8341–8353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy JM, Liao X, Hartwig JF, J. Am. Chem. Soc. 129, 15434–15435 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Miyaura N, Yanagi T, Suzuki A, Synth. Commun. 11, 513–519 (1981). [Google Scholar]
- 29.Yang H, Li Y, Jiang M, Wang J, Fu H, Chemistry 17, 5652–5660 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Leonori D, Aggarwal VK, Acc. Chem. Res. 47, 3174–3183 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Primer DN, Karakaya I, Tellis JC, Molander GA, J. Am. Chem. Soc. 137, 2195–2198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei CS, Jiménez-Hoyos CA, Videa MF, Hartwig JF, Hall MB, J. Am. Chem. Soc. 132, 3078–3091 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Oeschger RJ, Larsen MA, Bismuto A, Hartwig JF, J. Am. Chem. Soc. 141, 16479–16485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Characterization of unknown compounds and more detailed mechanistic studies are provided in the supplementary materials.