Abstract
Flooding due to extreme weather threatens crops and ecosystems. To understand variation in gene regulatory networks activated by submergence, we conducted a high-resolution analysis of chromatin accessibility and gene expression at three scales of transcript control in four angiosperms, ranging from a dryland-adapted wild species to a wetland crop. The data define a cohort of conserved submergence-activated genes with signatures of overlapping cis-regulation by four transcription factor families. Syntenic genes are more highly expressed than non-syntenic genes, yet both can possess the cis-motifs and chromatin accessibility associated with submergence upregulation. While the flexible circuitry spans the eudicot-monocot divide, the frequency of specific cis-motifs, extent of chromatin accessibility, and the degree of submergence-activation is more prevalent in the wetland crop and may have adaptive significance.
One Sentence Summary:
Conserved submergence-activated gene families display flexibility in regulatory circuitry.
Climate change has increased the frequency and intensity of floods that impact agricultural productivity. Of major crops, only rice (Oryza sativa) is resilient to waterlogging of roots and submergence of aerial tissue, due to adaptation to a semi-aquatic habitat. Other angiosperms experience intermittent flooding and are not adapted to these conditions. Submergence triggers signaling in plant cells as a consequence of entrapment of the gaseous hormone ethylene and depletion of available oxygen, (hypoxia) leading to inefficient anaerobic metabolism and energy starvation (1) To understand the variation in response to submergence, we studied rice as a representative monocot and flood resilient species, the legume Medicago truncatula, and two Solanum species, domesticated tomato (S. lycopersicum cv. M82) and its dryland-adapted wild relative S. pennellii (Fig. 1A). Roots are the first responders to flooding, and we thus monitored the early response of seedling apical root tips to complete seedling submergence. By monitoring the sentinel response gene family ALCOHOL DEHYDROGENASE (ADH), required for anaerobic production of ATP (1) (Fig. 1B), we identified 2 hours, the mid-point of maximal upregulation, as a physiologically relevant time to compare initiation of the submergence response across species.
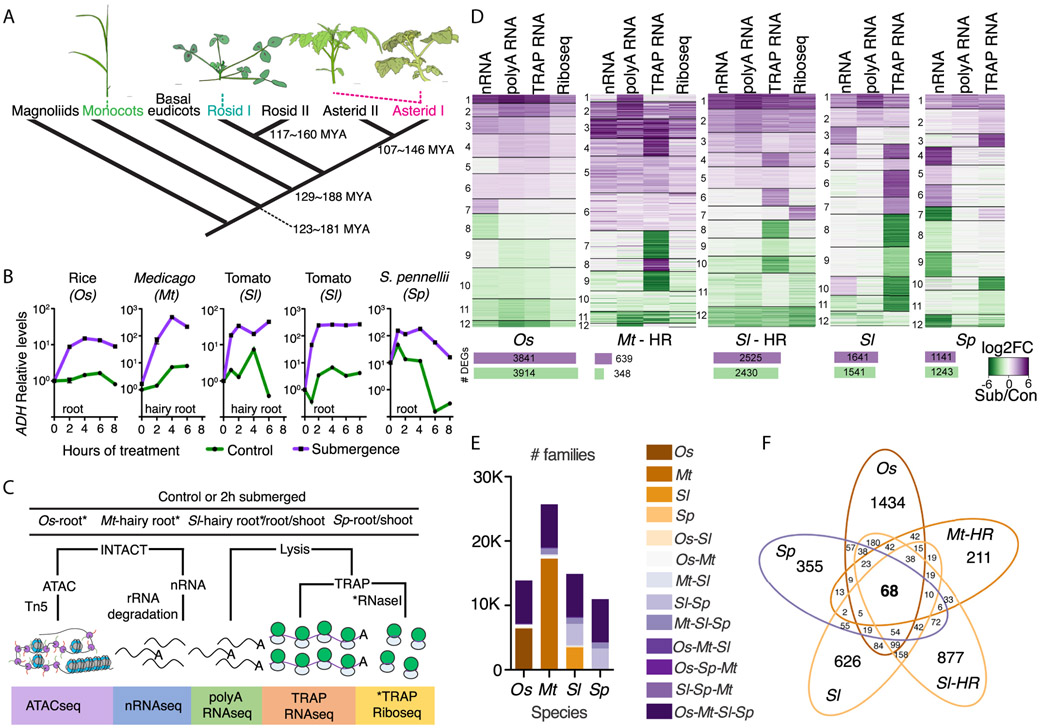
Fig. 1. Multi-tier evaluation of gene activity in four angiosperms identifies highly conserved submergence-upregulated genes.
(A) Relatedness of target species (19). (B) ALCOHOL DEHYDROGENASE (ADH) transcript levels of submerged seedlings. (C) Overview of experimental strategy. (D) Cluster analysis heatmap of log2 fold change (FC; submergence vs. control RNA) of differentially expressed genes (DEGs; ∣log2 FC∣>1 and padj<0.01). Below, Bars indicate number of up or down DEGs after submergence ∣log2 FC∣>1 and padj<0.05. (E) Gene families per species and their overlap. (F) Conserved submergence upregulated gene families (SURFs).
To conserve energy under hypoxia, stress-induced mRNAs are preferentially translated over transcripts associated with development in the model Arabidopsis thaliana (2-4). We therefore considered both transcriptional and post-transcriptional regulation under submergence across the species surveyed. To do so, we deployed Isolation of Nuclei TAgged in specific Cell Types (INTACT) (5) and Translating Ribosome Affinity Purification (TRAP) (6), using constitutive promoters. INTACT was used to profile chromatin accessibility by ATAC-seq (Assay for Transposase-Accessible Chromatin) (7), and measure the abundance of nuclear RNA (nRNA). TRAP was used to monitor ribosome-associated polyadenylated mRNA (TRAP RNA) and evaluate the position of individual ribosomes along transcripts (Ribo-seq) (8) (Fig. 1C, fig. S1-2). We also profiled total polyadenylated mRNA (polyA RNA). Multidimensional scaling analysis confirmed the reproducibility and distinctness of each of the RNA sub-populations and their changes following submergence (fig. S3, data S1-2).
Flood-adapted rice displayed the greatest plasticity in terms of the number of differentially up- and downregulated transcripts (Fig. 1D, fig. S4, data S3). Cultured hairy roots (Sl-HR) were used as a contrast to intact roots of tomato (Sl) plants, and were more responsive. The clustering of modulated RNAs resolved variation in regulation in all four species (Fig. 1D, figs. S5-9). Rice gene regulation was coordinated across scales (except in clusters 7 and 8 where transcripts were enriched or depleted in the nucleus). In M. truncatula and tomato, regulation of gene activity was more evident in the ribosome-associated RNAs, whereas in the dryland-adapted S. pennellii regulation was evident as nRNA enrichment or depletion.
Selection likely acts on species-specific traits and adaptation to specific environments that are largely regulated by a common set of gene families. The root meristem is frequently oxygen-deprived due to high metabolic activity and periodic soil inundation; therefore, its capacity to transiently upregulate anaerobic metabolism might be expected in all species. Yet, rice may have evolved a higher proportion of gene family members that are regulated by submergence than flooding-sensitive species. We leveraged gene families (9) to investigate conservation in submergence-responsive genes of the four species, focusing on the shared families (6685) plus those conserved between the two Solanums (3301) (Fig. 1E, data S4). Tabulation of the submergence-responsive gene family members of each species identifyied families with at least one member differentially controlled in any of the RNA populations evaluated (Fig. 1F, fig S10, data S5). This uncovered a set of 68 submergence-upregulated families (SURFs: 249 genes in Os, 121 in Mt, 137 in Sl, 181 in Sl-HR and 92 in Sp). The 68 SURFs include 17 of the 49 ubiquitously hypoxia-responsive genes of Arabidopsis seedlings (6), demonstrating evolutionarily conservation of gene families activated by submergence and hypoxia (data S5).
The 68 SURFs include one to 13 upregulated genes per family, leading us to investigate whether similar proportions of these families are elevated in each species (fig. S11, data S6). Consistent with overall numbers, rice had the highest and S. pennellii the lowest proportion of upregulated genes per family. The restrained response of wild tomato was evident from the 412 Solanum-specific gene families that were up-regulated in tomato but not in S. pennellii. This motivated exploration of the aerial tissue (shoot apex) response in the Solanums, which uncovered more gene families and family members upregulated in shoots of wild than domesticated tomato (fig. S12, data S7). The shoot response of S. pennellii showed greater overlap with Arabidopsis shoot-specific hypoxia-responsive genes (10). Distinctions between the two Solanums included genes involved in cell elongation and auxin signaling, which predominated in S. pennellii.
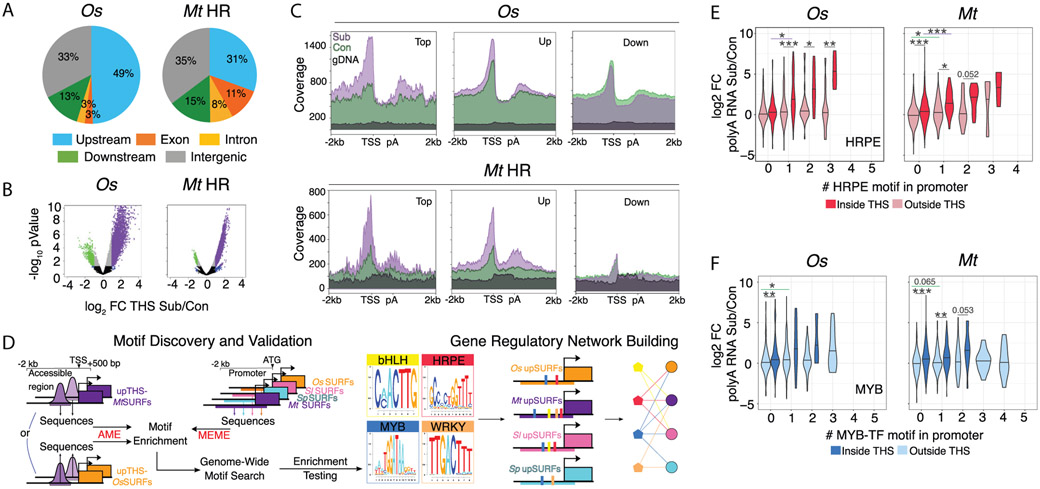
We reasoned that dynamics in chromatin accessibility and transcriptional activation may be coordinated and conserved for SURF members across species. ATAC-seq exposed open chromatin regions of rice and M. truncatula primarily within 1 kb upstream of the transcription start site (TSS) and downstream of the polyadenylation (pA) site of genes (Fig. 2A, data S8). By contrast, Solanum roots showed a majority of intergenic ATAC-seq reads (fig. S13). The rice and M. truncatula, transposase hypersensitive sites (THS) (11) uncovered a preference for opening of chromatin in response to submergence (Fig. 2B, fig. S13), with increases in 3,497 and 7,501 THSs, respectively. Highly submergence-upregulated genes had elevated accessibility 5’ of their TSS and 3’ of their pA sites (Fig. 2C, figs. S5-6,14), demonstrating nucleosome depletion accompanies activation of transcript production under submergence. Downregulated genes had lower chromatin accessibility overall, particularly in rice (Fig. 2C, figs. S5-6,14).
Fig. 2. Enhanced chromatin accessibility and motif enrichment in responsive genes.
(A) Accessible chromatin regions (transposase hypersensitive sites [THS]) measured by ATAC-seq. Categories: 2 kb upstream of the transcription start site (TSS), exons, introns, 1 kb downstream of polyadenylation (pA) site, and intergenic. (B) THS change in response to submergence. (C) Control (Con) and submergence (Sub) ATAC-seq reads on genes of upregulated (Top; cluster 1; Up) and downregulated (Down) clusters from Fig 1D. gDNA is ATAC-seq on naked DNA. (D) Discovery pipeline for enriched transcription factor motifs present in upregulated THSs and SURF promoters, using unsupervised (MEME) and supervised (TOMTOM, AME, FIMO) methods. (E) and (F) Distribution of log2 FC polyA RNA Sub/Con for SURFs arranged by presence and number of HRPE or MYB motif upstream of the ATG, inside or outside THSs. Student’s t test; * = p<0.05, ** = p<0.01, *** =p<0.001; values ≤0.1.
We exploited the ATAC-seq data to explore conservation in gene regulatory circuitry. A pipeline was developed to identify transcription factor (TF) binding site motif enrichment within promoters and their THS regions of the upregulated SURFs (Fig. 2D). Four significantly enriched TF motifs were identified. These included the Hypoxia Responsive Promoter Element (HRPE), transactivated by low oxygen-stabilized ethylene response group VII (ERFVII) TFs that upregulate genes key to anaerobic metabolism and flooding survival in Arabidopsis (12-14), a basic Helix-Loop-Helix (bHLH), a MYB, and a WRKY-type motif (Fig. 2D, figs. S15-16A, data S9). At least one of the four motifs was present in over 84% of the upregulated SURF genes of rice and M. truncatula and over 68% of those of the Solanums. HRPE and bHLH motifs predominated near the TSS in all species, with the MYB near the TSS in tomato and WRKY motifs more evenly distributed across the upstream region (fig S16B). Differential wiring of upregulated SURFs was evident from the HRPE enrichment in rice (55%) versus the MYB or bHLH motif enrichment in these three eudicots (fig. S16A; data S9).
Accessibility of chromatin in response to abiotic stress can be rapid and transient (15, 16). We hypothesized that concordance between a TF binding site and a THS would be representative of a more static regulatory architecture while discordance could reflect the transient propagation of a stress signal. Chromatin accessibility increased during submergence around HRPE and bHLH sites in rice and M. truncatula (Fig. S17). A more modest increase was observed for MYB and WRKY sites, potentially representing more rapid and/or transient regulatory interactions (Fig. S17). The co-occurrence of an HRPE and THS corresponded with more pronounced polyA RNA upregulation, with a similar trend observed for bHLH sites in rice and M. truncatula (Fig. 2E, fig. S18, data S10). In M. truncatula, the presence of a THS alone in the proximal promoter was associated with greater elevation of polyA RNA, and co-occurrence of a MYB and THS corresponded with higher upregulation than the presence of the motif alone (Fig. 2F, fig. S18). Repetitive motifs of the same type within accessible regions coincided with greater up-regulation than with repetitive motifs outside THSs. The incidence of multiple HRPE or WRKY motifs corresponded with higher upregulation in tomato, whereas only an HRPE or multiple bHLH motifs corresponded with upregulation in S.pennellii. These results establish a link between the four conserved motifs, chromatin accessibility and transcriptional activation under submergence.
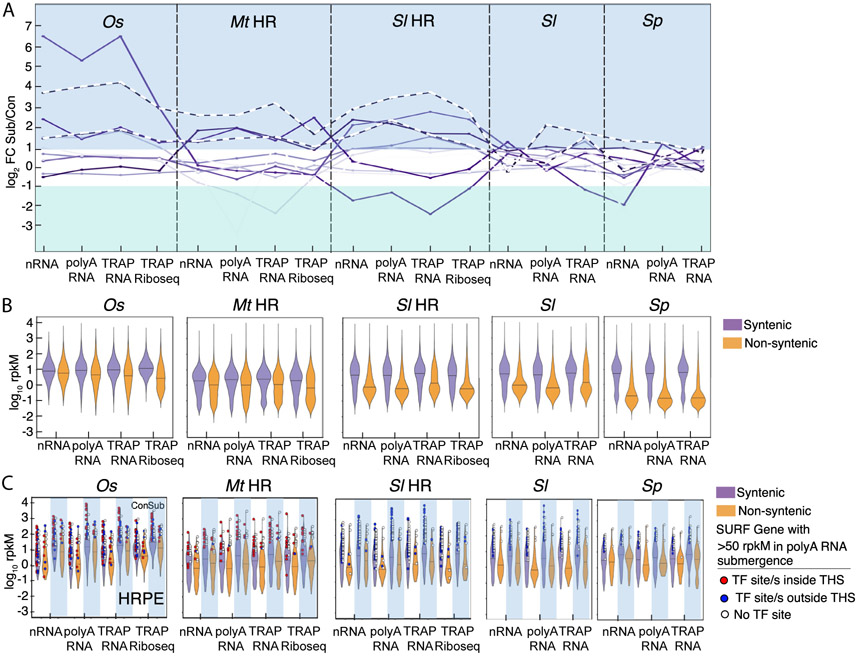
The discovery of the SURFs and four conserved cis-regulatory TF binding motifs in submergence-accessible chromatin regions motivated us to evaluate if the conservation prevails in genes maintained at syntenic chromosomal regions (syntelogs). To do so, the gene activity data were re-clustered for the differentially regulated syntelogs across the four species (711) that included 22 of the 68 SURFs (Fig. 3A, fig. S19, data S11). Syntelog clusters 2 and 3 had coordinated upregulation across the scales of gene activity in all species. These comprised seven SURFs with functions in anaerobic metabolism, nutrient transport, abscisic acid (ABA) perception and survival of extreme stress. The upregulated syntelogs included 32 and 53 SURFs in all three eudicots and the two Solanums, respectively (figs. S20-22, data S11).
Fig. 3. Syntenic genes are more highly expressed.
(A) Median log2 FC of syntenic genes across four species for eight upregulated clusters. Dashed lines indicate two clusters with conserved interspecies up-regulation. (B) Plot of log10 rpkM for all detected syntenic and non-syntenic genes under control condition. Rice synteny was evaluated to Brachypodium distachyon, M. truncatula to S. lycopersicum, and between Solanums. Variances between syntenic and non-syntenic genes are significant in every RNA population (F-test). (C) Control (white columns) and submergence (blue columns) plots for SURF genes. Highly expressed SURF genes under submergence (>50 rpkM) with a Hypoxia Responsive Promoter Element (HRPE) are depicted as a red or blue dot for those located within or outside a THS, respectively. Central horizontal lines indicate median values.
Next, we explored conservation of gene regulation on more recent evolutionary timescales by evaluating the activity of syntelogs of related species (Fig. 3B, fig. S23, data S12-14). Syntenic genes had higher transcript abundance than non-syntenic genes, as reported previously (17). This was evident in all RNA populations under both conditions, with the most pronounced difference between syntenic and non-syntenic genes in the Solanums. Rice and M. truncatula syntenic gene control regions had slightly higher chromatin accessibility than non-syntenic genes at the global scale (fig. S14), consistent with their higher expression. Transcript elevation was similar for syntenic and non-syntenic SURF genes, especially for the Solanums (Fig. 3C, fig. S24, data S14), indicating that upregulated non-syntenic genes have maintained or acquired features enabling their stress activation. Consistent with this, most highly expressed syntenic and non-syntenic SURF genes contained at least one of the four TF motifs recognized (80% rice, 80% M. truncatula, >70% Solanums) (Fig. 3C, fig. S24, data S15). Most TF motifs were coincident with THSs in rice and M. truncatula. Although the number of highly expressed but non-syntenic SURF genes was fewer than six in the Solanums, all from S. lycopersicum contained at least one motif. The four identified TF motifs are therefore a broadly conserved feature of both syntenic and non-syntenic submergence-responsive genes.
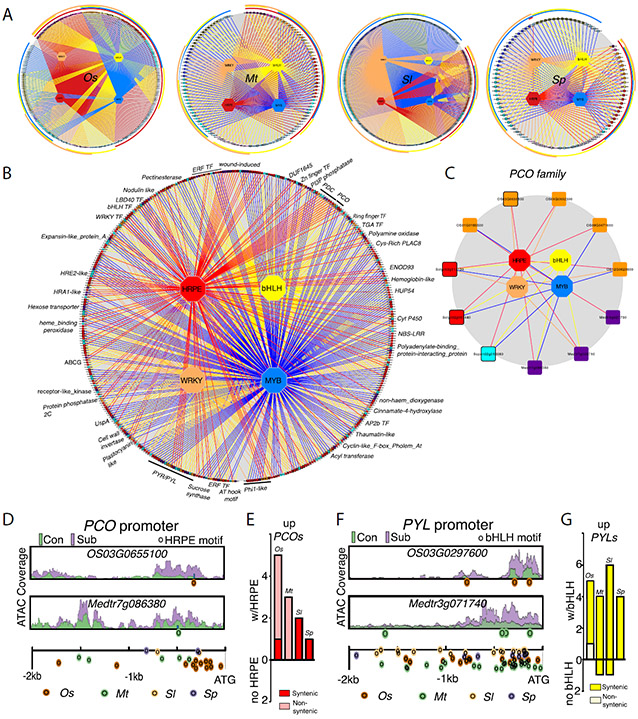
To appraise conservation in regulation across eudicots and monocots, we built networks that associate TF motif presence with each upregulated SURF gene for each species (Fig. 4A, figs. S25-S28, data S16). The individual species networks emphasize the presence of species-specific motif biases. The combinatorial nature of target gene regulation was also evident (overlapping outer circles of network) with over 70% of the genes with more than one of the four motifs. Syntenic upregulated SURF genes across the four species (represented with black borders) expose a single conserved putative regulatory network (Fig. 4B, fig. S29, data S16). This network illustrates conservation of TF motifs of syntelogs of responsive genes, in addition to the HRPE regulated by ERFVIIs.
Fig. 4. Conserved transcription factor motifs in SURFs and accompanying chromatin dynamics.
(A) Regulatory networks for upregulated SURF genes (expanded in figs. S25-S28). Hexagons, transcription factors (TFs); rectangles, genes; colored lines (edges), interactions of promoter and TF based on motif presence. Outer circles: genes grouped with shared motifs. Genes with black borders have a syntenic ortholog (rice to M. truncatula; M. truncatula to S. lycopersicum; and between Solanums). (B) Network for syntenic conserved SURF genes across species (expanded in fig. S29). Genes of alternating families have alternating grey or black borders. Families represented in three species are labeled. (C) Regulatory network of PLANT CYSTEINE OXIDASE (PCO) up-regulated genes. Syntenic orthologs have black borders. (D) and (F) Chromatin accessibility in promoters of syntenic PCO and PYL (PYRABACTIN RESISTANCE 1 (PYR1) / PYR1-LIKE (PYL) / REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) genes. ATAC coverage scale is the same for genes shown in each panel. Below: locations of HRPE or bHLH motifs for four species. (E) and (G) Number of upregulated genes containing motifs classified by syntenic and non-syntenic.
As oxygen levels decline below a threshold, constitutively synthesized ERFVIIs accumulate due to attenuation of their conversion into an N-degron for active turnover (1). The unified SURF network uncovered HRPE conservation across eudicots-monocots in promoters of genes essential to anaerobic metabolism and hypoxia survival including PLANT CYSTEINE OXIDASE (PCO) genes (Fig. 4C, fig. S30, data S17), which catalyze the oxygen-promoted degradation of ERFVIIs to temper the adaptive response (18). The upregulated SURF genes included ERFVIIs in all four species, with at least one with an HRPE motif, suggesting possible autoregulation (fig. S31).
The syntelog network also identified conservation of bHLH motif enrichment in genes not well associated with submergence (i.e., PYRABACTIN RESISTANCE 1 / PYR1-LIKE [PYL]) (Fig. 4B, fig. S32) and MYB motif enrichment in genes that contribute to hypoxia tolerance (14) (fig. S33-34). The upregulation of these genes often coincided with a TF motif within a region of submergence-enhanced chromatin accessibility (Fig. 4D-G, fig. S33), supporting functionality of the regulatory sequences. As for the ERFVIIs, the upregulated SURF genes included bHLH, MYB and WRKY family members (fig. S31).
Information from single genes is used in breeding or modifying crops for stress tolerance. The use of multi-scale gene regulatory information of gene families across flowering plant clades to infer regulatory networks demonstrates that conservation of flooding resilience mechanisms is complex and involves diverse regulatory mechanisms. Targeted manipulation of the four submergence-activated modules and seven SURF loci discovered here with the greatest interspecies conservation might be used to enhance flooding tolerance of susceptible crops.
Supplementary Material
Acknowledgements
We thank members of our labs Ralston Mataki, Sean Cabanlit, Elise Viox, Kelly Tran, Amin Addetia, Sonja Winte, Maureen Hummel, Travis Lee, Alex Mason and Hokuto Nakayama for support and discussions, Jérémie Bazin, Dan Koenig, Dan Kliebenstein, Timothy Bailey, Andrés Reynoso, Mike Covington and Sharon Gray for guidance.
Funding: Supported by United States National Science Foundation Plant Genome Research Program (IOS-1238243) to R.B.D., N.R.S., S.M.B. and J.B.-S., a Finnish Cultural Foundation fellowship to K.K. and an HHMI Faculty Scholar Fellowship to S.M.B.
Footnotes
Competing Interests: Authors declare no competing interests.
Data and material availability: Sequence data deposited in GEO (accession GSE128680), code and resources in http://plant-plasticity.github.io/data-and-code/. All other data are in the main paper or supplement.
Contacts for genetic materials: rice, J.B.-S.; Medicago, R.B.D.; tomato, S.B., S. pennellii, N.S..
References
- 1.Voesenek LACJ, Bailey-Serres J, Flood adaptive traits and processes: an overview. New Phytol. 206, 57–73 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J, Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J. 56, 743–755 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Sorenson R, Bailey-Serres J, Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 111, 2373–2378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juntawong P, Girke T, Bazin J, Bailey-Serres J, Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 111, E203–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deal RB, Henikoff S, The INTACT method for cell type–specific gene expression and chromatin profiling in Arabidopsis thaliana. Nat. Protoc 6, 56–68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustroph A et al. , Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 106, 18843–18848 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ, Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS, Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 324, 218–223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodstein DM et al. , Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klecker M et al. , A Shoot-Specific Hypoxic Response of Arabidopsis Sheds Light on the Role of the Phosphate-Responsive Transcription Factor PHOSPHATE STARVATION RESPONSE1. Plant Physiol. 165, 774–790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher KA et al. , Profiling of accessible chromatin regions across multiple plant species and cell types reveals common gene regulatory principles and new control modules. Plant Cell. 30, 15–36, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasch P et al. , Redundant ERF-VII Transcription Factors Bind to an Evolutionarily Conserved cis-Motif to Regulate Hypoxia-Responsive Gene Expression in Arabidopsis. Plant Cell. 28, 160–180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SC et al. , Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol. 190, 457–471 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Mustroph A et al. , Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 152, 1484–1500 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bargmann BOR et al. , TARGET: a transient transformation system for genome-wide transcription factor target discovery. Mol. Plant 6, 978–980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Para A et al. , Hit-and-run transcriptional control by bZIP1 mediates rapid nutrient signaling in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 111, 10371–10376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walley JW et al. , Integration of omic networks in a developmental atlas of maize. Science. 353, 814–818 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weits DA et al. , Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun 5, 3425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barba-Montoya J, dos Reis M, Schneider H, Donoghue PCJ, Yang Z, Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous Terrestrial Revolution. New Phytol. 218, 819–834 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynoso MA et al. , Nuclear transcriptomes at high resolution using retooled INTACT. Plant Physiol. 176, 270–281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao D et al. , Analysis of ribosome-associated mRNAs in rice Reveals the importance of transcript size and GC content in translation. G3 . 7, 203–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ron M et al. , Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol. 166, 455–469 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsley BT, Covington MF, Ichihashi Y, BrAD-seq: Breath Adapter Directional sequencing: a streamlined, ultra-simple and fast library preparation protocol for strand specific mRNA library construction. Front. Plant Sci 6, 366 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan JS, Reed A, Chen F, Stewart CN Jr, Statistical analysis of real-time PCR data. BMC Bioinformatics. 7, 85 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deal RB, Henikoff S, A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev. Cell 18, 1030–1040 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajic M, Maher KA, Deal RB, Identification of open chromatin regions in plant genomes using ATAC-seq. Methods Mol. Biol 1675, 183–201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynoso MA et al. , Nuclear transcriptomes at high resolution using retooled INTACT. Plant Physiol. 176, 270–281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynoso M et al. , Isolation of Nuclei in Tagged Cell Types (INTACT), RNA extraction and ribosomal RNA degradation to prepare material for RNA-seq. BIO-PROTOCOL. 8 (2018), doi: 10.21769/BioProtoc.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustroph A, Juntawong P, Bailey-Serres J, Isolation of plant polysomal mRNA by differential centrifugation and ribosome immunopurification methods. Methods Mol. Biol 553, 109–126 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Reynoso MA et al. , Translating Ribosome Affinity Purification (TRAP) followed by RNA sequencing technology (TRAP-SEQ) for quantitative assessment of plant translatomes. Methods Mol. Biol 1284, 185–207 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Juntawong P, Hummel M, Bazin J, Bailey-Serres J, Ribosome profiling: a tool for quantitative evaluation of dynamics in mRNA translation. Methods Mol. Biol 1284, 139–173 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Bazin J et al. , Global analysis of ribosome-associated noncoding RNAs unveils new modes of translational regulation. Proc. Natl. Acad. Sci. U. S. A 114, E10018–E10027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS, The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc 7, 1534–1550 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girke T, systemPipeR: NGS workflow and report generation environment. UC Riverside: https://github.com/tgirke/systemPipeR (2014) (available at http://www.bioconductor.org/packages/release/bioc/html/systemPipeR.html). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calviello L et al. , Detecting actively translated open reading frames in ribosome profiling data. Nat. Methods 13, 165–170 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Ritchie ME et al. , limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su S et al. , Glimma: interactive graphics for gene expression analysis. Bioinformatics. 33, 2050–2052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young MD, Wakefield MJ, Smyth GK, Oshlack A, Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenig D et al. , Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc. Natl. Acad. Sci. U. S. A 110, E2655–62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnhammer ELL, Östlund G, InParanoid 8: orthology analysis between 273 proteomes, mostly eukaryotic. Nucleic Acids Res. 43, D234–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons E, Freeling M, How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 53, 661–673 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Lyons E, Pedersen B, Kane J, Freeling M, The value of nonmodel genomes and an example using SynMap within CoGe to dissect the hexaploidy that predates the Rosids. Trop. Plant Biol 1, 181–190 (2008). [Google Scholar]
- 43.Langmead B, Salzberg SL, Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolger A et al. , The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat. Genet 46, 1034–1038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sijacic P, Bajic M, McKinney EC, Meagher RB, Deal RB, Changes in chromatin accessibility between Arabidopsis stem cells and mesophyll cells illuminate cell type-specific transcription factor networks. Plant J. 94, 215–231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H et al. , The Sequence Alignment/Map format and SAMtools. Bioinformatics. 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinz S et al. , Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinlan AR, Hall IM, BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anders S, Pyl PT, Huber W, HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramírez F et al. , deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salmon-Divon M, Dvinge H, Tammoja K, Bertone P, PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC Bioinformatics. 11, 415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey TL, Elkan C, Fitting a mixture model by expectation maximization to discover motifs in bipolymers (1994) (available at http://www.cs.toronto.edu/~brudno/csc2417_15/10.1.1.121.7056.pdf). [PubMed]
- 54.O’Malley RC et al. , Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell. 165, 1280–1292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weirauch MT et al. , Determination and inference of eukaryotic transcription factor sequence specificity. Cell. 158, 1431–1443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS, Quantifying similarity between motifs. Genome Biol. 8, R24 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLeay RC, Bailey TL, Motif Enrichment Analysis: a unified framework and an evaluation on ChIP data. BMC Bioinformatics. 11, 165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant CE, Bailey TL, Noble WS, FIMO: scanning for occurrences of a given motif. Bioinformatics. 27, 1017–1018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shannon P et al. , Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S, Stecher G, Tamura K, MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol 33, 1870–1874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynoso MA, Blanco FA, Bailey-Serres J, Crespi M, Zanetti ME, Selective recruitment of mRNAs and miRNAs to polyribosomes in response to rhizobia infection in Medicago truncatula. Plant J. 73, 289–301 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Gonzali S et al. , Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat Plants. 1, 15151 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Hartman S et al. , Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. bioRxiv (2019), p. 705194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schröder F, Lisso J, Müssig C, EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol. 156, 1620–1630 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibbs DJ et al. , Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature. 479, 415–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Licausi F et al. , HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J. 62, 302–315 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Giuntoli B et al. , A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in Arabidopsis. PLoS Biol. 12, e1001950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White MD et al. , Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nat. Commun 8, 14690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White MD, Kamps JJAG, East S, Taylor Kearney LJ, Flashman E, The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J. Biol. Chem 293, 11786–11795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pucciariello C, Parlanti S, Banti V, Novi G, Perata P, Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol. 159, 184–196 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeung E et al. , A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A 115, E6085–E6094 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR, Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol 61, 651–679 (2010). [DOI] [PubMed] [Google Scholar]
- 73.González-Guzmán M et al. , Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J. Exp. Bot 65, 4451–4464 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.