Studying anthropogenic bird extinctions shows that flight loss has evolved much more often than inferred from extant birds.
Abstract
Human-driven extinctions can affect our understanding of evolution, through the nonrandom loss of certain types of species. Here, we explore how knowledge of a major evolutionary transition—the evolution of flightlessness in birds—is biased by anthropogenic extinctions. Adding data on 581 known anthropogenic extinctions to the extant global avifauna increases the number of species by 5%, but quadruples the number of flightless species. The evolution of flightlessness in birds is a widespread phenomenon, occurring in more than half of bird orders and evolving independently at least 150 times. Thus, we estimate that this evolutionary transition occurred at a rate four times higher than it would appear based solely on extant species. Our analysis of preanthropogenic avian diversity shows how anthropogenic effects can conceal the frequency of major evolutionary transitions in life forms and highlights the fact that macroevolutionary studies with only small amounts of missing data can still be highly biased.
INTRODUCTION
Humans have substantially modified the world’s environments and have already caused the extinction of hundreds of vertebrate species (1). Well-known consequences of these impacts include the loss of phylogenetic diversity (PD) (2), the disappearance of key species for ecosystem functioning (3, 4), and the dissociation of species interactions (5). However, a less appreciated consequence of human-driven extinctions is the distortion of biological patterns (6–8). Such changes might limit our capacity to unveil underlying natural rules (9–12), leading to biased conclusions about how evolution works.
Anthropogenic biases may originate from the selective impact of humans, with some traits enhancing the vulnerability of species to human-driven extinctions (13). It is widely recognized, for instance, that larger mammals are more prone to going extinct than smaller mammals (14–17). This anthropogenic effect weakens multiple biological patterns related to body size, such as Bergmann’s rule (11, 18), which predicts that animals are larger at higher latitudes (19). Examples of how humans can affect observed natural phenomena are mainly restricted to biogeographical patterns of megafaunal extinction (5, 11, 12, 18), whereas the way in which extinctions can hide major evolutionary transitions is not well understood (20).
Birds are an excellent group to investigate how major evolutionary transitions might be obscured by human-driven extinctions. While they are generally considered to be the best-known major clade in terms of phylogeny, geographic distributions, and species traits (21–23), many human-related extinctions have occurred (24, 25). Although anthropogenically extinct species represent a low proportion of current biodiversity, they may exhibit different traits compared with living representatives (26), distorting the history of evolution depicted in the extant avifauna. One trait where this distortion could be particularly acute is flightlessness, the evolution of which renders species more vulnerable to hunting by humans and predation by human-introduced, non-native species such as rats and cats.
The loss of flight, or secondary flightlessness, has occurred independently in several clades of birds (27), generally accompanied by a suite of morphological, physiological, ecological, and genetic changes (28–31). Nevertheless, our capacity to study the real phylogenetic and geographical distribution of this phenomenon is limited, as the diversity of flightlessness has been reduced markedly by human-driven extinctions (Fig. 1). Previous studies have shown that flightless species are overrepresented among extinct species, but so far, these studies have been restricted to recent extinctions (27, 32) or particular regions, such as the Pacific islands (25, 33). Because human influence on biodiversity is globally widespread and can be traced back thousands of years (34), such studies may be underestimating the bias and, thereby, the effect of extinctions on our inference of evolutionary transitions.
Fig. 1. Trait selectivity during extinction, taking New Zealand as an example.

New Zealand was the island with the largest known diversity of flightless species, here represented by the heavy-footed moa (Pachyornis elephantopus), Lyall’s wren (Traversia lyalli), kakapo (Strigops habroptilus), and common kiwi (Apteryx australis). Flightless species have undergone extinction disproportionately more often than others, ever since the first settlements by Maori, and this trend may continue into the future. The drawing illustrates an imaginary transition in time from 126 thousand years ago (far left) to 2100 (far right). Illustration by I. Voet.
Here, we compile a comprehensive list of all bird species known to have gone extinct since the rise of humans (i.e., in the Late Pleistocene and Holocene) and use it to quantify the extent to which inferences about evolutionary transitions and rates of evolution to flightlessness are biased by anthropogenic extinctions. In addition, because flightless species are normally found in isolated and more vulnerable systems, such as islands, we use simulations to explore how trait- and geographic-dependent extinctions might interact to explain observed biases.
RESULTS
An exhaustive compilation of bird extinctions from the Late Pleistocene until the present revealed the known loss of 581 species from 85 different families, with substantial variation in taxonomic and geographical distribution (fig. S1). On the basis of the morphological descriptions, 166 of these species were considered flightless (or, at best, only weak flyers), representing 29% of the extinct birds. The complete list of known flightless birds therefore increases from 60 to 226 when both extant and extinct species are considered. Flightlessness was far more phylogenetically and geographically widespread before human impacts (Fig. 2). It was common in many of the island archipelagos (Fig. 2A), with remarkable hotspots in Hawaii (23 species) and New Zealand (26 species), including giant flightless geese and moa, respectively. Before human impacts, more than half of all bird orders had at least one flightless representative (23 orders out of 39), of which 16 orders still had a living representative before historic extinctions [500 years before the present (B.P.)]. In contrast, only nine orders include flightless species today (Fig. 2B). The evolutionary diversity of flightless forms has thus decreased markedly through time, from being present in 40 different families to just 12 today (Fig. 3). Just two families—the rails (Rallidae) and penguins (Spheniscidae)—account for 58% of extant flightless species, but represent 44% of the species when including extinct species.
Fig. 2. Geographical and phylogenetic distribution of flightless birds through time.
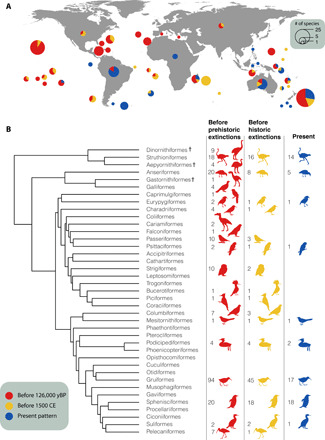
(A) The global distribution of flightless species is shown by the locations of circles, where the area of the circle represents the total diversity of known flightless species per archipelago and continent. The fraction of this diversity that is extant is shown in blue, the fraction representing historical extinctions (i.e., after 1500 CE) in yellow, and the fraction representing prehistoric extinctions (i.e., Late Pleistocene and Holocene up to 1500 CE) in red. (B) The phylogenetic distribution of flightlessness depicts a decrease in the number of orders with flightless species. The number of living flightless species for each order is shown for each time frame. Original silhouettes are deposited at phylopic.org under a public domain license. Entirely extinct orders are marked with †. yBP, years before the present.
Fig. 3. Occurrence of flightlessness among extant and extinct species.
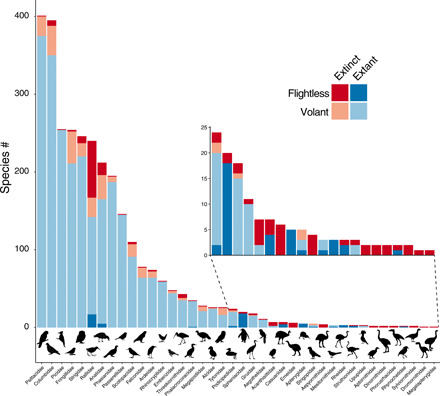
When including extinct species (red) together with extant species (blue), there are 27 bird families with flightless species (darker shade of red or blue). Silhouettes are available at phylopic.org under a public domain license.
Although extinct flightless species only represent 5% of the total number of bird species, the extinction of this subset leads to a more than fourfold underestimation of the rate of transition to flightlessness. When using data on extant species, flightlessness is estimated to have evolved at least 35 times in the extant phylogeny of birds, but this jumps to at least 150 transitions when we include human-caused extinct species in the analysis. Thus, the estimated rate of evolution when including Late Pleistocene and Holocene extinctions is more than four times higher (11.70 × 10−4 transitions/million years or Ma of evolutionary time, hereafter transitions/Ma) than estimates based on living species only (2.85 × 10−4 transitions/Ma). Estimates of rates of evolution based on two additional time frames—including historic extinctions (i.e., 500 years B.P.) reported by the International Union for Conservation of Nature (IUCN) Red List (35) and predicted in 100 years based on IUCN extinction risk probabilities from (36)—show that the bias in the estimated rate of evolution of flightlessness has been gradually increasing and is likely to increase further in the future as more threatened flightless species go extinct (Fig. 4). These observed differences—between the estimated rate of evolution of flightlessness at present (based solely on extant species) and the estimated rates including anthropogenic extinctions—are not artefacts of archipelago definition (fig. S2) or potential sampling biases in the fossil record (fig. S3). Even though flightless species are not distributed at random with respect to phylogeny and disproportionate numbers of extinct and extant flightless species are rails (Rallidae), our conclusions also hold when excluding this family: The estimated rate including anthropogenic extinctions is 7.04 × 10−4 transitions/Ma, still more than four times higher than the estimated rate at present (1.58 × 10−4 transitions/Ma) (fig. S4). It therefore represents a general pattern, not driven by a single clade.
Fig. 4. Inferred rate of evolution toward flightlessness in birds across different time frames.

In each case, the mean and 95% confidence interval for 100 estimations are shown, after repeating the analysis over a distribution of 100 phylogenetic trees. Prehistoric extinctions include known extinct species within the Late Pleistocene or Holocene (the last 126,000 years B.P.). Historic extinctions include species that went extinct after 1500 CE, whereas future patterns are predicted on the basis of the probability of extinctions in the next 100 years based on simulations of extinction risk based on the IUCN category.
The most obvious reason for the observed bias in the estimate of the evolutionary rate of flightlessness is higher extinction risk in flightless bird species (e.g., because they are easier to hunt or vulnerable to predation by introduced species). This is reflected in the IUCN threat categories, where the proportion of flightless species decreases from higher to lower categories of threat (fig. S5). However, other indirect mechanisms could lead to similar biases. For instance, the disproportionate extinction of island bird species (up to 80% of extinct known species), in which flightlessness is more prevalent, could also cause a disproportionate loss of flightless species. In addition, both mechanisms could interact; although flightless species are overall more prone to extinction, the probability is three times greater on islands than in nonisland settings (Fig. 5). Simulations that emulate nonrandomness in human-caused extinctions provide strong support for this interaction between region and trait selectivity: The combination of insularity and flightlessness provides the best predictor of the observed bias in evolutionary rates (simulated bias of 68%, on average, compared with a real bias of 75%; fig. S6), followed by an extinction model based on flightlessness alone (simulated bias of 65%).
Fig. 5. Proportions of extinct species in relation to the capacity for flight.
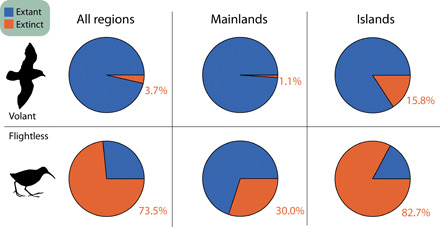
The percentage of extinct species is shown for volant and flightless species, including all bird species as well as distinguishing between island and mainland settings.
DISCUSSION
Our study highlights the fact that differences in extinction risk related to trait differences can substantially bias evolutionary patterns inferred from extant taxa. Extant species make up 95% of bird species in our study, but strong biases still arise from the imbalance in trait distributions between extant and extinct species. These results add further insight into the general problem of sampling biases in comparative phylogenetic analyses (37, 38) and highlight the need for better integration of paleontological, ecological, and evolutionary studies. Despite previous calls to restrict macroevolutionary analysis to species with genetic information (39), doing so could potentially lead to a greater bias if the excluded species represent a nonrandom sample of the total. As human-related extinctions have been shown to be highly selective in relation to species traits, evolutionary studies focusing on such traits may reflect anthropogenic impacts rather than fundamental biological rules.
Here, we show that the evolutionary path from the sky to the ground in birds was not nearly as rare as it appears from studying the extant avian phylogeny. Ecologically and phylogenetically diverse flightless birds occupied most of the world’s archipelagos when humans arrived, filling the niche of absent mammal species (28, 40). Although other flightless species also existed and went extinct in prehuman times (41, 42), anthropogenic extinctions are expected to be highly selective with respect to flightlessness: Because flightless species often evolved in response to the absence of mammals, they were particularly vulnerable to human arrival and the associated introduction of non-native mammals (43, 44). The extinctions of flightless birds on islands also resulted, in some cases, in the disappearance of important ecological roles that were unlikely to be replaced (45, 46). The impacts of these losses are likely to be underestimated, given that many species will have gone extinct without leaving a fossil record (24, 25), many of which are likely also to have been flightless (24, 25). The remaining flightless bird species now represent a tiny fraction of a once larger group with significant ecological importance for key ecosystem functions including seed dispersal, pollination, and herbivory (47, 48).
Flightless species—such as the iconic dodo (Raphus cucullatus)—are often caricatured as naïve animals, whose inevitable fate was to go extinct. Instead, these unique life forms should be regarded as a great example of convergent evolutionary shifts following the colonization of new environmental settings. Flightless birds showcase parallel transformations involving a suite of behavioral, morphological, and ecological changes that have become largely erased by human-driven extinctions.
MATERIALS AND METHODS
Data on anthropogenic extinctions
To obtain a list of all known bird extinctions during the rise of humans (i.e., from the Late Pleistocene onward), we reviewed the published literature on the topic. First, for historic extinctions (after 1500 CE), we extracted the information from the IUCN Red List of Threatened Species (Accessed on June 2019) (35), which includes 162 species that are categorized as extinct (EX) or extinct in the wild (EW). To search for older extinctions or undescribed species, we carried out a literature search using Google Scholar including the terms “geographical location AND (extinct OR fossil) AND (avian OR bird)” up to August 2019. For the geographical locations, we used all the main island archipelagos in the world as well as the continents. After accessing relevant studies, we also checked extinct species or archaeological sites cited within these papers. We complemented the search with scrutiny of relevant books on the subject (49–51). For the prehistoric extinctions, we only included species that were extinct after the last interglacial during the Late Pleistocene (126,000 years B.P.), which is determined by their presence in fossil deposits of later age or by contemporary records of the species. Although we distinguish between historic (after 1500 CE) and prehistoric (between 126,000 years B.P. and 1500 CE) extinctions in subsequent analyses, these terms are only temporal and do not imply different causes of extinction. Similarly, our list includes all extinctions dated within the mentioned time frame, assuming that all are related to human impacts. Although it is possible that some of the extinctions have natural causes (e.g., climatic changes or overcompetition by other lineages), this does not alter our conclusions: The observed bias we report arises when we compare the list of species that lived before and after the human impacts of Late Pleistocene and Holocene.
Species taxonomy and traits
For species taxonomic classification, we followed the Handbook of the Birds of the World and BirdLife International digital checklist of the birds of the world (52) and used the most recent evidence to classify extinct species. To classify extinct species into flightless or volant, we relied on authors’ morphological descriptions and inferences of flight ability. Species described as weak flyers were considered flightless in the main analysis, but considering them as volant does not change our conclusions (see the “Estimation of evolutionary transitions” section and fig. S2). Species with insufficient morphological data to assess flight ability were assumed to be volant, which ensures that our inferences of the magnitude of biases are conservative. In the case of extant species, we used the exhaustive classification from (23). For each species, we also recorded its geographical distribution and whether the species is an oceanic island endemic, considered to be so if it only occurs on islands that were not connected to the continent when the sea level decreased 120 m in the last glaciation (53). The complete list of extinct birds, their traits, and geographical locations are available in data file S1. A complete list of all extant bird species (N = 10,964), including their flight ability and island endemicity, is available in data file S2, whereas the list of islands where extant or extinct flightless species are found is available in data files S3 and S4.
Estimation of evolutionary transitions
To estimate the number of transitions toward flightlessness, we identified monophyletic flightless groups (e.g., entire orders, families, or genera of flightless birds). Assuming that flightlessness is irreversible, this approach gives us a minimum number of transitions and therefore could be considered a conservative estimate (i.e., it would not consider independent transitions within monophyletic clades). In species for which we did not have phylogenetic information and for which both flightless and volant genera exist, we assumed that genera within archipelagos are monophyletic entities, and hence, several flightless species from the same genus within an archipelago were considered to reflect a single transition. In the case of the white-throated rail (Dryolimnas cuvieri), only one of the subspecies (D. cuvieri aldabranus) is flightless, which was also considered an independent transition. To test the robustness of our analyses when inferring the number of transitions (N), we redefined archipelagos based on distances to avoid subjectivity in archipelago definition that could inflate transition rates. For instance, Madeira and the Canaries are traditionally considered distinct archipelagos, but they are closer to each other than islands within other archipelagos like the Azores. We thus built a cluster analysis of all islands based on geographical distances; classified archipelagos based on different distance thresholds every 100 km, from 100 to 5000 km; and recalculated transitions toward flightlessness in each new archipelago classification, which did not change our conclusions (fig. S2).
Estimation of evolutionary rates
To quantify the rate of evolution toward flightlessness, we divided the number of transitions (N) inferred in each bird family by the sum of all branch lengths of the clade, measured as Faith’s PD (54), which means that we assume that regaining the ability to fly is impossible once it is lost. This allows us to estimate the number of transitions relative to the amount of evolutionary time (i.e., independently evolving lineages). To quantify PD, we first calculated the total PD on a sample of 100 phylogenetic trees of all extant birds (21), from the Hackett’s backbone distribution (55) available at Birdtree.org. To estimate the PD of the missing species in the tree (e.g., known extinct species since the Late Pleistocene), for each family, we first fitted a logarithmic curve on PD as a function of the number of species, by resampling for each given number of species from 1 to the maximum number of species from the family present in the tree. Then, we used the terms of the function to infer the added PD (ΛPD) when adding the corresponding number of missing species (i.e., present in our list but not in the tree). We then added the ΛPD for each family to the total PD (fig. S7). As some fossil species from the Late Pleistocene could be direct ancestors of extant species, rather than distinct species, the inferred PD could, in principle, be overestimated. Nevertheless, because the cases where a Late Pleistocene extinction has the same genus and location as an extant species are very low (N = 48 species) compared with the total number of species in the tree that are used to compute PD (more than 11,000 species), the potential overestimated PD is marginal. The estimation of evolutionary rates was repeated 100 times, one for each tree, so we obtained a distribution of 100 inferences of evolutionary rates. When estimating evolutionary rates toward flightlessness of 100 years into the future, we used previously estimated extinction probabilities for each IUCN category (35). Then, for each phylogenetic tree, we simulated 10 future scenarios by randomly removing extant species based on their extinction probabilities. Therefore, in this case, we estimated 1000 evolutionary rates based on simulating extinctions 10 times in each of the 100 phylogenetic trees.
Simulation of a sampling bias to explain the observed patterns
The disproportionate number of flightless birds in extinct fauna, which caused the observed bias in the evolution of the trait, might come from a bias in the fossil record, where flightless birds have a greater probability of being preserved. We thus performed an additional simulation to test how many potentially missing volant species would need to be missing from the extinct record to remove the bias in evolutionary rates. To do so, we sequentially added species to random positions of the phylogeny and recalculated the number of transitions and rates toward flightlessness until the estimated rate met the observed rate. The analysis was run 1000 times, and we estimated the range of species that should be added to make the bias disappear. On the basis of these simulations, we found that the number of extinct volant species potentially missing from the fossil record would need to be as high as 60,000 to 80,000 species to remove the bias in the rates of evolution toward flightlessness (fig. S3). This number is unrealistic given the current standing diversity of birds, which is around 10,000.
Randomizations of extinctions within trait categories
To identify the mechanism responsible for the observed bias in the evolutionary rate of flightlessness, we performed four groups of randomizations, aiming to show the consequences of random versus nonrandom extinctions and whether island selectivity, rather than flightlessness selectivity in extinctions, is behind the observed bias. For instance, a higher extinction of flightless birds would tend to make the inferred evolutionary rate toward flightlessness decrease, but the same pattern could appear if island species, but not flightless species, have higher chances of going extinct (since flightlessness is more common on islands). We built four different randomization models by permutating the status (extant versus extinct) over the full list of species and then recalculating the rates of evolution toward flightlessness. Therefore, the total number of extinctions (N = 581) is the same in all the models, but the probability of extinction based on different traits will change among the models. In the first model (null model), permutations were done among all species, so any species had the same chance of going extinct. In the second model (island-dependent extinction model), permutations were done within island versus mainland groups, so the proportions of extinctions of island (N = 468) versus mainland (N = 113) species are maintained, and hence, island species have a higher probability of going extinct. In the third model (flightless-dependent extinction model), permutations were done within flightless (N = 166) and volant (N = 415) categories, whereas in the fourth model (island- × flightless-dependent extinction model), we fixed both flightlessness and insularity; therefore, volant-island (N = 314), flightless-island (N = 154), volant-continent (N = 101), and flightless-continent (N = 12) taxa went extinct (fig. S6). We ran each model 1000 times, each time recalculating the rate of evolution of flightlessness, and compared the bias in the estimate with the observed bias. We also repeated this analysis after excluding cases where a Late Pleistocene extinction could be an older form of an extant species (N = 48 species; see section on “Estimation of evolutionary rates”), but the conclusions do not change (fig. S8).
Supplementary Material
Acknowledgments
We thank all members of the Antonelli Lab who provided constructive feedback on the initial stages of this project and to R. Smith for help editing the manuscript before submission. We are also thankful to L. Valente, J. C. Ilera, and J. A. Alcover for providing information about some unpublished fossil material. Finally, we want to thank two anonymous reviewers for providing helpful comments that helped to improve the manuscript. Funding: This work has been funded by the Swedish Research Council (2017-03862) and a grant from Carl Tryggers Stiftelse för Vetenskaplig Forskning to S.F. A.A. was supported by the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Swedish Foundation for Strategic Research, and the Royal Botanic Gardens, Kew. F.S. was funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 838998. Author contributions: Conceptualization of the project was done by S.F. and F.S., with feedback input by M.J.S. and T.M.B. Data gathering and formal analysis were done by F.S. The original draft was written by F.S. and constructively reviewed by S.F., M.J.S., T.M.B., and A.A. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All data can be accessed from the Dryad Digital Repository (https://doi.org/10.5061/dryad.s1rn8pk66). Correspondence and requests for additional material should be addressed to F.S.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/49/eabb6095/DC1
REFERENCES AND NOTES
- 1.Ceballos G., Ehrlich P. R., Barnosky A. D., García A., Pringle R. M., Palmer T. M., Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis M., Faurby S., Svenning J.-C., Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proc. Natl. Acad. Sci. U.S.A. 115, 11262–11267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petchey O. L., Gaston K. J., Extinction and the loss of functional diversity. Proc. Biol. Sci. 269, 1721–1727 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhi Y., Doughty C. E., Galetti M., Smith F. A., Svenning J.-C., Terborgh J. W., Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galetti M., Moleón M., Jordano P., Pires M. M., Guimarães P. R. Jr., Pape T., Nichols E., Hansen D., Olesen J. M., Munk M., de Mattos J. S., Schweiger A. H., Owen-Smith N., Johnson C. N., Marquis R. J., Svenning J.-C., Ecological and evolutionary legacy of megafauna extinctions: Anachronisms and megafauna interactions. Biol. Rev. 93, 845–862 (2018). [DOI] [PubMed] [Google Scholar]
- 6.K. J. Gaston, T. M. Blackburn, in Macroecology: Concepts and Consequences (Blackwell, 2003), pp. 345–367. [Google Scholar]
- 7.Faurby S., Svenning J.-C., Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers. Distrib. 21, 1155–1166 (2015). [Google Scholar]
- 8.Torres-Romero E. J., Olalla-Tárraga M. Á., Untangling human and environmental effects on geographical gradients of mammal species richness: A global and regional evaluation. J. Anim. Ecol. 84, 851–860 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Murray B. R., Dickman C. R., Relationships between body size and geographical range size among australian mammals: Has human impact distorted macroecological patterns? Ecography 23, 92–100 (2000). [Google Scholar]
- 10.Di Marco M., Santini L., Human pressures predict species’ geographic range size better than biological traits. Glob. Change Biol. 21, 2169–2178 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Faurby S., Araújo M. B., Anthropogenic impacts weaken Bergmann’s rule. Ecography 40, 683–684 (2016). [Google Scholar]
- 12.Faurby S., Svenning J.-C., Resurrection of the island rule: Human-driven extinctions have obscured a basic evolutionary pattern. Am. Nat. 187, 812–820 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Purvis A., Agapow P.-M., Gittleman J. L., Mace G. M., Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A., Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Di Marco M., Boitani L., Mallon D., Hoffmann M., Iacucci A., Meijaard E., Visconti P., Schipper J., Rondinini C., A retrospective evaluation of the global decline of carnivores and ungulates: Global decline of carnivores and ungulates. Conserv. Biol. 28, 1109–1118 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Ripple W. J., Wolf C., Newsome T. M., Betts M. G., Ceballos G., Courchamp F., Hayward M. W., Van Valkenburgh B., Wallach A. D., Worm B., Are we eating the world’s megafauna to extinction? Conserv. Lett. 12, e12627 (2019). [Google Scholar]
- 17.Smith F. A., Elliott Smith R. E., Lyons S. K., Payne J. L., Body size downgrading of mammals over the late Quaternary. Science 360, 310–313 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Santini L., González-Suárez M., Rondinini C., Di Marco M., Shifting baseline in macroecology? Unravelling the influence of human impact on mammalian body mass. Divers. Distrib. 23, 640–649 (2017). [Google Scholar]
- 19.C. Bergmann, Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse (1848).
- 20.Gillespie R. G., Claridge E. M., Roderick G. K., Biodiversity dynamics in isolated island communities: Interaction between natural and human-mediated processes. Mol. Ecol. 17, 45–57 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Somveille M., Manica A., Butchart S. H. M., Rodrigues A. S. L., Mapping global diversity patterns for migratory birds. PLOS ONE 8, e70907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke R. S. C., Bates A. E., Eigenbrod F., Global trade-offs of functional redundancy and functional dispersion for birds and mammals. Glob. Ecol. Biogeogr. 28, 484–495 (2019). [Google Scholar]
- 24.Steadman D. W., Prehistoric extinctions of pacific island birds: Biodiversity meets zooarchaeology. Science 267, 1123–1131 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Duncan R. P., Boyer A. G., Blackburn T. M., Magnitude and variation of prehistoric bird extinctions in the Pacific. Proc. Natl. Acad. Sci. U.S.A. 110, 6436–6441 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyer A. G., Consistent ecological selectivity through time in Pacific island avian extinctions. Conserv. Biol. 24, 511–519 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Roff D. A., The evolution of flightlessness: Is history important? Evol. Ecol. 8, 639–657 (1994). [Google Scholar]
- 28.James H. F., Burney D. A., The diet and ecology of Hawaii’s extinct flightless waterfowl: Evidence from coprolites. Biol. J. Linn. Soc. 62, 279–297 (1997). [Google Scholar]
- 29.Campagna L., McCracken K. G., Lovette I. J., Gradual evolution towards flightlessness in steamer ducks*. Evolution 73, 1916–1926 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Wright N. A., Steadman D. W., Witt C. C., Predictable evolution toward flightlessness in volant island birds. Proc. Natl. Acad. Sci. U.S.A. 113, 4765–4770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sackton T. B., Grayson P., Cloutier A., Hu Z., Liu J. S., Wheeler N. E., Gardner P. P., Clarke J. A., Baker A. J., Clamp M., Edwards S. V., Convergent regulatory evolution and loss of flight in paleognathous birds. Science 364, 74–78 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Heinen J. H., van Loon E. E., Hansen D. M., Kissling W. D., Extinction-driven changes in frugivore communities on oceanic islands. Ecography 41, 1245–1255 (2018). [Google Scholar]
- 33.D. Steadman, M. L. Reaka-Kudla, D. E. Wilson, E. O. Wilson, Biodiversity II: Understanding and Protecting Our Biological Resources (Joseph Henry Press, 1996). [Google Scholar]
- 34.Sandom C., Faurby S., Sandel B., Svenning J.-C., Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B. 281, 20133254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IUCN, The IUCN Red List of Threatened Species (June 2019); https://www.iucnredlist.org.
- 36.Mooers A. Ø., Faith D. P., Maddison W. P., Converting endangered species categories to probabilities of extinction for phylogenetic conservation prioritization. PLOS ONE 3, e3700 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua X., Lanfear R., The influence of non-random species sampling on macroevolutionary and macroecological inference from phylogenies. Methods Ecol. Evol. 9, 1353–1362 (2018). [Google Scholar]
- 38.Marcondes R. S., Realistic scenarios of missing taxa in phylogenetic comparative methods and their effects on model selection and parameter estimation. PeerJ. 7, e7917 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabosky D. L., No substitute for real data: A cautionary note on the use of phylogenies from birth-death polytomy resolvers for downstream comparative analyses. Evolution 69, 3207–3216 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Wood J. R., Wilmshurst J. M., Richardson S. J., Rawlence N. J., Wagstaff S. J., Worthy T. H., Cooper A., Resolving lost herbivore community structure using coprolites of four sympatric moa species (Aves: Dinornithiformes). Proc. Natl. Acad. Sci. U.S.A. 110, 16910–16915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard H., A new avian fossil from Kern County, California. Condor 71, 68–69 (1969). [Google Scholar]
- 42.Tanaka T., Kobayashi Y., Kurihara K., Fiorillo A. R., Kano M., The oldest Asian hesperornithiform from the Upper Cretaceous of Japan, and the phylogenetic reassessment of Hesperornithiformes. J. Syst. Palaeontol. 16, 689–709 (2018). [Google Scholar]
- 43.Duncan R. P., Blackburn T. M., Extinction and endemism in the New Zealand avifauna. Glob. Ecol. Biogeogr. 13, 509–517 (2004). [Google Scholar]
- 44.Milberg P., Tyrberg T., Naive birds and noble savages - A review of man-caused prehistoric extinctions of island birds. Ecography 16, 229–250 (1993). [Google Scholar]
- 45.Boyer A. G., Jetz W., Extinctions and the loss of ecological function in island bird communities: Extinctions and functional diversity. Glob. Ecol. Biogeogr. 23, 679–688 (2014). [Google Scholar]
- 46.Sobral F. L., Lees A. C., Cianciaruso M. V., Introductions do not compensate for functional and phylogenetic losses following extinctions in insular bird assemblages. Ecol. Lett. 19, 1091–1100 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Carpenter J. K., Kelly D., Moltchanova E., O’Donnell C. F. J., Introduction of mammalian seed predators and the loss of an endemic flightless bird impair seed dispersal of the New Zealand tree Elaeocarpus dentatus. Ecol. Evol. 8, 5992–6004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood J. R., Wilmshurst J. M., Worthy T. H., Holzapfel A. S., Cooper A., A lost link between a flightless parrot and a parasitic plant and the potential role of coprolites in conservation paleobiology. Conserv. Biol. 26, 1091–1099 (2012). [DOI] [PubMed] [Google Scholar]
- 49.E. Fuller, Extinct Birds, Comstock. ISBN X. 80143954, 96–97 (2001).
- 50.D. W. Steadman, Extinction and biogeography of tropical Pacific birds (University of Chicago Press, 2006). [Google Scholar]
- 51.J. P. Hume, M. Walters, Extinct birds (A&C Black, 2012). [Google Scholar]
- 52.Handbook of the Birds of the World and BirdLife International, Handbook of the Birds of the World and BirdLife International digital checklist of the birds of the world. Version 3 (2018); http://datazone.birdlife.org/userfiles/file/Species/Taxonomy/HBW-BirdLife_Checklist_v3_Nov18.zip.
- 53.Siddall M., Rohling E. J., Almogi-Labin A., Hemleben C., Meischner D., Schmelzer I., Smeed D. A., Sea-level fluctuations during the last glacial cycle. Nature 423, 853–858 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Faith D. P., Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 (1992). [Google Scholar]
- 55.Hackett S. J., Kimball R. T., Reddy S., Bowie R. C. K., Braun E. L., Braun M. J., Chojnowski J. L., Cox W. A., Han K.-L., Harshman J., Huddleston C. J., Marks B. D., Miglia K. J., Moore W. S., Sheldon F. H., Steadman D. W., Witt C. C., Yuri T., A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/49/eabb6095/DC1


