Abstract
The neurotransmitter gamma-aminobutyric acid (GABA) is widely distributed in the mammalian central nervous system, where it acts as a major mediator of synaptic inhibition. GABA also serves as a neurotransmitter in a range of invertebrate phyla, including arthropods, echinoderms, annelids, nematodes, and platyhelminthes. This article reviews evidence supporting the neurotransmitter role of GABA in gastropod molluscs, with an emphasis on its presence in identified neurons and well-characterized neural circuits. The collective findings indicate that GABAergic signaling participates in the selection and specification of motor programs, as well as the bilateral coordination of motor circuits. While relatively few in number, GABAergic neurons can influence neural circuits via inhibitory, excitatory, and modulatory synaptic actions. GABA’s colocalization with peptidergic and classical neurotransmitters can broaden its integrative capacity. The functional properties of GABAergic neurons in simpler gastropod systems may provide insight into the role of this neurotransmitter phenotype in more complex brains.
Introduction
GABA is the major inhibitory neurotransmitter in the mammalian brain (Roberts, 1960, 1986a, b; Florey, 1961; Krnjević, 1970). Perturbation of GABAergic signaling has been implicated in numerous neurological disorders, including epilepsy, Parkinson’s disease, and Huntington’s disease (Watts et al., 2012; Möhler, 2013; Johnston et al., 2016). GABA also acts as a neurotransmitter in several invertebrate phyla, including arthropods (Kuffler and Edwards, 1958; Kravitz et al., 1963; Otsuka et al., 1967), echinoderms (Newman and Thorndyke, 1994), annelids (Ito et al., 1969; Cline, 1983, 1986), nematodes (del Castillo et al., 1964; Johnson and Stretton, 1987; McIntire et al., 1993), and platyhelminthes (Eriksson and Panula, 1994). Moreover, GABA has been shown to produce contractile responses in sponges (Porifera), raising the possibility that its signaling function preceded the appearance of nervous systems (Ellwanger et al., 2007; Elliott and Leys, 2010; Nickel, 2010). The conserved role of GABA as a neurotransmitter across phylogeny supports its ancient origins and ubiquitous function in the core operation of neural circuits.
The nervous systems of gastropod molluscs provide experimentally favorable models for investigating the organization of action (Kandel, 1976, 1979; Davis and Gillette, 1978; Chase, 2002), neuroendocrine regulation of behavior (Kupfermann, 1970; Roubos, 1976; Conn and Kaczmarek, 1989), principles of motor control (Kupfermann and Weiss, 1978; Getting and Dekin, 1985; Katz, 1995), and the cellular basis of learning and memory (Kandel, 1970, 2001; Crow and Alkon, 1980; Benjamin et al., 2008). These nervous systems contain large identifiable neurons that can be classified according to neurotransmitter phenotype (Pentreath et al., 1974; Ono and McCaman, 1980, 1984; Church and Lloyd, 1991). This article reviews evidence accumulated over the past 50 years that supports the role of GABA as a neurotransmitter in gastropod molluscs. GABAergic signaling by identified neurons in circuits that control behavior is emphasized because this role could provide insights into functions that are generalizable across phylogeny.
Biochemical Foundations
In his landmark review of invertebrate neurotransmitters, Gerschenfeld (1973, p. 81) concluded that the “possibility that GABA may play a role as a transmitter in the snail is very remote on the basis of present knowledge.” In several prior reports, GABA was shown to produce both excitatory and inhibitory responses upon application to snail neurons (Gerschenfeld and Tauc, 1961; Kerkut and Walker, 1961; Gerschenfeld and Lasansky, 1964; see Fig. 1A1, A2). However, Gerschenfeld’s reticence to confer neurotransmitter status reflected the failure of contemporaneous biochemical investigations to detect GABA or glutamic acid decarboxylase activity in snail ganglia (Roberts, 1960; Kerkut and Cottrell, 1962; Bradford et al., 1969).
Figure 1.
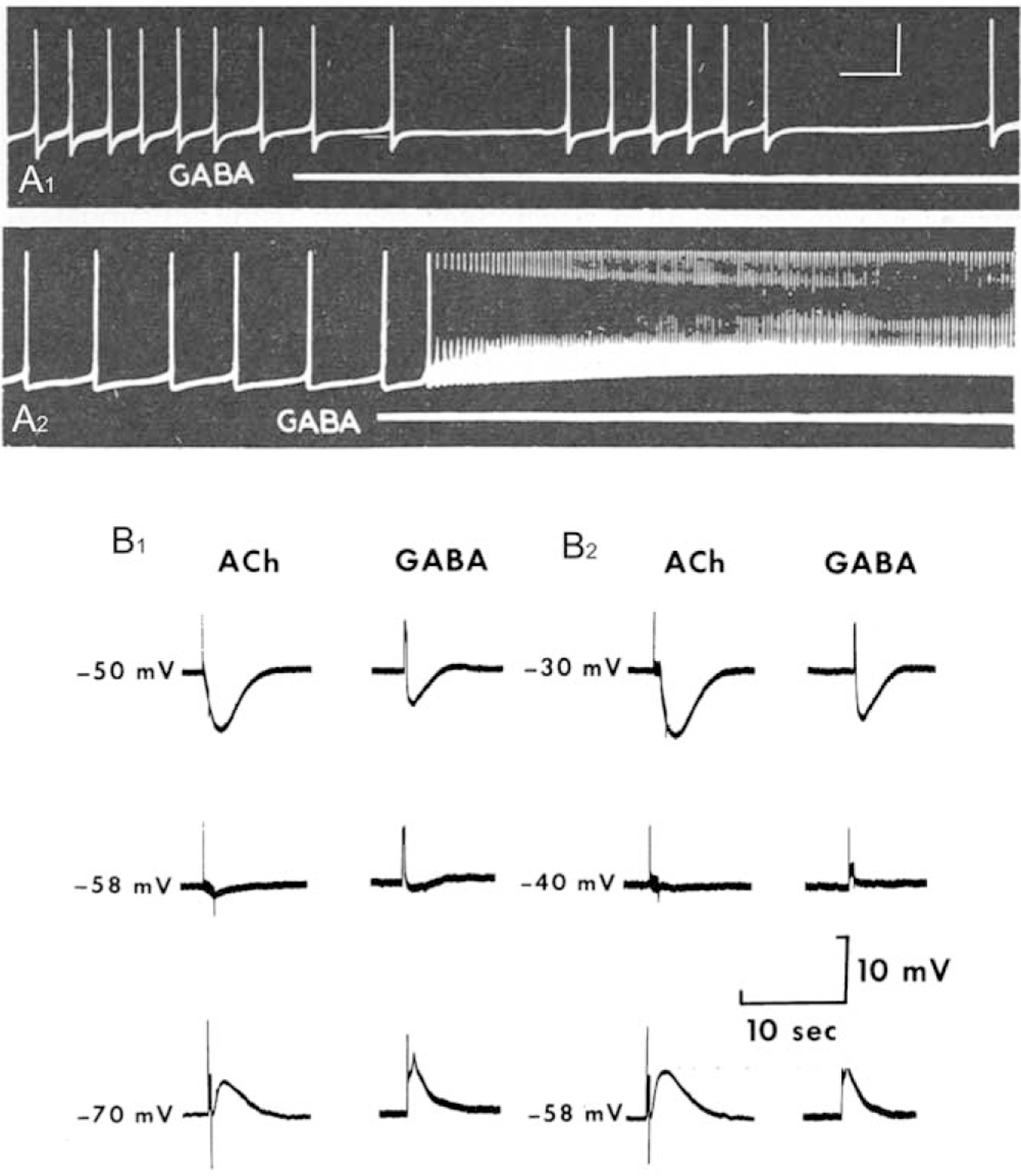
GABA as a neurotransmitter in gastropod molluscs. (A1, A2) Initial demonstration of inhibitory (A1) and excitatory (A2) actions of GABA on gastropod neurons. Perfusion of GABA (1 mmol L−1; white lines under recordings) while recording from neurons in the viscero-abdominal ganglia of Helix aspersa. Calibration bars: 2 s, 25 mV, from another panel in original paper, apply to both (A1) and (A2). Reprinted from Gerschenfeld and Lasansky, 1964. Int. J. Neuropharmacol. 3: 301–314, with permission from Elsevier. (B1, B2) Common properties of acetylcholine and GABA responses on cell R2 of Aplysia. (B1) In control solution, [Cl−] 5 593 mmol L−1, the reversal potential for both ACh and GABA was near 258 mV. (B2) When the external Cl2 concentration was reduced to 296 mmol L−1, the reversal potential for both drugs was shifted to −40 mV. ACh and GABA were delivered from independent iontophoretic pipettes. Reprinted from Yarowsky and Carpenter, 1978. J. Neurophysiol. 41: 531–541, with permission from the American Physiological Society.
Subsequent refinements to microanalytical separation and detection methods revealed low levels of GABA in individual ganglia of Helix pomatia (Osborne et al., 1971; Dolezalova et al., 1973). The presence of GABA in individual neurons was also reported in H. pomatia and Aplysia (Briel et al., 1971; Osborne et al., 1971; Cottrell, 1974). High-performance liquid chromatography enabled quantification of GABA levels in individual ganglia, establishing its differential regional distribution in the central nervous system (CNS) of Helisoma (Richmond et al., 1991). The demonstration of glutamic acid decarboxylase activity in the brain of H. pomatia provided a mechanism for GABA synthesis in the gastropod nervous system (Osborne et al., 1971). In Helisoma trivolvis, synthesis of 3H-GABA from 3H-glutamate was shown to occur predominantly in the buccal, cerebral, and pedal ganglia, consistent with its immunohistochemical localization (Richmond et al., 1991; see Neuronal Localization).
The capacity for GABA uptake was supported by the demonstration of a high-affinity (Km = 52 μmol L−1), sodium-dependent GABA transport mechanism in the CNS of Aplysia dactylomela (Zeman et al., 1975). Autoradiography disclosed GABA uptake and accumulation in the central ganglia of Aplysia (Zeman et al., 1975) and in the pond snails Planorbis corneus (Turner and Cottrell, 1978) and H. trivolvis (Richmond et al., 1991). In Aplysia, GABA accumulation occurred predominantly in glia, suggesting a functional role for glial uptake at GABAergic synapses (Zeman et al., 1975). In Helisoma, GABA uptake occurred in neurons that corresponded to cells labeled with GABA immunohistochemistry (Richmond et al., 1991).
Thus, during the years following Gerschenfeld’s 1973 review, evidence for the presence, synthesis, and uptake of GABA in gastropod nervous systems fulfilled several criteria required for its designation as a neurotransmitter. These foundations paved the way for studies aimed at characterizing GABA receptors and the localization of GABA to individual neurons.
GABA Receptors: Pharmacology and Molecular Biology
Focal delivery with iontophoretic ejection from micropipettes revealed properties of GABA receptors on gastropod neurons. Early investigations showed that GABA could produce both excitatory and inhibitory responses (Gerschenfeld and Lasansky, 1964; Walker et al., 1971, 1975; Takeuchi et al., 1977; Vehovsky et al., 1989). Testing individual identified neurons in Aplysia, Yarowsky and Carpenter (1977, 1978b) established five classes of GABA responses, including (1) a rapid Cl− dependent hyperpolarization, (2) a slower K+ dependent hyperpolarization, (3) a rapid curare sensitive depolarization, (4) a slower curare insensitive depolarization, and (5) a slow depolarization that resulted from a decreased K+ conductance. Several observations suggested that these receptors were utilized by synapses: (1) they were observed on only a subset of neurons; (2) characteristic response profiles were consistently observed on specific identified neurons; and (3) response types were localized to specific regions of reactive neurons. The slow K+ dependent responses were restricted to the neuropil, further supporting their involvement in synaptic signaling (Yarowsky and Carpenter, 1978b).
The rapid Cl− conductance increase elicited by GABA in the Aplysia visceral ganglion was found to share several characteristics with responses elicited by acetylcholine (Yarowsky and Carpenter, 1978a). Both responses reversed at −58 mV, and both exhibited similar permeability profiles (Fig. 1B1, B2). While both responses were blocked by the classical GABA antagonists picrotoxin and bicuculline, they did not cross-desensitize, and only the acetylcholine (ACh) response was blocked by α-bungarotoxin and strychnine. These results were consistent with the prescient hypothesis advanced by Swann and Carpenter (1975, p. 754) that “the ionophores associated with receptors to different neurotransmitters share many common properties, and may, in fact, be identical” (Yarowsky and Carpenter, 1978a; see also King and Carpenter, 1987, 1989).
The emergence of molecular biological and omics approaches confirmed that neurotransmitter receptors do, in fact, belong to large superfamilies that share common structural and functional properties (see Hille, 1989; Walker et al., 1996; Changeux, 2012). Early evidence for conservation of function of ligand-gated receptors emerged from studies of the GABAA receptor β subunit of Lymnaea stagnalis (Harvey et al., 1989, 1991). Co-expression of the Lymnaea β subunit with the bovine GABAA α1 subunit in Xenopus oocytes resulted in functional hetero-oligomeric receptors (Harvey et al., 1991).
More recently, genes encoding GABA receptors and other proteins involved in GABAergic signaling were identified in genomic and transcriptomic data generated from Aplysia californica (Moroz et al., 2006), Lymnaea stagnalis (Feng et al., 2009; Sadamoto et al., 2012), Biomphalaria glabrata (Adema et al., 2017), and Biomphalaria alexandrina (Mansour et al., 2017). Analysis of a Tritonia diomedea CNS transcriptome disclosed a β subunit of the GABAA receptor, a GABAB1 metabotropic receptor, glutamate decarboxylase, a vesicular GABA transporter, and a plasma membrane GABA transporter (Senatore et al., 2015). Genomic and transcriptomic approaches thus firmly established the presence of the molecular machinery for GABAergic synaptic signaling in gastropod nervous systems.
Neuronal Localization
Development of GABA-specific antibodies led to the histological localization of putative GABAergic neurons in several gastropod taxa. GABA-like immunoreactivity (GABAli) was initially localized to small neurons in the buccal, cerebral, and pedal ganglia in the terrestrial slug Limax maximus (Cooke and Gelperin, 1988). Similar distributions of GABAli neurons were observed in other panpulmonates, including the land snails Helix pomatia (Hernádi, 1994) and Helix aspersa (Ierusalimsky and Balaban, 2001) and the aquatic snails Helisoma trivolvis (Richmond et al., 1991) and Biomphalaria glabrata (Vaasjo et al., 2018). In Lymnaea stagnalis, a broader distribution of GABAli neurons was reported, including cells in the right parietal and visceral ganglion (Hatakeyama and Ito, 2000).
As part of a recent examination of GABA-dopamine colocalization in pulmonates (Vaasjo et al., 2018; see Colocalization of GABA and Dopamine), we confirmed the presence of a GABA-like epitope in neurons in the subesophageal ganglia of Lymnaea (Hatakeyama and Ito, 2000; Fig. 2A). One GABAli cell, a giant neuron in the ventromedial visceral ganglion, exhibited rhythmic spiking (Fig. 2B1). The axon of this cell bifurcated within the visceral ganglion and projected to the two pedal ganglia (Fig. 2C1–C3). Because of its size and position near the edge of the ganglion, this cell could usually be visualized from both ventral and dorsal aspects. We tentatively designate it VD1, according to the map of Benjamin and Winlow (1981). The large GABAli cell on the posteromedial edge of the right parietal ganglion (Fig. 2A, B2, D1–D3) is proposed to correspond to RPD2, a cell with electrophysiological properties and neurotransmitter contents in common with VD1 (Fig. 2B2, D1–D3; Soffe and Benjamin, 1980; Wildering et al., 1991; Kerkhoven et al., 1992).
Figure 2.
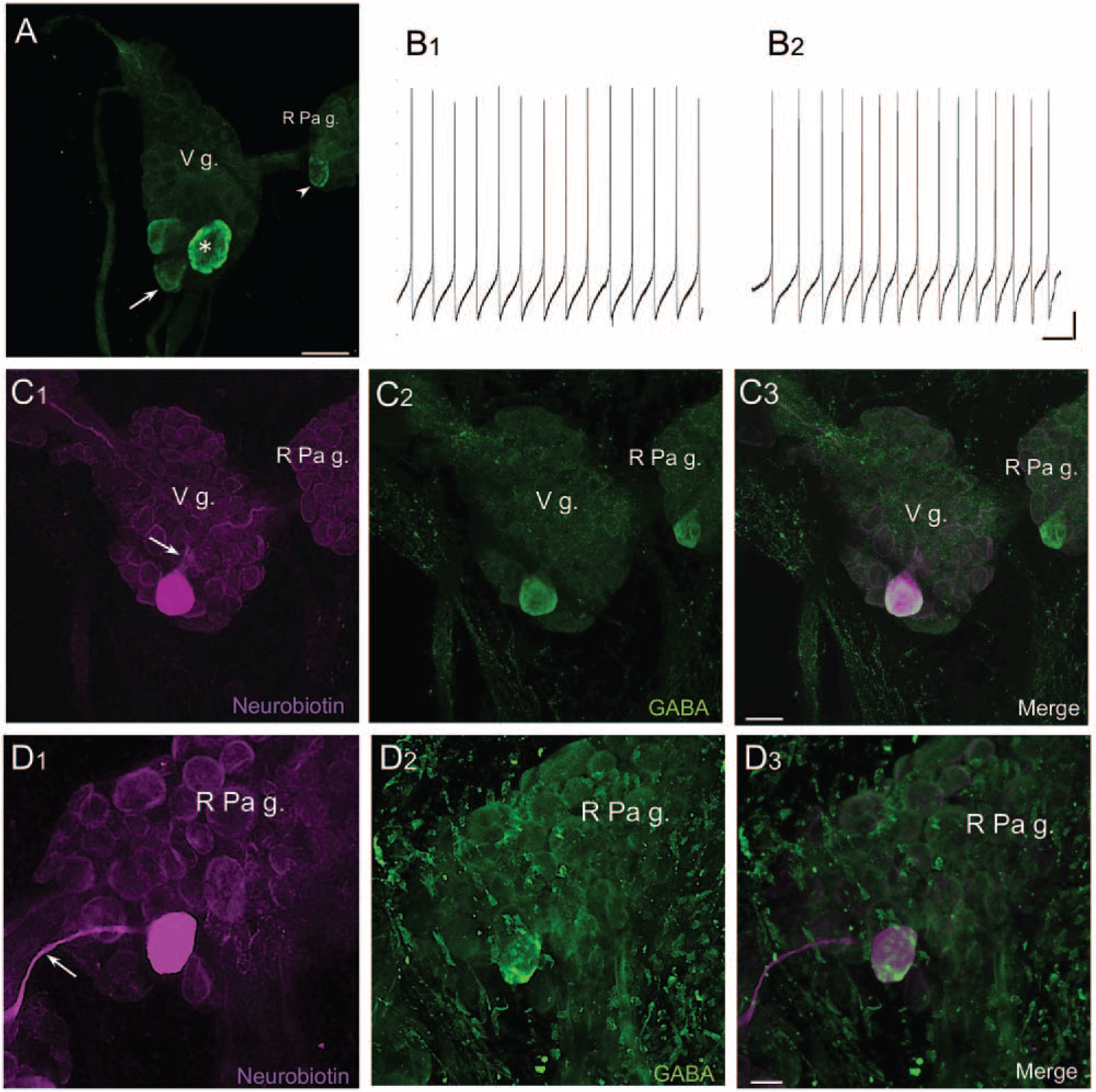
GABA-like immunoreactive (GABAli) neurons in the subesophageal ganglia of Lymnaea stagnalis. (A) GABAli neurons in the visceral ganglion (V g.) and right parietal ganglion (R Pa g.). A single large cell (20–30 μm, arrowhead) was located at the posterior edge of the right parietal ganglion, and a group of three cells (arrow) was located at the posterior pole of the visceral ganglion. One of the visceral ganglion cells was significantly larger (40–50 μm, asterisk). Calibration bar = 50 μm. (B1) Intracellular recording from the large putative VD1 neuron in the visceral ganglion disclosed rhythmic spiking activity. (B2) Repetitive impulses were also recorded from cell RPD2. Calibration bars = 2 s, 10 mV. (C1) Injection of the large visceral GABAli neuron with neurobiotin showed branching of its axon (arrow) and projections toward the right and left parietal ganglia. (C2) Same field of view as (C1), after processing for GABA-like immunoreactivity. (C3) Overlay of (C1) and (C2). Merge of magenta and green appears white. Calibration bar = 50 μm, applies to (C1–C3). (D1) Injection of RPD2 with neurobiotin labeled a projection toward the visceral ganglion (arrow). (D2) Same field of view as (D1) after processing for GABA-like immunoreactivity. (D3) Merge of (D1) and (D2). Calibration bar = 20 μm, applies to (D1–D3).
In each of the panpulmonate species examined, GABAergic fiber systems were largely confined to the CNS (Cooke and Gelperin, 1988; Richmond et al., 1991; Hatakeyama and Ito, 2000). Some exceptions include a projection of GABAli fibers to the lips of H. pomatia (Hernádi, 1994), a fiber system in the aorta of H. aspersa (Ierusalimsky and Balaban, 2001), and a projection to the base of the tentacle in Biomphalaria (Vaasjo et al., 2018). Fibers were located in each of the interganglionic connectives, and major GABAli tracts were present in the commissures connecting the paired buccal, cerebral, and pedal hemiganglia. Collectively, the patterns of GABAli in panpulmonates supported a role of GABAergic signaling in the central regulation and selection of behaviors, as well as in the bilateral coordination of sensorimotor systems (Arshavsky et al., 1993).
General features of the distribution of GABAli in panpulmonates were also observed in other gastropod groups. GABAli neurons were detected in the buccal, cerebral, and pedal ganglia of the marine euopisthobranchs Clione limacina (Arshavsky et al., 1993) and Aplysia californica (Soinila and Mpitsos, 1991; Díaz-Ríos et al., 1999) and in the nudibranchs Tritonia diomedea, Melibe leonina, Dendronotus iris, and Hermissenda crassicornis (Gunaratne et al., 2014; Gunaratne and Katz, 2016; Webber et al., 2017). A detailed comparison of GABAli in the buccal ganglia of nudibranchs revealed a consistent pattern across species with widely varying feeding behaviors (Gunaratne and Katz, 2016). GABAli in a sister Nudipleura species, Pleurobranchea californica (Pleurobranchomorpha), was highly divergent, however, leading to the proposal that it could represent a derived feature related to its specialized cannibalistic feeding behavior (Gunaratne and Katz, 2016).
GABAergic Synaptic Signaling and Regulation of Behavior
Involvement of GABA in the regulation of gastropod feeding was initially suggested by electrophysiological and behavioral studies. Application of GABA to the buccal ganglion of Limax maximus was reported to suppress the intensity of feeding motor programs elicited by stimulation to the lips (Cooke et al., 1985). Injection of GABA into the hemocoel of Clione limacina, however, evoked elements of its complex predatory behavior, including tentacle protraction, mouth opening, and rhythmic movements of the buccal mass (Arshavsky et al., 1991, 1993). In the isolated CNS, GABA activated (1) motor neurons in the cerebral ganglion responsible for protraction of the tentacles, or buccal cones (see also Norekian and Satterlie, 1993); (2) the feeding rhythm generator in the buccal ganglion; and (3) efferent input to statocyst receptor cells. Excitatory actions on the buccal motor network were mimicked by baclofen and were proposed to reflect activation of GABAB-like receptors (Arshavsky et al., 1993; see also Richmond et al., 1994). Collectively, GABA appeared capable of coordinating multiple motor systems required to achieve the highly complex feeding behavior of Clione (Arsharvsky et al., 1993). GABA produced similar organizational effects in the land snail Helix lucorum, where it promoted feeding movements and inhibited the neural circuit controlling an incompatible behavior: defensive withdrawal (Bravarenko et al., 2001).
The prey capture component of Clione feeding provided the first demonstration of GABAergic synaptic signaling by identified gastropod neurons (Norekian, 1999; Fig. 3A1, A2; Table 1). A pair of GABAli neurons (cerebral A interneuron [Cr-Aint], Arshavsky et al., 1993; Norekian and Satterlie, 1993) was shown to drive a prolonged afterdischarge in the network that controls the buccal cone appendages. Each Cr-Aint projects a prominent axon through the cerebral commissure and produces excitation of its contralateral counterpart via electrical coupling and excitatory GABAergic synapses. Each Cr-Aint also produces prolonged self-excitatory GABAergic afterpotentials. The electrical coupling and recurrent excitation between the two Cr-Aint cells, as well as their autaptic self-excitation, were proposed to drive the long-lasting afterdischarge in the cerebral A motor neurons that project to the prey capture appendages (Norekian, 1993, 1999).
Figure 3.
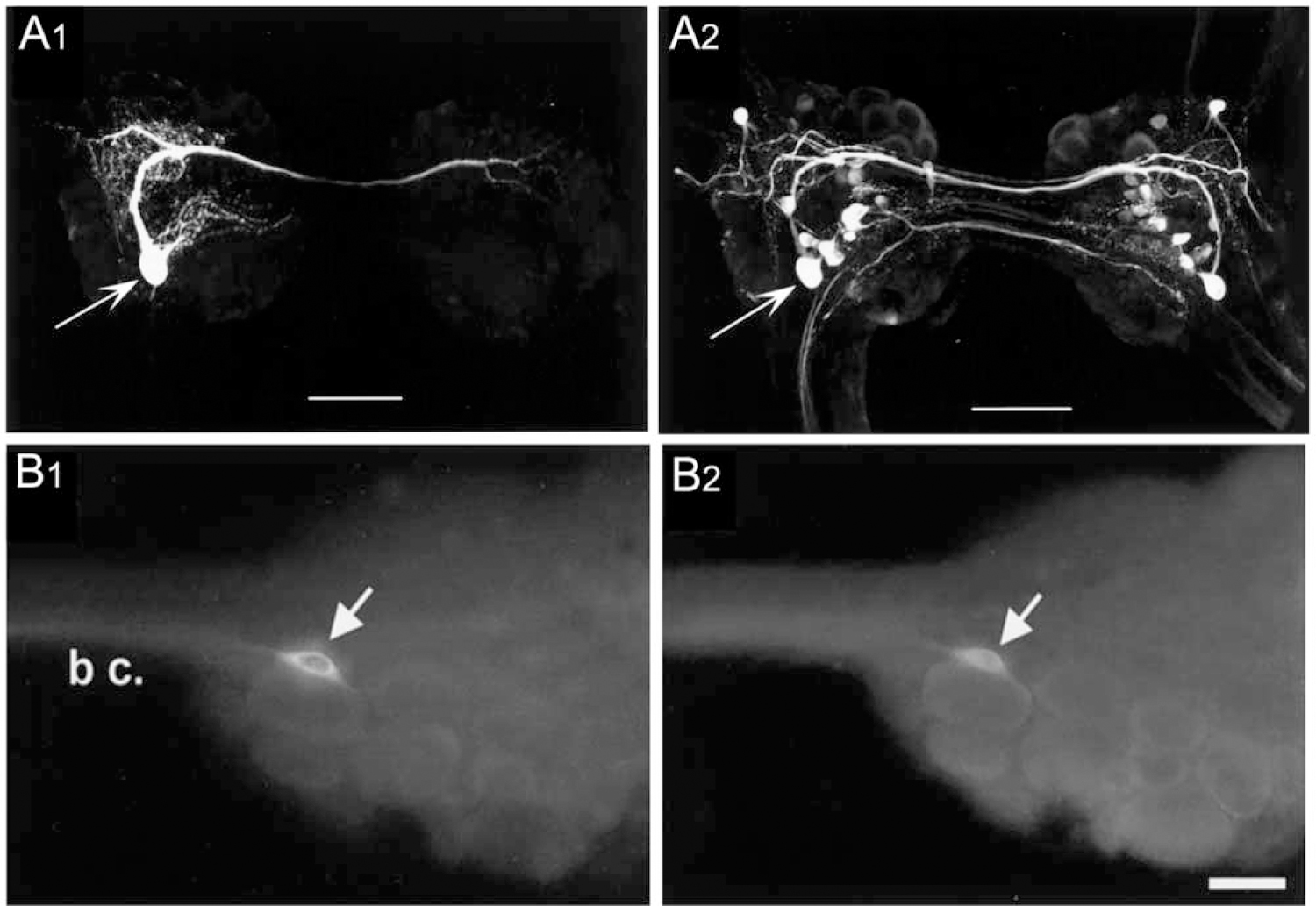
Identified GABAergic neurons. (A1, A2) Identified GABAergic neuron cerebral A interneuron (Cr-Aint) in the prey capture circuit of Clione limacia. (A1) A single CR-Aint (arrow) was filled with neurobiotin and visualized with Texas Red-labeled avidin. (A2) GABA-like immunoreactivity, same cerebral ganglion as (A1). The cell body of Cr-Aint is indicated by the arrow. Scale bars = 200 μm. Reprinted from Norekian, 1999. J. Neurosci. 19: 1863–1875, with permission from the Society for Neuroscience. (B1, B2) Colocalization of THli and GABAli in neuron B20 in the buccal ganglion of Aplysia californica. (B1) THli was observed in one neuron (arrow) on the rostral surface of each buccal hemiganglion (only left hemiganglion shown) near the buccal commissure (b c.). (B2) GABAli was present in the same cell (arrow). Calibration bar = 40 μm, applies to both (B1) and (B2). Reprinted from Díaz-Ríos and Miller, 2002. J. Comp. Neurol. 445: 29–46, with permission from John Wiley.
Table 1.
Identified GABAergic neurons in gastropods
| Species | Neuron | Cotransmitter | Commissural | BCI | CBI | Reference |
|---|---|---|---|---|---|---|
| Clione limacina | ||||||
| Cr-Aint | ✓ | Norekian, 1999 | ||||
| Cr-BM | ✓ | Norekian and Malyshev, 2005 | ||||
| Aplysia californica | ||||||
| B34 | ACha | ✓ | ✓ | Jing and Weiss, 2003 | ||
| B40 | ✓ | ✓ | Jing and Weiss, 2003 | |||
| B20 | Dopamine | ✓ | ✓ | Díaz-Ríos et al., 2002 | ||
| B65 | Dopamine | ✓ | Díaz-Ríos et al., 2002 | |||
| CBI-3 | APGWa | ✓ | Jing and Weiss, 2003 | |||
| CBI-11 | FCAP, ACha | ✓ | Wu et al., 2003, 2014 | |||
| Aplysia kurodai | ||||||
| CBM3 | ✓ | Narusuye et al., 2005 | ||||
| Helisoma trivolvis | ||||||
| N1aa | Dopamine | ✓ | Vaasjo et al., 2018 | |||
| N1ba | Dopamine | ✓ | ✓ | Vaasjo et al., 2018 | ||
| Lymnaea stagnalis | ||||||
| VD1a | VD1/RPD2 peptides | This article | ||||
| RPD2a | VD1/RPD2 peptides | This article |
BCI, buccal-cerebral interneuron; CBI, cerebral-buccal interneuron.
Requires confirmation.
GABAergic neurons are also present in the feeding system of Aplysia, where they play multiple roles in the regulation of motor activity. Two commissural GABAergic interneurons in each buccal hemiganglion, termed B34 and B40, participate in shaping feeding motor programs (Hurwitz et al., 1997; Jing and Weiss, 2001, 2002; Jing et al., 2003; Sasaki et al., 2009). Both B34 and B40 project axons across the buccal commissure and exert their strongest synaptic actions in the contralateral hemiganglion. They also project to the cerebral ganglion via the contralateral cerebral-buccal connective (CBC), but their role as buccal-cerebral interneurons (BCIs) remains largely unexplored. In postsynaptic buccal cells, B34 and B40 produce chloride-mediated rapid inhibitory postsynaptic potentials (IPSPs) that are desensitized by GABA and the GABAA agonist muscimol, blocked by the GABAA antagonists picrotoxin and bicuculline, and augmented by the GABA uptake inhibitor nipecotic acid (Jing et al., 2003).
Rapid IPSPs produced by B34 and B40 influence the multi-functional two-phase (radula protraction/retraction) motor program toward its ingestive conformation by prolonging the protraction phase of the program (Jing et al., 2003). Rapid GABAergic IPSPs elicited by B40 in interneurons and motor neurons also bias motor programs toward ingestion by coordinating closure of the radula with its retraction phase, resulting in an inward displacement of food toward the esophagus (Jing et al., 2003). Slower and more long-lasting excitatory signals from B40 are mediated via GABAB-like receptors on radula closer motor neurons (Dacks and Weiss, 2013). Because these synaptic responses also bias the circuit toward its ingestive conformation, it was concluded that inhibitory and excitatory GABAergic signals originating from a single interneuron can act over multiple timescales to influence motor circuit output in a coherent fashion (Dacks and Weiss, 2013).
In addition to its intraganglionic role in bilateral coordination of feeding in Aplysia, GABAergic signaling also contributes to motor program generation and specification via interganglionic projections from the cerebral ganglion. In Aplysia californica, approximately 13 bilaterally paired cerebral-buccal interneurons (CBIs) project to the buccal ganglion via each CBC (Rosen et al., 1991). Two CBIs, termed CBI-3 and CBI-11, exhibit GABA-like immunoreactivity (Jing et al., 2003; Wu et al., 2003). While CBI-3 and CBI-11 are not highly effective initiators of buccal motor programs, they both can influence these programs toward their ingestive mode (Jing et al., 2003; Wu et al., 2003, 2014).
CBI-3 biases feeding motor patterns toward an ingestive configuration through signaling by at least two colocalized neurotransmitters, the neuropeptide APGWamide and GABA (Morgan et al., 2002; Jing et al., 2003). Rapid IPSPs produced by CBI-3 in the egestive buccal interneuron B20 (see Colocalization of GABA and Dopamine) were blocked by picrotoxin and bicuculline (Jing et al., 2003). CBI-3 also produces rapid excitatory postsynaptic potentials (EPSPs) in B40, resulting in feedforward inhibition of egestive motor patterns. It was hypothesized that the rapid IPSPs and EPSPs produced by CBI-3 in the buccal ganglion are both mediated by GABA (Jing and Weiss, 2003). Further characterization of the CBI-3-elicited EPSPs could test this proposal.
A GABAli CBI, designated CBM3, was identified in Aplysia kurodai and was proposed to correspond to CBI-3 of A. californica (Narusuye et al., 2005). Calcium imaging demonstrated longer-lasting responses in CBM3 when the lips were exposed to a palatable seaweed versus an aversive sea-weed. This differential response to sensory stimuli supports the role of GABAergic CBIs in biasing motor programs toward their ingestive conformation (Narusuye et al., 2005).
Stimulation of GABAli CBI-11 can also bias motor programs toward their ingestive configuration in A. californica (Wu et al., 2003, 2014). Fast picrotoxin-sensitive IPSPs in buccal motor neuron B3 produced by CBI-11 were mimicked by local application of GABA (Wu et al., 2003). GABA produced a picrotoxin-sensitive hyperpolarization of B3 that reversed at the same membrane potential as the IPSPs elicited by stimulation of CBI-11 (Wu et al., 2003). Further studies have emphasized the capacity of CBI-11 to specify buccal motor programs elicited by other CBI program initiators (Wu et al., 2014). Its ability to bias motor programs toward the ingestive conformation was attributed to its co-expression of the feeding circuit activating peptide (FCAP) family of neuropeptides (Wu et al., 2014). As described above for CBI-3, CBI-11 also produced rapid EPSPs in the ingestive GABAergic buccal interneuron B40. These EPSPs were blocked by hexamethonium, leading to the proposal that CBI-11 signaling could be mediated by acetylcholine, in addition to GABA and FCAP (Wu et al., 2014; see Table 1).
A GABAergic CBI identified in Clione limacina, termed Cr-BM, shares several characteristics with CBI-11 of A. californica (Norekian and Malyshev, 2005). Cr-BM promotes ingestive motor programs by coordinating movements of three structures: the buccal cones used for prey capture, the chitinous hooks used to extract the prey from its shell, and the radula. Depolarizing and hyperpolarizing postsynaptic potentials from Cr-BM to buccal motor neurons and interneurons were occluded by GABA and blocked by picrotoxin and bicuculline. It was proposed that Cr-BM is homologous to CBI-11 of Aplysia but that it has assumed control of additional circuits that are unique to the carnivorous feeding behavior of Clione (Norekian and Malyshev, 2005).
Colocalization of GABA and Dopamine
GABA-like immunoreactivity was reported to be colocalized with tyrosine hydroxylase-like immunoreactivity (THli) in five neurons in the buccal ganglion of Aplysia (Díaz-Ríos et al., 2002). Two of the GABAli-THli neurons corresponded to the previously characterized bilateral pair of dopaminergic B20 BCIs (Teyke et al., 1993; Fig. 3B1, B2). Like the GABAergic B40 and B34 interneurons previously described, B20 fires during the initial protraction phase of buccal motor programs. In contrast to B40 and B34, however, B20 produces EPSPs, rather than IPSPs, in the radula closer motor neuron B8. By promoting radula closure during the protraction phase, B20 biases motor programs toward their egestive conformation, pushing inedible material in the outward direction (Jing and Weiss, 2001; Proekt et al., 2004). The EPSPs produced by B20 in B8 were blocked by sulpiride and were occluded by dopamine, indicating that they are mediated by dopamine (Díaz-Ríos and Miller, 2005). These EPSPs were augmented by GABA and baclofen, suggesting that co-released GABA could act as a modulator by activating GABAB receptors (Díaz-Ríos and Miller, 2005; Fig. 4A1, A2). Pharmacological experiments provided further support for this modulatory role of co-released GABA at the B20-to-B8 synapse, and they implicated a postsynaptic site for this action (Svensson et al., 2014). Potentiation of dopamine-induced currents in B8 by GABA and baclofen was blocked by the GABAB antagonist phaclofen, the non-specific G-protein inhibitor GDPbS, and the protein kinase C (PKC) inhibitor chelerythrine (Fig. 4B1, B2). Together, these observations support the proposal that GABAergic potentiation of B20-to-B8 synaptic signals reflects a postsynaptic G-protein-mediated activation of PKC in B8 (Svensson et al., 2014).
Figure 4.
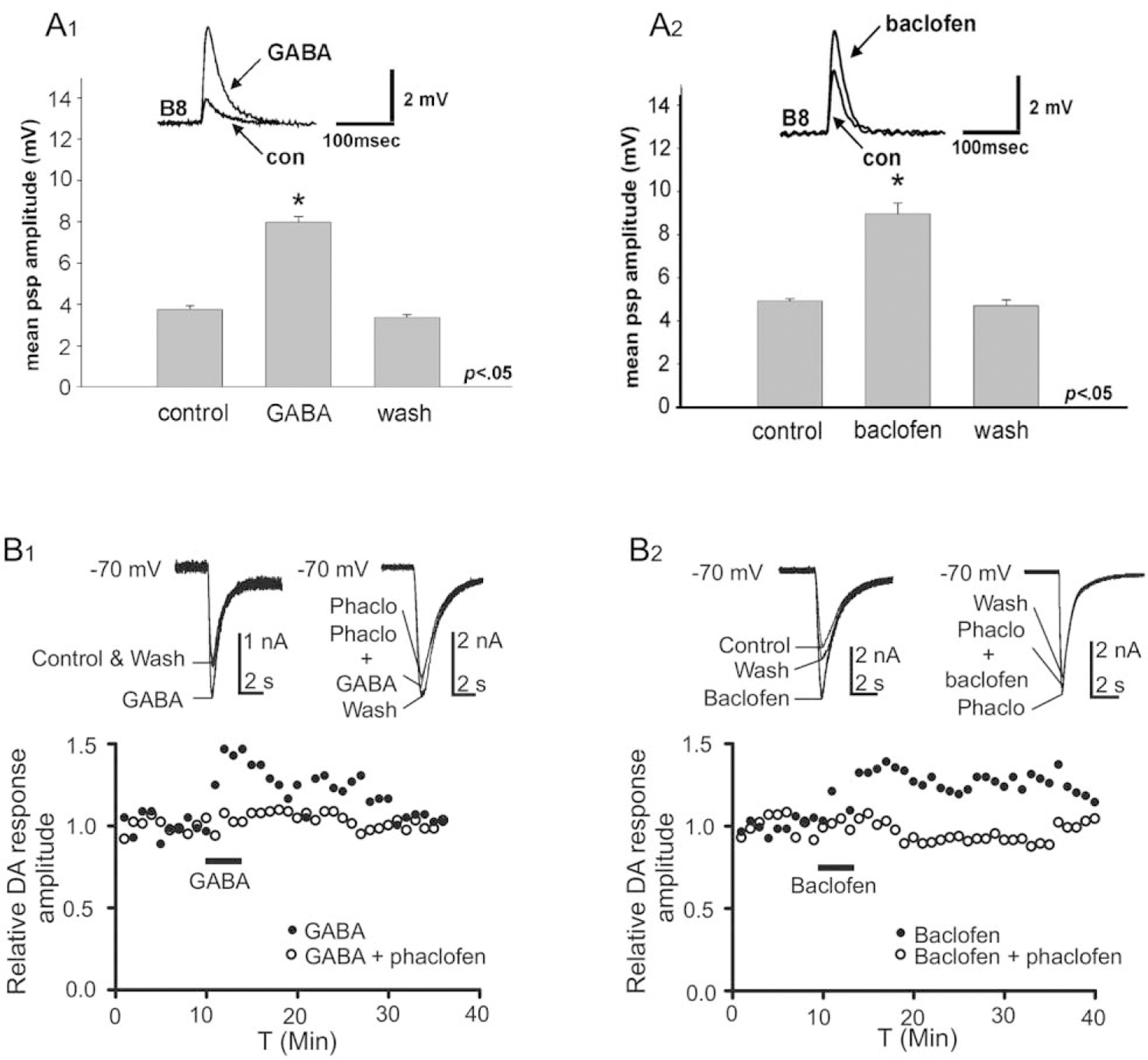
GABA modulates rapid dopaminergic signaling from B20 to the radula closer motor neuron B8. (A1) Bath application of GABA (1 mmol L−1) increased the amplitude of excitatory postsynaptic potentials (EPSPs) produced in B8 by impulses evoked in B20 (con, control). (A2) GABAB agonist baclofen (1 mmol L−1) also augmented the B20-to-B8 EPSP. Reprinted from Díaz-Ríos and Miller, 2005. J. Neurophysiol. 93: 2142–2156, with permission from the American Physiological Society. (B1, B2) GABA and baclofen potentiate dopamine (DA) currents in B8. (B1) Perfusion of GABA (100 μmol L−1) augmented the inward current evoked by dopamine puffed from a micropipette onto the soma of B8. This potentiation was blocked by the GABAB antagonist phaclofen (Phaclo, 100 mmol L−1). (B2) Perfusion of baclofen (100 μmol L−1) also potentiated the inward current evoked by dopamine in B8. The effect of baclofen was also blocked by phaclofen. All experiments were conducted in the presence of tetrodotoxin (10 μmol L−1) to suppress impulses and synaptic activity. Reprinted from Svensson et al., 2014. J. Neurophysiol. 112: 22–29, with permission from the American Physiological Society.
GABAergic modulation of dopaminergic B20-to-B8 signaling was further examined with repetitive stimuli that mimicked the natural form of bursting in motor programs (Díaz-Ríos and Miller, 2006). GABA and baclofen enhanced two forms of intrinsic synaptic plasticity—facilitation and summation—exhibited by the EPSPs evoked in B8 by trains of impulses in B20. These observations led to the proposal that co-released GABA could influence feeding motor programs via “homosynaptic modulatory metaplasticity” (Díaz-Ríos and Miller, 2006, p. 223).
A second pair of GABAli-THli buccal neurons was identified as interneuron B65 (Díaz-Ríos et al., 2002). B65 was characterized previously as a bilaterally paired catecholaminergic neuron that projects an axon across the buccal commissure and exerts its strongest synaptic actions in the contralateral hemiganglion (Kabotyanski et al., 1998). Unlike B34, B40, and B20, B65 does not project to the CBC. Firing B65 is sufficient to evoke fully coordinated motor programs in which it fires during the protraction phase (Kabotyanski et al., 1998). EPSPs produced by B65 in protraction phase interneurons were occluded by dopamine and blocked by sulpiride, indicating that dopamine also acts as the mediator of synaptic signals by this dual-transmitter interneuron (Due et al., 2004). GABA did not mimic or occlude synaptic potentials produced by B65 (Due et al., 2004). GABA and baclofen did reduce the amplitude of EPSPs generated by B65 in the retraction phase interneuron B4/B5, again suggesting its modulatory role in neurons in which it is colocalized with dopamine (Díaz-Ríos and Miller, 2005).
Recently, GABAli-THli colocalization was observed in five neurons in the buccal ganglia of three pulmonate snails: Biomphalaria glabrata, Helisoma trivolvis, and Lymnaea stagnalis (Vaasjo et al., 2018). As in Aplysia, one unpaired GABAli-THli cell was present near the buccal commissure. Interestingly, the unpaired GABAli-THli cell was located in the right buccal hemiganglion in the sinistral snails Biomphalaria and Helisoma and in the left buccal ganglion in the dextral Lymnaea (Vaasjo et al., 2018).
The presence of GABAli-THli neurons in the buccal ganglia of pulmonates indicates that colocalization of the classical neurotransmitters GABA and dopamine in feeding central pattern generator (CPG) interneurons preceded the divergence of euopisthobranch and panpulmonate taxa. It also supports the hypothesis that heterogastropod feeding CPG networks exhibit a common universal plan (Murphy, 2001; Wentzell et al., 2009). Two Helisoma catecholaminergic protraction phase interneurons, termed N1a and N1b, were proposed to be homologous to the B65 and B20 interneurons of Aplysia (Murphy, 2001). These homologies (N1a∶B65 and N1b∶ B20) were based on cell location, morphology, synaptic connections, CPG function, and dopaminergic phenotype. While the localization of GABAli-THli neurons in the Helisoma buccal ganglion appears to add the GABA-dopamine phenotype to the shared properties of these cells, electrophysiological and dye fill confirmation will be required for unequivocal verification (Table 1).
Conclusions and Future Directions
This overview spans more than 50 years of investigation leading to our present understanding of GABA as a neurotransmitter in gastropods. The protracted advance of this inquiry may be contrasted with the earlier characterization of GABAergic neurotransmission in crustaceans and insects, where GABA serves a major role in neuromuscular signaling (Usherwood and Grundfest, 1964; Otsuka et al., 1967; Sattelle, 1990; see Edwards et al., 1999). In the nematode Caenorhabditis elegans, where GABA also functions as a neuromuscular transmitter, genetic analyses have produced a deep understanding of inhibitory and excitatory GABAergic signaling (Jorgensen, 2005). Several properties of the GABAergic phenotype of gastropods, however, including its presence in influential interneurons that are embedded in premotor circuits, are shared by the GABAergic nervous systems of vertebrates. Emerging findings are also disclosing diverse cotransmitter profiles for GABAergic neurons in the brains of mammals, including GABA’s colocalization with dopamine in neurons of the olfactory bulb, retina, ventral tegmental area, and substantia nigra (Maher and Westbrook, 2008; Hirasawa et al., 2012; Tritsch et al., 2012, 2014, 2016). The identified GABAergic and GABA-DA interneurons of gastropods should inform further investigation of these neuronal phenotypes in more complex brains.
This survey underscores the versatility of GABA as a neurotransmitter in gastropod molluscs. Although it is present in a limited number of neurons, GABAergic signaling can decisively influence circuits via inhibitory and excitatory synaptic actions that span a broad temporal range. The diversity of GABAergic signaling observed in gastropods may be compared with other taxa. While the GABAA receptors of mammals are selectively permeable to anions (Enna and Bowery, 2013), the rapid excitatory GABAergic postsynaptic potentials observed in gastropods appear to reflect the presence of cation-permeable channels, as observed in nematodes (Beg and Jorgensen, 2003; Jorgensen, 2005). GABAB receptors, which are also predominantly inhibitory in vertebrate nervous systems (Pinard et al., 2010; Enna and Bowery, 2013), are proposed to underlie excitatory modulation in the feeding networks of Clione (Arshavsky et al., 1993), Helisoma (Richmond et al., 1994), and Aplysia (Díaz-Ríos and Miller, 2005; Svensson et al., 2014). Interestingly, GABAB receptors mediate an increase of excitatory transmitter release at a crustacean neuromuscular junction (Gutovitz et al., 2001), suggesting that excitatory GABAB modulation may reflect both pre- and postsynaptic actions in invertebrates (see also Swensen et al., 2000). Finally, the increasing recognition of GABA as a cotransmitter in the mammalian CNS should also stimulate investigation into the generality of GABAB-mediated homosynaptic modulatory metaplasticity (Díaz-Ríos and Miller, 2006).
Although GABA is not present in gastropod motor neurons, it can specify both qualitative and quantitative features of motor programs (Richmond et al., 1994; Moccia et al., 2009; Dacks and Weiss, 2013). Likewise, despite its limited role as a sensory neurotransmitter, GABA can regulate transmission of sensory information (see Alkon et al., 1993; Arshavsky et al., 1993; Jin et al., 2009). The versatility of GABAergic signaling in gastropods is further demonstrated by its association with diverse cotransmitters. In the Aplysia feeding system, it can partner with neuropeptides, for example, APGWamide in CBI-3 and FCAP in CBI-11, or with other small molecule neurotransmitters, for example, dopamine in B20 and B65 and acetylcholine in CBI-11 and B34 (Hurwitz et al., 2003; see Table 1). Further study is likely to disclose additional cotransmitters in GABAergic neurons (see Díaz-Ríos and Miller, 2005; Wu et al., 2014).
The localization of GABA-like immunoreactive neurons enabled early investigators to deduce several attributes of GABA’s function (Cooke and Gelperin, 1988; Richmond et al., 1991; Arshavsky et al., 1993). GABA’s presence in the paired buccal, cerebral, and pedal ganglia suggested its involvement in coordinating bilateral networks. The subsequent identification of GABAergic commissural neurons with predominant contralateral synaptic actions substantiated this proposal (Norekian, 1999; Jing and Weiss, 2003; Díaz-Ríos and Miller, 2005). The presence of GABAli fibers in the connectives between ganglia also suggested its involvement in the control and integration of motor systems, another premise that was confirmed by behavioral studies (Arshavsky et al., 1993; Bravarenko et al., 2001) and with the identification of GABAergic CBIs and BCIs in the Aplysia and Clione feeding systems (Jing et al., 2003; Wu et al., 2003; Norekian and Malyshev, 2005).
The present state of knowledge concerning GABA as a neurotransmitter in gastropods suggests several functional properties that may be relevant to more complex nervous systems. First, its participation in bilateral motor systems ensures that the two sides of the organism perform actions in synchrony. In vertebrates, such coordination may be relevant to certain motor systems, such as breathing and chewing, where the two sides act in unison, and not to others, such as walking and swimming, where movements on the two sides alternate. Second, the GABAergic systems of gastropods participate in circuits that can transform temporal input-out relations. Whether through bilateral reverberating excitatory afterdischarges, as in the prey capture of Clione, or triggering the multi-phasic feeding motor programs of Aplysia, GABAergic signaling can generate prolonged responses to brief stimuli. Finally, the anatomical features of the gastropod GABAergic systems suggest a role in efference copy or read-out of motor activity to higher centers. Bilateral coordination, temporal transformation, and efference copy are tasks of information processing that must be met by all complex nervous systems. The participation of GABAergic signaling in meeting these demands in the simpler gastropod nervous systems should guide investigation of this major neurotransmitter system in the vertebrate brain. This knowledge could also increase our understanding of the pathologies that can occur when this system is compromised.
Acknowledgments
MWM’s research is supported by the National Institutes of Health: RCMI MD007600, MBRS GM087200, COBRE P20GM103642; the National Science Foundation: DBI-1337284, HRD-1137725, OISE 1545803; the National Academy of Sciences (NAS): U.S.-Egypt Science and Technology (S&T) Joint Fund 2000007152; and the Science and Technology Development Fund (Egypt): USC17-188. This article is derived from the Subject Data funded in whole or part by NAS and the U.S. Agency for International Development (USAID). Any opinions, findings, conclusions, or recommendations expressed are those of the authors alone and do not necessarily reflect the views of USAID or NAS. Mariela Rosa Casillas and Paola Méndez de Jesus contributed to the experimental results (Fig. 2) included in this article.
Abbreviations:
- BCI
buccal-cerebral interneuron
- CBC
cerebral-buccal connective
- CBI
cerebral-buccal interneuron
- CNS
central nervous system
- CPG
central pattern generator
- Cr-Aint
cerebral A interneuron
- DA
dopamine
- EPSP
excitatory postsynaptic potential
- FCAP
feeding circuit activating peptide
- GABA
gamma-aminobutyric acid
- GABAli
GABA-like immunoreactivity
- IPSP
inhibitory postsynaptic potential
- PKC
protein kinase C
Literature Cited
- Adema CM, Hillier LW, Jones CS, Loker ES, Knight M, Minx P, Oliveira G, Raghavan N, Shedlock A, Rodrigues do Amaral L et al. 2017. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun 8: 15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL, Anderson MJ, Kuzirian AJ, Rogers DF, Fass DM, Collin C, Nelson TJ, Kapetanovic IM, and Matzel LD 1993. GABA-mediated synaptic interaction between the visual and vestibular pathways of Hermissenda. J. Neurochem 61: 556–566. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Deliagina TG, Gamkrelidze GN, Orlovsky GN, Panchin YV, Popova LB, and Shupliakov OV 1993. Pharmacologically induced elements of the hunting and feeding behavior in the pteropod mollusk Clione limacina. I. Effects of GABA. J. Neurophysiol 69: 512–521. [DOI] [PubMed] [Google Scholar]
- Arshavsky YL, Gamkrelidze GN, Orlovsky GN, Panchin YV, and Popova LB 1991. Gamma-aminobutyric acid induces feeding behaviour in the marine mollusc, Clione limacina. NeuroReport 2: 169–172. [DOI] [PubMed] [Google Scholar]
- Beg AA, and Jorgensen EM 2003. EXP-1 is an excitatory GABA-gated cation channel. Nat. Neurosci 6: 1145–1152. [DOI] [PubMed] [Google Scholar]
- Benjamin PR, and Winlow W 1981. The distribution of three wide-acting synaptic inputs to identified neurons in the isolated brain of Lymnaea stagnalis (L.). Comp. Biochem. Physiol. A Physiol 70: 293–307. [Google Scholar]
- Benjamin PR, Kemenes G, and Kemenes I 2008. Non-synaptic neuronal mechanisms of learning and memory in gastropod molluscs. Front. Biosci 13: 4051–4057. [DOI] [PubMed] [Google Scholar]
- Bradford HF, Chain EB, Cory HT, and Rose SPR 1969. Glucose and amino acid metabolism in some invertebrate nervous systems. J. Neurochem 16: 969–978. [DOI] [PubMed] [Google Scholar]
- Bravarenko N, Ierusalimsky VN, Korshunova TA, Malyshev AY, Zakharov IS, and Balaban PM 2001. Participation of GABA in establishing behavioral hierarchies in the terrestrial snail. Exp. Brain Res 141: 340–348. [DOI] [PubMed] [Google Scholar]
- Briel G, Neuhoff V, and Osborne NN 1971. Determination of amino acids in single identifiable nerve cells of Helix pomatia. Int. J. Neurosci 2: 129–136. [DOI] [PubMed] [Google Scholar]
- Changeux J-P 2012. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem 287: 40207–40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase R 2002. Behavior and Its Neural Control in Gastropod Molluscs Oxford University Press, New York. [Google Scholar]
- Church PJ, and Lloyd PE 1991. Expression of diverse neuropeptide cotransmitters by identified motor neurons in Aplysia. J. Neurosci 11: 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H 1983. 3H-GABA uptake selectively labels identifiable neurons in the leech central nervous system. J. Comp. Neurol 215: 351–358. [DOI] [PubMed] [Google Scholar]
- Cline H 1986. Evidence for GABA as a neurotransmitter in the leech. J. Neurosci 6: 2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, and Kaczmarek LK 1989. The bag cell neurons of Aplysia: a model for the study of the molecular mechanisms involved in the control of prolonged animal behaviors. Mol. Neurobiol 3: 237–273. [DOI] [PubMed] [Google Scholar]
- Cooke IRC, and Gelperin A 1988. Distribution of GABA-like immunoreactive neurons in the slug Limax maximus. Cell Tissue Res 253: 77–81. [DOI] [PubMed] [Google Scholar]
- Cooke IRC, Delaney K, and Gelperin A 1985. Complex computation in a small neural network. Pp. 173–191 in Memory Systems of the Brain, Weinberger NM, McGaugh JL, and Lynch G, eds. Guilford Press, New York. [Google Scholar]
- Cottrell GA 1974. Serotonin and free amino acid analysis of ganglia and isolated neurons of Aplysia dactylomela. J. Neurochem 22: 557–559. [DOI] [PubMed] [Google Scholar]
- Crow TJ, and Alkon DL 1980. Associative behavioral modification in Hermissenda: cellular correlates. Science 209: 412–414. [DOI] [PubMed] [Google Scholar]
- Dacks AM, and Weiss KR 2013. Release of a single neurotransmitter from an identified interneuron coherently affects motor output on multiple time scales. J. Neurophysiol 109: 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WJ, and Gillette R 1978. Neural correlate of behavioral plasticity in command neurons of Pleurobranchaea. Science 199: 801–804. [DOI] [PubMed] [Google Scholar]
- del Castillo J, de Mello WC, and Morales T 1964. Inhibitory action of γ-aminobutyric acid (GABA) on Ascaris muscle. Experientia 20: 141–145. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, and Miller MW 2005. Rapid dopaminergic signaling by interneurons that contain markers for catecholamines and GABA in the feeding circuitry of Aplysia. J. Neurophysiol 93: 2142–2156. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, and Miller MW 2006. Target-specific regulation of synaptic efficacy in the feeding central pattern generator of Aplysia: potential substrates for behavioral plasticity? Biol. Bull 210: 215–229. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Suess E, and Miller MW 1999. Localization of GABA-like immunoreactivity in the central nervous system of Aplysia californica. J. Comp. Neurol 413: 255–270. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Oyola E, and Miller MW 2002. Colocalization of γ-aminobutyric acid-like immunoreactivity and catecholamines in the feeding network of Aplysia californica. J. Comp. Neurol 445: 29–46. [DOI] [PubMed] [Google Scholar]
- Dolezalova H, Giacobini E, and Stepita-Klauco M 1973. An attempt to identify putative neurotransmitter molecules in the central nervous system of the snail. Int. J. Neurosci 5: 53–59. [DOI] [PubMed] [Google Scholar]
- Due MR, Jing J, and Weiss KR 2004. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci. Lett 358: 53–57. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, and Krasne FB 1999. Crustacean studies and the early history of GABA. Trends Neurosci 22: 347. [DOI] [PubMed] [Google Scholar]
- Elliott GRD, and Leys SP 2010. Evidence for glutamate, GABA and NO in coordinating behaviour in the sponge, Ephydatia muelleri (Demospongiae, Spongillidae). J. Exp. Biol 213: 2310–2321. [DOI] [PubMed] [Google Scholar]
- Ellwanger K, Eich A, and Nickel M 2007. GABA and glutamate specifically induce contractions in the sponge Tethya wilhelma. J. Comp. Physiol. A. Sens. Neural Behav. Physiol 193: 1–11. [DOI] [PubMed] [Google Scholar]
- Enna SJ, and Bowery N 2013. The GABA Receptors Humana Press, New York. [Google Scholar]
- Eriksson KS, and Panula P 1994. Gamma-aminobutyric acid in the nervous system of a planarian. J. Comp. Neurol 345: 528–536. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Zhang Z, van Kesteren RE, Straub VA, van Nierop P, Jin K, Nejatbakhsh N, Goldberg JI, Spencer GE, Yeoman MS et al. 2009. Transcriptome analysis of the central nervous system of the mollusc Lymnaea stagnalis. BMC Genomics 10: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey, E., ed. 1961. Nervous Inhibition Pergamon Press, New York. [Google Scholar]
- Gerschenfeld HM 1973. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol. Rev 53: 11–19. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, and Lasansky A 1964. Action of glutamic acid and other naturally occurring amino acids on snail neurons. Int. J. Neuropharmacol 3: 301–314. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, and Tauc L 1961. Pharmacological specificities of neurons in an elementary nervous system. Nature 189: 924–925. [DOI] [PubMed] [Google Scholar]
- Getting PA, and Dekin MS 1985. Mechanisms of pattern generation underlying swimming in Tritonia. IV. Gating of central pattern generator. J. Neurophysiol 53: 466–480. [DOI] [PubMed] [Google Scholar]
- Gunaratne CA, and Katz PS 2016. Comparative mapping of GABA-immunoreactive neurons in the buccal ganglia of Nudipleura molluscs. J. Comp. Neurol 524: 1181–1192. [DOI] [PubMed] [Google Scholar]
- Gunaratne CA, Sakurai A, and Katz PS 2014. Comparative mapping of GABA-immunoreactive neurons in the central nervous systems of nudibranch molluscs. J. Comp. Neurol 522: 794–810. [DOI] [PubMed] [Google Scholar]
- Gutovitz S, Birmingham JT, Luther JA, Simon DJ, and Marder E 2001. GABA enhances transmission at an excitatory glutamatergic synapse. J. Neurosci 29: 5935–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Vreugdenhil E, Barnard EA, and Darlison MG 1989. Cloning of genomic cDNA sequences encoding an invertebrate γ-aminobutyric acidA receptor subunit. Biochem. Soc. Trans 18: 1990–1991. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Vreugdenhil E, Zaman SH, Bhandal NS, Usherwood PNR, Barnard EA, and Darlison MG 1991. Sequence of a functional invertebrate GABAA receptor subunit which can form a chimeric receptor with a vertebrate a subunit. EMBO J 10: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama D, and Ito E 2000. Distribution and developmental changes in GABA-like immunoreactive neurons in the central nervous system of the pond snail, Lymnaea stagnalis. J. Comp. Neurol 418: 310–322. [PubMed] [Google Scholar]
- Hernádi L 1994. Distribution and anatomy of GABA-like immunoreactive neurons in the central and peripheral nervous system of the snail Helix pomatia. Cell Tissue Res 277: 189–198. [DOI] [PubMed] [Google Scholar]
- Hille B 1989. The Sharpey-Schafer Lecture. Ionic channels: evolutionary origins and modern roles. Q. J. Exp. Physiol 74: 785–804. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Betensky RA, and Raviola E 2012. Corelease of dopamine and GABA by a retinal dopaminergic neuron. J. Neurosci 33: 1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz I, Kupfermann I, and Susswein AJ 1997. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J. Neurophysiol 78: 1305–1319. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Kupfermann I, and Weiss KR 2003. Fast synaptic connections from CBIs to pattern-generating neurons in Aplysia: initiation and modification of motor programs. J. Neurophysiol 89: 2120–2136. [DOI] [PubMed] [Google Scholar]
- Ierusalimsky VN, and Balaban PM 2001. Ontogenesis of the snail, Helix aspersa: embryogenesis timetable and ontogenesis of GABA-like immunoreactive neurons in the central nervous system. J. Neurocytol 30: 73–91. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kuriyama H, and Tashiro N 1969. Effects of γ-aminobutyric acid and picrotoxin on the permeability of the longitudinal somatic muscle of the earthworm to various anions. J. Exp. Biol 51: 363–375. [DOI] [PubMed] [Google Scholar]
- Jin NG, Tian L-M, and Crow T 2009. 5-HT and GABA modulate intrinsic excitability of type I interneurons in Hermissenda. J. Neurophysiol 102: 2825–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, and Weiss KR 2001. Neural mechanisms of motor program switching in Aplysia. J. Neurosci 21: 7349–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, and Weiss KR 2002. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J. Neurosci 22: 6228–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Wu J-S, Park J-H, and Weiss KR 2003. Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J. Neurosci 23: 5283–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, and Stretton AO 1987. GABA-immunoreactivity in inhibitory motor neurons of the nematode Ascaris. J. Neurosci 7: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Adams HP, and Fatemi A 2016. Neurobiology of Disease Oxford University Press, New York. [Google Scholar]
- Jorgensen EM 2005. GABA. In WormBook, The C. elegans Research Community, ed. [Online]. Available: http://www.wormbook.org/chapters/www_gaba/gaba.html [2018, November 27].
- Kabotyanski EA, Baxter DA, and Byrne JH 1998. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J. Neurophysiol 79: 605–621. [DOI] [PubMed] [Google Scholar]
- Kandel ER 1970. Nerve cells and behavior. Sci. Am 223: 57–67. [DOI] [PubMed] [Google Scholar]
- Kandel ER 1976. Cellular Basis of Behavior W. H. Freeman, San Francisco. [Google Scholar]
- Kandel ER 1979. Behavioral Biology of Aplysia: A Contribution to the Comparative Study of Opisthobranch Molluscs W. H. Freeman, San Francisco. [Google Scholar]
- Kandel ER 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038. [DOI] [PubMed] [Google Scholar]
- Katz PS 1995. Intrinsic and extrinsic neuromodulation of motor circuits. Curr. Opin. Neurobiol 5: 799–808. [DOI] [PubMed] [Google Scholar]
- Kerkhoven RM, Croll RP, Ramkema MD, Van Minnen J, Bogerd J, and Boer HH 1992. The VD1/RPD2 neuronal system in the central nervous system of the pond snail, Lymnaea stagnalis studied by in situ hybridization and immunocytochemistry. Cell Tissue Res 267: 551–559. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, and Cottrell GA 1962. Amino acids in the blood and nervous system of Helix aspersa. Comp. Biochem. Physiol 5: 227–230. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, and Walker RJ 1961. The effect of drugs on the neurones of the snail Helix aspersa. Comp. Biochem. Physiol 3: 143–160. [DOI] [PubMed] [Google Scholar]
- King WM, and Carpenter DO 1987. Distinct GABA and glutamate receptors may share a common channel in Aplysia neurons. Neurosci. Lett 82: 343–348. [DOI] [PubMed] [Google Scholar]
- King WM, and Carpenter DO 1989. Voltage-clamp characterization of Cl2 conductance gated by GABA and L-glutamate in single neurons of Aplysia. J. Neurophysiol 61: 892–899. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Kuffler SW, and Potter DD 1963. Gamma-aminobutyric acid and other blocking compounds in Crustacea. III. Their relative concentrations in separated motor and inhibitory axons. J. Neurophysiol 26: 739–751. [DOI] [PubMed] [Google Scholar]
- Krnjević K 1970. Glutamate and gamma-aminobutyric acid in the brain. Nature 228: 119–124. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, and Edwards C 1958. Mechanism of gamma-aminobutyric acid (GABA) and its relation to synaptic inhibition. J. Neurophysiol 21: 589–610. [DOI] [PubMed] [Google Scholar]
- Kupfermann I 1970. Stimulation of egg laying by extracts of neuroendocrine cells (bag cells) of abdominal ganglion of Aplysia. J. Neurophysiol 33: 877–881. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, and Weiss KR 1978. The command neuron concept. Behav. Brain Sci 1: 3–39. [Google Scholar]
- Maher BJ, and Westbrook GL 2008. Co-transmission of dopamine and GABA in periglomerular cells. J. Neurophysiol 99: 1559–1564. [DOI] [PubMed] [Google Scholar]
- Mansour TA, Habib MR, Rodríguez LCV, Vázquez AH, Alers JM, Ghezzi A, Croll RP, Brown CT, and Miller MW 2017. Central nervous system transcriptome of Biomphalaria alexandrina, an intermediate host for schistosomiasis. BMC Res. Notes 10: 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Kaplan J, and Horvitz HR 1993. The GABAergic nervous system of Caenorhabditis elegans. Nature 364: 337–341. [DOI] [PubMed] [Google Scholar]
- Moccia F, Di Cristo C, Winlow W, and Di Cosmo A 2009. GABAA- and AMPA-like receptors modulate the activity of an identified neuron within the central pattern generator of the pond snail Lymnaea stagnalis. Invertebr. Neurosci 9: 29–41. [DOI] [PubMed] [Google Scholar]
- Möhler H 2013. Pharmacology of GABA and Glycine Neurotransmission Springer, New York. [Google Scholar]
- Morgan PT, Jing J, Vilim FS, and Weiss KR 2002. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J. Neurophysiol 87: 49–61. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L et al. 2006. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell 127: 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AD 2001. The neuronal basis of feeding in the snail, Helisoma, with comparisons to selected gastropods. Prog. Neurobiol 63: 383–408. [DOI] [PubMed] [Google Scholar]
- Narusuye K, Kinugawa A, and Nagahama T 2005. Responses of cerebral GABA-containing CBM neuron to taste stimulation with seaweed extracts in Aplysia kurodai. J. Neurobiol 65: 146–156. [DOI] [PubMed] [Google Scholar]
- Newman SJ, and Thorndyke MC 1994. Localization of gamma aminobutyric acid (GABA)-like immunoreactivity in the echinoderm Asterias rubens. Cell Tissue Res 278: 177–185. [DOI] [PubMed] [Google Scholar]
- Nickel M 2010. Evolutionary emergence of synaptic nervous systems: What can we learn from the non-synaptic, nerveless Porifera? Invertebr. Biol 129: 1–16. [Google Scholar]
- Norekian TP 1993. Cerebral neurons underlying prey capture movements in the pteropod mollusc, Clione limacina. II. Afterdischarges. J. Comp. Physiol. A Sens. Neural Behav. Physiol 172: 171–181. [DOI] [PubMed] [Google Scholar]
- Norekian TP 1999. GABAergic excitatory synapses and electrical coupling sustain prolonged discharges in the prey capture neural network of Clione limacina. J. Neurosci 19: 1863–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norekian TP, and Malyshev AY 2005. Coordinated excitatory effect of GABAergic interneurons on three feeding motor programs in the mollusk Clione limacina. J. Neurophysiol 93: 305–315. [DOI] [PubMed] [Google Scholar]
- Norekian TP, and Satterlie RA 1993. FMRFamide and GABA produce functionally opposite effects on prey-capture reactions in the pteropod mollusk Clione limacina. Biol. Bull 185: 248–262. [DOI] [PubMed] [Google Scholar]
- Ono JK, and McCaman RE 1980. Identification of additional histaminergic neurons in Aplysia: improvement of single cell isolation techniques for in tandem physiological and chemical studies. Neuroscience 5: 835–840. [DOI] [PubMed] [Google Scholar]
- Ono JK, and McCaman RE 1984. Immunocytochemical localization and direct assays of serotonin-containing neurons in Aplysia. Neuroscience 11: 549–560. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Briel G, and Neuhoff V 1971. Distribution of GABA and other amino acids in different tissues of the gastropod mollusc Helix pomatia, including in vitro experiments with 14C-glucose and 14C-glutamic acid. Int. J. Neurosci 1: 265–272. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Kravitz EA, and Potter DD 1967. Physiological and chemical architecture of a lobster ganglion with particular reference to gamma-aminobutyrate and glutamate. J. Neurophysiol 30: 725–752. [DOI] [PubMed] [Google Scholar]
- Pentreath VW, Berry MS, and Cottrell GA 1974. Anatomy of the giant dopamine-containing neuron in the left pedal ganglion of Planorbis corneus. Cell Tissue Res 161: 369–384. [DOI] [PubMed] [Google Scholar]
- Pinard A, Seddik R, and Bettler B 2010. GABAB receptors: physiological functions and mechanisms of diversity. Pp. 231–256 in GABAB Receptor Pharmacology: A Tribute to Norman Bowery, Blackburn TP and Enna SJ, eds. Academic Press, Amsterdam. [Google Scholar]
- Proekt A, Brezina V, and Weiss KR 2004. Dynamic basis of intentions and expectations in a simple neuronal network. Proc. Natl. Acad. Sci. U.S.A 101: 9447–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Bulloch AGM, Bauce L, and Lukowiak K 1991. Evidence for the presence, synthesis, immunoreactivity, and uptake of GABA in the nervous system of the snail Helisoma trivolvis. J. Comp. Neurol 307: 131–143. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Murphy AD, Lukowiak K, and Bulloch AGM 1994. GABA regulates the buccal motor output of Helisoma by two pharmacologically distinct actions. J. Comp. Physiol. A Sens. Neural Behav. Physiol 174: 593–600. [Google Scholar]
- Roberts E, ed. 1960. Inhibition in the Nervous System and Gamma-Aminobutyric Acid Pergamon Press, New York. [Google Scholar]
- Roberts E 1986a. GABA: the road to neurotransmitter status. Pp. 1–39 in Benzodiazepine/GABA Receptors and Chloride Channels: Structural and Functional Properties, Olsen RW and Venter JC, eds. Alan R. Liss, New York. [Google Scholar]
- Roberts E 1986b. What do GABA neurons really do? They make possible variability generation in relation to demand. Exp. Neurol 93: 279–290. [DOI] [PubMed] [Google Scholar]
- Rosen SC, Teyke T, Miller MW, Weiss KR, and Kupfermann I 1991. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J. Neurosci 11: 3630–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubos EW 1976. Neuronal and non-neuronal control of the neurosecretory caudo-dorsal cells of the freshwater snail Lymnaea stagnalis (L.). Cell Tissue Res 168: 11–31. [DOI] [PubMed] [Google Scholar]
- Sadamoto H, Takahashi H, Okada T, Kenmoku H, Toyota M, and Asakawa Y 2012. De novo sequencing and transcriptome analysis of the central nervous system of the mollusc Lymnaea stagnalis by deep RNA sequencing. PLoS One 7: e42546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Brezina V, Weiss KR, and Jing J 2009. Distinct inhibitory neurons exert temporally specific control over activity of a motoneuron receiving concurrent excitation and inhibition. J. Neurosci 29: 11732–11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelle DB 1990. GABA receptors of insects. Adv. Insect Physiol 22: 1–113. [Google Scholar]
- Senatore A, Edirisinghe N, and Katz PS 2015. Deep mRNA sequencing of the Tritonia diomedea brain transcriptome provides access to gene homologues for neuronal excitability, synaptic transmission and peptidergic signaling. PLoS One 10: e0123514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffe SR, and Benjamin PR 1980. Morphology of two electrotonically-coupled giant neurosecretory neurons in the snail, Lymnaea stagnalis. Comp. Biochem. Physiol. A Physiol 67: 35–46. [Google Scholar]
- Soinila S, and Mpitsos GJ 1991. Immunohistochemistry of diverging and converging neurotransmitter systems in mollusks. Biol. Bull 181: 484–499. [DOI] [PubMed] [Google Scholar]
- Svensson E, Proekt A, Jing J, and Weiss KR 2014. PKC-mediated GABAergic enhancement of dopaminergic responses: implication for short-term potentiation at a dual-transmitter synapse. J. Neurophysiol 112: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, and Carpenter DO 1975. Organisation of receptors for neuro-transmitters on Aplysia neurones. Nature 258: 751–754. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Golowasch J, Christie AE, Coleman MJ, Nusbaum MP, and Marder E 2000. GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis. J. Exp. Biol 203: 2075–2092. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yokoi I, and Hiramatsu M 1977. Structure-activity relationships of GABA and its relatives on the excitability of an identified molluscan giant neurone (Achatina fulica Férussac). Comp. Biochem. Physiol. C Comp. Pharmacol 56: 63–73. [DOI] [PubMed] [Google Scholar]
- Teyke T, Rosen SC, Weiss KR, and Kupfermann I 1993. Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res 630: 226–237. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, and Sabatini BL 2012. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu C, and Sabatini BL 2014. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. eLife 3: e01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, and Sabatini BL 2016. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci 17: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, and Cottrell GA 1978. Cellular accumulation of amines and amino acids in the central ganglia of a gastropod mollusc, Planorbis corneus: an autoradiographic study. J. Neurocytol 7: 759–776. [DOI] [PubMed] [Google Scholar]
- Usherwood PN, and Grundfest H 1964. Inhibitory postsynaptic potentials in grasshopper muscle. Science 143: 817–818. [DOI] [PubMed] [Google Scholar]
- Vaasjo LO, Quintana AM, Habib MR, Méndez de Jesus PA, Croll RP, and Miller MW 2018. GABA-like immunoreactivity in Biomphalaria: colocalization with tyrosine hydroxylase-like immunoreactivity in the feeding motor systems of panpulmonate snails. J. Comp. Neurol 526: 1790–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehovszky A, Bikisch AJ, Krogsgaard-Larsen P, and Walker RJ 1989. Pharmacological profile of gamma-aminobutyric acid (GABA) receptors of identified central neurons from Helix aspersa. Comp. Biochem. Physiol. C Comp. Pharmacol 92: 391–399. [Google Scholar]
- Walker RJ, Crossman AR, Woodruff GN, and Kerkut GA 1971. The effect of bicuculline on the gamma-aminobutyric acid (GABA) receptors of neurones of Periplaneta americana and Helix aspersa. Brain Res 34: 75–82. [DOI] [PubMed] [Google Scholar]
- Walker RJ, Aranza MJ, Kerkut GA, and Woodruff GN 1975. The action of gamma-aminobutyric acid (GABA) and related compounds on two identifiable neurones in the brain of the snail Helix aspersa. Comp. Biochem. Physiol. C Comp. Pharmacol 50: 147–154. [PubMed] [Google Scholar]
- Walker RJ, Brooks HL, and Holden-Dye L 1996. Evolution and overview of classical transmitter molecules and their receptors. Parasitology 113: S3–S33. [DOI] [PubMed] [Google Scholar]
- Watts RL, Standaert DG, and Obeso JA 2012. Movement Disorders, 3rd ed. McGraw Hill, New York. [Google Scholar]
- Webber MP, Thomson JWS, Buckland-Nicks J, Croll RP, and Wyeth RC 2017. GABA-, histamine-, and FMRFamide-immunoreactivity in the visual, vestibular and central nervous systems of Hermissenda crassicornis. J. Comp. Neurol 525: 3514–3528. [DOI] [PubMed] [Google Scholar]
- Wentzell MM, Martínez-Rubio C, Miller MW, and Murphy AD 2009. Comparative neurobiology of feeding in the opisthobranch seal slug, Aplysia, and the pulmonate snail, Helisoma: evolutionary considerations. Brain Behav. Evol 74: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildering WC, Janse C, and de Vlieger TA 1991. The role of pacemaker properties and synaptic input in generation and modulation of spiking activity in a pair of electrically coupled peptidergic neurons. Brain Res 556: 324–328. [DOI] [PubMed] [Google Scholar]
- Wu JS, Jing J, Díaz-Ríos M, Miller MW, Kupfermann I, and Weiss KR 2003. Identification of a GABA-containing cerebral-buccal interneuron-11 in Aplysia californica. Neurosci. Lett 341: 5–8. [DOI] [PubMed] [Google Scholar]
- Wu JS, Wang N, Siniscalchi MJ, Perkins MH, Zheng YT, Yu W, Chen SA, Jia RN, Gu JW, Qian YQ et al. 2014. Complementary interactions between command-like interneurons that function to activate and specify motor programs. J. Neurosci 34: 6510–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarowsky PJ, and Carpenter DO 1977. GABA mediated excitatory responses on Aplysia neurons. Life Sci 20: 1441–1448. [DOI] [PubMed] [Google Scholar]
- Yarowsky PJ, and Carpenter DO 1978a. A comparison of similar ionic responses to γ-aminobutyric acid and acetylcholine. J. Neurophysiol 41: 531–541. [DOI] [PubMed] [Google Scholar]
- Yarowsky PJ, and Carpenter DO 1978b. Receptors for gamma-aminobutyric acid (GABA) on Aplysia neurons. Brain Res 144: 75–94. [DOI] [PubMed] [Google Scholar]
- Zeman GH, Myers PR, and Dalton TK 1975. Gamma-aminobutyric acid uptake and metabolism in Aplysia dactylomela. Comp. Biochem. Physiol. C Comp. Pharmacol 51: 291–299. [Google Scholar]


